Abstract
The changes in blood flow in shin skin were analyzed by laser Doppler flowmetry during transcutaneous electrical spinal cord stimulation (TSCS) by subthreshold bipolar pulses with a frequency of 30 Hz in 12 healthy subjects. It was shown that TSCS in the area of the T11 and L1 vertebrae led to a significant increase in skin blood flow. With a stimulus intensity of 90% of the motor threshold, the microcirculation rate increased by more than 85% compared to the baseline. The findings indicate that the stimulation of blood flow in skin by TSCS is mainly due to the antidromic stimulation of sensory nerve fibers. Nitric oxide (NO) is an important mediator contributing to vasodilation and increased cutaneous blood flow in TSCS; NO is of mostly endothelial origin. It was shown that high-conductivity Ca2+-sensitive K+ channels are involved in the process of vasodilation in skin during TSCS. The interaction between NO- and Ca-mediated mechanisms in shin skin was observed during TSCS, resulting in increased vasodilation and blood flow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
According to the data from the World Health Organization, from 250 000 to 500 000 people worldwide suffer spinal cord (SC) injury annually; as a result, the number of patients with SC injury in developed countries is more than 100 per 100 000 people [1]. The classical manifestations of SC injury are severe somatic symptom disorders, namely, the impairment or absence of motor and sensory functions below the level of injury. At the same time, SC injury is also accompanied by impaired control of the processes and functions regulated by the autonomic nervous system. One of the most important disorders of autonomic regulation is impaired functions of the cardiovascular system (CVS) (bradycardia, hypotension, hypothermia in the acute phase and, later on, dysregulation of coronary blood flow, orthostatic hypotension, altered control of skin blood flow and, as a consequence, impaired thermoregulation and responses to physical loads) [2, 3].
The severity of impairment of CVS functions is determined by the level of injury. SC injury, even at a low level, such as T12, is accompanied by the loss of supraspinal and spinal control of sympathetic neurons localized in the lower lumbar ganglia, which innervate the vessels of the lower extremities, and by the absence of peripheral vasomotor responses [2]. At the same time, is has been reported that the spinal neural nets after SC injury are able to generate some activity [3]; in particular, sympathetic preganglionic neurons also generate spontaneous activity in spite of the absence of supraspinal activation [4].
Therapy for SC injury is a serious medical and social issue; the search for methods that could substantially relieve their condition is continued. In particular, the method of electrical stimulation (ES) of SC for the rehabilitation of motor function after SC injury has evolved considerably in recent decades; this method makes it possible not only to control the tone of skeletal muscles of the lower extremities but also to induce stepping movements [5]. However, it should be noted that SC ES was proposed for clinical practice more than 50 years ago by Shealy et al., first of all, for treating ischemic disease not amenable to other types of therapy [6]. The method proved to be quite efficient and was usually applied in the case of impossibility of extremity revascularization in patients with endarteritis and Raynaud’s disease. However, the treatment procedure was invasive and required the insertion of four to eight electrodes into the epidural spinal space between the 8th and 11th thoracic vertebrae and the 1st and 2nd lumbar vertebrae [6, 7]. The complicated procedures of electrode implantation and fixation were often accompanied by hardware (electrode migration, loss of contact, hematomas) and infectious complications with a frequency of occurrence of 36.3 and 6.7%, respectively [8, 9].
The method of transcutaneous electrical spinal cord stimulation (TSCS) developed in recent years is fundamentally different as it is noninvasive. This method involves the placing of electrodes on skin above particular segments of the spinal cord and allows modulating the contractions of skeletal muscles of the lower extremities and, with certain parameters of electrical pulses, inducing stepping movements in healthy volunteers and in patients with paraplegia as a result of SC injury [5, 10, 11]. The authors of the method have established that multi-segment TSCS is accompanied by the convergence of descending and ascending effects on the neural networks controlling postural–locomotion functions. In these studies, the attention of researchers was focused primarily on investigation of the effects of TSCS on the neural networks controlling skeletal muscles. However, the analysis of effects has shown that TSCS, affecting the dorsal roots of SC, activates not only the neural networks controlling skeletal muscles but also other SC structures. Later, we studied the effect of TSCS on blood flow in the skin of hallux [12]. Skin tissue was chosen for estimating the effects of TSCS on blood flow not only because of research convenience, which is also important, but also for other reasons: in skin, there are all types of regulation of microcirculation [13] but no working hyperemia of skeletal muscles that could mask neurogenic regulatory responses. In addition, the range of changes in skin blood flow is enormously high compared to other tissues: in humans, from almost zero in the cold up to 6–7 L/min under extreme thermal loads [14].
We showed that TSCS was accompanied by enhanced skin perfusion; in addition, the amplitude of microcirculatory oscillations increased within a frequency range of 0.05–0.1 Hz [12], demonstrating their neurogenic origin [15]. The aim of this study was to investigate the effects and mechanisms of action of TSCS inducing no motor response on the blood flow in shin skin where, in contrast to the skin of the plantar surface of hallux, there is a more complex system for regulation of vasomotor responses [16].
METHODS
Research participants were 12 volunteers aged 27–42 years (5 men and 7 women). All subjects were healthy, had no cardiovascular or metabolic diseases, did not take any medicines, and were 24-h smoke free.
Stimulating electrodes (cathodes) were placed at two levels between the spinous processes of the neighboring vertebra Т11–Т12 and L1–L2; anodes were mounted above the iliac crests. The procedure and equipment of TSCS have been described previously [17]. At the first stage, we determined the minimum intensity of the current inducing motor responses in leg muscles to single 1-ms unipolar pulses in stimulation of each of the two levels of the spinal cord, i.e., the motor threshold (MT). The MT was from 40 to 100 mA in different subjects. Surface electromyography was used to analyze motor response. The m. biceps femoris, m. rectus femoris, m. gastrocnemius lateralis and m. tibialis anterior activities were recorded on both legs. EMG activity was recorded using H124SG electrodes (Covidien-ARBO-Kendall), A-M Systems (United States), and the LabChart software (United States).
The effect of continuous TSCS at the Т11 and L1 levels was recorded at the second stage of research. 30 Hz bipolar pulses with a 5 kHz frequency filling were used; the series duration was 0.5–1 min. The current intensity was gradually increased from 0 mA to 90% of the individual MT with a step of 5–10 mA. The parameters and mechanisms of regulation of skin blood flow were assessed using a LAKK-M multifunctional laser diagnostic system (LAZMA, Russian Federation). The temperature in the experimental room was maintained at a level of 22–23°С. The tip of the fiber optic probe of the diagnostic system was applied to skin on the anterior surface of the lower third of the leg. Effective perfusion index was calculated by the equation Meff = M × Amax, where M is the index of microcirculation and Amax is the maximum averaged oscillation amplitude with the dominant value among oscillation amplitudes of all active frequency ranges [15].
The mechanisms of blood flow regulation activated by TSCS were determined at the third stage of research. The following agents were used: N(G)‑nitro-L-arginine methyl ester (L-NAME, Sigma), 20 mM; tetraethylammonium chloride (TEA, Sigma), 20 mM; EMLA cream (AstraZeneca, Wilmington) containing 2.5% lidocaine and 2.5% prilocaine. L-NAME and TEA were dissolved in distilled water. The preparations were introduced into skin by iontophoresis with an Elfor Prof electrophoretic system (Nevoton, Russian Federation). Iontophoresis was performed at a current of 1 mA for 2 min. Previous research showed that the 2-min iontophoresis of 0.9% NaCl solution with the above current strength did not cause any significant changes in the parameters of skin blood flow. EMLA cream was applied directly to skin over about 4 cm2. Application of the agents was accompanied by continuous TSCS according to the protocol used at the second stage of research.
The mathematical processing of LDF-grams was carried out using the standard LAKK-M software (v. 3.0.2.376). Amplitude–frequency characteristics were analyzed by wavelet transform algorithm using the bundled software. Statistical data processing was performed with Microsoft Excel and Statistica for Windows, v. 10. The results of analysis are presented as the mean of a data set (M) and the values of the standard deviation. Statistically significant differences between the mean values were assessed by Student’s t‑test. The results were considered to be statistically significant at p < 0.05.
RESULTS
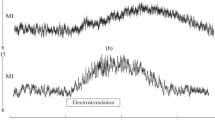
Single-pulse TSCS did not lead to any significant changes in skin perfusion. Continuous TSCS with the current amplitude above 20% of MT was accompanied by a rapid increase in skin microcirculatory index (MI). The value and rate of increase in skin MI became greater with the increase in stimulation current. The maximum skin blood flow was obtained within about 30 s of TSCS. The typical record of shin skin perfusion during TSCS with the current amplitude of 90% of MT is shown in Fig. 1; the averaged data on MI value in shin skin during TSCS with the stimulus amplitudes of 30, 60, and 90% of MT are presented in Table 1. MI in shin skin was nonsignificantly higher during stimulation at the L1 level compared to the T11 level at the same current amplitude (in percent of MT).
The wavelet analysis of LDF-grams recorded during TSCS showed an increase in the amplitude of microcirculation oscillations in the neurogenic range; these reactions were most marked during TSCS with the amplitude of 90% of MT. During TSCS in the L1 region with the stimulus amplitude of 90% of MT, the effective perfusion index in shin skin increased by 93.7%.
Since our study has shown no significant differences between the effects of TSCS at the Т11 and L1 levels on skin blood flow, hereinafter we present the data on TSCS only at the L1 level. Figure 2 shows the results of testing skin blood flow after skin pretreatment with EMLA cream, which blocks sensory nerves [18]. On EMLA-treated skin areas, the vasodilator response to TSCS was lower. EMLA held down the TSCS-induced increase in blood flow by 73.0 ± 5.7% at the stimulation current intensity of 30% of MT, by 54.3 ± 4.2% at 60% of MT, and by 41.4 ± 3.8% at 90% of MT, relative to the value of MI increase in the intact skin.
The microcirculatory flow index in intact shin skin and shin skin pretreated with EMLA during TSCS at the L1 level by continuos stimuli of the different intensity (in % of MT): a, 30%; b, 30% against EMLA; c, 60%; d, 60% against EMLA; e, 90%; f, 90% against EMLA. * The difefrences are significant, р < 0.01.
When studying the role of sensory nerves in reflex vasodilation in human skin, Wong showed that vasodilation is partially determined by nitrogen oxide (NO) [18]. Therefore, in some experiments we electrophoretically introduced L-NAME (NO synthase inhibitor) into skin prior to TSCS. The results of these experiments are shown in Fig. 3. The treatment of skin with L-NAME resulted in significant reduction of TSCS-induced hyperemia. The L-NAME-induced decrease in hyperemia during TSCS was 58.8 ± 4.11% and 38.6 ± 3.32% of hyperemia in intact skin at a stimulus intensity of 30% and 90% of MT, respectively.
The microcirculatory flow index in shin skin during TSCS at the current amplitude of (a) 30% of MT and (b) 90% of MT at the L1 level and in intact skin (ES), against the inhibition of NO production (L-NAME), against the blocking of BKCa channels (TEA), and upon combined application of L-NAME and TEA. * The differnces are significant, р < 0.01.
Hodges et al. [19] studying the mechanisms of reactive hyperemia in skin demonstrated the significant role of high-conductance Ca-sensitive K+ channels (BKCa channels) of smooth muscle cells in vasodilation. Taking into account the results of their and other studies, we have investigated the role of BKCa channels of smooth muscle cells of skin microvessels in the vasodilator effect of TSCS. BKCa channels were blocked before TSCS by means of iontophoretic introduction of TEA into skin [20]. The results of this series of experiments are shown in Fig. 3. The MI value in skin after the iontophoresis with TEA did not vary significantly. TSCS after the iontophoresis with TEA resulted in enhanced skin blood flow; however, against the background of TEA, the increase in MI was much less compared to that during TSCS of intact skin. The inhibitory effect of TEA was observed only with TSCS stimuli of 60 and 90% of MT; stimulation with the current of 30% of MT in the presence of TEA caused nonsignificant changes in MI.
Simultaneous iontophoresis with L-NAME and TEA yielded the maximum inhibitory effect (during stimulation with the intensity of 90% of MT, the MI increased by 19.9 ± 2.14% of the MI increase in intact skin). It should be noted that the inhibitory effect of L-NAME + TEA combination was higher compared to the sum of individual inhibitory effects of L-NAME and TEA (Fig. 3).
DISCUSSION
Spinal cord injury leads not only to the impairment of motor and sensory functions below the level of injury, but also to the loss of supraspinal control of autonomic functions. Preganglionic neurons and interneurons involved in the regulation of autonomic functions lose their connection to rostroventrolateral medulla neurons and, via the latter, to suprabulbar structures performing the integration of somatic–autonomic responses [4, 21, 22]. A considerable part of these neurons have a spontaneous activity, which is regulated through bulbospinal connections. The absence of bulbar effects results in disinhibition of preganglionic neurons, which is accompanied, in the case of SC injuries at the Т4–Т6 level, by life-threatening dysreflexia hypertension [23].
In the past decade, SC ES has been widely used for postural regulation and initiation of stepping movements in patients with SC injuries [1, 5, 24]. At the same time, SC ES, in addition to its effect of SC neural networks responsible for somatic functions, exerts a marked influence on SC structures regulating autonomic functions. The same happens in the case of noninvasive TSCS [10, 11]. In our previous work, when studying the locomotor responses of subjects to TSCS with supra-threshold stimuli, we simultaneously measured blood flow in the skin of hallux [12]. At the same time, muscle responses to the stimuli caused certain difficulties of recording skin blood flow by the LDF (laser Doppler flowmetry) technique. The objective of the present research was to investigate the parameters of blood flow and the mechanisms of its regulation in shin skin during TSCS with the parameters below MT. The measurement of skin blood flow during SC ES, as an indicator of SC ES effect on autonomic functions, is not only convenient but also maximally informative when studying the effects of SC ES on blood circulation. Skin blood flow can be measured over a long period using a noninvasive technique without activation of regulatory mechanisms in the vessels of the microcirculatory bed (MCB). The blood vessels of shin skin, in contrast to the plantar skin of hallux, are characterized by more complex innervations represented by sympathetic adrenergic, sympathetic cholinergic and sensory peptidergic fibers [16]. It is no less important that now skin is generally viewed as an adequate universal model for studying microcirculatory blood flow in different pathological states, because its changes adequately reflect systemic vascular responses and the dynamics of regulatory mechanisms [25, 26].
This study has shown that shin skin perfusion was much lower compared to skin perfusion of the plantar surface of the hallux [12]. In resting subjects, the microcirculatory flow index (MFI) in shin skin varied from 5.2 to 7.6 PU. The wavelet transform of LDF-grams showed that the amplitude of oscillations of microcirculatory blood flow in the myogenic, neurogenic and endothelium-dependent ranges in resting subjects was nearly the same, with minor individual peculiarities. Continuous TSCS in the region of Т11 and L1 vertebrae by subthreshold stimuli with the current amplitudes of 30, 60, and 90% of MT resulted in the marked increase in MT in shin skin. At the same time, the increase in perfusion with electrodes mounted in the L1 region was slightly greater compared to the effect of stimulation in the Т11 region. In our opinion, the minor differences in the responses of skin MCR during TSCS in the region of Т11 and L1 vertebrae are accounted for by the fact that, in contrast to epidural stimulation, where the effects of stimuli are rather local (with cathode and anode mounted on the same electrode matrix on the dura mater of the spinal cord), during TSCS, when cathode and anode are mounted at a considerable distance on the subject’s body, the exciting effects of the stimuli not only occur directly under the electrode, but, spreading over the skin, subcutaneous fat and muscles, capture also the neighboring roots of the spinal cord. As a result, the stimulatory effect is exerted on several inputs in the lumbosacral intumescence located at the level of Т10–L1 vertebrae.
The degree of increase in skin perfusion was determined by the amplitudes of acting stimuli. With an increase in current intensity, the magnitude of vasodilator response in skin also increased, resulting in perfusion increase at a current intensity of 90% of MT by 71.1 and 85.2% compared to the initial value (for stimulation in the Т11 and L1 areas, respectively) (Table 1). TSCS with different current intensities was accompanied by the changes in not only the intensity of hyperemia, but also the rate of its escalation. During the stimulation with the amplitude of 90% of MT, the rate of MFI increase was maximal (Fig. 1). The mean root square deviation of MFI increased simultaneously with the increase in perfusion in the microcirculatory bed, demonstrating the activation of regulatory mechanisms of microcirculation [15]. As it has been noted previously, the wavelet analysis of LDF-gram recorded during TSCS showed an increase in the oscillation amplitude of microcirculatory blood flow in the neurogenic range. The above changes in perfusion parameters demonstrate intensification of the regulation of microcirculatory blood flow by neural structures [12, 15]. In addition, the oscillation amplitude of skin microcirculation in the endothelium-dependent range also significantly increased. TSCS was accompanied by an increase not only in MFI, but also in effective perfusion index, which in some cases is more informative than MFI.
Human skin exhibits the maximum range of blood flow oscillations compared to other organs: from the minimum value approaching zero under the conditions of strong cooling to 6–7 L/min under the conditions of hyperthermia [16, 27, 28]. Hundreds of studies deal with the mechanisms of temperature-dependent vasodilation in skin [11, 19, 27–29]; however, this issue has not been fully clarified up to now. Moreover, the authors of different articles often express almost contrary opinions about the significance of a particular vasodilation mechanism for skin blood flow regulation [19, 28]. The same situation is also revealed by analysis of the published data on the mechanisms of increasing skin blood flow during TSCS [7–9, 30]. Some researchers believe that SC ES-induced vasodilation may result from inhibition of the activity of sympathetic neurons [31, 32]. The second theory, which most experts adhere to, states that SC ES antidromically activates sensory fibers and induces the release of vasodilators in nerve endings, resulting in enlarged lumen of resistance vessels and increased blood inflow [33–35]. In recent years, it has also been suggested that sympathetic cholinergic nerves are significant for the regulation of blood flow in non-glabrous (hairy) skin [9, 16].
In the present study, the mechanisms of TSCS-induced vasodilation in skin have been analyzed by means of several tests using inhibitors and blockers. Skin in situ was pretreated with EMLA cream. The cream contains lidocaine and prilocaine, which block the ion channels of sensory nerves and are extensively used for studying the mechanisms of blood flow regulation in skin [19]. EMLA considerably reduced perfusion increment in skin during TSCS at all values of stimulating current (Fig. 2). The results of this test lead to the conclusion that vasodilation in calf skin during TSCS occurs partially due to antidromic activation of afferent fibers in the dorsal roots causing the release of vasodilator substances in the sensory nerve endings of skin. The involvement of sensory nerves in enhanced skin blood flow during TSCS is also confirmed by a considerable decrease in the oscillation amplitude of microcirculatory blood flow in the neurogenic range in EMLA-treated skin compared to intact skin [15].
Thus, we have tested the hypothesis that the TSCS-induced skin perfusion increment occurs for the most part due to antidromic activation of sensory nerve fibers. However, what exactly exerts a strong inhibitory effect on smooth muscle cells (SMC) of skin vessels, resulting in their relaxation and, as a consequence, vasodilation and hyperemia, is still an open question. Some works have shown that sensory nerve endings contain several compounds with vasodilator properties [36]. It is known that calcitonin gene-related peptide (CGRP) is a strong microvascular vasodilator present in nerve endings. We could not determine the exact role of CGRP in TSCS-induced vasodilation, because the classical antagonist of the CGRP receptor (CGRP 8–37) used in animal experiments is not permitted to be used in medical practice. However, there was a possibility of assessing the mediated effect of this peptide. CGRP is reported to increase the content of nitrogen oxide (NO) in microvascular tissue [37]. NO is considered to be an important component involved in vasodilation induced by the epidural stimulation of SC [9, 33, 34]. In this research, we have used electrophoresis of skin with L-NAME, which is a nonspecific NO synthase inhibitor [33, 37]. The measurement of microcirculatory blood flow in skin after L-NAME electrophoresis under thermally neutral conditions showed the absence of any significant changes compared to the microcirculation in intact skin. However, in the case of TSCS after L-NAME electrophoresis, skin perfusion increment was significantly lower: 10.3 ± 0.77 PU on the average against 12.5 ± 0.86 PU in intact skin. The findings demonstrate the involvement of NO in TSCS-induced vasodilation in shin skin. There are grounds to believe that NO formed during TSCS is mostly of endothelial origin. This statement is confirmed by: (1) the published data that L-NAME effectively inhibits endothelial NO synthase and (2) the increase in the oscillation amplitude of microcirculatory blood flow in the endothelium-dependent range recorded during TSCS, which is evidence of enhanced activity of endothelium in skin microvessels [15].
The blockade of NO synthesis in our experiments reduced the extent of TSCS-induced vasodilation in shin skin but not eliminated it, suggesting the involvement in TSCS of other mechanisms leading to relaxation of vascular SMC and vasodilation. In literature, there are data on the involvement of different types of K+ channels of endothelial and smooth muscle cells in vasodilation in skin under the conditions of hyperthermia [38]. In particular, high-conductance Ca-sensitive K+-channels of SMC were shown to play an important role in hyperthermic vasodilation in skin. High-conductivity Ca-sensitive K+ channels (BKCa channels) are localized in the membrane of vascular SMC, and their activation leads to K+ release from the cell, membrane hyperpolarization, and SMC relaxation [39]. Lorenzo and Minson obtained the data demonstrating that human BKCa channels are the key inducers of reactive hyperemia in skin, causing up to 45% of hyperemia [21]. Altogether, these data show the important role of BKCa channels in different regulatory effects on skin microcirculation. Therefore, we have conducted a series of experiments to determine whether BKCa channels are involved in the vasodilator effect of TSCS on skin. The iontophoresis with TEA, being a nonspecific inhibitor of BKCa channels, was used. The iontophoresis with TEA slightly decreased skin blood flow in the state of rest; however, this decrease was statistically nonsignificant. TSCS after the iontophoresis with TEA increased skin blood flow at all current intensities, but this increase was less than the effect of TSCS on intact skin. At a current strength of 30% of MT, skin perfusion changed little (Fig. 3); the inhibitory effect of TEA was greater as the current increased to 90% of MT (Fig. 3). The results confirm the involvement of BKCa channels in vasodilator effects of TSCS in shin skin.
The results of combined application of TEA and L‑NAME also seem to be important. The iontophoresis of these two preparations considerably enhanced their inhibitory effect on TSCS-induced vasodilation; the reduction of blood flow increment in this case was greater compared to the sum of individual inhibitory effects of L-NAME and TEA. We believe that the results of joint application of TEA and L-NAME are also important. Simultaneous iontophoresis of these two preparations considerably enhanced their inhibitory effect on TSCS-induced vasodilation; the reduction of blood flow increment in this case was greater compared to the sum of individual inhibitory effects of L-NAME and TEA. The mechanisms of vasodilation triggered by sensory nerves are somehow integrated with the signaling pathways initiated by the activation of BKCa channels, probably via sympathetic cholinergic innervation [18, 40]. However, this potential vasodilation mechanism requires separate studies.
CONCLUSIONS
The basic results of this research are the following:
(1) TSCS at the Т11 and L1 levels by continuous electrical pulses at a frequency of 30 Hz and intensity of 30–90% of the intensity inducing motor response increases perfusion in shin skin.
(2) The findings demonstrate that the enhanced microcirculatory blood flow in skin during TSCS is caused mainly by antidromic activation of sensory nerve fibers.
(3) It has been shown that NO is a key component mediating the vasodilator effect of TSCS on skin.
(4) It has been demonstrated that the TSCS-induced vasodilation in skin involves high-conductivity Ca2+-sensitive K+ channels.
(5) The interaction between NO- and BKCa-mediated mechanisms in skin during TSCS resulting in increased vasodilation has been revealed.
REFERENCES
Rouanet, C., Reges, D., Rocha, E., et al., Traumatic spinal cord injury: current concepts and treatment update, Arq. Neuro-Psiquiatr., 2017, vol. 75, no. 6, p. 387
Garstang, S.V. and Miller-Smith, S.A., Autonomic nervous system dysfunction after spinal cord injury, Phys. Med. Rehabil. Clin. North Am., 2007, vol. 18, no. 2, p. 275.
Krassioukov, A. and Claydon, V.E., The clinical problems in cardiovascular control following spinal cord injury: an overview, Prog. Brain Res., 2006, vol. 152, p. 223.
Schramm, L.P., Spinal sympathetic interneurons: their identification and roles after spinal cord injury, Prog. Brain Res., 2006, vol. 152, p. 27.
Harkema, S., Gerasimenko, Y., Hodes, J., et al., Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study, Lancet, 2011, vol. 377, no. 9781, p. 1938.
Stojanovic, M.P. and Abdi, S., Spinal cord stimulation, Pain Phys., 2002, vol. 5, no. 2, p. 156.
Jivegård, L.E., Augustinsson, L.E., Holm, J., et al., Effects of spinal cord stimulation (SCS) in patients with inoperable severe lower limb ischemia: a prospective randomized controlled trial, Eur. J. Vasc. Endovasc. Surg., 1995, vol. 9, no. 4, p. 421.
Pedrini, L. and Magnoni, F., Spinal cord stimulation for lower limb ischemic pain treatment, Interact. Cardiovasc. Thorac. Surg., 2007, vol. 6, no. 4, p. 495.
Naoum, J.J. and Arbid, E.J., Spinal cord stimulation for chronic limb ischemia, Method. Debakey Cardiovasc. J., 2013, vol. 9, no. 2, p. 99.
Gerasimenko, Y., Gorodnichev, R., Moshonkina, T., et al., Transcutaneous electrical spinalcord stimulation in humans, Ann. Phys. Rehabil. Med., 2015, vol. 58, no. 4, p. 225.
Gerasimenko, Y., Gorodnichev, R., Puhov, A., et al., Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans, J. Neurophysiol., 2015, vol. 113, no. 3, p. 834.
Lobov, G.I., Shcherbakova, N.A., Gorodnichev, R.M., et al., Effect of transcutaneous electrical spinal cord stimulation on the blood flow in the skin of lower limbs, Hum. Physiol., 2017, vol. 43, no. 5, p. 518.
Johnson, J.M., Minson, C.T., and Kellogg, D.L., Jr., Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation, Compr. Physiol., 2014, vol. 4, no. 1, p. 33.
Smith, C.J. and Johnson, J.M., Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: neural control of skin blood flow and sweating in humans, Auton. Neurosci., 2016, vol. 196, p. 25.
Krupatkin, A.I. and Sidorov, V.V., Funktsional’naya diagnostika sostoyaniya mikrotsirkulyatorno-tkanevykh sistem (Rukovodstvo dlya vrachei) (Functional Diagnostics of Microcirculatory Tissue System State: Handbook for Physicians), Moscow: Librokom, 2013.
Wong, B.J. and Hollowed, C.G., Current concepts of active vasodilation in human skin, Temperature (Austin), 2016, vol. 4, no. 1, p. 41.
Grishin, A.A., Moshonkina, T.R., Solopova, I.A., et al., A five-channel noninvasive electrical stimulator of the spinal cord for rehabilitation of patients with severe motor disorders, Biomed. Eng., 2017, vol. 50, no. 5, p. 300.
Wong, B.J., Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans, Am. J. Physiol.-Regul. Integr. Comp. Physiol., 2013, vol. 304, no. 8, p. R651.
Hodges, G.J., Traeger, J.A., Tang, T., et al., Role of sensory nerves in the cutaneous vasoconstrictor response to local cooling in humans, Am. J. Physiol. Heart Circ Physiol., 2007, vol. 293, p. H784.
Carter, S.J. and Hodges, G.J., Sensory and sympathetic nerve contributions to the cutaneous vasodilator response from a noxious heat stimulus, Exp. Physiol., 2011, vol. 96, p. 1208.
Lorenzo, S. and Minson, C.T., Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves, J. Physiol., 2007, vol. 585, p. 295.
Turner, A., Kumar, N., Farnham, M., et al., Rostroventrolateral medulla neurons with commissural projections provide input to sympathetic premotor neurons: anatomical and functional evidence, Eur. J. Neurosci., 2013, vol. 38, no. 4, p. 2504.
Rabchevsky, A.G., Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury, Prog. Brain Res., 2006, vol. 152, p. 265.
Shah, P., Sureddi, S., Alam, M., et al., Unique spatiotemporal neuromodulation of the lumbosacral circuitry shapes locomotor success after spinal cord injury, J. Neurotrauma, 2016, vol. 33, no. 18, p. 1709.
Bagno, A. and Martini, R., Wavelet analysis of the Laser Doppler signal to assess skin perfusion, Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society, Piscataway, NJ: Inst. Electr. Electron. Eng., 2015, p. 7374.
Holowatz, L.A., Thompson-Torgerson, C.S., Kenney, W.L., et al., The human cutaneous circulation as a model of generalized microvascular function, J. Appl. Physiol., 2008, vol. 105, no. 1, p. 370.
Rowell, L.B., Reflex control of regional circulations in humans, J. Auton. Nerv. Syst., 1984, vol. 11, no. 2, p. 101.
Charkoudian, N., Fromy, B., and Saumet, J.L., Reflex control of the cutaneous circulation after acute and chronic local capsaicin, J. Appl. Physiol., 2001, vol. 90, p. 1860.
Hodges, G.J., Del Pozzi, A.T., McGarr, G.W., et al., The contribution of sensory nerves to cutaneous vasodilatation of the forearm and leg to local skin heating, Eur. J. Appl. Physiol., 2015, vol. 115, no. 10, p. 2091.
Wu, M., Linderoth, B., and Foreman, R.D., Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies, Auton. Neurosci., 2008, vol. 138, nos. 1–2, p. 9.
Linderoth, B., Herregodts, P., and Meyerson, B.A., Sympathetic mediation of peripheral vasodilation induced by spinal cord stimulation: animal studies of the role of cholinergic and adrenergic receptor subtypes, Neurosurgery, 1994, vol. 35, p. 711.
Tanaka, S., Barron, K.W., Chandler, M.J., et al., Local cooling alters neural mechanisms producing changes in peripheral blood flow by spinal cord stimulation, Auton. Neurosci., 2003, vol. 104, no. 2, p. 117.
Carter, S.J. and Hodges, G.J., Sensory and sympathetic nerve contributions to the cutaneous vasodilator response from a noxious heat stimulus, Exp. Physiol., 2011, vol. 96, no. 11, p. 1208.
Croom, J.E., Foreman, R.D., Chandler, M.J., and Barron, K.W., Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP, Am. J. Physiol., 1997, vol. 272, p. 950.
Tew, G.A., Klonizakis, M., Moss, J., et al., Role of sensory nerves in the rapid cutaneous vasodilator response to local heating in young and older endurance-trained and untrained men, Exp. Physiol., 2011, vol. 96, no. 2, p. 163.
van Beek, M., van Kleef, M., Linderoth, B., et al., Spinal cord stimulation in experimental chronic painful diabetic polyneuropathy: delayed effect of high-frequency stimulation, Eur. J. Pain, 2017, vol. 21, no. 5, p. 795.
Wu, M., Komori, N., Qin, C., et al., Roles of peripheral terminals of transient receptor potential vanilloid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation, Brain Res., 2007, no. 1156, p. 80.
Félétou, M., Endothelium-dependent hyperpolarization and endothelial dysfunction, J. Cardiovasc. Pharmacol., 2016, vol. 67, no. 5, p. 373.
Fujii, N., Louie, J.C., McNeely, B.D., et al., K+ channel mechanisms underlying cholinergic cutaneous vasodilation and sweating in young humans: roles of KCa, KATP, and KV channels? Am. J. Physiol.-Regul. Integr. Comp. Physiol., 2016, vol. 311, no. 3, p. R600.
Fujii, N., Meade, R.D., Minson, C.T., et al., Cutaneous blood flow during intradermal NO administration in young and older adults: roles for calcium-activated potassium channels and cyclooxygenase? Am. J. Physiol.-Regul. Integr. Comp. Physiol., 2016, vol. 310, no. 11, p. R1081.
Funding
The study was supported by the Russian Foundation for Basic Research (project no. 16-29-08277) and the Program of Basic Research of the Presidium of the Russian Academy of Sciences no. 1.42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Bioethics Committee of the Pavlov Institute of Physiology, Russian Academy of Sciences (St. Petersburg), and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Additional information
Translated by E.V. Makeeva
Rights and permissions
About this article
Cite this article
Lobov, G.I., Gerasimenko, Y.P. & Moshonkina, T.R. Mechanisms of Vasodilation in Skin during Lumbar Transcutaneous Spinal Cord Stimulation. Hum Physiol 45, 389–396 (2019). https://doi.org/10.1134/S0362119719040091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119719040091