Abstract
The potato Solanum tuberosum L. has the greatest diversity of cultivated and wild relatives, whose genetic nature has been studied insufficiently. The aim of the present study was to examine the resistance to PVY in S. chacoense Bitt. and S. pinnatisectum Dun. and to search for DNA markers linked to resistance genes. For the first time, representative populations of two diploid tuber-bearing Solanum species were evaluated by the resistance to PVY and the presence of DNA markers linked to the Ry (extreme resistance) or Ny (hypersensitivity) genes localized on the long arm of chromosome 9. Nine accessions of S. chacoense and six accessions of S. pinnatisectum represented by 168 and 170 genotypes, respectively, from the VIR collection were assessed by resistance to artificial infection with PVY. The differences in segregation of two wild potato species into phenotypic classes with response to PVY infection have been established. The diversity of visible reactions of S. chacoense plants after PVY infection differs from the uniform type of symptoms observed in S. pinnatisectum. DNA analysis was performed in 170 S. chacoense genotypes and 44 S. pinnatisectum genotypes using the Ry186, S1d11, and CT220 markers linked to genes determining viral resistance in potato varieties, breeding clones, or species of the genus Solanum L. Most genotypes of S. chacoense and S. pinnatisectum have amplified CT220 marker linked to the Nxphu gene and the Sw-5 gene in the IvP35 line of the species S. phureja Juz. et Buk. and tomato, respectively. The STS Ry186 marker linked to the Rychc gene in Japanese varieties created on the basis of S. chacoense was identified in a small number of S. chacoense genotypes; however, no association between the DNA marker and resistance phenotype was detected. The CAPS S1d11/AcsI marker, which distinguishes between the PVY resistant and susceptible genotypes of both wild potato species, was developed. The plants of two wild-growing tuber-bearing species of Solanum L. were first examined for the presence of the GBSS gene-specific PCR marker (the gene encoding granules-bound starch synthase). A significant part of the genotypes (15% in S. pinnatisectum and 25% in S. chacoense) did not reveal the genetic marker regulating amylose biosynthesis in the forming starch granules. No significant differences in the ability to form tubers depending on the genotypes with or without GBSS marker, indicating the possible presence of an alternative allelic variant of the GBSSI gene in S. chacoense and S. pinnatisectum species, were detected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Together with other species forming tubers and morphologically similar in aboveground organs, the agriculturally cultivated potato (Solanum tuberosum L. subsp. tuberosum) belongs to the subsection Potatoe G. Don. of section Petota Dumort. of genus Solanum L. In comparison with other important agricultural plants, potato demonstrates the greatest variety in cultural and wild relatives [1]. The natural habitats of the species of section Petota Dumort. of genus Solanum L. belong to different ecological and geographical areas in a vast territory stretching from the southern regions of the United States (38° N) to the coast of Chile and the Chiloé Archipelago (41° S) within 16 countries of Central and South America [2].
Tuber-bearing species of the genus Solanum L. possess a wide range of traits determining resistance to an unfavorable environment, which are absent in commercially cultivated potatoes, including resistance to diseases and pests. The main method of potato breeding is interspecies hybridization, which provides the introgression of genetic material (responsible for the inheritance of a target trait) from related Solanum species into the genome of cultivated potato [3]. Potato is a vegetatively propagated culture susceptible to viral diseases such as potato virus Y (PVY) infection resulting in serious economic losses [4]. PVY-resistant forms were identified among wild-growing tuber-bearing Solanum spp. The genetic studies of wild relatives of Solanum L. were conducted in a limited number of species. The molecular-genetic screening of potato collections significantly expands the knowledge on the content of R genes in wild potato forms [5].
Selection for PVY resistance is based on extreme resistance controlled by the Ry gene or on hypersensitivity determined by the Ny gene [6]. An extreme PVY resistance is controlled by the Rysto (source—S. stoloniferum Schlechtd.), Rychc (S. chacoense Bitt.), and Ryadg (S. tuberosum ssp. andigena Juz. et Buk.) genes transferred into Russian and foreign varieties and breeding clones from different sources [3, 6]. The hypersensitivity to distinct strains of PVY in several European varieties and breeding clones was inherited from S. demissum Lindl., S. chacoense, and S. tuberosum [6] and detected in the S. sparsipilum (Bitt.) Juz. et Buk. [7]. The genes determining an extreme resistance and hypersensitivity reside on potato chromosomes 4, 9, 11, and 12.
In contemporary breeding, the use of marker-assisted-selection (MAS) to identify forms with the genes that determine valuable traits is of great importance. An intensification of potato breeding based on MAS technology represents one of the priorities of contemporary genetic and breeding research. Flanking DNA markers have been developed to identify the Ry and Ny genes [6]. The results of screening of breeding material in the United States and Canada by the Rysto and Ryadg gene markers indicate the necessity of more specific novel markers of genes for extreme resistance to PVY [8, 9]. A molecular screening of genetic collections and Russian breeding varieties revealed a different frequency in forms with the Rysto, Rychc, and Ryadg genes depending on the breeding center from which the studied material originated [10–12]. However, published results of the analysis of varieties and breeding clones using molecular markers were not always accompanied by information on the PVY resistance of the examined varieties and breeding clones.
The initial samples, including the S. stoloniferum, S. andigenum, S. chacoense, and S. demissum forms, used as a source of genetic material in breeding for PVY resistance have not been preserved. A validation of DNA markers is mainly carried out using varieties and breeding clones of hybrid origin. Therefore, the study of genetic nature of PVY resistance in tuber-bearing species of the genus Solanum L. and the search for DNA markers significantly linked to the targeted loci in wild potato relatives represent a specific interest. The aim of the present study is to examine the PVY resistance in S. chacoense and S. pinnatisectum and to search for DNA markers linked to resistance genes. For the first time, the representative populations of two diploid tuber-bearing Solanum spp. were characterized by PVY resistance and the presence of DNA markers linked to the Ry and Ny genes located in the same region of chromosome 9.
MATERIALS AND METHODS
Material of study is accessions of the species S. chacoense kk-2732, 2861, 3060, 7394, 19769, 21848, 21849, 21854, and 22638 and of the species S. pinnatisectum kk-4455, 4459, 15254, 19157, 21955, and 23569 from the collection of the All-Russian Institute of Plant Genetic Resources (St. Petersburg, Russia). A seed progeny (25–30 seedlings of each sample) and plants arising from first tuber generation were studied.
Phytopathological analysis. The assessment of PVY resistance was carried out by standard methods of artificial infection, including mechanical inoculation (all plants of S. pinnatisectum and S. chacoense) and grafting (S. pinnatisectum plants resistant to mechanical inoculation). The source of infection was Detskosel’skii variety of potato and a clone of S. chacoense k‑21321 affected by the ordinary (PVYO) and necrotic (PVYN) strains, respectively. The Nicotiana tabacum L. (Samsun variety) was used for the accumulation of viral infection, inoculum preparation and as a rootstock in grafting test. A mechanical inoculation of S. chacoense and S. pinnatisectum plants was conducted twice with an interval of 7–8 days. The total suspension of sap of tobacco plants infected with ordinary PVYO and necrotic PVYN strains was used. The results were evaluated two to three weeks after the second infection by visual symptoms of viral infection and via enzyme-linked immunosorbent assay (ELISA) in the “double sandwich” modification [13]. The diagnostic kits from the Lorkh All-Russian Scientific Research Institute of Potato Farming (Korenevo, Moscow oblast) were used. The N. tabacum L. plants previously infected or not infected with PVY served as a control. Diagnostics of PVY lesions was performed twice: in seedlings and in plants of the first tuber generation.
Molecular methods of analysis. DNA was extracted from fresh leaves using CTAB buffer and 2-mercaptoethanol according to Doyle et al. [14]. For DNA isolation, 100–150 mg of the material was crushed by freezing in liquid nitrogen and subsequent homogenization in an automatic mill. The reaction PCR mixture consisted of 2.5 µL of 10× Taq buffer with MgCl2, 1 µL of dNTPs (10 mM), 0.5 µL of each primer (10 mM), 0.5 µL of Taq polymerase (5 U/µL), and 2 µL of DNA (10–30 ng/µL). The volume was adjusted to 25 µL by deionized water.
The Ry186, S1d11, and CT220 markers linked to the genes determining viral resistance and localized on the long arm of chromosome 9 were used [6]. The STS Ry186 marker was developed by Takeuchi et al. (2008) and demonstrated a recombination frequency of 0.203% with Rychc gene [cit. 15]. This marker appeared to be linked to the Rychc gene in the Japanese varieties Saikai 35 and Konafubuki (the last one was developed on the basis of S. chacoense accession extremely resistant to PVY) [15, 16] and to the Ny-Smira gene controlling hypersensitivity of the Sarpo Mira variety to the PVYNTN strain, which causes necrotic lesions of tubers [17]. The CAPS S1d11 marker is linked to the Ny-1 gene determining hypersensitivity of the Albatros, Koga, Neptun, Niagara, and Sekwana varieties to the PVYN strain [18]. The RFLP CT220 marker is linked to the Nxphu gene located in the same region on the chromosome 9, which determines hypersensitivity to potato virus X (PVX) of the IvP35 line of tuber-bearing S. phureja Juz. et Buk. [19] and to the Sw-5 gene controlling tomato resistance to tomato bronze or spotted wilt virus (TSWV) [20].
The PCR analysis of the Ry186 marker was performed with the primers (5'-TGGTAGGGATATTTTCCTTAGA-3', 5'-GCAAATCCTAGGTTATCAACTCA-3') and under the conditions proposed by Mori et al. [15]. The PCR protocol consisted of one cycle (10 min at 94°C), 35 cycles (30 s at 94°C, 30 s at 55°C, 1 min at 72°C), and one cycle (5 min at 72°C). The GBSS marker of the GBSSI gene (granule-bound starch synthase) was used as a positive control in PCR of the Ry186 marker according to recommendations of Mori et al. [15]. The Bashkirskii and Belosnezhka varieties with detected Ry186 marker were included in the analysis [10].
The PCR analysis of the CT220 marker was performed with the primers (5'-AAGCGAATTATCTGTCAAC-3' and 5'-GTTCCTGACCATTACAAAAGTAC-3') proposed by van der Voort et al. [21]. The PCR conditions were as follows: one cycle of denaturation (95°C for 3 min), 35 cycles (95°C for 30 s, 60°C for 45 s, 72°C for 90 s), and one cycle of elongation (72°C for 10 min).
The PCR results of the Ry186 and CT220 markers were analyzed via electrophoretic separation in 1.5% agarose gel.
The PCR analysis of the S1d11 marker was conducted with the primers (5'-GCCAAAAAGGGTAGGAAAAATG-3' and 5'-TCATCTTCACGAATCGGACTAAA-3') according to the protocol suggested by Szajko et al. [18]: one cycle of denaturation (95°C for 3 min), 35 cycles (95°C for 25 s, 54°C for 35 s, 72°C for 90 s), and one cycle of elongation (72°C for 5 min).
The sequencing of the S1d11 and CT220 markers included purification of the PCR product followed by its concentration using AMPure XP magnetic beads according to the Agencourt AMPure XP protocol [22]. Preparation of probes for sequencing was carried out with BigDye Terminator v3.1 (Thermo Fisher Scientific). The reaction mixture was adapted for the Nanophore-05 genetic analyzer (Syntol, Russia) and included 1.2 µL 5× Sequencing Buffer, 0.3 µL Ready Reaction Mix, 0.3 µL (10 mM) primer, 4.5 µL of deionized water, and 2 µL of purified PCR product. The required concentration of the PCR product was adjusted according to the BigDyeTM Terminator v3.1 Cycle Sequencing Kit User Guide [23]. The PCR program and further purification of the PCR product using 125 mM EDTA were carried out in accordance with the manufacturer’s recommendations.
The PCR product of the S1d11 marker was subjected to the restriction analysis with AcsI endonuclease, which contained 1 µL SEBuffer W, 0.1 µL AcsI (20 U/µL), 1 µL BSA (1 mg/mL), 2.9 µL of deionized water, and 5 µL of PCR product (10–20 ng/µL). The reaction mixture was incubated at 50°C for 3 h followed by enzymatic inactivation at 80°C (20 min). Restriction results were analyzed via electrophoresis in 1.5% agarose gel. The length of fragments was determined with GeneRuler 50 bp DNA Ladder (Thermo Fisher Scientific).
Statistical analysis was conducted using Statistica Basic Academic 13 (JSC StatSoft, Russia). The association between DNA markers and PVY resistance was assessed via contingency tables with Pearson’s chi-square (χ2) test with Yates’s correction for continuity. The rejection of the H0 hypothesis for two data sets occurs at p < 0.05.
RESULTS
Phytopathological Assessment
The plants of 338 genotypes of two diploid Solanum spp. including 168 genotypes of nine accessions of S. chacoense and 170 genotypes of six accessions of S. pinnatisectum were evaluated for PVY resistance and interspecies differences in response to artificial viral infection were established (Table 1). The families of S. chacoense plants demonstrated a variety of visible symptoms a week after the mechanical inoculation, including development of mottling, wrinkled mosaic or point necrosis on inoculated leaves, vein necrosis on inoculated and upper leaves, complet necrosis of leaves and/or shoots, leaves fall off, and plant death in some cases. Leaf fall and/or plant death was observed after mechanical inoculation in S. pinnatisectum plant families.
As a result of necrosis distribution, only some plants of S. chacoense kk-2732, 3060, 7394, 21848, and 21854 accessions died, whereas infection of S. pinnatisectum caused a massive death of plants from kk-15254, 19157, and 23569 accessions (Table 1). The absence of visible symptoms of disease was characteristic of most plants in S. pinnatisectum kk-4455, 4459 and 21955 accessions, whereas in S. chacoense the family of 19769 accession was the most notable at number of plants without symptoms of viral infection. The majority of S. chacoense plants of the k-22638 accession demonstrated a local reaction to the infection, while in the families of k-2861 and k-21849 a systemic reaction was manifested in a significant part of the plants. Among the studied S. chacoense accessions, the greatest diversity of responses to PVY infection was characteristic of the kk-2732, 3060 and 21854 families, each of which consisted of visually healthy plants and plants with local or systemic reaction, mosaic, or fast necrosis resulting in death of plants.
The data on ELISA confirm the differences in PVY resistance between S. chacoense and S. pinnatisectum. As a result of two-year screening of two wild relatives, among 91 genotypes of S. chacoense 12 resistant, 21 hypersensitive, and 58 susceptible to PVY were detected, while 43 S. pinnatisectum genotypes consisted of 34 resistant and 9 susceptible ones. The S. chacoense plants without symptoms or with necrotic response to viral infection demonstrated the presence of PVY in the sap of 27–100% of the examined genotypes depending on the sample. Hypersensitive S. chacoense genotypes were identified in the families of kk-2732, 2861, 3060, 19769, and 21854 (Table 1). The diagnostics performed by ELISA in S. pinnatisectum plants without symptoms of viral infection established a negative reaction of all plants of the k-21955 and a positive reaction (PVY presence) in the sap of 7–73% of the analyzed genotypes in the remaining accessions. The S. pinnatisectum genotypes extremely resistant to PVY were detected in the families of kk-4455, 4459, 19157, 21955, and 23569. Several genotypes of the S. pinnatisectum k-15254 and S. chacoense k-21854 had no symptoms of viral infection; however, a positive ELISA reaction to PVY was identified, thus indicating their tolerance to the virus.
Analysis of DNA Polymorphism Linked to Resistance Genes
The analysis conducted with DNA markers was carried out in 170 S. chacoense genotypes and 44 S. pinnatisectum genotypes. In addition to the examined S. chacoense, two clones (3-41-6 and 3-42-2) of S. chacoense k-19759 accession previously identified in the VIR collection and used as sources of PVY resistance for the development of interspecies hybrids—donors of breeding and valuable potato traits—were tested for the presence of DNA markers. The polymorphism of three DNA markers linked to PVY resistance in potato and tomato and localized in a short segment on chromosome 9 was analyzed [6]. They comprised CAPS S1d11 marker linked to the hypersensitivity Ny-1 gene [18], STS Ry186 marker linked to the Rychc and Ny-Smira genes [16, 17], and RFLP CT220 marker linked to the loci of the Nxphu and Sw-5 genes controlling resistance to PVY and TSWV respectively in the family of Solanaceae Juss [19, 20]. In the present study, the plants of two wild tuber-bearing Solanum L. species were primarily examined for the presence of the GBSSI gene marker (granule-bound starch synthase gene), which was recommended as a positive control in PCR to identify the Ry186 marker [15].
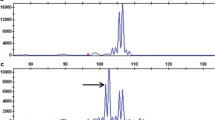
The detection of CAPS S1d11 marker polymorphism in potato varieties was performed with MnlI restriction endonuclease. The wild potato species might possess different polymorphic sites; thus, prior to massive screening of samples, the sequencing of the S1d11 PCR product was conducted in six S. chacoense accessions contrasting in PVY resistance. As a result, two polymorphic sites being the targets for AcsI restriction endonuclease were identified in the amplified sequence (386 bp in length). The obtained CAPS S1d11/AcsI marker divided 95 genotypes of Solanum spp. into two groups differing in restriction profile (+) and (–) (Fig. 1). Genotypes with a fragment of ~256 bp in length (+) after restriction analysis demonstrated a significant correlation with resistance. The genotypes not having this fragment after restriction analysis (–) were PVY-sensitive ones. An association of identified groups with PVY resistance in the case of artificial infection appeared to be statistically significant (Table 2).
The CT220 marker was amplified in the majority of S. chacoense and S. pinnatisectum genotypes (85 and 98%, respectively). The sequencing of CT220 PCR product in eight samples contrasting in resistance and the analysis of obtained sequences failed to detect nucleotide polymorphism in S. chacoense and S. pinnatisectum genotypes, which could be used as a target for restriction endonucleases.
The Ry186 marker was amplified in the Bashkirskii and Belosnezhka varieties in seven S. chacoense genotypes (six in the k-7394 family and one in the k-2732 family) and was absent in S. pinnatisectum. Moreover, the fragment of the GBSS marker used as a positive control in PCR was detected in the majority of S. chacoense (75%) and S. pinnatisectum (85%) genotypes, including 11 of 14 S. chacoense genotypes resistant to PVY but not having the Ry186 marker. No significant differences in the ability to form tubers were observed between the genotypes bearing or not bearing the GBSS marker.
No association of CT220 and Ry186 markers with PVY resistance was established in plants of two Solanum species (Table 2).
DISCUSSION
For the first time, representative populations of two wild-growing tuber-bearing Solanum L. species have been characterized by PVY resistance and the presence of DNA markers mapped to the potato chromosome 9. The differences in the segregation of the two wild potato species into phenotypic classes as a result of PVY infection have been established. The variety of visible reactions of S. chacoense plants distinguishes this species from S. pinnatisectum plants with similar symptoms of PVY infection.
The S. chacoense species belongs to series Yungasensа Corr., whereas S. pinnatisectum belongs to series Pinnatisecta (Rudb.) Hawkes; the morphological differences are expressed between their representatives and these series inhabit different ecological and geographical regions. The S. chacoense species grows in South America—Argentina, Bolivia, Brazil, Paraguay, Peru, and Uruguay. The geographic range of S. pinnatisectum is located on another continent—in North America, Mexico. The species have different endosperm balance numbers (EBNs) and belong to different clades according to the molecular genotyping [2]; i.e., they are evolutionarily distant wild tuber-bearing relatives of Solanum L. The differences observed in the response to PVY infection reflect the dissimilarity of the genetic nature of the two Solanum spp.
DNA analysis conducted using three markers linked to the various genes controlling in potato and its relatives resistance to viruses and in tomato to TSWV demonstrated a consistency in the distribution of each DNA marker in the examined genotypes of two tuber-bearing wild Solanum L. species. The CT220 marker was amplified in the vast majority of S. chacoense and S. pinnatisectum genotypes, although we failed to detect any polymorphism in the amplified DNA sequence. This marker is described as linked to the Sw-5 gene cluster, which was primarily identified as the dominant gene locus controlling high level of resistance of tomato cultivar Stevens (developed on the basis of the wild species Solanum peruvianum Mill.) to TSWV tospovirus. Later, tomato with Sw-5 cluster was found to be resistant to a wide range of tospoviruses. Five paralogs encoding proteins with NB and LRR domains in the cluster together with the immune receptor against TSWV were identified [24]. The DNA sequence corresponding to the CT220 marker in genotypes phylogenetically distant from Solanum spp. identified in the present study indicates its conservative structure in the genus Solanum L.
The Ry186 marker linked to the Rychc gene in potato varieties of Japanese selection, whose PVY resistance was provided by introgression of S. chacoense genetic material, was rare in the genotypes of this species. The Ry186 marker was absent in another examined tuber-bearing species—S. pinnatisectum. Detection of the Ry186 marker in Russian potato varieties [10] requires the addition of the results of the offspring segregating analysis to confirm its diagnostic value. The S. chacoense forms with contrast PVY response identified in the present study represent the basis for further study, mapping, and identification of the R genes.
A combined molecular and phytopathological screening of a large sample of S. chacoense and S. pinnatisectum made it possible to develop the CAPS S1d11/AcsI marker, which can reliably distinguish the genotypes of two related tuber-bearing species by PVY resistance. Validation of the S1d11/AcsI marker on genetically diverse material, including sources of PVY resistance in the VIR collection, will clarify its universality and the possibility of its use in practical breeding.
The massive screening of two wild tuber-bearing Solanum L. species failed to detect the marker of the GBSSI gene (granule-bound starch synthase gene) in a significant part (15–25%) of Solanum spp. genotypes. The absence of the DNA marker of one gene associated with starch metabolism in S. chacoense and S. pinnatisectum plants is interesting, although the probability of unsuccessful PCR cannot be completely excluded. The GBSSI (granule-bound starch synthase) gene controlling the amylose biosynthesis in the forming starch granules was identified and characterized in many potato varieties, and its inactivation makes it possible to obtain potatoes with tubers predominantly containing amylopectin [25]. No significant differences in tuber formation between the genotypes with and without the GBSS marker were observed in both wild species. Our findings on the detection of the GBSS marker can be explained by the presence of the allelic variant of the GBSSI gene in wild diploid species S. chacoense and S. pinnatisectum.
REFERENCES
Vincent, H., Wiersema, J., Kell, S., et al., A prioritized crop wild relative inventory to help underpin global food security, Biol. Conserv., 2013, vol. 167, pp. 265—275. https://doi.org/10.1016/j.biocon.2013.08.011
Spooner, D., Ghislain, M., Simon, R., et al., Systematics, diversity, genetics, and evolution of wild and cultivated potatoes, Bot. Rev., 2014, vol. 80, pp. 283—383. https://doi.org/10.1007/s12229-014-9146-y
Ross, H., Potato breeding—problems and perspectives, Journal Plant Breeding Supplement, Horn, W. and Robbelen, G., Eds., Berlin: Paul Parey, vol. 13, 1986, pp. 82—86.
Tsedaley, B., A review paper on potato virus Y (PVY) biology, economic importance and its managements, J. Biol.,Agric. Healthcare, 2015, vol. 5, no. 9, pp. 110—126.
Sokolova, E., Pankin, A., Beketova, M., et al., SCAR markers of the R-genes and germplasm of wild Solanum species for breeding late blight-resistant potato cultivars, Plant Genet. Resour., 2011, vol. 9, no. 2, pp. 309—312. https://doi.org/10.1017/S1479262111000347
Valkonen, J.P.T., Gebhardt, C., Zimnoch-Guzowska, E., and Watanabe, K., Resistance to potato virus Y in potato, in Potato Virus Y: Biodiversity, Pathogenicity, Epidemiology and Management, Springer-Verlag, 2017, pp. 207—241. https://doi.org/10.1007/978-3-319-58860-5_8.
Moury, B., Caromel, B., Johansen, E., et al., The helper component proteinase cistron of potato virus Y induces hypersensitivity and resistance in potato genotypes carrying dominant resistance genes on chromosome IV, MPMI, 2011, vol. 24, no. 7, pp. 787—797.https://doi.org/10.1094/MPMI-10-10-0246
Fulladolsa, A., Navarro, F., Kota, R., et al., Application of marker assisted selection for potato virus Y resistance in the University of Wisconsin Potato Breeding Program, Am. J. Potato Res., 2015, vol. 92, pp. 444—450. https://doi.org/10.1007/s12230-015-9431-2
Nie, X., Lalany, F., Dickison, V., et al., Detection of molecular markers linked to Ry genes in potato germplasm for marker-assisted selection for extreme resistance to PVY in AAFC’s potato breeding program, Can. J. Plant Sci., 2016, vol. 96, pp. 737—742.https://doi.org/10.1139/cjps-2015-0335
Biryukova, V.A., Shmyglya, I.V., Abrosimova, S.B., et al., Search for sources of pathogen resistance genes among samples of breeding and genetic collections of the All-Russian Research Institute of Agriculture using molecular markers, Zashch. Kartofelya, 2015, no. 1, pp. 3—7.
Gavrilenko, T.A., Klimenko, N.S., Antonova, O.Yu., et al., Molecular screening of potato varieties bred in the northwestern zone of the Russian Federation, Vavilovskii Zh. Genet. Sel., 2018, vol. 22, no. 1, pp. 35—45. https://doi.org/10.18699/VJ18.329
Rogozina, E.V., Terent’eva, E.V., Potokina, E.K., et al., Multiplex PCR-based identification of potato genotypes as donors in breeding for resistance to diseases and pests, S.-kh.Biol., 2019, vol. 54, no. 1, pp. 19—30. https://doi.org/10.15389/agrobiology.2019.1.19rus
Clark, M. and Adams, A., Characteristics of the microplate methods of enzyme-linked immunosorbent assay for the detection of plant viruses, J. Gen. Virol., 1977, vol. 34, pp. 475—483.
Doyle, J.J. and Doyle, J.L., Isolation of plant DNA from fresh tissue, Focus, 1990, vol. 12, no. 13, pp. 39—40.
Mori, K., Sakamoto, Yu., Mukojima, N., Tamya, S., et al., Development of a multiplex PCR method for simultaneous detection of diagnostic DNA markers of five disease and pest resistance genes in potato, Euphytica, 2011, vol. 180, no. 3, pp. 347—355.https://doi.org/10.1007/s10681-011-0381-6
Sato, M., Nishikawa, K., Komura, K., and Hosaka, K., Potato virus Y resistance gene, Ry chc, mapped to the distal end of potato chromosome 9, Euphytica, 2006, vol. 149, no. 3, pp. 367—372. https://doi.org/10.1007/s10681-006-9090-y
Tomczynska, I., Jupe, F., Hein, I., et al., Hypersensitive response to potato virus Y in potato cultivar Sarpo Mira is conferred by the Ny-Smira gene located on the long arm of chromosome IX, Mol. Breed., 2014, vol. 34, no. 2, pp. 471—480. https://doi.org/10.1007/s11032-014-0050-2
Szajko, K., Strzelczyk-Zyta, D., and Marczewski, W., Ny-1 and Ny-2 genes conferring hypersensitive response to potato virus Y (PVY) in cultivated potatoes: mapping and marker-assisted selection validation for PVY resistance in potato breeding, Mol. Breed., 2014, vol. 34, no. 1, pp. 267—271. https://doi.org/10.1007/s11032-014-0024-4
Tommiska, T., Hämäläinen, J., Watanabe, K., and Valkonen, J., Mapping of the gene Nxphu that controls hypersensitive resistance to potato virus X in Solanum phureja IvP35, Theor. Appl. Genet., 1998, vol. 96, no. 6—7, pp. 840—843.
Brommonschenkel, S.H. and Tanksley, S.D., Map-based cloning of the tomato genomic region that spans the Sw-5 tospovirus resistance gene in tomato, Mol. Gen. Genet., 1997, vol. 256, pp. 121—126.
van der Voort, J.R., van der Vossen, E., Bakker, E., et al., Two additive QTLs conferring broad-spectrum resistance in potato to Globodera pallida are localized on resistance gene clusters, Theor. Appl. Genet., 2000, vol. 101, no. 7, pp. 1122—1130. https://doi.org/10.1007/s001220051588
https://www.beckmancoulter.com/wsrportal/techdocs?docname=B37419.
http://tools.thermofisher.com/content/sfs/manuals/cms_081527.pdf.
de Oliveira, A.S., Boiteux, L.S., Kormelink, R., and Resende, R.O., The Sw-5 gene cluster: tomato breeding and research toward orthotospovirus disease control, Front. Plant Sci., 2018, vol. 9, p. 1055. https://doi.org/10.3389/fpls.2018.01055
Slugina, M.A. and Kochieva, E.Z., The use of carbohydrate metabolism genes for potato (Solanum tuberosum L.) improvement (review), S.-kh.Biol., 2018, vol. 53, no. 3. pp. 450—463. https://doi.org/10.15389/agrobiology.2018.3.450rus
ACKNOWLEDGMENTS
We are grateful to E.N. Yurkina for the maintenance of cloned plants of S. chacoense and S. pinnatisectum.
Funding
The present study was supported by the Russian Foundation for Basic Research (project no. 18-016-00138) “Molecular Genetic Study of Loci Associated with Late Blight and Potato Virus Y Resistance of the Species and Hybrids of Solanum L. Section Petota.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Kazantseva
Rights and permissions
About this article
Cite this article
Rogozina, E.V., Ulianich, P.S., Volkov, V.A. et al. Genetic Diversity of Solanum pinnatisectum Dun. and Solanum chacoense Bitt. by Resistance to Potato Virus Y and Results of DNA Analysis. Russ J Genet 55, 1330–1337 (2019). https://doi.org/10.1134/S1022795419110139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795419110139