Abstract
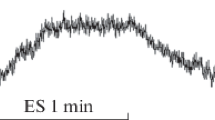
Changes in the blood flow in the shin skin were observed by laser Doppler flowmetry after transcutaneous electrical spinal cord stimulation (TSCS) by subthreshold bipolar pulses with a frequency of 30 Hz in 12 healthy subjects. It was found that TSCS in the area of the T11 and L1 vertebrae led to a significant increase in skin blood flow. The microcirculation rate increased by more than 85% relative to the baseline at a stimulus intensity of 90% of the motor threshold. Cutaneous blood flow activization by TSCS is implemented mainly through the antidromic stimulation of sensory nerve fibers. Nitric oxide (NO) is an important mediator that contributes to vasodilation and increase in cutaneous blood flow upon TSCS. NO is predominantly of endothelial origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently [8–10], a method of transcutaneous electrical spinal cord stimulation (TSCS) has been developed, the most important benefit of which being that it is non-invasive. This leads to the absence of complications typical of epidural electrical spinal cord stimulation. Another important advantage of the new method is that it could be used to study the functions of the spinal cord in a healthy person. Further development of the spinal cord stimulation technique was associated with the development of multi-segmental stimulation. There is a convergence of the descending and ascending effects on the neural networks that control postural and locomotor functions in the process of multi-segment TSCS [8, 9]. It is assumed that this interaction leads to a stimulating effect on the primary sympathetic neurons and the spinal cord (SC) interneurons involved in the regulation of autonomic functions. Our earlier study of the TSCS effects on the blood flow in the skin of the hallux showed that, upon TSCS, skin perfusion increases due to modulation of the nerve control of the vascular tone [3].
The aim of this study was to investigate the effects and mechanisms of the action of TSCS through stimuli that are below the threshold sufficient to trigger a motor response on the blood flow in the skin of the shin, in which, unlike the bare toe skin, a more complex system of regulation of vasomotor reaction functions exists [11].
The study involved 12 volunteers, 27–42 years old (five men and seven women). Stimulating electrodes (cathodes) were located epicutaneously along the midline of the spinal cord between the spinous processes of adjacent vertebrae at the T11–T12 and L1–L2 levels, while the anodes were placed epicutaneously above the crests of the iliac bones. The TSCS protocol, the equipment used, and the recording of the results by the laser Doppler flowmetry (LDF) were described in detail earlier [1]. Initially, the minimum intensity of the current causing the motor responses in the leg muscles to single pulses with a duration of 1 ms was determined for each level of the stimulation. This parameter is called the motor threshold (MT). Then the effect of continuous TSCS at the T11 and L1 levels was analyzed. Bipolar pulses with a frequency of 30 Hz, modulated with a frequency of 5 kHz, with a series duration of 0.5–1 min were applied. The intensity of the current was gradually increased from the minimum to 90% of the value of the individual MT. To evaluate the parameters and mechanisms of skin blood flow regulation, a LAKK M multifunctional laser diagnostic complex (NPP Lazma, Russia) was used [3]. The tip of the light guide probe of the diagnostic complex was placed on the skin of the anterior surface of the lower third of the shin. Emla® cream (AstraZeneca, United Kingdom) containing 2.5% lidocaine and 2.5% prilocoine was used to study the mechanisms of vasodilation caused by TSCS. The cream was applied to a skin area of about 4 cm2. After application, continuous TSCS was performed.
The standard software built into the LAKK-M complex (version 3.0.2.376) was used for mathematical processing of LDF-grams. The Statistica v. 10 applied package was used for statistical processing of the results. The statistical significance of differences between the means was assessed using Student’s t test. The differences were considered significant at p ≤ 0.05.
At rest, the microcirculation index (MI) of the shin skin in different subjects varied in the range of 5.2–7.6 perfusion units. TSCS (frequency, 30 Hz) by subthreshold stimuli with a current strength of 90% of the MT in the region of the T11 and L1 vertebrae led to a pronounced increase in MI in the shin skin. With a mean value of shin skin perfusion at rest of 6.62 ± 0.39 perfusion units, TSCS with an intensity of 90% of the MT increased blood flow by 74.3% at the level of T11, and by 88.2% at the level of L1 (Table 1). We believe that the small differences in the reaction of the vessels of the shin skin during TSCS in the T11 and L1 areas are explained by the following. During epidural stimulation, the cathode and anode are located on the dorsal surface of the SC and the stimuli act on limited groups of SC neurons [10]. TSCS, on the contrary, is characterized by the cathode and anodes on the subject’s body being far apart from each other. Thus, the stimulating effect of the stimuli manifests itself directly under the electrode and, spreading through the skin, subcutaneous fatty tissue, and muscles, simultaneously captures the neighboring roots of the spinal cord [14]. As a result, several entrances to the lumbosacral enlargement are exposed to the stimulating effect, which leads to the coverage of several SC segments by the neuron excitation.
Simultaneously with an increase in the perfusion in the skin microvasculature, the amplitude of blood flow oscillations also increased, which indicated the activation of regulatory mechanisms in the vessel wall [2]. Wavelet analysis of LDF-grams recorded during TSCS showed an increase in the amplitude of microcirculation oscillations in the neurogenic and endothelium-dependent ranges, which indicates the effect of TSCS on the nervous and endothelium-mediated mechanisms of modulation of the skin vasculature tone [2, 3, 5].
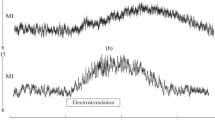
Hundreds of papers report the study of vasodilation mechanisms in the skin, but there is no consensus about this issue so far [7, 11]. Unlike hyperthermic vasodilation in the skin, TSCS-induced vasodilatation has been poorly studied [12], and the mechanisms of vasodilatation caused by TSCS have hardly been studied at all due to the novelty of the noninvasive electrostimulation of the spinal cord. We tested the previously suggested hypothesis about the stimulating effect of TSCS on the sensory nerve fibers of the SC posterior roots and the retrograde transfer of the stimulation to the sensory nerve endings in the skin [3]. For this purpose, the shin skin at the site of the study was pretreated with EMLA cream. The cream contains lidocaine and prilocaine, the anesthetic effect of which is mediated by blocking the ion channels of the sensory nerves. This cream is widely used to study the mechanisms of blood flow regulation in the skin [11]. In our study, the use of EMLA cream significantly reduced the increase in perfusion in the skin during TSCS in the T11 and L1 zones (Table 1).
In addition to reducing skin perfusion, EMLA cream also had an effect on the modulation of blood flow in the skin during TSCS. Wavelet analysis showed that the structure of blood flow oscillations in the skin microvasculature changed: the amplitude of blood flow oscillations greatly decreased in the range of 0.02–0.052 Hz and the amplitude of oscillations significantly decreased in the range of 0.0095–0.02 Hz. This is evidence of the inhibition of the neurogenic component of the blood flow modulation and the decrease in the effectiveness of endothelium-dependent regulation of vascular tone in the shin skin [2, 5].
The results of our study allow us to conclude that a significant part of the vasodilation effect in the shin skin during TSCS is caused by antidromic activation of afferent fibers in the dorsal roots of the spinal cord, which leads to the release of vasodilator substances in sensory nerve endings of the skin. It has been shown that the skin sensory nerves endings produce several compounds with vasodilator properties [4]. In particular, calcitonin gene–related peptide (CGRP) is a powerful vasodilator located in the sensory nerve endings, contributing to the formation of significant amounts of NO in the vessel wall [15]. Nitric oxide is considered to be an important component involved in vasodilation induced by epidural stimulation of SC [6, 12]. There are reasons to believe that NO produced during TSCS has endothelial origin. This is confirmed by the increase in the amplitude of microcirculation oscillations in the endothelium-dependent range recorded in the process of TSCS, which indicates an increase in the activity of the endothelium in the microvasculature of the skin and its significant decrease during TSCS combined with EMLA cream application [2].
In conclusion, we consider it necessary to note that inhibition of the activity of sensory nerves in the shin skin in our study reduced the TSCS-induced vasodilation but did not eliminate it completely. We suggest that TSCS stimulates not only sensory nerve fibers, but also SC structures that control nonadrenergic sympathetic neurons that innervate the blood vessels of the skin [7]. Studies of many laboratories demonstrate the importance and complexity of this mechanism of vasodilation, which functions in human hairy skin [3, 7, 15]. Evidence of the involvement of this mechanism in TSCS-induced vasodilation in the skin is the subject of our future research.
REFERENCES
Grishin, A.A., Moshonkina, T.R., Solopova, I.A., Gorodnichev, R.M., and Gerasimenko, Yu.P., Med. Tekh., 2016, no. 5, pp. 8–11.
Krupatkin, A.I. and Sidorov, V.V., Funktsional’naya diagnostika sostoyaniya mikrotsirkulyatorno-tkanevykh system: Rukovodstvo dlya vrachei (Functional Diagnosis of the Condition of Microcirculatory–Tissue Systems: A Physician’s Guide), Moscow: Librokom, 2013.
Lobov, G.I., Shcherbakova, N.A., Gorodnichev, R.M., Grishin, A.A., Gerasimenko, Yu.P., and Moshonki-na, T.R., Hum. Physiol., 2017, vol. 43, no. 5, pp. 518–523.
van Beek, M., van Kleef, M., Linderoth, B., et al., Eur. J. Pain, 2017, vol. 21, no. 5, pp. 795–803.
Bernjak, A., Cui, J., Iwase, S., et al., J. Physiol., 2012, vol. 590, no. 2, pp. 363–375.
Carter, S.J. and Hodges, G.J., Exp. Physiol., 2011, vol. 96, no. 11, pp. 1208–1217.
Charkoudian, N., J. Appl. Physiol., 2010, vol. 109, no. 4, pp. 1221–1228.
Gerasimenko, Y., Gorodnichev, R., Moshonkina, T., Sayenko, D., and Gad, P., Ann. Phys. Rehabil. Med., 2015, vol. 58, no. 4, pp. 225–231.
Gerasimenko, Y., Gorodnichev, R., Puhov, A., Moshonkina, T., Savochin, A., Selionov, V., Roy, R.R., Lu, D.C., and Edgerton, V.R., J. Neurophysiol., 2015, vol. 113, no. 3, pp. 834–842.
Harkema, S., Gerasimenko, Y., Hodes, J., et al., Lancet, 2011, vol. 377, no. 9781, pp. 1938–1947.
Hodges, G.J., Traeger, J.A., Tang, T., et al., Am. J. Physiol. Heart. Circ. Physiol., 2007, vol. 293, no. 1, pp. 784–789.
Naoum, J.J. and Arbid, E.J., Methodist Debakey Cardiovasc. J., 2013, vol. 9, no. 2, pp. 99–102.
Pedrini, L. and Magnoni, F., Interact. Cardiovasc. Thorac. Surg., 2007, vol. 6, no. 4, pp. 495–500.
Sayenko, D.G., Atkinson, D.A., Floyd, T.C., et al., Neurosci. Lett., 2015, vol. 609, pp. 229–234.
Wu, M., Komori, N., Qin, C., et al., Brain Res., 2007, vol. 1156, pp. 80–92.
ACKNOWLEDGMENTS
This study was supported by the Russian Foundation for Basic Research (project no. 16-29-08277) and Program for Basic Research of the Presidium of the Russian Academy of Sciences no. 1.42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Additional information
Translated by I. Shipounova
Rights and permissions
About this article
Cite this article
Lobov, G.I., Gerasimenko, Y.P. & Moshonkina, T.R. Mechanisms of Blood Flow Regulation in the Skin during Stimulation of the Spinal Cord in Humans. Dokl Biol Sci 485, 27–29 (2019). https://doi.org/10.1134/S0012496619020030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012496619020030