Abstract
Changes in blood supply to the skin of the anterior-lateral surface of the shin of 12 healthy subjects were detected. The analysis was performed using laser Doppler flowmetry during transcutaneous electrical spinal cord stimulation (TSCS) by subthreshold bipolar pulses with a frequency of 30 Hz. The TSCS at T7 and L1 vertebrae level leads to a significant increase in cutaneous blood flow. With a stimulus intensity of 90% of the motor threshold, the increase in skin perfusion during stimulation at L1 was about 74%, and during stimulation at T7, 38%, relative to the baseline. We suggest that vasodilation and hyperemia of the skin during TSCS occur mainly due to the antidromic stimulation of sensory nerve fibers. Nitric oxide (NO) is an important modulator that promotes vasodilation in TSCS. It is released by the nerve endings and the layer of endothelial cells. Inhibition of cystathionine-γ-lyase significantly reduces the increase in skin blood flow during TSCS. Therefore, it was concluded that H2S, as well as NO, is also involved in the vasodilation in the skin during TSCS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The spinal level of regulation of vascular tone is critical for regulating the metabolism of organs and tissues and for the functioning of the cardiovascular system (CVS) in general. The centers of the sympathetic nervous system are located in the thoracic and lumbar spinal cord, innervating the smooth muscle cells of blood vessels, as well as modulating the activity of neurons of the cervical nodes and affecting the work of the heart. A spinal cord injury in humans is accompanied by severe multiple dysfunctions of CVS functions: hypotension, hypothermia, dysregulation of coronary blood flow, orthostatic hypotension, and circulatory disorders in the skin [1].
Experimental studies of spinal mechanisms of regulation of peripheral blood circulation in humans, as a rule, are carried out exclusively in clinical conditions, with the participation of patients. Such studies involving healthy people were unavailable for ethical reasons until recently. The development of the method of transcutaneous electrical spinal cord stimulation (TSCS) of a person [2] removed this limitation, since the most important advantage of this method is the absolute non-invasiveness and, consequently, the absence of infectious and hardware complications [3]. It has been proven that TSCS selectively affects neural networks at different levels of the spinal cord [4].
Previously, we examined the mechanisms of regulation of peripheral blood flow in humans, in which neural networks of the lumbar spinal cord are involved. For this purpose, TSCS was used at the level of the T11–T12 and L1–L2 vertebrae and blood flow was recorded in the skin of the toes and the skin of the shin [5, 6]. The skin as a tissue for assessing the effects of TSCS on blood flow was chosen not only because of the convenience of the study, but also because all types of microcirculatory blood flow regulation are present in the skin and the local mechanisms of its modulation are well studied [7]. It has been proved that nitric oxide (NO) is involved in the dilatation of skin vessels during electrical stimulation of the spinal cord [6, 8]. However, the question of the source of this NO is not yet resolved: there is no convincing evidence whether NO has neuronal or endothelial origin.
In recent years, it has been shown that in addition to NO, an important role in modulating blood flow in the skin is played by hydrogen sulfide (H2S), which is a potent vasodilator [9]. Two enzymes producing H2S were found in the wall of skin vessels: cystathionine-γ-lyase and 3-mercaptopyruvate sulfurtransferase. These enzymes are localized in the cytosol or in the mitochondria of blood vessel cells [10]. In addition, the level of H2S in the skin can also be increased non-enzymatically from various forms of storage of sulfur, such as thiosulfate, thiocysteine and sulfite [10]. In addition, it became known that there are complex relationships between these two gas transmitters (NO and H2S) that can increase or decrease the vasodilation effect [9].
It was shown that electrical stimulation of the spinal cord at the lumbar [11] and at the thoracic level [12] causes the same vasomotor effect in patients with spinal cord injury. It prevents orthostatic hypotension and normalizes blood circulation in the cerebral vessels. One of our tasks was to compare the spinal mechanisms of regulation of peripheral blood circulation in humans, implemented at the lumbar and thoracic level.
In this connection, the following tasks were set in this study: (1) to study the characteristics of the reactions of the microcirculatory bed of the tibial skin in healthy volunteers during TSCS of the segments located at different levels of the spinal cord: in the thoracic region (level of the T7–T8 vertebrae) and in the lumbar region (level of the L1–L2 vertebrae), (2) to determine the source (s) of NO in the skin, which promotes vasodilation and hyperemia in TSCS, and (3) to study the role of H2S in TSCS-induced skin hyperemia.
METHODS
The study involved 12 volunteers aged 24–45 years (7 men, 5 women). All of them were healthy, had no cardiovascular or metabolic diseases, did not take any medications, and did not smoke during the previous day. The temperature in the room in which the study was conducted was maintained at 22–23°С.
Stimulating electrodes (cathodes) were placed cutaneous, along the midline of the spine, at two levels between the spinous processes of the adjacent vertebrae T7–T8 (hereinafter, level T7) and L1–L2 (hereinafter, level L1); the anodes were placed above the iliac crests. The technique of carrying out TSCS and the equipment used were described in detail earlier [6]. At the first stage, the motor threshold (MT) was determined. MT is the minimum intensity of the current causing motor responses of the leg muscles to single monopolar impulses of 1 ms duration upon stimulation of each of the two levels of the spinal cord. The MT varied from 45 to 110 mA in different volunteers. Surface electromyography (EMG) was used to determine the motor response. The activity of m. biceps femoris, m. rectus femoris, m. gastrocnemius lateralis, and m. tibialis anterior was registered in both legs. EMG activity was recorded using H124SG electrodes (Covidien-ARBO-Kendall, Germany), A-M Systems (AM-Systems, United States) and the Lab Chart software (ADInstruments, United States).
After evaluating the MT using the multifunctional LACC M laser diagnostic complex (LAZMA, Russia), we determined the microcirculation index (MI, which is blood flow (perfusion of tissue by blood) per unit time in the probed volume, measured in perfusion units, units PU). This is initial blood flow in the skin of the anterolateral surface of the lower third of the shin. Laser Doppler flowmetry (LDF) has a number of advantages compared to other methods for assessing blood flow in the skin: the signal is continuous, has a good temporal resolution, the measurement area is very small (high spatial resolution), the method measures blood flow at a shallow depth, i.e. only in the skin, without affecting the subcutaneous structures with their specific blood supply [7, 13]. The impact of the continuous TSCS on the Т7 and L1 levels on the blood flow was registered after the recording of the initial blood flow. The bipolar pulses with a frequency of 30 Hz, filled with a frequency of 5 kHz, were used; the duration of the series is 1 min. A current intensity of 30, 60, and 90% of the individual MT was used.
In the final part of the work, the mechanisms of blood flow regulation activated in TSCS were investigated. The following substances were used: 7-nitroindazole (7-NI) (Sigma-Aldrich, United States), which is a selective inhibitor of neuronal synthase NO, and DL-propargylglycine (PAG) (Sigma-Aldrich, United States), which is an irreversible inhibitor of the cystathionine-γ-lyases. 7-NI and PAG were dissolved in distilled water. The drugs were introduced into the skin iontophoretically, and the Elfor-Prof electrophoresis apparatus (Nevoton, Russia) was used. The current strength during iontophoresis was 1 mA, the duration of iontophoresis was 2 minutes. The effect of the iontophoresis with 0.9% NaCl solution with the indicated current strength on the skin blood flow was analyzed previously. Iontophoresis of a 0.9% NaCl solution for 2–3 min did not cause significant changes in skin blood flow parameters. Upon completion of 7-NI or PAG iontophoresis, continuous TSCS was performed according to the protocol used in the second stage of the study. LDF-gram (LDF recording of blood flow in the skin) was recorded for 2 min before iontophoresis and for 1 min after 7-NI or PAG iontophoresis.
The LDF-grams were mathematically analyzed with the standard software provided with the LACC M complex (version 3.0.2.376). To analyze the amplitude-frequency characteristics, a wavelet transform was used, which was carried out by a program supplied by the manufacturer with the device. The wavelet analysis of LDF-grams allows us to estimate the amplitude of blood flow fluctuations in different frequency ranges and to calculate the values of neurogenic, myogenic, or endothelium-dependent components in the general tone of the studied blood vessels of the skin [13]. Statistical data processing was performed using Microsoft Excel tables and the Statistica for Windows v.10 software package. The results of the analysis are presented by mean values of data (M) and values of standard error. The normality of the distribution of the obtained data was determined by the Shapiro–Wilk W test. The statistical significance of differences in the means was estimated basing on Student’s t test. The results were considered statistically significant for р < 0.05.
RESULTS
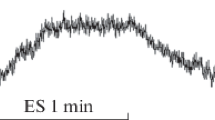
Continuous TSCS with a current amplitude of 30% of the MT was accompanied by a relatively slow and low amplitude increase in MI in the shin skin. At this magnitude of the current, the MI increase rate and its amplitude at continuous TSCS at the T7 and L1 levels differed insignificantly. An increase in the stimulating current up to 60% of the MT led to an increase in the amplitude of the increase in the MI of the tibial skin (Table 1). At the same time, the MI increase rate and its amplitude at TSCS at the L1 level were significantly higher compared to TSCS at the T7 level. The maximum changes in the amount of blood flow in the shin skin were recorded with TSCS, which was 90% of MT. It is important to note that during stimulation at the L1 level, the growth rate of MI and its amplitude were significantly higher compared to the speed obtained during stimulation of SC at the T7 level. At the same time, it should be emphasized that in all subjects with TSCS at the L1 level, despite the high growth rate of MI, blood flow began to decrease immediately after the termination of TSCS, and at TSCS at the T7 level, the increase in blood flow continued for another 15–20 s after termination of TSCS (Fig. 1).
Processing of the LDF-gram data recorded during the TSCS by means of wavelet analysis showed a pronounced increase in the amplitude of blood flow oscillations at frequencies of ~0.01 and ~0.06 Hz (Fig. 2). The first high-amplitude wave is of endothelial origin, and the second is caused by activation of sensory nerve fibers [13]. The most pronounced increase in the amplitude of oscillations in the neurogenic range was recorded with TSCS with an amplitude of 90% MT both during stimulation in the L1 region and during stimulation in the T7.
The amplitude-frequency spectrum of blood flow fluctuations in the shin skin of patient B. (a) At rest and (b) with TSCS at the L1 level with a current intensity of 90% of MT. The arrows indicate the oscillation peaks with a frequency of ~0.01 Hz (endothelial genesis) and ~0.06 Hz (caused by activation of sensory nerves). The abscissa axis is the frequency of blood flow oscillations (Hz), the ordinate axis is the oscillation amplitude (conventional units).
The local mechanisms promoting vasodilation in ESM, as already indicated in the introduction, are complex and not sufficiently studied. As for the mechanisms activated in the skin with TSCS, such information is practically absent. In order to identify the source of NO released in the wall of the blood vessels of the skin during TSCS, a selective inhibitor of neuronal synthase NO, 7NI, was used. The results of electrical stimulation after 7NI iontophoresis are presented in Fig. 3. The same figure shows blood flow data for TSCS after PAG iontophoresis. In both cases, the increase in blood flow during TSCS significantly decreased after the use of inhibitors.
Changes in the microcirculation index in the skin of the anterior-lateral part of the shin during TSCS with a current intensity of 90% of MT after iontophoresis of inhibitors: a, during stimulation in the T7 region; b, during stimulation in the L1 region. MI is presented in % of the initial level in intact skin. Significant differences: * р < 0.05; # р < 0.01.
DISCUSSION
As in our previous study of changes in blood flow in the shin skin with lumbar TSCS [6], in this study, continuous TSCS with subthreshold stimuli was accompanied by an increase in blood flow in the shin skin, while the degree of increase in MI correlated with the amplitude of the applied current. As in [6] stimulating electrodes were placed at the close distance from each other (Т11 and L1), the TSCS results after the stimulation of the neighboring regions of the spinal cord differed insignificantly. In the present study, the stimulating electrodes were located far enough from each other (T7 and L1), therefore, in TSCS, the increase in blood flow in the first or second region was very different (Table 1). During TSCS in the L1 region with a current of 90% of the MT, the increase in blood flow was 74% compared to the initial one, while during TSCS in the T7 region with a current of the same magnitude, only 38%. It can be assumed that the difference in the reactions of the skin blood supply system is explained by the fact that, by stimulating different segments of the spinal cord, we affected different parts of the regulation of vascular blood flow. The sympathetic neurons located in the ganglia in the T7 region innervate mainly the organs of the lower part of abdominal cavity and the pelvis and produce a relatively small number of nerve fibers going to the vessels of the lower extremities. The sympathetic nerve fibers (including cholinergic) of the lower ganglia “connect to the lumbosacral plexus and are distributed along the lower limb along the main nerves, sciatic and femoral, and from this the innervation extends to the vessels of the thigh and shin” [14]. A similar distribution is observed for sensory nerve fibers. Due to the innervation characteristics of the vessels of the lower extremities, the stimulation effect of the lower segments of the spinal cord is more pronounced.
The second feature identified during the study is the differences in the rate of increase in MI during TSCS in T7 and L1 regions. However, to answer the question, what is the reason for the differences in the rate of increase in MI and the duration of the increase in blood flow with TSCS at T7 and L1 at the present time is not possible. An additional special series of studies is needed.
An analysis of the literature indicates that the local mechanisms leading to an increase in blood flow in the skin with ESSC and thermal exposure are not well understood [15, 16]. Several studies have found that part of reflex vasodilation in human skin is caused by the release of NO at the ends of sensory nerves [17]. At the same time, other authors prefer the role of endothelial synthase NO in the mechanisms of cutaneous reflex vasodilation [18]. Previously, we have also shown the important role of NO in increasing blood flow in the skin during TSCS. In the previous work, we used L-NAME, which is a non-specific inhibitor of all NO synthases, which created certain difficulties in identifying the source of NO produced during TSCS [6]. In this work, in order to determine the role of nerves in the formation and release of NO during TSCS, we used 7-Nitroindazole, which is a selective inhibitor of neuronal synthase NO. 7-NI was injected into the skin by electrophoresis immediately before TSCS and blood flow measurement. In the skin treated with 7-NI, TSCS led to a decrease in the rate of hyperemia development compared to intact skin; the amplitude of MI increase was also lower (Fig. 2). However, if we compare the decrease in MI growth during TSCS with an intensity of 90% of MT after pretreatment with L-NAME [6] and with 7-NI under the same experimental conditions, it is clearly seen that L-NAME had a greater inhibitory effect on the growth of MI at TSCS in the L1 region. L-NAME inhibits both types of NO synthases and this leads to a decrease in blood flow by ~40% relative to the increase upon stimulation of 90% of MT [6]. 7-NI, which selectively inhibits only neuronal synthase, causes a decrease in blood flow by ~20% relative to growth at 90% of MT (Fig. 2). Thus, we can conclude that in TSCS, NO formed in the wall of blood vessels has both neurogenic and endothelial origin. As is known, activation of sensory nerves stimulates the release of neuronal NO and the calcitonin gene related peptide (CGRP) in the nerve endings [19], and the latter can activate endothelial synthase NO, as was found in the culture of some tissues [20] and directly in the wall of microvessels [21]. Direct interaction of neuronal and endothelial synthases NO is also possible, as was shown in the myocardium [22]. The NO synthesis blockade in our experiments, both with L-NAME and with 7-NI, only reduced the value of the TSCS-induced vasodilation in the shin skin, but did not cancel it, which implies the activation of other signaling pathways during the TSCS smooth muscle cells of blood vessels of the skin, vasodilation and hyperemia.
In the final series of experiments, we tested the hypothesis that H2S may be involved in the implementation of the vasodilation effect of TSCS. As mentioned earlier, H2S is found in many tissues and is believed to be one of the gas transmitters [23]. H2S has a rather low barrier to intercellular transport and can function well as a paracrine signaling molecule. In the last decade, H2S has been shown to have many physiological effects on the vessel wall. H2S is produced by vascular cells and has antioxidant, antiapoptotic, anti-inflammatory, and vasoactive properties [24].
The main mechanism by which H2S affects the activity of signaling proteins is the persulfidation of reactive cysteine residues on target proteins to form a persulfide group (-SSH) [23]. Depending on the nature of the target protein, the effect of H2S may take from a few seconds to several days. For example, persulfidation of ATP-sensitive K-channels leads to hyperpolarization and relaxation of smooth muscle cells, which occurs within a few seconds. It is recognized that the main effect of H2S in the vasculature is vasodilating, although in some cases a vasoconstrictor effect has been identified [25].
In our study, we performed TSCS after iontophoresis in the shin skin of the DL-Propargylglycine, which is an irreversible inhibitor of cystathionine-γ-lyase, the main enzyme that produces H2S in the vascular wall. After PAG iontophoresis, the amplitude of MI growth was significantly lower compared to intact skin (Fig. 3). The data obtained undoubtedly indicate the involvement of H2S in the vasodilation process during TSCS, but it is rather difficult to explain the activation of cystathionine-γ-lyase during TSCS. In this regard, [23] is extremely interesting, in which it was shown that NO donors increase the relaxation of smooth muscles caused by H2S. We can consider the following hypothetical scheme explaining the participation of H2S in TSCS-induced vasodilation of the shin skin vessels: through atidromic activation of the sensory nerve endings TSCS leads to the release of neuronal NO (proved [19]), which, acting on cystathionine-γ-lyase, leads to its activation. In addition, NO produced by endothelium can also stimulate cystathionine-γ-lyase (discussed above). Activation of cystathionine-γ-lyase leads to the formation of H2S, which has an additional vasodilation effect, using other signaling pathways other than signaling pathways activated by NO [26, 27].
CONCLUSIONS
(1) TSCS at the Т7–Т8 vertebrae level, as well as during stimulation at the L1–L2 level, by continuous electric pulses with a frequency of 30 Hz and an intensity of 30–90% of the motor threshold, leads to an increase in perfusion of the shin skin.
(2) The magnitude and rate of change in blood flow during stimulation in the L1–L2 region significantly differ from that in stimulation in the Т7–Т8 region, which demonstrates the possibility of using TSCS for targeted exposure to different sections of blood flow regulation.
(3) The increase in the microcirculation in the shin skin during TSCS has been proven to be caused mainly by antidromic activation of the sensor nerve fibers, producing NO by their endings.
(4) It was found that, along with neuronal NO, the vasodilation effect in the skin of the shin during TSCS is modulated by endothelial NO.
(5) It has been shown for the first time that H2S produced by cystathionine-γ-lyase is involved in the process of vasodilation in the skin with TSCS.
REFERENCES
Garstang, S.V. and Miller-Smith, S.A., Autonomic nervous system dysfunction after spinal cord injury, Phys. Med. Rehabil. Clin. N. Am., 2007, vol. 18, no. 2, p. 275.
Harkema, S., Gerasimenko, Y., Hodes, J., et al., Effect of epidural stimulation of thelumbosacral spinal cord on voluntary movement, standing, and assisted step-ping after motor complete paraplegia: a case study, Lancet, 2011, vol. 377, no. 9781, p. 1938.
Gerasimenko, Y., Gorodnichev, R., Moshonkina, T., et al., Transcutaneous electrical spinal cord stimulation in humans, Ann. Phys. Rehabil. Med., 2015, vol. 58, no. 4, p. 225.
Gerasimenko, Y., Gorodnichev, R., Puhov, A., et al., Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans, J. Neurophysiol., 2014, vol. 113, no. 3, p. 834.
Lobov, G.I., Shcherbakova, N.A., Gorodnichev, R.M., et al., Effect of transcutaneous electrical spinal cord stimulation on the blood flow in the skin of lower limbs, Hum. Physiol., 2017, vol. 43, no. 5, p. 518.
Lobov, G.I., Gerasimenko, Yu.P., and Moshonkina, T.R., Mechanisms of vasodilation in skin during lumbar transcutaneous spinal cord stimulation, Hum. Physiol., 2019, vol. 45, no. 4, p. 389.
Johnson, J.M., Minson, C.T., and Kellogg, Jr., D.L., Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation, Compr. Physiol., 2014, vol. 4, no. 1, p. 33.
Ozüm, Ü., Akyol, M., Balaban, H., et al., Effect of cervical spinal cord electrical stimulation on nitric oxide levels in brain and dermal tissues: an evaluation using by real-time nitric oxide measurement, Acta Neurochir. (Wien), 2012, vol. 154, no. 9, p. 1641.
Branco, L.G., Soriano, R.N., and Steiner, A.A., Gaseous mediators in temperature regulation, Compr. Physiol., 2014, vol. 4, no. 4, p. 1301
Streeter, E., Hart, J., and Badoer, E., An investigation of the mechanisms of hydrogen sulfide-induced vasorelaxation in rat middle cerebral arteries, Naunyn-Schmiedebergs Arch. Pharmakol., 2012, vol. 385, no. 10, p. 991.
Aslan, S.C., Legg Ditterline, B.E., Park, M.C., et al., Epidural spinal cord stimulation of lumbosacral networks modulates arterial blood pressure in individuals with spinal cord injury-induced cardiovascular deficits, Front. Physiol., 2018, vol. 9, p. 565.
Phillips, A.A., Squair, J.W., Sayenko, D.G., et al., An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury, J. Neurotrauma, 2018, vol. 35, no. 3, p. 446.
Krupatkin, A.I. and Sidorov, V.V., Funktsional’naya diagnostika sostoyaniya mikrotsirkulyatorno-tkanevykh sistem (Rukovodstvo dlya vrachei) (Functional Diagnostics of Microcirculatory Tissue System State: Handbook for Physicians), Moscow: Librokom, 2013.
Dellon, A.L., Höke, A., Williams, E.H., et al., The sympathetic innervation of the human foot, Plast. Reconstr. Surg., 2012, vol. 129, no. 4, p. 905.
van Duijnhoven, N.T., Janssen, T.W., Green, D.J., et al., Effect of functional electrostimulation on impaired skin vasodilator responses to local heating in spinal cord injury, J. Appl. Physiol., 2009, vol. 106, no. 4, p. 1065.
Smith, C.J., Craighead, D.H., and Alexander, L.M., Effects of vehicle microdialysis solutions on cutaneous vascular responses to local heating, J. Appl. Physiol., 2017, vol. 123, no. 6, p. 1461.
Wong, B.J., Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans, Am. J. Physiol.-Regul. Integr. Comp. Physiol., 2013, vol. 304, no. 8, p. R651.
Bruning, R.S., Santhanam, L., Stanhewicz, A.E., et al., Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin, J. Appl. Physiol., 2012, vol. 112, no. 12, p. 2019.
Croom, J.E., Foreman, R.D., Chandler, M.J., and Barron, K.W., Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP, Am. J. Physiol., 1997, vol. 272, no. 2, p. H950.
Yan, L., Yinghui, T., Bo, Y., et al., Effect of calcitonin gene-related peptide on nitric oxide production in osteoblasts: an experimental study, Cell Biol. Int., 2011, vol. 35, no. 8, p. 757.
Wu, M., Komori, N., Qin, C., et al., Roles of peripheral terminals of transient receptor potential vanilloid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation, Brain Res., 2007, vol. 1156, p. 80.
Idigo, W.O., Reilly, S., Zhang, M.H., et al., Regulation of endothelial nitric-oxide synthase (NOS) S-glutathionylation by neuronal NOS: evidence of a functional interaction between myocardial constitutive NOS isoforms, J. Biol. Chem., 2012, vol. 287, no. 52, p. 43 665.
Hosoki, R., Matsuki, N., and Kimura, H., The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide, Biochem. Biophys. Res. Commun., 1997, vol. 237, no. 3, p. 527.
Bełtowski, J., Hydrogen sulfide in pharmacology and medicine. An update, Pharmacol. Rep., 2015, vol. 67, no. 3, p. 647.
Kimura, H., The physiological role of hydrogen sulfide and beyond, Nitric Oxide, 2014, vol. 41, p. 4.
Zhao, W., Zhang, J., Lu, Y., and Wang, R., The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener, EMBO J., 2001, vol. 20, no. 21, p. 6008.
Li, L., Rose, P., and Moore, P.K., Hydrogen sulfide and cell signaling, Annu. Rev. Pharmacol. Toxicol., 2011, vol. 51, p. 169.
Funding
The study was financially supported by the Program “Fundamental Scientific Research for the Long-Term Development and Ensuring the Competitiveness of Society and the State” (Topic 63.4, (0113-2019-0006)), Russian Foundation for Basic Research, project no. 16-29-08277 and the Basic Research Program of the Presidium of the Russian Academy of Sciences no. 1.42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare no apparent or potential conflicts of interest related to the publication of this article.
Statement of compliance with standards of research involving humans as subjects. All studies were carried out in accordance with the principles of biomedical ethics formulated in the Helsinki Declaration of 1964 and its subsequent updates and approved by the local bioethical committee of the Institute of Physiology, RAS (St. Petersburg). Each study participant submitted voluntary written informed consent, signed by him after explaining to him the potential risks and benefits, as well as the nature of the forthcoming study.
Additional information
Translated by I. Shipounova
Rights and permissions
About this article
Cite this article
Lobov, G.I., Gerasimenko, Y.P. & Moshonkina, T.R. Modulation of Blood Flow in the Skin of Human Legs during Transcutaneus Electrical Stimulation of the Spinal Cord. Hum Physiol 46, 384–390 (2020). https://doi.org/10.1134/S0362119720040088
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119720040088