Abstract
The structure-related morphological characteristics and fractional composition of Al–2B composite powders obtained by mechanical alloying have been studied as depending on the physicochemical conditions of the process. Quantitative data on the morphology, microstructure, and particle size distribution functions of the composite powders have been obtained at different stages of their mechanical activation in different grinding fluids. The effects of surfactants added to the grinding fluids on the characteristics of the composite powders have been analyzed at different stages of their preparation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The use of the energy-saturated composite metal powders as reagents is a promising method of creating new functional materials for thermoreactive chemical processes. Such powders are commonly prepared by mechanical alloying, which comprises joint treatment of components in activating mills [1–7].
The Al–B system may be presented as an example of such a powder, which is known to be used for producing high-efficiency energy-saturated thermoreactive composites of this type. Preliminary studies of the thermodynamic and microstructural behaviors of such powders in the course of mechanical activation [8–11] have shown that they can be used as intermediate products for implementation of corresponding solid-state reactions [12].
The process of the synthesis of such composite system with the optimum thermoreactive characteristics must provide, in addition to a desired composition, the complete absence of intermetallides and products of interaction with components of a grinding fluid, the minimum boron content outside the composite granules, and a required set of the granulometric and microstructural parameters of the resulting powder [13]. However, the elaboration of such a process requires additional investigations. In particular, scientific problems are encountered in connection with revealing the relation between structure-related morphological, granulometric, and microstructural characteristics of a composite powder and synthesis conditions.
The evolution of the structure-related morphological characteristics and fractional composition of Al‒2B composite powders during mechanical alloying in a grinding fluid represented by pure hexane was studied in our previous work [14]. The goal of this work was to study the influence of the composition of a grinding fluid on the granulometric and microstructural characteristics of Al–2B composite powders at different regimes of their mechanosynthesis.
MATERIALS AND METHODS
Powders of ASD-4 aluminum (volume-average particle size of 20–30 μm) and B-99V amorphous boron (volume-average particle size of 2–4 μm) were used in the experiments. Pure hexane and solutions of oleic acid (2.5 wt %), paraffin (3 wt %), and turpentine (3 wt %) in hexane were used as grinding fluids. Mixtures of the powders preliminarily prepared with the stoichiometric composition were mechanically treated in an AGO-2U laboratory water-cooled planetary-type ball mill. Balls made of ShKh-15 alloy with a diameter of 6 mm and a total mass of 100 g served as grinding bodies. The mass of a mixed powder in an individual experiment was 10 g, the rate of cylinder rotation was 1062 rpm; and the time of the mechanical treatment of an individual powder sample was varied from 3 to 21 min.
The structural and morphological studies of the synthesized powder samples were carried out using Camebax and JSM-6460LA scanning electron microscopes equipped with attachments for energy-dispersive X-ray spectroscopic analysis.
The phase composition of the products was controlled by X-ray diffractometry with Empyrean and Stoe Humber G670 setups.
The granulometric characteristics of powdered intermediate products were determined with a Microtrac S3500 high-resolution liquid-phase laser diffractometer. To destroy loosely packed aggregates of microparticles in the intermediate products, corresponding powder samples were preliminarily subjected to ultrasonic vibrodispersion.
RESULTS AND DISCUSSION
Al–2B composite powders are synthesized in several stages. At the initial stage of the mechanical treatment of a mixture in any grinding fluid, micron and submicron solid boron particles are intensely incorporated into the near-surface layers of plastic aluminum particles, which have relatively larger sizes. At this stage, two competitive processes occur simultaneously: the fracture and cold welding of composite particles. Cold welding of powder particles dominates during the treatment for time t = 3–5 min. The composite particles, which, at this stage, consist mainly of aluminum and have the initial spherical shape, grow and are transformed into anisometric plates of an irregular shape. Their average size increases by several times. However, as the time of the mechanical treatment increases, the growth of the composite particles dramatically decelerates due to the fracture (fragmentation) of the largest ones. The shape of the fragments approaches the isometric one and is characterized by an acute-angled habit. This morphology of the fragments suggests the quasi-brittle fracture of composite particles at this stage of the mechanical treatment in all grinding fluids including those containing the surfactant additives.
After the mechanical treatment for 7–9 min, the relative content of boron in composite particles is stabilized independently of the composition of the grinding fluid; further, it remains unchanged, with the surface layers of the particles appearing to be noticeably supersaturated with boron. The content of residual free boron in the powder is low. This is indirectly confirmed by the absence of dust formation during the vibration treatment of dry samples, which is observed for submicron and micron powders of pure boron.
Figure 1 shows the electron micrographs taken using the characteristic X-ray radiation of aluminum and boron from the samples of the initial mixture and a composite powder (synthesis time of 7 min, 2.5% oleic acid solution in hexane as a grinding fluid).
According to the X-ray diffraction data, aluminum borides are almost absent in all studied samples. The content of oxide phases in the composite is several times higher than that in the initial mixture. The latter fact is explained by the oxidation of the composite in the course of sample preparation for the analysis and enhanced chemical activity of mechanically activated composite particles.
The total content of mechanical degradation products of grinding fluid components and the products of their interaction with aluminum and boron was determined from the weight fraction of carbon in the composite. When using solutions of paraffin and turpentine in hexane as grinding fluids, the carbon content in composite powder samples was as high as 8–10%. A much lower content of carbon was observed in samples mechanically activated in an oleic acid solution. After the mechanical treatment of the mixed powder in pure hexane, composite particles were almost free of carbon. In addition, the analysis of the X-ray diffraction data has shown the absence of crystalline carbide phases in the composite powders. Nevertheless, under these conditions, the possibility of the formation of carbides in the X-ray amorphous state cannot be excluded.
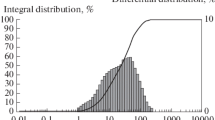
In all considered cases, the statistical properties of the ensemble of particles are, with a satisfactory accuracy, described by the superposition of two lognormal distributions reflecting fine and coarse fractions of the powder system. At the initial stages of the synthesis, the character of the evolution of the powder fractional composition is qualitatively the same for all used grinding fluids. After the mechanical treatment for time t = 7–9 min, the particle size distribution functions, which are initially bimodal due to the great difference between the dispersities of the initial boron and aluminum powders, approach unimodal ones. Therewith, the most probable sizes of the particles of the coarse and fine fractions become closer to each other. The subsequent evolution of the particle size distribution functions essentially depends on the grinding fluid composition. The aforementioned tendency remains preserved for the mechanical treatment of the mixed powder in pure hexane and paraffin and turpentine solutions. On the contrary, an opposite process occurs during the mechanical treatment of the system in the oleic acid solution, and the distributions acquire the bimodal character again (see Fig. 2).
It follows from the experimental data that the most probable size of the particles in the coarse fraction of the composite powder remains almost unchanged with time, in contrast to its weight factor and the half-width of its distribution. At the same time, the most probable size of the particles in the fine fraction markedly grows at the initial stage of the mechanical treatment; then, it decreases to about 2 μm.
The quantitative characteristics of the aforementioned distributions strongly depend on the grinding fluid composition. In particular, at mechanical treatment time t = 21 min, the volume-average particle size of the powder treated in hexane is 45 μm; in the paraffin and turpentine solutions, it is equal to 25 μm; and, in the oleic acid solution, it is 16 μm. Figure 3 illustrates the influence of the synthesis time and the fluid composition on the specific surface area of the composite powders.
The data obtained enable us to formulate the following scheme of the evolution of the fractional composition of the composite powder. In the course of the saturation with boron and the shear strengthening and hardening of the material, the plasticity of the composite particles decreases, while their fracture acquires a quasi-brittle character and markedly accelerates. The fracture of the composite particles occurs predominantly along interfaces. As a result, the saturation of the surface layers of the fragmented products with boron and a decrease in the rate of the parallel enlargement of the particles via their welding are observed. The stabilization of the volume-average size and component composition of the particles as a result of equilibrating the rates of fragmentation and cold welding occurs almost simultaneously. The strong influence of oleic acid added to the grinding fluid on the granulometric composition of the composite particles may be explained by the joint action of two factors, namely, the deceleration of welding due to the adsorption of amphiphilic oleic acid molecules on the surface of the particles and the acceleration of the particle fragmentation due to the adsorption-induced reduction in their strength (Rehbinder effect [15, 16]).
CONCLUSIONS
(1) The structure-related morphological characteristics and fractional composition of Al–2B composite powders synthesized by mechanical alloying at different physicochemical and time conditions of the process have been studied.
(2) It has been found that the synthesis of the composite has a pronounced two-stage character. At the first stage, solid finely dispersed boron particles are intensely incorporated into aluminum particles and composite particles grow. As the synthesis time increases, the main statistical characteristics of the particle size distribution functions vary nonmonotonically.
(3) The fractional composition of the composite powder results from the equilibration of the rates of particles fragmentation and their agglomeration via the “cold welding”. The relative intensities of these processes are controlled by the degree of saturation of the composite particle surface with boron and depend on the presence of surfactant additives in a grinding fluid.
REFERENCES
Suryanarayana, C., Mechanical Alloying and Milling, New York: Marcel Dekker, 2004.
Kuz’mich, Yu.V., Kolesnikova, I.G., Serba, V.I., and Freidin, B.M., Mekhanicheskoe legirovanie (Mechanical Alloying), Moscow: Nauka, 2005.
Grigor’eva, T.F., Baranova, A.P., and Lyakhov, N.Z., Mekhanokhimicheskii sintez v metallicheskikh sistemakh (Mechanochemical Synthesis in Metallic Systems), Novosibirsk: Parallel’, 2008.
High Energy Ball Milling Mechanochemical Processing of Nanoparticles, Sopicka-Lizer, M., Ed., Oxford-Cambridge-New Delhi: Woodhead, 2010.
James, S.L., Adams, C.J., Bolm, C.J., Braga, C., Collier, D., and Friscic, P., Chem. Soc. Rev., 2012, vol. 41, p. 413.
V Int. Conf. “Fundamental Bases of Mechanochemical Technologies”, FBMT-18. Book of Abstracts, Lyakhov, N., Šepelák, V., Shakhtshneider, T., and Dudina, D., Eds., Novosibirsk: IPC NSU, 2018.
Zoz, H., Ren, H., Reichardt, R., and Benz, H.U., Abstracts of Papers, Mechanical Alloying: Principle,Development and Current Activities (Part I−VII), Thermec2000, Int. Conf. on Processing & Manufacturing of Advanced Materials, Las Vegas, USA, 2000.
Mirković, D., Gröbner, J., Schmid-Fetzer, R., Fabrichnaya, O., and Lukas, H.L., J. Alloys Compd., 2004, vol. 384, p. 168.
Abenojar, J., Martinez, M.A., and Velasco, F., J. Alloys Compd., 2006, vol. 422, p. 67.
Ma, Y., Zhang, H., Zhao, F., Jiang, C., Wei, L., and Pei, C., Micro Nano Lett., 2014, vol. 9, p. 132.
Adila, S., Karatib, A., and Murty, B.S., Ceram. Int., 2018, vol. 44, p. 20105.
Arkhipov, V., Savel’eva, L., and Zolotorev, N., MATEC Web Conf., 2015, vol. 23, p. 01005.
Streletskii, A.N., Borunova, A.B., Kolbanev, I.V., Si-vak, M.V., and Dolgoborodov, A.Yu., Gorenie Vzryv, 2017, vol. 10, no. 2, p. 100.
Malkin, A.I., Klyuev, V.A., Ryazantseva, A.A., and Savenko, V.I., Colloid J., 2019, vol. 81, p. 695.
Encyclopedia of Materials Science and Engineering, Oxford, 1986, p. 1462.
Shchukin, E.D., Savenko, V.I., and Malkin, A.I., Lektsii po fiziko-khimicheskoi mekhanike (Lectures on Physicochemical Mechanics), Moscow: Nobel’ Press, 2015.
ACKNOWLEDGMENTS
The authors are grateful to the Center for Collective Use of the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, for providing the opportunity to use its equipment in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors declare that they have no conflict of interest.
Additional information
Translated by L. Tkachenko
Rights and permissions
About this article
Cite this article
Malkin, A.I., Aliev, A.D., Klyuev, V.A. et al. The Influence of Technological Medium Composition on the Structure-Related Morphological Characteristics of Al–2B Composite Powders. Colloid J 82, 403–407 (2020). https://doi.org/10.1134/S1061933X20040079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X20040079