Abstract
The aim of the present paper is to review the recent progress in the synthesis of in situ particle reinforced aluminum composites using thermal, mechanical and combined mechanical-thermal activation of aluminothermic reduction reactions. The combination of combustion synthesis (CS) and mechanosynthesis (MS) is the most recent development in the processing of advanced materials like micro and nano aluminum based composites. The combined mechanical thermal synthesis (MTS) has widened the possibilities for both CS and MS. MTS holds great potential for commercial viability and offers exciting processing route for the synthesis of advanced materials. Enhanced reaction kinetics and extended concentration limits in MTS are demonstrated by illustrating the synthesis of aluminum based nanocomposite involving Al–CeO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The possibility of taking the advantage of particular properties of the constituent materials to meet specific demands is the most important motivation for the development of composites. Metal matrix composites (MMCs) are the materials where rigid ceramic and/or intermetallic reinforcements are embedded in a ductile metal or alloy matrix. MMCs combine best properties of its constituent materials i.e. metallic properties (ductility and toughness) with ceramic or intermetallic characteristics (high strength and modulus). It is now well recognized that metal matrix composites (MMCs) have a high potential for advanced structural applications.
Aluminum is the most popular matrix for the metal matrix composites (MMCs). The Al alloys are quite attractive due to their low density, their capability to be strengthened by precipitation, their good corrosion resistance, their high thermal and electrical conductivity, and their high damping capacity. Aluminum matrix composites (AMCs) have been widely studied since the 1920s and in the 1980s, transportation industries began to develop discontinuously reinforced AMCs. They are very attractive for their isotropic mechanical properties (higher than their unreinforced alloys) and their low costs.
AMCs are finding more and more applications and their usage is increasing continuously over the past 25 years. This is due to the development and better understanding of different processing techniques resulting in reproducible microstructures and properties. Also the developments in the processing of nano to micron sized reinforcements attracted material scientists towards the processing of advanced AMCs. The discontinuously reinforced AMCs, specifically the particulate-reinforced AMCs, are of particular interest due to their isotropic nature and ease of fabrication [1–3].
Traditionally, the particulate-reinforced AMCs are produced by processing techniques such as powder metallurgy, preform infiltration, spray deposition and conventional melting casting route. In all the above techniques, prior to the composite fabrication, the reinforcement (usually in particulate form) is prepared and combined with the matrix material (either in molten or powder form). Thus, traditional AMCs can be viewed as ex situ AMCs. Ex situ particulate-reinforced AMCs are suffered with uneven distribution of reinforcement and non-wetting of the reinforcement and the matrix due to surface contamination of the reinforcements. To overcome the inherent problems that are associated with traditionally processed composites, a relatively new processing technique is developed, where the reinforcement is formed in situ by chemical reaction.
In situ composite is the material, where the reinforcing phase is formed within the parent phase by controlled melt growth, chemical reaction, transformation and deformation. The interface between matrix and reinforcement produced are relatively stable and impurity-free. Moreover, the in situ formed reinforcing particles are finer in size and their distribution in the matrix is more uniform resulting in better mechanical properties than traditionally processed ex situ composites. In situ processed composites are considered technologically more advanced materials and are finding widespread applications [4].
In heterogeneous systems like composites the properties are influenced by the chemical nature of the components, morphology of particles, their spatial distribution and interface interaction. The method for manufacturing in situ composites can play an important role in controlling the properties of resulting composites. In spite of a variety of methods available, processing of AMCs following these techniques is rather complicated. The researchers are continuously pursuing for a more effective and economical synthesis route for in situ AMCs, which has resulted in various synthesis routes in last several years. In situ particulate-reinforced AMCs can be processed by a large number of techniques which can be grouped on the basis of physical state of the reactant phases, namely liquid–liquid, liquid–solid and solid–solid reactions [1, 4].
Particulate-reinforced aluminum matrix composites (AlMCp) are considered for potential light-weight, high-performance applications in aircraft and more recently engine parts of automobiles. However, it is the economy of processing the composite that will decide the processing route. To lower the cost and to improve the properties of the composite, solid–solid reactions for the processing of in situ aluminum based composites involving aluminothermic reactions can be exploited. Here oxides such as CuO, NiO, ZnO, TiO2, and SiO2 as well as some ores are utilized to react in situ with the aluminum matrix to obtain the particle reinforcements. Such reinforcements possess much cleaner interfaces and thereby stronger binding strength with the matrix, compared with those added externally. However, such reactions \((\hbox{Al}+\hbox{MO}\rightarrow\hbox{Al}_{2}\hbox{O}_{3}+\hbox{M})\) are limited and the underlying thermodynamics and kinetics of reactions dictate the composition of the composites as well as processing variables [4].
High energy ball milling (HEBM) can increase the reactivity of the system, i.e., it can induce chemical reactions in variety of powder mixtures. The process is called mechanical alloying when used in metallurgical systems. Material processing based on mechanical alloying is widely used to prepare metastable phases, amorphous alloys, composites, and nanostructured materials [5, 6]. The mechanical and thermal activation (MTS) process for the synthesis of in situ composites using aluminothermic reactions represents one of the most exciting recent advancements and holds a great promise for commercial viability.
Keeping this in view, the present article provides a review on various solid–solid processing methods involving aluminothermic reactions for the synthesis of aluminum based in situ composites. Specifically emphasis is on the recent progress in in situ AlMCp synthesis based on mechanical activation approaches. Example of enhanced reaction rates and synthesis of in situ nanocomposite materials are provided to illustrate the progress brought about by the MTS process.
Many exothermic reactions are studied in both SHS and MS. However, a quantitative understanding of the effects of HEBM on the subsequent thermal processing (MTS) in reactions exhibiting moderate exothermicity is still warranted. For all the processing routes the ignition and propagation of aluminothermic reduction reactions for the synthesis of in situ aluminum based composites are governed by the thermodynamic principles of combustion synthesis (CS) reactions (SHS). Hence, the thermodynamics of SHS reactions is discussed in detail in the present review.
Synthesis routes (solid–solid interaction)
Aluminum based in situ composites involving aluminothermic reduction reactions are processed by
Thermal activation of chemical reactions (CS)
Combustion synthesis (CS) generally termed as, self propagating high temperature synthesis, is a useful technique to produce metal, ceramic and intermetallic composites taking advantage of the extreme heat generated by the exothermic reaction during the formation of some inorganic materials. The chemical energy of a reaction is converted to the thermal energy, when a compact of the constituent elemental powders is ignited. Once ignited, the combustion wave propagates and will be self sustaining due to a large amount of enthalpy release at the reaction front. Combustion is self sustaining when the reaction is highly exothermic and the rate of heat dissipation is less than the rate of heat generation. The advantages of CS technique include low energy requirement, the relative simplicity of the process and equipment, higher purity of the products, and low cost. Moreover, the high thermal gradients and rapid cooling rates during CS can produce metastable phases [8, 9].
An early application of CS is in the ‘thermite’ reduction of metal oxide powders with aluminum powder yielding either metal or an alloy of the metal and alumina following a reaction scheme: \(\hbox{Al}+\hbox{MO}\rightarrow\hbox{Al}_{2}\hbox{O}_{3}+\hbox{M}\) [10].
The following three steps are involved in almost all CS reactions resulting in synthesis of in situ composites.
-
Mixing of the reactant powders thoroughly using a mixture
-
Green compaction of the mixed powders in the pellet form
-
Initiation of the combustion
On the basis of initiation of combustion, combustion reaction can be conducted in two modes, namely self propagating combustion (SHS) and simultaneous combustion (SC) modes. In propagating combustion mode, combustion is initiated by the external source and combustion sweeps through the compact with a high velocity, provided the heat of reaction is sufficiently high. In contrast, in simultaneous combustion mode the combustion takes place simultaneously throughout by heating the compact to the ignition temperature of the exothermic reaction. Simultaneous combustion or thermal explosion is carried out when the heat of the reaction is moderate and the combustion temperature is low. Simultaneous combustion or thermal explosion mode of CS is the widely used for the synthesis of metal matrix composites. The propagating mode of CS is used for the intermetallic and ceramic matrix composites [8–11]. Figure 1 shows the schematic representation of a time temperature plot for propagating mode of a CS reaction.
Schematic representation of the temperature–time curve during a SHS reaction [8]. “Reprinted from Progress in Materials Science, Vol number 39, Combustion synthesis of advanced materials: Part 1. Reaction parameters John J. Moore, H. J. Feng, pp. 243–273, Copyright (Year) 1995, with permission from Elsevier”
Thermodynamics of CS reactions
SHS reaction
As shown in Fig. 1 the high combustion temperature associated with SHS reaction is a measure of exothermicity of the reaction. For a typical aluminothermic reaction
There are four important temperature points which can provide important thermodynamic information about the SHS reaction and they are
-
T 0: initial temperature of reactants and products
-
T ig: temperature at which SHS reaction is initiated in the propagation mode
-
T ad: maximum temperature of the combustion under adiabatic conditions
-
T c: maximum measured combustion temperature
Figure 2 shows the schematic representation of the enthalpy–temperature plot for reactants and products in a self propagating combustion reaction system that involves no phase changes in reactants and products. From the figure the following basic thermodynamic expressions can be derived.
Schematic representation of the enthalpy–temperature plot for reactants and products in a reaction system that involves no phase changes in reactants and products [8]. “Reprinted from Progress in Materials Science, Vol number 39, Combustion synthesis of advanced materials: Part 1. Reaction parameters John J. Moore, H. J. Feng, pp. 243–273, Copyright (Year) 1995, with permission from Elsevier”
H(R) is the amount of heat supplied to the reactants at T 0 (at constant pressure) for bringing them to the ignition temperature T ig and it is expressed as following:
The heat of reaction at (T ig) evolves due to the combustion and it is shown as following (considering T 0 = 298 K)
In SHS mode ΔH (T ig) is utilized in heating the reactants from T 0 to the T ig and products from T 0 to the T ad.
where
Substituting Eqs. 2, 3 and 5 in Eq. 4 we get the following equation:
By observing Eq. 4 and neglecting the effect of temperature on C p, one can establish a relationship between ΔH or and C p of the reaction. Thus, for a reaction to be self sustaining experimentally it is found that \([\Delta H/ C_{\rm p}]_{298} \ge 2,000\,\hbox{K}\) or \(T_{\rm ad}\ge 1,800\,\hbox{K}\) [12].
Simultaneous combustion reaction (SC)
Combustion synthesis of materials by simultaneous combustion reaction involves the heating of reactants in a furnace to the ignition temperature of the reaction. The following equation represents heating of the sample (representing Eq. 1) in a furnace neglecting the phase changes,
In SC the reaction heat is exclusively absorbed by the products as shown in Eq. 9. Comparing Eqs. 6 and 9, it is found that T ad is much higher in SC compared to that in SHS.
Combustion synthesis of in situ aluminum based composites
The CS, especially the SHS process, is extensively used for the production of intermetallic and ceramic matrix composites. The SHS processing of in situ AMCs is not feasible. However, reports on in situ processing of AMCs using SC are limited. The problems associated with CS of AMCs can be summarized as follows:
-
Combustion synthesis of in situ composites for the production of AMCs involving aluminothermic reduction reactions can be written as
$$ \hbox{Al} +\hbox{MO} + X \hbox{Al} \rightarrow X\hbox{Al} + \hbox{M}+ \hbox{Al}_{2}\hbox{O}_{3}$$(10)where X Al in the reaction is the matrix and M is in solution of aluminum or forming intermetallics. X Al in the reaction is the inert matrix acting as a diluent which may cause reduction in the adiabatic temperature of the reaction and results in the damping of combustion wave. Thus, the stoichiometry of the reactant mixture is an important parameter which significantly affects the combustion synthesis, specifically SHS reactions [8, 9].
-
Another problem is the generation of high fraction of the ceramic reinforcement, which is not always desirable [1].
-
Moreover, the structural imperfections of the materials produced through the SHS reaction, such as micro-porosity, lamination and cracking arising from (1) the rapid evolution of impurity gases or volatile reactants and/or products during the combustion reaction, (2) the change in volume that occurs between the reactants and products, (3) the porosity that is present in the green reactant mixture, e.g., 65% theoretical density, and/or (4) the diffusion of vacancies to produce micro pores make the process unreliable. These imperfections can get exacerbated by the existence of any non-uniformity present in the green compacts [8, 9].
Gotman et al. [13] are the first to report on the synthesis of in situ aluminum based composites following the SHS route. In their study, powder blends of Al–Ti–C, Al–Ti–B, Al–Ti–B4C, and Al–Ti–C–B are prepared by ball milling. Cylindrical compacts (32 mm diameter and 100 mm height) with about 36–38% theoretical density are placed in a reactor with thermally insulated walls. On top of each compact, 15 g of an igniting Ti–C mixture (1:1 mole ratio) is placed. The mixture is ignited by passing electric current through a tungsten wire. For all four compositions investigated, very low combustion velocities (1 mm/s) are observed. The propagation of the combustion wave is unstable, indicating that the rate of heat dissipation into the “inert” aluminum matrix is approaching the rate of heat generation. The non-steady-state oscillatory motion of the wave during combustion results in a typical layered structure of the reaction products. The heat required to maintain the propagation front of these reactions with the formation of 30 vol% TiC and/or TiB2, appears to be close to the lower limit required for the self-sustaining synthesis process.
Wang and Shi [14] are the first to report on the synthesis of in situ aluminum based composites following simultaneous combustion route involving aluminothermic reduction reaction. They have studied Al + TiO2 system with 20 and 25 vol% of TiO2 additions to aluminum to produce alumina particle reinforced aluminum matrix composites. The powders are mixed, cold pressed at 1,000 MPa and then heated to different temperatures between 400 °C and 800 °C, under flowing argon. Differential scanning calorimetric (DSC) experiments on powder blends show exotherms corresponding to the reduction of titanium from its oxide and formation of an intermetallic with aluminum. For the sample with 25 vol% of TiO2 the exothermic maximum appears at 882 °C and for the sample with 20 vol% of TiO2 the exotherm appeared at 917 °C. It is also reported that with a decrease in the TiO2 the exotherm is shifting towards the high temperature due to the diluting effect of aluminum. The in situ synthesis of metal matrix composites in the Al–TiO2 system can be written as following:
Peng et al. [15] have reported on the synthesis of in situ aluminum based composites following simultaneous combustion route involving aluminothermic reduction reaction. In their experiments, TiO2 (35 wt%)/Al bulk material has been prepared by squeeze casting method using anatase TiO2 powder and pure aluminum ingot as the raw materials. Subsequently, the squeeze-cast TiO2/Al bulk material has been heat treated according to the differential thermal analysis (DTA) results; the reaction between TiO2 and aluminum occurs to form the final Al3Ti–A12O3–Al in situ composite. They have also reported that the interfaces in the in situ composite are atomically flat, smooth, and free of any interfacial phases resulting in the good interfacial bonding between matrix and the reinforcements.
Huang et al. [16] have prepared in situ reinforced AMCs following a novel modified simultaneous combustion technique. In their experiments Al + CuO and Al + SiO2 powder blends with excess aluminum ranging from 0 to 16 mol are mixed in a mortar and cold pressed in a steel die. The compacts are dried after wrapping in an aluminum foil in an oven at 333 K. Pure aluminum in liquid state at 1173 K is held in a graphite crucible and compacts are introduced in to the melt at regular intervals. Indication of in situ reaction is observed by a dazzling light emanating from the surface of the melt. Compacts are introduced in to the melt one by one and when the reactions are over the melt is stirred, degassed and cast in to molds. Thermodynamic analysis is also reported for the reaction which is shown below:
The higher is the value of X, the lower is the heat Q released from the reaction (12). When the value of X is too high, the compact in molten aluminum will not explode and remain as compact that will undergo a reactive sintering process. In this study, a different amount of diluent aluminum is mixed with the reactants. Then the reaction is observed to be triggered and then complete in less than 1 s. Thus the reaction can be roughly regarded as an adiabatic system. From the calculation, the influence of the additional aluminum as diluent on the adiabatic temperature of reaction (12) is shown in Fig. 3.
Effect of diluent amount x on adiabatic temperature Tad of the reaction, theoretical calculation [16]. “Reprinted from Materials Science and Engineering A351, Study on the fabrication of Al matrix composites strengthened by combined in situ alumina particle and in situ alloying elements, Zan-Jun Huang, Bin Yang, Hua Cui, Ji-Shan Zhang, pp 15–22, 2003, with permission from Elsevier”
Mechanical activation of chemical reactions (MS)
The mechanochemistry/mechanochemical synthesis (MS) utilizes the mechanical energy to activate chemical reactions and structural changes [5, 17]. Recently, the aluminothermic reduction reactions induced by the high-energy ball milling (HEBM) are gaining importance because of the potential applications like the synthesis of microcrystalline and nanocrystalline in situ MMCs [17, 18]. The mechanical activation of the chemical reactions by the HEBM often changes the reaction mechanism and produces unique metastable materials, which cannot be prepared by the conventional techniques [19]. Also, they are nanocrystalline in nature and consequently they exhibit better properties and performance over their conventional coarse-grained counterparts.
The HEBM (also known as mechanical alloying when alloying takes place between the constituent materials) involves the repeated cold welding, fracturing and rewelding of powder particles [20]. The process of the HEBM starts with the preparation of a powder blend with an appropriate proportion of powders charged in the grinding bowels, called vials, with the grinding medium. The powder blend is milled for the desired length of time until the composition of each powder particle is same as the proportion of initial powder mix. The important components of HEBM are raw materials, the milling device, and the milling parameters. The HEBM is carried out in the planetary mills or vibratory mills. The mechanical activation of powders in the mills takes place by a shearing action and/or impact of high velocity balls with powder [5, 21]. The kinetics of chemical reactions induced by HEBM depends significantly on the mechanical energy transferred to the powder blend during milling. The energy transfer is mainly affected by milling parameters such as type of mill, milling speed, type of milling media, ball to powder ratio, dry or wet milling and duration of milling.
In most of the solid–solid chemical reactions, the product phases are formed at the interfaces of the reactants. The rate controlling step in these solid–solid reactions is the diffusion of the reactants through the product phase and is therefore dependant on the initial particle size, temperature and product morphology. In thermally activated reactions the reactants are spatially separated during the course of the reaction. Mechanosynthesis can substantially increase the reaction rate by the repeated welding and fracturing of powder particles allowing fresh surfaces to come into contact repeatedly. This allows the reaction to proceed without the necessity for the diffusion through the product layer. Also, the high defect densities induced by mechanical activation greatly enhances the diffusion rates. As a result, reactions that normally require high temperatures will take place at lower temperatures during the HEBM without any externally applied heat, thus enabling the retention of nanostructure [22]. Several MS reactions involving displacement reactions between a reactive metal like aluminum and metal oxide are reported to prepare in situ microcrystalline and nanocrystalline composite materials [18, 22]. Milling introduces lattice defects, where chemical reactions progress faster, as well as follow unusual routes and yield unexpected products [23–26].
Depending on the milling conditions and enthalpy of reaction, the following different reaction kinetics is possible:
-
1.
Progressive reaction: The reaction may extend to a very small volume during each collision, resulting in a gradual transformation.
-
2.
Combustion reaction: A self-propagating combustion reaction is initiated after a definite amount of milling.
The self-propagating combustion reaction is ignited during high-energy ball milling when the reaction enthalpy is sufficiently high. Combustion type reactions require a critical mechanical energy (milling time) for the combustion reaction to be initiated. Combustion type reactions in most of the cases produce coarse product particles owing to the rapid evolution of reaction heat [23–26]. A progressive reaction, where gradual transformation kinetics is observed, is the most likely to occur in the synthesis of AMCps involving aluminothermic reactions. The combustion reactions can be converted to the progressive type reactions by adding the diluent powders to the reactants. AMCs are synthesized by mechanosynthesis following the reaction scheme
Excess aluminum (X Al) is helpful in converting the reaction from a combustion to a progressive reaction. This is also helpful in retaining the nanostructure of the product phases which is the inherent nature of the mechanosynthesis.
Thermodynamic considerations
Aluminothermic reduction reactions between aluminum powder and the oxide(s) of a less reactive metal(s) are the first solid-state combustion reactions and the most widely investigated processes in mechanosynthesis. The general equation representing an oxide–metal displacement reaction involving aluminum as reductant is shown below
The reaction is thermodynamically favored in the indicated direction, if the heat of formation (per oxygen atom) is larger for the oxide of aluminum than for the oxide of M resulting the reaction exothermic. The mechanosynthesis reaction may progress if the reaction is highly exothermic. However the progress of the reaction in the indicated direction is greatly affected by the specific heat of the product phases and the milling conditions [27]. The metal oxide that can be reduced to metal using aluminum as reductant during aluminothermic reaction is selected on the basis of the thermodynamic principle of CS as discussed in the previous section. Reactions of the type (14) may proceed in the indicated direction during CS provided that for the reaction \([\Delta H/C_{\rm p}]_{298} \ge 2,000\,\hbox{K}\) or \(T_{\rm ad} \ge 1,800\,\hbox{K}.\) In contrast, the minimum T ad required for the mechanosynthesis is reduced to approximately 1,300 K during milling [28, 29]. A reduction of T ad from 1,800 K to 1,300 K during the mechanical activation is quite considerable. This reduction in T ad by mechanical activation broadens the scope of reactions in the sense that moderately high exothermic powder mixers can be processed i.e., reactions which are not possible by conventional routes like CS can be processed by mechanical activation. Also, the reduction in T ad from 1,800 K to 1,300 K during mechanosynthesis for the processing of in situ composites is helpful in increasing the amount of excess aluminum in highly exothermic reactions, which is required to form the matrix phase in AMCs (Table 2).
For a reaction of the type (14) [28]
Since the ball milling involves only solid state reactions,
HEBM process parameters
The mechanical activation of reactants participating in a reaction is effected by the HEBM process parameters, e.g., the milling temperature, grinding ball diameter, ball to powder ratio, use of a process control agent, and relative proportion of the reactants [5, 21, 22]. The process parameters like the process control agent, ball to powder ratio, milling media and relative proportion of reactants are extensively studied with reference to the mechanosynthesis reactions.
Process control agent (PCA)
The HEBM process essentially involves two processes namely cold welding between particles and fracturing of cold welded particles under a high energy collision. The optimum balance between the cold welding and fracturing is essential for the successful solid-state reactions during the HEBM. The cold welding as well as the fracturing are essential to keep the particles together (welding) with clean interfaces generated during milling (fracturing) resulting in a reduced diffusion distance. However, in most material systems this balance is controlled by the addition of a surfactant called process control agent. Thus PCA is added to achieve an optimum balance between the welding and fracture. In the case of ductile materials like aluminum the PCA addition is required to prevent an excessive welding as well as to prevent the oxidation. The PCAs are mostly organic materials like stearic acid, methanol or toluene [30]. The addition of PCA in the case of the mechanosynthesis reactions has the following effects:
-
PCA acts like diluent which either delays or completely suppresses the reaction.
-
Also the PCA inhibits welding process resulting in reduced kinetics.
Lu et al. [30] have reported that the milling in the presence of the PCA can drastically effect the solid state reactions in the Al–Mg system. In their study the extent of the formation of Al3Mg2 during milling of elemental powders of Al and Mg as a function of amount of PCA after 5 h of milling is reported. They found that the amount of the PCA may strongly affect the formation of the new compound. The reaction kinetics is improved when the amount of the PCA is less.
Das et al. [31] studied CuO + Al system for the synthesis of copper based composites with in situ Al2O3 as reinforcement. They have conducted a dry milling (without PCA) and a wet milling (with toluene as the PCA) and compared the reaction kinetics of mechanosynthesis reactions. The results are quite interesting that the mechanosynthesis reaction is suppressed between CuO and Al in presence of toluene even after an extended duration of the mechanical activation. Also, it is reported that the mechanosynthesis reaction is completed within 1 h in the case of dry milling. These findings are extremely useful in evaluating the effects of PCA on the mechanochemical reaction between CuO and Al as presented in Table 3.
Jain et al. [32] reported the same pattern of results for the Fe2O3 + Al system for the synthesis of iron based composites with the in situ Al2O3 as a reinforcement for the magnetic applications. In their study the stoichiometric mixture of Fe2O3 and aluminum powder blend, for the potential reaction \(\hbox{Fe}_{2}\hbox{O}_{3} + 2\hbox{Al} \rightarrow 2 \hbox{Fe} + \hbox{Al}_{2}\hbox{O}_{3},\) are milled with and without toluene. During wet milling the reduction reaction started after 4 h and completed within 25 h. However, during the dry milling the same reaction is completed within few minutes. It is important to note that nanostructured composite is produced only during the wet milling of the reactants.
Relative proportion of the reactants
In the mechanical activation of the chemical reactions involving a metal oxide and aluminum as reductant, the composition of the starting mixture corresponds to the stoichiometry of the desired reaction. However, about 10–15% stoichiometric excess of the reductant has been used in some of the investigations conducted to compensate for the surface oxidation of the reactive reductant powder particles, e.g., aluminum [5]. Some reports claim that for reaching the completion of the reaction it requires an addition of 25–30% stoichiometric excess of reductant [33]. What ever may be the reason for which the excess reductant is added, the addition of excess reductant results in increasing the heat capacity without contributing to the reaction heat resulting in the decrease of T ad. If the other conditions are similar, a reaction requires longer activation time before ignition.
There are very few investigations which discuss the effect of off-stoichiometry on the reaction. Takacs et al. [34, 35] studied a dry milling route for the \(\hbox{Fe}_{3}\hbox{O}_{4} + \hbox{Al}\) system: following the reaction scheme
The effect of off-stoichiometry has been studied by milling mixtures corresponding to \(3\hbox{Fe}_{3}\hbox{O}_{4} + X\hbox{Al},\) where \(2 \le X \le 16\) . The stoichiometric mixture gets ignited after an activation period of 10 min. Off-stoichiometry delays the ignition or completely suppresses the combustive reaction. The results are summarized in Table 4.
Thus, off-stoichiometry prolongs the ignition stage of the reaction and influences the reaction products. Also it is observed that the combustion type reaction is transformed to a progressive type reaction with an increase in the excess reductant.
Ball to powder ratio and milling media
The time required for the reduction reaction to be completed decreases with an increase in the ball-to-powder weight ratio (BPR). The BPR is always proportional to the rate of specific energy input. The inverse proportionality between the charge ratio and the ignition time is confirmed by Schaffer and McCormick for the reduction of CuO with Fe. But Takacs et al. have reported that it is difficult to initiate a self-sustaining reaction if the amount of powder is very small for the same material system [22]. When the milling is carried out with a different milling media, the time required for the initiation and completion of reaction get changed. Different milling media (vial and balls) are available like alumina, zirconia, steel, cemented WC in the order of increasing density. It is generally observed that with an increase in the density of the milling media, the intensity of the milling increases reducing the time for the initiation and the completion of the reactions. However, the reaction front gets extinguished by the heat loss to the milling media. Therefore, the degree of milling is not only dependent on the density of the milling medium (vial and balls), but also the weight and specific heat of the milling medium. However, the BPR and type of milling media require further studies.
Mechanosynthesis of in situ aluminum based composites
Mechanically activated aluminothermic reduction reaction for the synthesis of the in situ AMCps has number of advantages. During Mechanosynthesis of the in situ aluminum based composites involving aluminum and a metal oxide, the metal oxide is directly reduced to metal/alloy forming in situ ceramic reinforcements without first converting the metal oxide to their respective metals. Moreover, the formation of the nanostructure resulting in the formation of the nanocomposites during mechanosynthesis is an added advantage resulting in attractive properties. The HEBM is considered as methods for producing materials with unique microstructures. Analogous in some ways to rapid solidification processing, the HEBM offers a non-equilibrium method at a cost which is low relative to some other processing techniques. The Material processing may take place at room temperature which can have advantages over high temperature synthesis, in particular for synthesis of composites with nanostructure [18].
However, several disadvantages are evident for mechanosynthesis as a processing method for the production of in situ AMCs [36, 37]:
Low productivity: Low productivity of the high energy ball mills, which makes it difficult to introduce mechanochemistry as a large scale technology. The advent of horizontal attritors with different types of milling media in recent years is able to overcome this problem to a certain extent.
Powder contamination: The contamination from the milling media and reaction of the milled products with the atmosphere in which synthesis is performed are quite common. The contamination from the reaction with the atmosphere can be avoided or minimized by milling in a high purity inert atmospheres or vacuum. The Powder handling after milling must also be performed under an inert atmosphere until the consolidation is completed.
Decomposition of the surfactant: The decomposition of the organic surfactants during milling or on subsequent thermal mechanical treatment can contaminate the powder (e.g., carbon).
Large thermal mass: The reaction front gets cooled down due to the increase in the thermal mass, i.e., milling media and the PCA. An increase in the thermal mass can effectively suppress a reaction with a large exothermicity.
Limited science content: The science base for HEBM is quite limited [36]. Although it is known that the technique works and therefore it is useful, no one is very clear about how and why the technique works. This is because the HEBM is a complex, stochastic process and the number of variables involved is quite high. These include the type of the mill, size, shape, and weight of the grinding medium, velocity, angle and frequency of media impacts, ball-to-powder weight ratio, milling atmosphere, purity, size, shape, and hardness of the powder particles, milling time, milling temperature, and type and amount of the PCA.
Relative proportion of reactants: An addition of stoichiometric excess aluminum is required for the synthesis of the AMCs using MS. Amount of excess aluminum not only suppresses the reaction, but also modifies the reaction route complicating the understanding of the process.
The forerunner of mechanical activation of aluminothermic reactions for the synthesis of in situ AMCs is the investigation by Wu et al. on the aluminum and CuO system [38]. In their study pure aluminum and CuO powders are mixed to give powder blends of compositions Al + 5% CuO and Al + 10% CuO. Mechanical activation is performed in a ball mill (QM-1SP planetary ball mill) under argon atmosphere using hardened steel vials with 250:1 ball to powder ratio. The solid state PCA, i.e., 1 wt% of stearic acid is used for controlling the agglomeration of particles (Fig. 4).
The extent of formation of \(\hbox{Al}_{3}\hbox{Mg}_{2}\) as a function of the PCA after 5 h of MA [30]. “Journal of Alloys and Compounds 290, Li Lu, Y.F. Zhang, Influence of process control agent on interdiffusion between Al and Mg during mechanical alloying, pp 279–283, 1999, with permission from Elsevier”
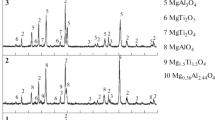
Figure 5 shows the XRD patterns of the Al–10CuO powder blend activated for different times. It is observed that the intensities of the CuO peaks decrease with increase in milling time (as illustrated in Fig. 5a, b). After 15 h of milling, the CuO peaks get vanished and only the Al peaks are detected (Fig. 5c). Increasing the milling time to 17 h, new peaks corresponding to \(\hbox{Al}_{4}\hbox{Cu}_{9}\) have appeared (Fig. 5d), and their intensity has increased on further milling to 56 h (Fig. 5e). No changes are found on further milling up to 115 h. The absence of an exothermic peak corresponding to the reaction between aluminum and CuO in the DTA heating trace of the powder mixture after 56 h of ball milling suggests that the reaction is completed within this period.
XRD patterns of the Al–10CuO mixed powders after different milling time [38]. “Reprinted from Journal of Alloys and Compounds 299, J.M. Wu, Z.Z. Li, Nanostructured composite obtained by mechanically driven reduction reaction of CuO and Al powder mixture, pp 9–16, 2000, with permission from Elsevier”
Considering the phase evolution of the Al–10CuO mixed powders (Fig. 5), the following reaction scheme can be suggested: \(2\hbox{Al} + 3\hbox{CuO}\cdots\hbox{Cu}\cdots\hbox{O}\cdots2\hbox{Al} \rightarrow 3\hbox{Cu} + \hbox{Al}_{2}\hbox{O}_{3}.\) During the initial stages of ball milling, CuO particles are dispersed in the Al matrix and are refined to 10–50 nm size by further milling, and thus increasing the contact area of the CuO and Al reactants. Large quantities of grain boundaries induced by ball milling and defects in the boundary of CuO and Al provide short-circuit diffusion paths for the atoms or ions. The product layers and the reactants are separated dynamically, minimizing the deleterious effects of the product barriers. The peaks corresponding to \(\hbox{Al}_{2}\hbox{O}_{3}\) are not detected in XRD patterns which may due to its amorphous nature or low concentration (about 3 wt%) to be detected by XRD.
Shengqi et al. [39] have also reported on the Al + CuO system without the addition of a PCA. In their study pure aluminum and CuO powders are mixed to give powder blends of compositions with aluminum weight percentage of 20, 35, 57, 70 and 85. As the amount of Al increases, along with the reduction reaction, the synthesis reaction occurs simultaneously. The reaction products are \(\hbox{Cu} + \hbox{Al}_{2}\hbox{O}_{3},\) \(\hbox{Cu}_{9} \hbox{Al}_{4}+ \hbox{Al}_{2}\hbox{O}_{3},\) \(\hbox{Al}_{2}\hbox{Cu} + \hbox{Al}_{2}\hbox{O}_{3}\) or \(\hbox{Al (Cu)} + \hbox{Al}_{2}\hbox{O}_{3}\), respectively, as the aluminum content is increased. The change in the reaction scheme and the reaction products with an increase in the amount of aluminum is also reported by Takacs et al. in \(\hbox{Fe}_{3}\hbox{O}_{4} + \hbox{Al}\) system following a dry milling route [34]. Their results are summarized in Table 4.
Mechanical–thermal activation of chemical reactions
Recently, the combination of mechanical activation (HEBM) and thermal activation (CS) of chemical reactions, i.e., aluminothermic reduction for the synthesis of the in situ AMCps have widened the possibilities for both the methods [40]. The combined mechanical thermal synthesis (MTS) HEBM, i.e., mechanical activation of reactions is used as an intermediate step to enhance the kinetics of a reaction during subsequent thermal treatment. It is observed that the mechanical activation induced by a HEBM can substantially reduce the reaction temperature required for completing the reactions. Also, the potential limitation of HEBM, e.g., contamination effects, can be minimized if the time for intensive mechanical activation is sufficiently short, and this is possible when the mechanocomposites are used as precursors for the traditional synthesis methods [40, 41]. The productivity of a ball mill can be increased sufficiently by reducing the activation time during the HEBM. In this way all the advantages of the mechanochemical approach are conserved while the limitations may be reduced significantly.
In the subject of our interest, i.e., synthesis of AMCps combined MTS, the milling of the reactants for short time followed by a thermal treatment can minimize the dilution effects arising from the addition of stoichiometric excess aluminum. Also it is observed from the previous discussion, a highly exothermic reaction can also be suppressed during milling due to an increase in thermal mass, i.e., the milling media and PCA. Therefore, the synthesis of in situ AMCs by either the CS or MS is a difficult task. The combination of mechanical thermal activation is a viable route for the synthesis of in situ AMCs using aluminothermic reduction reactions.
A new term, the mechanical and thermal synthesis (MTS) process, is catching up recently to describe the aforementioned new process and to reflect the integration of MTS in synthesizing desired AMCps. Furthermore, the powder product from the MTS process is nanostructured rather than the coarse-grained particles [42, 43].
The MTS process consists of the following steps:
-
Mechanical activation of the reactants (aluminum and metal oxide) at room temperature by the HEBM to produce the mechanocomposites consisting of aluminum and metal oxide.
-
Thermal activation of the mechanocomposites by a conventional heating process to initiate and complete the reduction reaction.
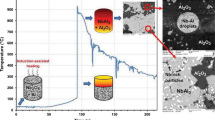
Mechanical–thermal synthesis of Al–\(\hbox{Ce/Al}_{2}\hbox{O}_{3}\) nanocomposites [44, 45]
Al-24 wt% CeO2 has been subjected to MTS with a view to synthesize in situ Al–\(\hbox{Ce/Al}_{2}\hbox{O}_{3}\) nanocomposite. The chemical reaction involved here can be expressed as following:
A powder blend of ceria (Alfa Aesar, \( < 1.0\,\mu\hbox{m},\) 99.5%) and aluminum (Loba Chemie \( < 20\,\mu\hbox{m},\) 99.7%) has been prepared in such a way that the composite with 9 wt% contribution of alumina in Al-20 wt% of cerium matrix would be expected if reaction (1) is followed. The blend has been subjected to the HEBM in a Fritsch Pulverisette P5 planetary ball mill at a speed of 300 rpm using hardened chrome steel grinding media with 10 mm diameter balls and ball to powder ratio of 10:1. Toluene has been used as a process control agent to avoid /minimize oxidation of the reaction product(s) by the mill atmosphere. The phase evolution at different stages of milling has been studied by X-ray diffraction. Simultaneous differential thermal analysis and thermogravimetric (DTA/TG) measurements have been carried out within the temperature range of 50–1,200 °C using a heating rate of 10 °C/min. Scanning electron microscopy (SEM) observations of the powder particle size, morphology and elemental mapping using the EDS have been performed.
The XRD results after 0 and 100 h of milling are shown in Fig. 6. Finally, after 100 h of milling there is hardly any evidence of reaction, since only peaks for aluminum and ceria are present. Reduction reaction by the mechanical activation is not expected since the calculated enthalpy of stoichiometric reaction between ceria and aluminum is ΔH r = −44.2 kJ/mol of \(\hbox{Al}_{2}\hbox{O}_{3}\) and [\(\Delta H/C_{\rm p}]_{298} = 301\,\hbox{K}.\) Moreover, diluting effect of aluminum further dampens the chances of reduction reaction during mechanical activation. However, powders milled for longer times (>30 h) show some magnetic particles, possibly iron, coming from milling medium.
When the mechanically activated powders are subjected to a thermal treatment, there is, in general, a decrease in the reaction onset temperature. The lowering of the reaction temperature in the case of prior ball milling may be due to (i) an establishment of good interfacial contact between the reactants, (ii) a refinement of the crystallite size and strain to enhance the reactivity of the components, and (iii) a decrease in the particle size. Also, an increase in the defect density is one of the key factors in modifying the diffusion path. The diffusion along/through the defects is prominent. The differential thermal analysis (DTA) is a useful tool in studying the effect of mechanical activation on subsequent thermal processing. The DTA traces are shown in Fig. 7. There are no thermal events below 500 °C for any extent of prior milling. Clearly the major enhancement in the reaction is achieved within 5 h of milling and the reaction turns to be a solid-state reaction after 30 h of milling, which can be observed in Fig. 8.
In order to study the response to the thermal treatment after the mechanical activation, the milled powders are heated to 650 °C. Figure 9 compares the XRD patterns for 0 and 50 h powder with the heat treated 50 h milled samples. XRD pattern for the heat treated 50 h milled samples shows the peaks for elemental cerium and intermetallic compounds like \(\hbox{Al}_{11}\hbox{Ce}_{3}\) and Al4Ce, as shown in Fig. 10. They can be attributed to the reduction reaction.
Conclusions
Synthesis of in situ aluminum based composites by the aluminothermic reduction reactions between aluminum and metal oxide requires stoichiometric excess aluminum. Excess aluminum often suppresses the reactions during the CS and MS. Our work on Al + CeO2 system shows that a combination of thermal activation with mechanical activation is quite attractive for the technological applications. The combined mechanical and then thermal activations facilitates a reduction reaction, even in the concentration region where the combustion synthesis and mechanosynthesis never observed. The mechanical–thermal synthesis broadens the scope of metal oxides with aluminum for the synthesis of s variety of in situ composites.
Finally the combination of MTS can overcome the shortcomings of the CS and mechanosynthesis as individual processes and their advantages can be extended for the synthesis of the micro/nano composites.
References
Tjong SC, Ma ZY (2000) Mater Sci Eng 29:49
Girot FA, Quenisset JM, Naslain R (1987) Compos Sci Technol 30:155
Torralba JM, da Costa CE, Velasco F (2003) J Mater Process Technol 133:203
Daniel BSS, Murthy VSR, Murty GS (1997) J Mater Process Technol 68:132
Suryanarayana C (2001) Prog Mater Sci 46:1
Thostenson ET, Li C, Chou T-W (2005) Compos Sci Technol 65:491
Gale WF, Tottemeier TC (eds) (2003) Smithells metals reference book, 8th edn. Elseiver, Butterworth, USA
Moore JJ, Feng HJ (1995) Prog Mater Sci 39:243
Moore JJ, Feng HJ (1995) Prog Mater Sci 39:275
Merzhanov G (1995) Ceram Int 21:371
Patil KC, Aruna ST, Mimani T (2002) Curr Opin Solid State Mater Sci 6:507
Zhu P, Li JCM, Liu CT (2003) Mater Sci Eng A 357:248
Gotman I, Koczak MJ, Shtessel E (1994) Mater Sci Eng A 187:189
Wang D, Shi Z (2004) J Adv Mater 36:56
Peng HX, Wang DZ, Geng L, Yao CK (1997) Scripta Mater 37(2):199
Huang Z-J, Yang B, Cui H, Zhang J-S (2003) Mater Sci Eng A 351:15
Zhang DL (2004) Prog Mater Sci 49:537
Matteazzi P, Le Caer G (1992) J Am Ceram Soc 75:2749
Shingu PH, Ishihara KN (1995) JIM 36:96
Prabhu B, Suryanarayana C, An L, Vaidyanathan R (2006) Mater Sci Eng A 425:192
Murthy BS, Ranganathan S (1998) Int Mater Rev 43:101
Takacs L (2002) Prog Mater Sci 47:355
(Sam) Froes FH, Trindade B (2004) J Mater Sci 39:5019
Cocco G, Mulas G, Schiffini L (1995) JIM 36:150
McCormick PG (1995) JIM 36:161
Nagumo M (1995) JIM 36:170
Schaffer GB, McCormick PG (1989) Scripta Metall 23:835
Schaffer GB, McCormick PG (1990) Metall Trans 21A:2789
Botta FWJ, Tomasi R, Pallone EMJA, Yavari AR (2001) Scripta Mater 44:1735
Lu L, Zhang YF (1999) J Alloys Compd 290:279
Das D, Samntha A, Chattopadhyay PP (2006) Development of bulk nano-Al2O3 dispersed Cu-matrix composite using ball milled precursor. ICAMMP, IIT-Kharagpur, India
Jain M et al (2004) Synthesis of Fe-Al2O3 nanocomposite through reactive milling. ISAMAP, IIT-Kharagpur, India
Venugopal T, Prasad Rao K, Murty BS (2005) Mater Sci Eng A 393:382
Takacs L (1992) Mater Lett I3:119
Takacs L (1993) Nanostructured Mater 2:241
Ivanov E, Suryanarayana C (2000) J Mater Synth Process 8:235
Grigorieva TF, Barinova AP, Lyakhov NZ (2003) J Nanoparticle Res 5:439
Wu JM, Li ZZ (2000) J Alloys Compd 299:9
Xi S, Qu X, Ma M, Zhou J, Zheng X, Wang X (1998) J Alloys Compd 268:211
Grigorieva TF, Korchagin M, Lyakhov NZ (2002) KONA 20:144
Osso D, Tillement O, Mocellin A, Le Caer G, Babushkin O, Lindback T (1995) J Eur Ceram Soc 15:1207
Shaw LL (2001) Mater Manufact Process 16:405
Li J, Li F, Hu K (2004) J Mater Process Technol 147:236
Reddy BSB, Das K, Pabi SK, Das S (2007) Mater Sci Eng A 445–446:341
Reddy BSB, Karabi Das, Pabi SK, Das Siddhartha (in press) Preparation of Al–Ce/Al2O3 nanocomposite powder by high-energy ball milling and subsequent heat treatment, PMAI-2006, Hyderabad, India
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, B.S.B., Das, K. & Das, S. A review on the synthesis of in situ aluminum based composites by thermal, mechanical and mechanical–thermal activation of chemical reactions. J Mater Sci 42, 9366–9378 (2007). https://doi.org/10.1007/s10853-007-1827-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-1827-z