Abstract
The causal agent of late blight, the oomycete Phytophthora infestans Mont de Bary, is characterized by a high degree of variability, as a result of which new races of the pathogen are able to overcome the resistance of long-term cultivated potato cultivars. Primitive cultivated potato species belong to the primary gene pool, the representatives of which easily cross with Solanum tuberosum L., and their use for breeding is promising. The objective of this study was to identify the genotypes of wild and primitive cultivated potato species from the VIR collection carrying the Rpi genes. For the first time, accessions of primitive cultivated and wild potato species (105 genotypes from the VIR collection) were analyzed for resistance to late blight and the presence of SCAR markers of the Rpi genes (RB/blb1, Rpi-blb2, R2-like, Rpi-vnt1.3). In the cultivated species S. stenotomum subsp. stenotomum, high (0.71) frequency of one of the two marker fragments of the RB/blb1 gene (Rpi-sto1), originally characterized in the wild North American species S. bulbocastanum, which belongs to the tertiary gene pool of potato species, was detected. In the species S. phureja and S. stenotomum subsp. goniocalyx, high frequency (0.71–0.88) of the Rpi-vnt1.3 gene marker, originally characterized in the wild South American species S. venturii, was found. For the first time, in primitive potato species, the fragment sequences, presumptive homologs of the Rpi-vnt1 and RB/blb1 genes, were characterized. In S. ajanhuiri, S. stenotomum, and S. phureja, three sequence variants homologous to Rpi-vnt1.3 were identified. The possible role of the detected polymorphism of the Rpi-vnt1.3 marker fragments in ensuring the resistance of primitive cultivated species to late blight is speculated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Late blight (the causal organism Phytophthora infestans (Mont.) de Bary) is one of the most destructive diseases in potato. An outbreak of this disease was the cause of the Ireland’s famine in the middle of the 19th century [1]. The most effective method of combating this disease is the cultivation of resistant cultivars. These cultivars are developed as a result of the introgression of the resistance genes (Rpi) through interspecific hybridization and subsequent selection. To date, over 20 late blight resistance genes have been identified in potato. In practical breeding, accessions with Rpi genes are mainly used, the sources of which are wild potato species from North and Central America, S. bulbocastanum Dunal, S. demissum Lindl., and S. stoloniferum Schltdl. [2]. Resistance genes were also found in other wild species, namely, the North American S. cardiophyllum Lindl. and S. pinnatisectum Dunal, as well as the South American S. berthaultii Hawkes, S. mochiquense Ochoa and S. venturii Hawkes & Hjert., and others [3]. P. infestans is characterized by high intrapopulation genetic variability of isolates [4], which is phenotypically manifested in the formation of new (more virulent, aggressive) races of the pathogen. Owing to the intensive race-forming process, it is impossible to obtain stably resistant potato cultivars, and effective breeding for this trait requires new, previously unused resistance genes. The sources of new resistance genes can be resistant primitive cultivated species that are representatives of the primary gene pool, which greatly facilitates the process of gene introgression, on account of the absence of biological barriers for crossing [5].
The late blight pathogen genome has been sequenced [6, 7]. Using molecular genetic approaches, considerable progress has been made in the study of P. infestans populations, molecular mechanisms of host–pathogen interaction, genetic control of pathogen virulence, and host resistance [8]. However, there is still no consensus on where the center of origin of the potato late blight pathogen is located, i.e., in central Mexico or the South American Andes [9]. It is noteworthy that both possible centers of origin of P. infestans are located within the territories designated as centers of diversity for tuber-bearing species of the section Petota Dumort. of the genus Solanum L., relatives of the cultivated potato. In South America, the highest species diversity of wild and cultivated potatoes was found in Peru, and the secondary center of potato biodiversity is located in Mexico [10].
Tuber-bearing species of the genus Solanum occupy an extended range extending from 38° N to 41° S and grow in a wide range of vertical zonation, namely, from 0 to 5000 m above sea level [10]. Within this territory, four centers of origin of phytophthora-resistant forms of wild and cultivated potato species were identified [11]. The study of the genetic control of late blight resistance trait in potato species that have developed in different parts of the common range of the section Petota is an important step toward understanding their phylogeny and determination of their relationships. Comparative analysis of the Rpi genes and assessment of the degree of their structural similarity will make it possible to shed new light on the evolutionary pathways of genetic determinants of plant resistance to the P. infestans pathogen. Sequencing of the genome of the doubled monoploid clone of S. phureja, DM1-3 516 R44 [12], and its study activated interest in the group of cultivated potato species (landraces cultivated by the indigenous population in different regions of the Andes). A bioinformatic search identified 435 NBS-LRR genes (Nucleotide binding site, leucine rich repeats) in the reference genome sequence. This family includes all the main R genes of plants, which are crucial in providing plant resistance to pathogens of different nature [13]. Using the methods of classical genetics, in the S. stenotomum × S. phureja interspecific hybrid, the Rpi-phu1 gene was identified, which was located on chromosome 9. The Rpi-phu1 gene is characterized by a wide spectrum of action, ensuring the resistance of both leaves and tubers to late blight [14, 15]. In the wild potato S. venturii from Argentina, at the same locus of chromosome 9, the Rpi-vnt1.1 late blight resistance gene (and the Rpi‑vnt1.2 and Rpi-vnt1.3 allelic variants) was identified, which was a homolog of the Tm-22 gene, which provides resistance of tomato (S. lycopersicum L.) to tomato mosaic virus [16]. The Rpi-vnt1.1- and Rpi-vnt1.3-encoded proteins demonstrate 73% amino acid sequence identity to the Tm-22 gene-encoded protein [17]. Resistance to a wide range of P. infestans strains is provided by the genes of the wild Mexican species S. bulbocastanum, RB/blb1 [18], Rpi-blb2 [19, 20], and Rpi-blb3 [21]. The DM1-3 516 R44 reference sequence shows cluster organization of the resistance genes, including homologs of the Rpi-vnt1 gene on chromosome 9, those of the Rpi-blb2 gene on chromosome 6, and the most representative (55 R genes) cluster on chromosome 4 [13]. A large family of genes located on chromosome 4 that provide resistance to late blight includes R2, R2-like, Rpi-abpt, Rpi-blb3, Rpi-edn1.1, Rpi-hjt1.1, Rpi-hjt1.2, Rpi-hjt1.3, Rpi-snk1.1, and Rpi-snk1.2 [22].
The diversity of primitive cultivated potato species, determined by their cultivation in very different climatic conditions [23], suggests the presence of other, previously unknown resistance genes to various diseases, including late blight. In particular, according to the studies of Gabriel et al. [24], in S. phureja, phytophthora-resistant accessions are quite common, which substantiates the need for a more comprehensive analysis of this group.
The objective of this study was to identify genotypes carrying late blight resistance genes (Rpi genes) among primitive cultivated potato species, as well as previously uncharacterized accessions of wild potato species from the VIR collection. Since the range of known Rpi genes is extremely wide, intragenic SCAR markers of the genes the homologs of which were previously found in primitive cultivated potato species, as well as wild potato species closest to cultivated ones [14, 25], were selected for the study. This is particularly so with the RB/blb1, Rpi-blb2, and Rpi-vnt1 genes, the homologous sequences of which were identified in the reference potato genome during bioinformatic analysis [13, 26].
For the first time, a representative sample of cultivated and wild tuber-bearing species of the genus Solanum L. from the South American and North American centers of biodiversity was screened. Among the group of primitive cultivated potato species, the presence of markers of all studied resistance genes, except for R2-like, was demonstrated. Comparison of data on the presence of SCAR markers and polymorphism of their sequences with the results of laboratory infection of potatoes with late blight made it possible to suggest which of the studied genes could be involved in the resistance development and to identify accessions the resistance of which was not determined by any of the studied genes.
MATERIALS AND METHODS
The study was carried out using accessions of diploid primitive cultivated potato species, including S. ajanhuiri Juz. & Bukasov, S. stenotomum subsp. goniocalyx (Juz. & Bukasov) Hawkes, S. stenotomum subsp. stenotomum, and S. phureja, as well as accessions of three North American and eight South American wild potato species from the VIR collection (Appendix 1). For ease of comparison with published sources, the species affiliation of the collection accessions is presented in accordance with the most common taxonomy of the section Petota Dumort of genus Solanum L. suggested by J. Hawkes [27]. A total of 105 genotypes of tuber-bearing species of the genus Solanum were examined. The majority (75 genotypes) belong to the group of primitive cultivated potato species, 13 genotypes belong to three North American wild species (S. brachystotrichum, S. lesteri, and S. bulbocastanum), and the remaining 17 genotypes are representatives of eight wild South American potato species. The studied genotypes of wild-growing species were obtained from seeds of collection accessions and are preserved as clones by obtaining tubers from greenhouse plants. Cultivated species are maintained by tuber propagation in the field.
Cultivated species were analyzed for the first time for resistance to late blight and the presence of DNA markers of R genes. Accessions of the wild species included in the study were not previously assessed for resistance to late blight, but were involved in other studies, in particular, in screening for resistance to potato golden cyst nematode [28].
Laboratory Assessment of Late Blight Resistance
Plants were grown in a greenhouse in 500-cm3 plastic pots (one tuber in each pot). For evaluation, five leaflets of the middle formation were taken from plants more than 60 days old after planting in a double biological replication.
Laboratory screening of potato accessions for resistance to late blight was carried out according to the standard method [29]. For infection, an inoculum based on the MP1841 isolate obtained from the Institute for Plant Breeding and Acclimatization, Mlochow, Poland (IHAR-Mlochow) was used. The isolate contains all 11 virulence genes (1.2.3.4.5.6.7.8.9.10.11). The inoculum was incubated for 30 min at a temperature of 10–12°C to stimulate the release of zoospores. The concentration of sporangia in the inoculum corresponded to 50 000 units/mL. The leaves were placed on wet filter paper the abaxial side down, and 30 µL of inoculum was applied between the central and lateral veins. One day after inoculation, the leaves were turned the abaxial side up. During the entire period of inoculation, in the climatic box, constant conditions of 16°C were maintained [30]. The degree of damage was assessed on the sixth day after infection on a 9‑point scale [31]. Accessions with scores from 1 to 3 (damage to more than 25% of the infected leaf surface area) were considered susceptible (S) to late blight, with scores from 4 to 6 (from 5 up to 25%) were considered moderately resistant (MR), and with scores from 7 to 9 (less than 5%) were considered resistant (R). The experiment was carried out in duplicate, and resistance was assessed by the average values in both repetitions. The Nevskii (susceptible) and Sudarynya (resistant) cultivars served as experimental control.
Screening of Primitive Cultivars and Wild Potato Species Using SCAR Markers of the RB/blb1, Rpi-blb2, R2-like, and Rpi-vnt1.3 Resistance Genes
DNA was extracted from young potato leaves in duplicate using CTAB buffer according to the protocol of Gavrilenko et al. [32]. Fragments of putative homologs of resistance genes in primitive species were amplified using Rpi-blb1, Rpi-sto1, Rpi-blb2, R2-like, and Rpi-vnt1.3 primers specific to SCAR markers according to the protocols suggested by the authors (Table 1) using Taq polymerase (Dialat, Moscow).
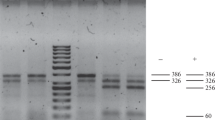
The PCR products were visualized in 1.7% agarose gel, stained with ethidium bromide, and documented in the BioDocII system (Biometra GmbH, Germany).
Sequencing of Putative Rpi-vnt1 and RB/blb1 Homolog Fragments in Primitive Potato Cultivars
In seven accessions of primitive cultivated potato species (k-9911, k-3558, k-9345, k-8873, k-17618, k‑9301, and k-1120), contrasting in resistance to the pathogen, the fragments obtained using Rpi-vnt1.3 primers were sequenced. In two phytophthora-susceptible accessions of S. stenotomum subsp. stenotomum, k-7366 and k-10478, fragments obtained using Rpi-sto1 and Rpi-blb1 primers were sequenced. Amplicons of both types were first isolated from the PCR mixture using the Cleanup Standard kit, then ligated into the pAL-TA vector according to the Evrogen protocol (http://evrogen.ru/kit-user-manuals/pAL-TA.pdf). The E. coli DH5α strain was used for transformation. The protocol is presented in detail in the VIR guidelines [36]. Two fragments of each accession were sequenced in two directions using the equipment of the Genomic Technologies, Proteomics and Cell Biology Collective Use Center of the All-Russian Research Institute for Agricultural Microbiology, Pushkin, St. Petersburg, on an ABI 3500xl Genetic Analyzer (Applied Biosystems, United States). The resulting sequences were aligned and analyzed using the MEGA version 11 software program [37]. The fragments were identified by the degree of similarity with the sequences deposited in the NCBI GenBank international database of nucleotide sequences and in the BLAST search engine (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The nucleotide sequences were deposited in the GenBank database under the accession numbers for the Rpi-vnt1.3 gene sequences, ON322726–ON322739; for the RB/blb1 gene sequences, ON515750.
RESULTS
Among the accessions of each potato cultivated species, genotypes resistant (moderately resistant) to late blight were found (Fig. 1), which points to the prospects for studying this group of the Solanum gene pool. Among the studied accessions of North American wild species, representatives of S. brachystotrichum (Bitt.) Rydb., there were no genotypes resistant to late blight, while resistant forms were found among other species (Fig. 2). Among the South American wild species, only some genotypes of S. doddsii Corell and S. leptophyes Bitter were moderately resistant; the studied genotypes of all other species were susceptible to late blight.
The occurrence of SCAR markers of the RB/blb1, Rpi-blb2, R2-like, and Rpi-vnt1.3 genes in cultivated and wild potato species was different (Figs. 1, 2). The most noticeable differences were in the marker distributions among North American wild and primitive cultivated potato species. Owing to the small number of accessions studied in South American wild potato species, they are presented in Fig. 2 as a combined group. The specificity of the SCAR marker distributions in representatives of different potato species was revealed. The R2 area 1/2 marker of the R2-like gene is present in all the studied North American wild species, while among the studied South American species it was found only in two accessions of the wild species S. doddsii and was completely absent from cultivated potato species. The opposite pattern is typical of the Rpi-blb2 marker. It was found in representatives of South American wild and cultivated potato species (S. alandiae, S. doddsii, S. kurtzianum, S. sparsipilum, S. yungasense, S. ajanhuirii, and S. stenotomum subsp. goniocalyx) and was absent from the studied accessions from North America (Figs. 1, 2). The Rpi-vnt1.3 marker was found in South American potato species. Namely, with a high frequency (0.71–0.88), it was found in cultivated species S. phureja and S. stenotomum subsp. goniocalyx and was also found in the wild species S. doddsii, S. kurtzianum, S. neocardenasii, and S. spegazzinii, but was found only in a single representative of North American species, S. brachystotrichum. The Rpi-sto1 marker is found in all primitive cultivated potato species, including with a high frequency (0.55–0.71) in accessions of two subspecies of S. stenotomum, but of all the wild species studied, it was found only in a single accession of S. bulbocastanum.
Within the group of primitive cultivated species, there are considerable differences in the frequency of two markers of the RB/blb1 gene. The Rpi-sto1 marker, found in all accessions of primitive species, is most frequent in S. stenotomum subsp. stenotomum and extremely rare in S. phureja; the second marker, Rpi-blb1, was found only in two genotypes of S. stenotomum subsp. stenotomum (Fig. 1). The Rpi-blb2 marker was found in representatives of two other species, S. ajanhuirii and S. stenotomum subsp. goniocalyx (Fig. 1).
In S. phureja, two genotypes resistant (k-8873, k‑17618) and five moderately resistant (k-9345, k-11547, k-16896, k-19321, k-23516) to late blight were found (Table 2). Both resistant accessions are of Peruvian origin, four of the five moderately resistant accessions are of Colombian origin, and one more moderately resistant accession was obtained in England by crossing accessions from Bolivia and Colombia. Accessions of S. phureja from Bolivia and Ecuador were susceptible to late blight. In the studied S. phureja accessions, no correlation between the presence of markers and resistance indices was found. The Rpi-vnt1.3 fragment was amplified in accessions resistant, moderately resistant, and susceptible to late blight (Fig. 1). Two moderately resistant accessions of S. phureja (k-11547, k-23516) also contained one of the two markers of the RB/blb1 gene, Rpi-sto1.
In S. stenotomum subsp. stenotomum, one resistant (k-11020) and three moderately resistant (k-8354, k-9278, k-17486) genotypes were identified. Three of these genotypes are of Peruvian origin; one moderately resistant genotype (k-9278) was obtained in England by crossing two accessions from Bolivia. In two moderately resistant accessions of this species, none of the used markers was found. A single resistant genotype of S. stenotomum subsp. stenotomum (k-11020), as well as S. phureja accessions, carries the Rpi-vnt1.3 marker. It was demonstrated that moderately resistant accession k-8354 contained the Rpi-sto1 marker fragment designed for the coiled-coil domain (CC) of the RB/blb1 gene, but there was no Rpi-blb1 marker, specific to the LRR domain (leucine-rich repeat) of the same gene [2]. At the same time, two susceptible genotypes of S. stenotomum subsp. stenotomum (k-7366 and k-10478) had both the RB/blb1 gene markers and the Rpi-vnt1.3 marker (Fig. 1).
The studied genotypes of S. ajanhuiri differ in their response to infection with the pathogen, albeit each genotype carries the Rpi-blb2 marker. In addition, in resistant S. ajanhuiri k-9900, the Rpi-vnt1.3 SCAR marker was found, and moderately resistant S. ajanhuiri k-9911 was found to contain the Rpi-sto1 marker (Fig. 1). Among the studied S. stenotomum subsp. goniocalyx accessions, only one moderately resistant genotype (k-9922) was identified, in which the Rpi-sto1 and Rpi-vnt1.3 markers were found.
Among representatives of South American wild potato species, two moderately resistant genotypes, S. doddsii (k-19817) and S. leptophyes (k-5764), were identified. In S. doddsii, only one SCAR marker, Rpi-blb2, was identified, while in S. leptophyes, no markers were found.
Among the accessions of North American wild potato species, S. bulbocastanum and S. lesteri, one moderately resistant and two resistant genotypes were found. All accessions of S. brachystotrichum were susceptible to late blight. Two genotypes of S. bulbocastanum k-24868 and three genotypes of S. lesteri k-24475 (including one moderately resistant) did not contain any of the studied markers. The resistant accession of S. bulbocastanum contained both markers of the RB/blb1 gene, as well as a marker of the R2-like gene. It can be suggested that it is the RB/blb1 gene that ensures the resistance of this S. bulbocastanum genotype to late blight, since among all wild species, this is the only accession that has both marker fragments of the gene.
In accessions of primitive cultivated species contrasting in resistance, amplicon sequences obtained using Rpi-sto1, Rpi-blb1, and Rpi-vnt1.3 primers were studied.
The nucleotide sequence of the Rpi-sto1 fragment in the S. stenotomum subsp. stenotomum k-10478 (GenBank: ON515750) is highly similar to the reference sequence of the CC domain (coiled-coil) of the RB/blb1 gene in S. stoloniferum (GenBank: EU884421.1), presented in the BLAST information retrieval database. Eight SNPs in the exon region and a rather large deletion (in the region between positions 574 and 629 of the reference gene) in the noncoding region were found (Fig. 3). At the same time, no homology was found between the nucleotide sequence of the other amplicon obtained using Rpi-blb1 primers and the reference sequence of the LRR domain of the RB/blb1 gene (GenBank: EU884421.1).
Sequence alignment of the Rpi-sto1 fragment from the S. stenotomum subsp. stenotomum k-10478 (GenBank: ON515750) and the CC domain fragment of the RB/blb1 gene reference sequence from S. stoloniferum (GenBank: EU884421.1). Abbreviated species names for Figs. 3–5 are given according to Z. Huaman and R. Ross, 1985 [38].
Using the Rpi-vnt1.3 primer pair, in most accessions of primitive species, fragments of the expected length of about 600 bp were amplified. Selectively, fragments amplified in seven genotypes of cultivated species contrasting in resistance to late blight were isolated, cloned, and sequenced (see Materials and Methods). The fragments obtained are shown in Fig. 4.
Sequence alignment of the CC domain fragment of the Rpi-vnt1.3 resistance gene homologs in clones of cultivated species: S. ajanhuiri (k-9911) (GenBank: ON322726, ON322727), S. stenotomum subsp. goniocalyx (k-3558) (GenBank: ON322728, ON322729), S. phureja (k-9345 (GenBank: ON322730, ON322731), k-8873 (GenBank: ON322732, ON322733), k-17618 (GenBank: ON322734, ON322735)), and S. stenotomum subsp. stenotomum (k-9301 (GenBank: ON322736, ON322737), k-11020 (GenBank: ON322738, ON322739)). Nucleotide numbering corresponds to the reference sequence of the Rpi-vnt1.3 gene (GenBank: FJ423046.1).
The obtained sequences are similar to the Rpi-vnt1.1 (GenBank: FJ423044), Rpi-vnt1.2 (GenBank: FJ423045), and Rpi-vnt1.3 (GenBank: FJ423046.1) gene fragments of S. venturii [16, 17] and pseudogenes of the Rpi-vnt1 type in S. phureja (GU338337.1) and S. stenotomum (GU338321.1, GU338322.1, and GU338323.1) [39]. A similarity to the gene fragment encoding the RPP13-like protein in the whole genome sequence of the doubled monoploid DM1-3 516 R44 (GenBank: XM_015315064.1) was also revealed.
In total, three sequence variants were found, the degree of similarity of which to the Rpi-vnt1.3 reference sequence (FJ423046.1) was 90.2, 97.7, and 94.9%, respectively. The first variant was found only in accessions resistant to late blight, including k-9911 of S. ajanhuiri (GenBank: ON322726, ON322727); k‑9345 (GenBank: ON322730, ON322731), k-8873 (GenBank: ON322733), and k-17618 (GenBank: ON322735) of S. phureja; and k-11020 (GenBank: ON322739) of S. stenotomum subsp. stenotomum. Compared to the Rpi-vnt1.3 reference sequence, in the studied fragment, 52 SNPs were found, including 22 transitions (12 G ↔ A and 10 T ↔ C), as well as 30 transversions (11 A ↔ T, 4 T ↔ G, 7 G ↔ C, and 8 A ↔ C). Twenty-eight nucleotide substitutions resulted in the amino acid substitutions. The second Rpi-vnt1.1 fragment sequence variant was found only in one amplicon of the k-11020 resistant accession of S. stenotomum subsp. stenotomum (GenBank: ON322738). This variant differed from the similar fragment in the FJ423046.1 sequence in 21 SNPs, including 10 transitions (5 G ↔ A and 5 T↔C) and 11 transversions (3 A ↔ T, 2 T ↔ G, 5 G ↔ C, and 1 A ↔ C). Fourteen nucleotide substitutions were found to be nonsynonymous. Despite considerable differences, both variants identified have traditional CC domain structure, which is highly similar to the Rpi-vnt1.3 gene domain. It is known that amino acid sequences of CC domains include repeats of seven amino acids (heptads) with hydrophobic residues located in positions 1 and 4 and polar amino acid residues in positions 5 and 7 [40]. The putative amino acid sequences found in pathogen-resistant accessions have the same structure. Nonsynonymous substitutions at the key points of the heptads were not detected (Fig. 5).
Alignment of presumptive protein sequences of coiled-coil (CC) fragments of the Rpi-vnt1.3 domain (ACJ66594.1) and presumptive homologous protein sequences of the studied accessions of S. ajanhuiri (k-9911) (GenBank: ON322726, ON322727), S. phureja (k-8873 (GenBank: ON322733), k-9345 (GenBank: ON3227230, ON322731), and k-17618 (GenBank: ON322735)), and S. stenotomum subsp. stenotomum (k-11020) (GenBank: ON322739, ON322738). Presumptive coiled-coil domains are framed. Amino acid numbering corresponds to the Rpi-vnt1.3 sequence (ACJ66596.1).
The third variant of the Rpi-vnt1.1 fragment was found in both resistant and susceptible accessions and was a pseudogene fragment, since it had a five-nucleotide deletion in the CC domain, which led to a frameshift and, consequently, to the formation of a stop codon (Fig. 4).
DISCUSSION
Phytopathological screening of 71 collection accessions of primitive tuber-bearing species (S. ajanhuiri, S. stenotomum subsp. goniocalyx, S. stenotomum subsp. Stenotomum, and S. phureja) using P. infestans isolate MP1841 as an inoculum provided identification of 14 resistant and moderately resistant accessions. In general, the frequency of resistant and moderately resistant genotypes in the sample corresponded to the published data on resistance to late blight of the accessions of S. phureja [24] and other primitive species [11].
Using SCAR markers of the Rpi genes, the homologs of which were previously identified in the S. phureja duplicated monoploid clone DM1-3 516 R44 [13, 26], the presence of marker fragments of these genes and their variability in the accessions of S. phureja and those of closely related cultivated potato species were studied. Possible association between the resistance to late blight of representatives of wild and cultivated potato species and the presence of the RB/blb1, Rpi-blb2, and Rpi-vnt1.3 gene fragments providing resistance to late blight in S. bulbocastanum and S. venturii was analyzed [15–20]. No association between the presence/absence of the Rpi gene SCAR amplicons and the resistance of cultivated potato species to the late blight pathogen P. infestans was found.
The R2-like gene marker fragment was not amplified in any of the accessions of primitive species, while it was found in most of the accessions of North American wild potato species. This gene was first identified in cultivated potatoes (clone SW93-1015), into which it was presumably introgressed from S. demissum [41], which grows in North and Central America. Comparison of the R2-like sequence (GenBank: FJ536323.1) with gene homologs in the whole genome nucleotide sequence of the reference potato genome (NW_006239540.1) [42] revealed considerable differences in sequences. In particular, several SNPs (from three to eight in different homologs) were found in the primer annealing region of the R2 area 1/2 marker. In any case, no correlation between the presence of this marker and resistance was found in any of the studied groups.
Both markers of another effective late blight resistance gene, RB/blb1 (Rpi-sto1 and Rpi-blb1), were found only in one resistant S. bulbocastanum genotype and in two susceptible S. stenotomum subsp. stenotomum, while they were not found in any of the studied accessions of South American wild species. At the same time, the RB/blb1 gene fragment (Rpi-sto1 marker) was often found in primitive cultivated species. Previously, in the study of the NBS-LRR family, which includes most of the known genes for resistance to various plant diseases, including late blight, a number of sequences of the reference potato genome (S. phureja DM1-3 516 R44) were found to be similar in some regions to RB/blb1 [13]. As for the R2-like gene, sequences of the RB/blb1 gene and its homologs in the reference genome of doubled monoploid clone DM1-3 516 R44 have a number of substantial differences. As expected, the most substantial differences were found in the Rpi-blb1 marker primer annealing region, up to 15 nucleotide substitutions. On the other hand, the presence of both of the RB/blb1 gene markers in some S. bulbocastanum and S. stenotomum subsp. stenotomum accessions points to the similarity of primer annealing regions in these accessions.
The marker fragment of another gene, Rpi-blb2, originally found in S. bulbocastanum, was not observed in accessions of wild North American species and was detected only in single primitive cultivated potato species, which can also be explained by nucleotide polymorphism between the S. bulbocastanum gene sequence (GenBank: DQ122125. 1) and its homologs in primitive species, especially in primer annealing regions. The degree of similarity between Rpi-blb2 and similar sequences in DM1-3 516 R44 does not exceed 90%.
The results of the search for homologs of the RB/blb1 and Rpi-blb2 genes in wild potato species are consistent with the data reported elsewhere. Namely, homologs of the Rpi-blb2 gene were found in the South American species S. alandiae and S. okadae [2], while they were absent from the North American species S. cardiophyllum, S. jamesii, S. lesteri, S. pinnatisectum, S. polyadenium, S. polytrichon, S. stoloniferum, S. trifidum, and S. verrucosum [43]. For the further study of the RB/blb1 gene homologs in representatives of tuber-bearing species of the genus Solanum, it is recommended to use an extended sample of accessions of South American wild species. In the present study, the Rpi-sto1 and Rpi-blb1 markers were not found in S. alandiae, which is consistent with the data of Muratova et al. [2], and were not found in accessions of S. doddsii, S. kurtzianum, S. leptophyes, S. neocardenasii, S. sparsipilum, S. spegazzinii, and S. yungasense. At the same time, the RGA1F/R marker, amplified with another primer pair, was found in the South American species S. chacoense and S. huancabambense, as well as in the North American species S. cardiophyllum, S. jamesii, S. lesteri, S. pinnatisectum, S. polyadenium, S. polytrichon, S. stoloniferum, S. trifidum, and S. verrucosum [43].
Using primers designed to mark effective resistance gene, Rpi-vnt1, first discovered in the wild-growing species S. venturii and its homolog, the Rpi-phu1 gene [16], marker fragments were obtained in almost all studied accessions of primitive potato species. However, no correlation between the presence of the marker and resistance was observed. The authors who previously studied late blight resistance genes in S. venturii identified a series of genes with similar sequences located in a single cluster on chromosome 9 [17]. Close to this cluster, the well-known tomato mosaic virus resistance gene Tm-22 is located, as well as the late blight resistance gene of S. phureja, Rpi-phu1. The high degree of similarity of Rpi-vnt1.1 and Rpi-vnt1.3 and their relative similarity to Tm-22 suggested their common origin. In the present study, N‑terminal sequences of the coiled-coil (CC) domain in primitive potato cultivars were analyzed and a number of variations was identified. One of the variants is a pseudogene, as it carries a five-nucleotide deletion, which leads to substantial changes in the protein structure. In resistant accessions of S. ajanhuiri, S. phureja, and S. stenotomum subsp. stenotomum, other sequence variants were found that do not have stop codons in this region. The putative amino acid sequences of these fragments have a structure typical of the CC domain and, possibly, are parts of functional genes. The simultaneous presence of several variants of similar sequences in the same genotype can be explained by the complex structure of resistance gene loci of the CC-NBS-LRR type with similar sequences arranged in tandem. On the basis of the identified SNPs in the found variants, in the future, a PCR marker can be developed for screening an extended sample of primitive potato species from the VIR collection and assessing the correlation of variant sequences like Rpi-vnt1 with phytophthora resistance.
Late blight resistance of primitive cultivated species S. ajanhuiri, S. stenotomum, and S. phureja and wild potato species seems to be caused by different genetic determination. In the present study, the majority of resistant and moderately resistant accessions of primitive cultivated species showed correlation between the resistance to the pathogen and the presence of one of the Rpi-vnt1 allelic variants. Bioinformatic search for homologs of other known resistance genes RB/blb1, Rpi-blb2, and R2-like along with the study of their polymorphism and possible association with the trait in primitive cultivated potato species is promising. The most interesting accessions for further study are S. stenotomum subsp. stenotomum, resistant to late blight, but not possessing any of the investigated SCAR markers of the Rpi genes. They can serve as sources of new, previously unknown resistance genes.
REFERENCES
Ristaino, J.B., Tracking historic migrations of the Irish potato famine pathogen, Phytophthora infestans, Microbes Infect., 2002, vol. 4, no. 13, pp. 1369—1377. https://doi.org/10.1016/s1286-4579(02)00010-2
Muratova (Fadina), O.A., Beketova, M.P., Kuznetsova, M.A., et al., South American species Solanum alandiae Card. and S. okadae Hawkes et Hjerting as potential sources of potato late blight resistance genes, Tr. Prikl. Bot., Genet. Sel., 2020, vol. 181, no. 1, pp. 73—83. https://doi.org/10.30901/2227-8834-2020-1-73-83
Kim, H.J., Lee, H.R., Jo, K.R., et al., Broad spectrum late blight resistance in potato differential set plants MaR8 and MaR9 is conferred by multiple stacked R genes, Theor. Appl. Genet., 2012, vol. 124, pp. 923—935. https://doi.org/10.1007/s00122-011-1757-7
Samen, F.M., Secor, G.A., and Gudmestad, N.C., Variability in virulence among asexual progenies of Phytophthora infestans, Phytopathology, 2003, vol. 93, no. 3, pp. 293—304. https://doi.org/10.1094/PHYTO.2003.93.3.293
Bradeen, J.M., Haynes, K.G., and Kole, C., Introduction to Potato: Genetics, Genomics and Breeding of Potatoes, Enfield, NH: Sci. Publ., 2011, pp. 1—19.
Haas, B., Kamoun, S., Zody, M., et al., Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans, Nature, 2009, vol. 461, pp. 393—398. https://doi.org/10.1038/nature08358
Lee, Y., Cho, K.S., Seo, J.H., et al., Improved genome sequence and gene annotation resource for the potato Late Blight pathogen Phytophthora infestans, Mol. Plant—Microbe Interact., 2020, vol. 33, no. 8, pp. 1025—1028. https://doi.org/10.1094/MPMI-02-20-0023-A
Khavkin, E.E., Plant—pathogen molecular dialogue: evolution, mechanisms and agricultural implementation, Russ. J. Plant Physiol., 2021, vol. 68, pp. 197—211. https://doi.org/10.1134/S1021443721020072
Martin, M.D., Vieira, F.G., Ho, S.Y.W., et al., Genomic characterization of a South American Phytophthora hybrid mandates reassessment of the geographic origins of Phytophthora infestans, Mol. Biol. Evol., 2016, vol. 33, pp. 478—491. https://doi.org/10.1093/molbev/msv241
Spooner, D.M., Ghislain, M., Simon, R., et al., Systematics, diversity, genetics, and evolution of wild and cultivated potatoes, Bot. Rev., 2014, vol. 80, pp. 283—383. https://doi.org/10.1007/s12229-014-9146-y
Budin, K.Z., Genetic foci of Solanum species, Petota Dumort, resistant to Phytophthora infestans (Mont.) De Bary, Genet. Resour. Crop Evol., 2002, vol. 49, pp. 229—235. https://doi.org/10.1023/A:1015549214779
Potato Genome Sequencing Consortium, Genome sequence and analysis of the tuber crop potato, Nature, 2011, vol. 475, pp. 189—195. https://doi.org/10.1038/nature10158
Lozano, R., Ponce, O., Ramirez, M., et al., Genome-wide identification and mapping of NBS-encoding resistance genes in Solanum tuberosum group phureja, PLoS One, 2012, vol. 7, no. 4. https://doi.org/10.1371/journal.pone.0034775
Sliwka, J., Jakuczun, H., Lebecka, R., et al., The novel, major locus Rpi-phu1 for late blight resistance maps to potato chromosome IX and is not correlated with long vegetation period, Theor. Appl. Genet., 2006, vol. 113, no. 4, pp. 685—695. https://doi.org/10.1007/s00122-006-0336-9
Śliwka, J., Jakuczun, H., Kamiński, P., et al., Marker-assisted selection of diploid and tetraploid potatoes carrying Rpi-phu1, a major gene for resistance to Phytophthora infestans, J. Appl. Genet., 2010, vol. 51, pp. 133—140. https://doi.org/10.1007/BF03195721
Foster, S., Park, T.H., Pel, M., et al., Rpi-vnt1.1, a Tm-22 homolog from Solanum venturii, confers resistance to potato late blight, Mol. Plant—Microbe Interact., 2009, vol. 22, pp. 589—600. 10.1094/MPMI-22-5-0589
Pel, M.A., Foster, S.J., Park, T.H., et al., Mapping and cloning of late blight resistance genes from Solanum venturii using an interspecific candidate gene approach, Mol. Plant—Microbe Interact., 2009, vol. 22, pp. 601—615. https://doi.org/10.1094/MPMI-22-5-0601
Song, J., Bradeen, J.M., Naess, S.K., et al., Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight, Proc. Natl. Acad. Sci. U.S.A., 2003, vol. 100, no. 16, pp. 9128—9133. https://doi.org/10.1073/pnas.1533501100
van der Vossen, E.A., Gros, J., Sikkema, A., et al., The Rpi-blb2 gene from Solanum bulbocastanum is an Mi-1 gene homolog conferring broad-spectrum late blight resistance in potato, Plant J., 2005, vol. 44, pp. 208—222. https://doi.org/10.1111/j.1365-313X.2005.02527.x
Orbegozo, J., Roman, M.L., Rivera, C., et al., Rpi-blb2 gene from Solanum bulbocastanum confers extreme resistance to late blight disease in potato, Plant Cell Tiss. Organ Cult., 2016, vol. 125, pp. 269—281 https://doi.org/10.1007/s11240-016-0947-z
Zhu, S., Li Y., Vossen, J.H., et al., Functional stacking of three resistance genes against Phytophthora infestans in potato, Transgenic Res., 2012, vol. 21, no. 1, pp. 89—99. https://doi.org/10.1007/s11248-011-9510-1
Aguilera-Galvez, C., Champouret, N., Rietman, H., et al., Two different R gene loci co-evolved with Avr2 of Phytophthora infestans and confer distinct resistance specificities in potato, Stud. Mycol., 2018, vol. 89, pp. 105—115. https://doi.org/10.1016/j.simyco.2018.01.002
Rogozina, E.V. and Gurina, A.A., Composition of the collection of primitive cultivated species within the Solanum L. section Petota Dumort. and current trends in their studies, Tr. Prikl. Bot., Genet. Sel., 2020, vol. 181, no. 3, pp. 190—202. https://doi.org/10.30901/2227-8834-2020-3-190-202
Gabriel, J., Plata, G., Cadima, X., and Franco, J., Solanum phureja Juz et Buk.: valuable source of genetic resistance to potato Late Blight (Phytophthora infestans (Mont.) de Bary), Rev. Latinoam. Papa, 2013, vol. 17, pp. 131—142
Blossei, J., Uptmoor, R., Thieme, R., et al., Insights into the genetic basis of the pre-breeding potato clones developed at the Julius Kühn Institute for high and durable late blight resistance, Plant Genet. Resour.: Charact. Util., 2021, vol. 1, no. 4. https://doi.org/10.1017/S1479262121000447
Jupe, F., Pritchard, L., Etherington, G.J., et al., Identification and localisation of the NB-LRR gene family within the potato genome, BMC Genomics, 2012, vol. 13, no. 75. https://doi.org/10.1186/1471-2164-13-75
Hawkes, J.G., The Potato: Evolution, Biodiversity and Genetic Resources, London: Belhaven Press, 1990.
Mironenko, N.V., Rogozina, E.V., Gurina, A.A., et al., Wild relatives and interspecific hybrids of potato as source materials in breeding for resistance to golden nematode, Tr. Prikl. Bot., Genet. Sel., 2020, vol. 181, no. 4, pp. 173—184. https://doi.org/10.30901/2227-8834-2020-4-173-184
Brylińska, M. and Śliwka, J., Laboratory assessment of potato resistance to Phytophthora infestans, Plant Breed. Seed Sci., 2017, vol. 76, pp. 17—23. https://doi.org/10.1515/plass-2017-00016
Khyutti, A.V., Rybakov, D.A., Gavrilenko, T.A., and Afanasenko, O.S., Resistance to late blight causal agent and golden potato nematode of modern cultivars of seed potatoes and their phytosanitary status in different agroclimatic zones of the European part of Russia, Vavilovskii Zh. Genet. Sel., 2020, vol. 24, no. 4, pp. 363—375. https://doi.org/10.18699/VJ20.629
Vleeshouwers, V.G., Van Dooijeweert, W., Keizer, L.C., et al., A laboratory assay for Phytophthora infestans resistance in various Solanum species reflects the field situation, Eur. J. Plant Pathol., 1999, vol.105, pp. 241—250. https://doi.org/10.1023/A:1008710700363
Gavrilenko, T., Antonova, O., Shuvalova, A., et al., Genetic diversity and origin of cultivated potatoes based on plastid microsatellite polymorphism, Genet. Resour. Crop Evol., 2013, vol. 60, no. 7, pp. 1997—2015. https://doi.org/10.1007/s10722013-9968-1
Haesaert, G., Vossen, J.H., Custers, R., et al., Transformation of the potato variety Desiree with single or multiple resistance genes increases resistance to late blight under field conditions, Crop Prot., 2015, vol. 77, pp. 163—175. https://doi.org/10.1016/J.CROPRO.2015.07.018
Wang, M., Allefs, S., van den Berg, R.G., et al., Allele mining in Solanum: conserved homologues of Rpi-blb1 are identified in Solanum stoloniferum, Theor. Appl. Genet., 2008, vol. 116, no. 7, pp. 933—943. https://doi.org/10.1007/s00122-008-0725-3
Lenman, M., Ali, A., Mühlenbock, P., et al., Effector-driven marker development and cloning of resistance genes against Phytophthora infestans in potato breeding clone SW93-1015, Theor. Appl. Genet., 2016, vol. 129, no. 1, pp. 105—115. https://doi.org/10.1007/s00122-015-2613-y
Alpatieva, N.V., Antonova, O.Yu., and Radchenko, E.E., et al., PTsR-diagnostika vrednykh organizmov guara (metodicheskie ukazaniya) (PCR Diagnostics for Harmful Organisms of Guar (Guidelines)), St. Petersburg: Vseross. Inst. Rastenievod., 2019. https://doi.org/10.30901/978-5-907145-44-3
Tamura, K., Stecher, G., Kumar, S., MEGA11: Molecular Evolutionary Genetics Analysis version 11, Mol. Biol. Evol., 2021, vol. 38, no. 7, pp. 3022—3027. https://doi.org/10.1093/molbev/msab120
Huaman, Z. and Ross, R., Updated listing of potato species names, abbreviations and taxonomic status, Am. Potato J., 1985, vol. 62, no. 11, pp. 629—641. https://doi.org/10.1007/BF02854438
Pel, M.A., Mapping, isolation and characterization of genes responsible for late blight resistance in potato, PhD Thesis Wageningen University, The Netherlands, 2010.
Mason, J.M. and Arndt, K.M., Coiled coil domains: stability, specificity, and biological implications, ChemBioChem, 2004, vol.5, pp. 170—176. https://doi.org/10.1002/cbic.200300781
Plich, J., Tatarowska, B., Lebecka, R., et al., R2-like gene contributes to resistance to Phytophthora infestans in Polish potato cultivar Bzura, Am. J. Potato Res., 2015, vol. 92, pp. 350—358. https://doi.org/10.1007/s12230-015-9437-9
Veilleux, R.E., Genetic stocks used for potato genome sequencing, in The Potato Genome Compendium of Plant Genomes, Cham: Springer-Verlag, 2017. https://doi.org/10.1007/978-3-319-66135-3_4
Tiwari, J.K., Devi, S., Sharma, S., et al., Allele mining in Solanum germplasm: cloning and characterization of RB-homologous gene fragments from late blight resistant wild potato species, Plant Mol. Biol. Rep., 2015, vol. 33, no. 5, pp. 1584—1598. https://doi.org/10.1007/s11105-015-0859-9
ACKNOWLEDGMENTS
Sequencing of the Rpi gene fragments was carried out using the equipment of the Genomic Technologies, Proteomics, and Cell Biology Resource Center of the All-Russian Research Institute for Agricultural Microbiology, Pushkin, St. Petersburg.
Funding
This study was supported by the Russian Science Foundation, grant no. 22-26-00111 “Potato Late Blight Resistance Genes in the Context of the Evolution of Cultivated and Wild Tuber-Bearing Species of Solanum L.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement on the welfare of animals. This article does not contain any research using animals as a subject.
Statement of compliance with standards of research involving humans as subjects. This article does not contain any research involving humans as a subject.
Conflict of interest. The authors declare that they have no conflicts of interest.
Additional information
Translated by N. Maleeva
Supplementary Information
Rights and permissions
About this article
Cite this article
Gurina, A.A., Alpatieva, N.V., Chalaya, N.A. et al. Homologs of Late Blight Resistance Genes in Representatives of Tuber-Bearing Species of the Genus Solanum L.. Russ J Genet 58, 1473–1484 (2022). https://doi.org/10.1134/S1022795422120043
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795422120043