Abstract
Background
The stress hyperglycemia ratio (SHR), adjusted for average glycemic status, is suggested for assessing actual blood glucose levels. Its link with adverse outcomes is known in certain populations, yet its impact on sepsis patients’ prognosis is unclear. This study explores the association between SHR and mortality in sepsis.
Methods
We included 13,199 sepsis patients in this study and categorized SHR into distinct groups. Additionally, we utilized restricted cubic spline analysis to evaluate the correlation between SHR as a continuous variable and mortality. The primary outcome was 1-year all-cause mortality. Logistic regression and Cox proportional hazards models were employed to assess the associations between the SHR and both in-hospital mortality and 1-year mortality, respectively.
Results
Among the study participants, 4,690 (35.5%) patients died during the 1-year follow-up. After adjusting for confounding variables, we identified a U-shaped correlation between SHR and 1-year mortality. Using an SHR of 0.99 as the reference point, the hazard ratio for predicted 1-year mortality increased by 1.17 (95% CI 1.08 to 1.27) per standard deviation above 0.99, whereas each standard deviation increase predicted the hazard ratio of 0.52 (95% CI 0.39 to 0.69) below 0.99. Furthermore, we found that SHR could enhance the predictive performance of conventional severity scores.
Conclusion
There exists a U shaped association between SHR and mortality in sepsis patients, where both low and high SHR values are associated with an increased risk of poor outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis, a life-threatening condition resulting from a dysregulated host response to infection, remains a pervasive global health concern [1]. Its prevalence and morbidity rates are on the rise, and both contribute significantly to the challenges faced by healthcare systems worldwide [2, 3]. Furthermore, sepsis is linked to an unacceptably high mortality rate, with a 90-day mortality rate of more than 35% [4, 5]. It is estimated that approximately 2.8 million deaths worldwide can be attributed to sepsis each year [2, 6]. In recent years, researchers and clinicians have been tirelessly exploring various factors that contribute to sepsis outcomes, with a particular focus on identifying markers and predictors of mortality [7,8,9].
Stress hyperglycemia, marked by a transient surge in blood glucose levels in response to illness-induced stress, is a prevalent occurrence in critically ill individuals [10]. Moreover, several studies have demonstrated that hyperglycemia is associated with an increased risk of adverse outcomes [11, 12]. Nevertheless, it is important to recognize that admission blood glucose (ABG) measurement may not provide a comprehensive portrayal of the acute hyperglycemic state, as these values can be influenced by an individual’s chronic glucose status [13]. Consequently, numerous research inquiries have emerged, proposing the stress hyperglycemia ratio (SHR) as an invaluable metric for characterizing the acute hyperglycemic condition and as a prognostic indicator of adverse outcomes among critically ill patients [14,15,16,17].
However, our current knowledge regarding the association between SHR and mortality in patients with sepsis remains limited. While high blood glucose in patients without diabetes contributes to sepsis diagnosis, current sepsis severity models and prognostic scores do not account for blood glucose [18, 19]. Moreover, although several studies have demonstrated the prognostic value of stress hyperglycemia in sepsis [20, 21], the relationship between SHR and mortality in sepsis continues to be undefined. This study seeks to address this critical gap in our knowledge, shedding light on the potential significance of SHR as a prognostic marker in sepsis and ultimately contributing to improved patient care and outcomes in this challenging clinical context.
Methods
Study population
This study was a retrospective, observational cohort study. The dataset used in this study originates from the Medical Information Mart for Intensive Care IV (MIMIC-IV), a publicly accessible database compiled from electronic health records at the Beth Israel Deaconess Medical Center (BIDMC). Data for MIMIC-IV were collected from patients who received care at the emergency department or intensive care units at BIDMC during the period spanning from 2008 to 2019. As a result, the MIMIC-IV database comprises a substantial cohort, encompassing a total of 256,878 unique individuals. This rich and extensive dataset forms the basis for our research, allowing us to explore and analyze critical aspects of healthcare and patient outcomes in a real-world clinical setting. The author (Le Li) obtained the necessary authorization to access the database. It is important to highlight that our study is centered on the analysis of a third-party, openly accessible database, which has already undergone institutional review board (IRB) approval. As a consequence, our own institution’s IRB review process was determined to be exempt. Furthermore, in this dataset, a variety of methods such as de-identification, date shifting, and format conversion have been meticulously implemented to effectively erase any identifiable patient information. Patients aged 18 years or older, diagnosed with sepsis according to the Sepsis-3 criterion (infection with organ dysfunction) [22], were eligible for inclusion in this study. Conversely, individuals who did not receive blood glucose or glycosylated hemoglobin A1c (HbA1c) measurements were excluded. SHR was calculated as SHR = ABG (mg/dL)/(28.7 × HbA1c (%) − 46.7). Based on the SHR value, patients were divided into seven groups with an interval of 0.25, ranging from < 0.50 to ≥ 1.75 [16].
Data collection
Data pertaining to baseline characteristics within the first 24 h of ICU admission were extracted from the MIMIC-IV database. These included demographic information such as sex and age and essential clinical parameters such as weight and urine output (UO). Measures of illness severity, including the Sequential Organ Failure Assessment (SOFA), quick Sequential Organ Failure Assessment (qSOFA), Simplified Acute Physiology Scores II (SAPS II), and Logistic Organ Dysfunction System (LODS), were also retrieved. In addition, vital signs such as systolic blood pressure (SBP), heart rate, temperature, and respiratory rate, and general laboratory tests (white blood cell count [WBC], serum creatinine [SCr], blood urea nitrogen [BUN]) were documented. Medical history variables encompassed insulin usage, antibiotic administration, mechanical ventilation (MV), and renal replacement treatment (RRT). Comorbidities were identified based on documented ICD-9 codes and included conditions such as hypertension, diabetes mellitus (DM), congestive heart failure (CHF), acute myocardial infarction (AMI), old myocardial infarction (OMI), stroke, acute kidney injury (AKI), chronic kidney disease (CKD), liver disease (including hepatitis and liver cirrhosis), anemia, cancer, and dyslipidemia.
In this study, variables exhibiting more than 40% missing values were excluded. For variables with less than 5% missing data, mean imputation was employed as a method for data completion. For variables missing 5% to 40% of their values, multiple imputation was conducted utilizing the multivariate imputation by chained equations (MICE) package in R, providing a robust approach to addressing missing data [23].
Outcomes
The primary endpoint in this study was the one-year all-cause mortality rate after discharge. The secondary endpoint pertained to in-hospital mortality, encompassing fatalities occurring in both the ICU and general ward settings. Mortality data for discharged patients were retrieved from the US Social Security Death Index.
Statistical analysis
The participants were divided into seven groups based on the SHR value. The Kolmogorov–Smirnov test is employed to assess whether continuous variables exhibit normality. Continuous variables following a normal distribution were summarized with mean ± standard deviation, while nonnormally distributed variables were described using the median and interquartile range (IQR). For categorical variables, we provide the quantity and percentage frequency. To assess group differences in continuous variables, we used Wilcoxon t tests, and for categorical variables, we employed chi-square or Fisher’s exact tests as appropriate. To examine the association between SHR and one-year mortality, we conducted univariate and multivariate Cox regression analyses, presenting hazard ratios (HRs) along with their 95% confidence intervals (CIs). The Schoenfeld residuals method is used to test the proportional hazards assumption of the Cox regression model. We utilized Kaplan–Meier survival analysis to assess the primary outcome event incidence rates across SHR-defined groups, with intergroup differences evaluated using the log-rank test. For evaluating the relationship between SHR and in-hospital mortality, we conducted univariate and multivariate logistic regression analyses, and the findings were reported in terms of odds ratios (ORs) accompanied by their respective 95% CIs. The reference group for this analysis was defined as the SHR interval with the lowest incidence rate. Furthermore, we employed a restricted cubic spline (RCS) regression model with four assumed knots to delineate the associations between the SHR and HR or OR.
In the context of the multivariate regression model, we proactively addressed the potential concern of overfitting. To do so, we assessed the degree of multicollinearity among variables by calculating variance inflation factors (VIF). Variables with VIF values exceeding or equal to 5 were systematically excluded from the model to mitigate multicollinearity-related issues. Consequently, the variables incorporated into our models for adjustment encompassed a wide range of factors. These included demographics (age, sex, weight, UO), comorbidities (CHF, DM, hypertension, pneumonia, stroke, AKI, CKD, liver disease, cancer, dyslipidemia, anemia), laboratory tests (WBC, SCr, BUN), medical treatments (insulin and antibiotic use, MV, RRT). We selected variables for multivariable-adjustment based on their clinical relevance and established associations with the outcome, as evidenced in prior literature [16], ensuring a robust analysis by accounting for potential confounders.
Additionally, we conducted subgroup analyses by stratifying outcomes based on variables such as age, sex, and the presence of comorbidities, including DM, hypertension, pneumonia, AMI, stroke, and AKI. These subgroup analyses were carried out using regression models that were comprehensively adjusted to account for potential confounding factors. Certain subgroup analyses aim to explore the diverse impacts of SHR based on demographic variables and relevant health conditions, aiming for a more targeted and clinically applicable insight.
To assess whether the inclusion of SHR improves the predictive accuracy of adverse outcome events when integrated with existing severity of illness scores (SAPS II, SOFA score, qSOFA, LODS score), we computed the area under the curve (AUC). We then conducted comparisons between the different models using the DeLong test.
Results
Baseline characteristics
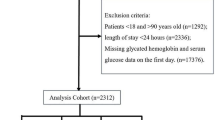
After a meticulous review of 27,139 sepsis patients admitted to the ICU from the MIMIC-IV database, a total of 13,199 qualified patients were ultimately included in this study (Figure S1). The median age of the study cohort was 68.3 years (with an interquartile range of 58.7–77.8), and among these individuals, 8021 (60.8%) were male. The participants were stratified into seven distinct groups (groups 1–7) based on their SHR levels: < 0.50 (n = 373), 0.50–0.74 (n = 1550), 0.75–0.99 (n = 4816), 1.00–1.24 (n = 3015), 1.25–1.49 (n = 1553), 1.50–1.74 (n = 796), and ≥ 1.75 (n = 1096). Among these seven groups, the distribution of one-year mortality rates exhibits a U shaped pattern, with the lowest one-year mortality rate observed in Group 3 (Fig. 1). Baseline characteristics grouped according to SHR value are shown in Table 1. To provide additional context, Table S1 offers a comparison of baseline characteristics between survivors and non-survivors during the in-hospital period, and Table S2 presents a similar comparison for the 1-year follow-up. In addition, the comparison of baseline characteristics between included and excluded patients was conducted to evaluate the selection bias (Table S3).
Clinical outcomes for all-cause mortality
After a 1-year follow-up period, a total of 4690 patients (35.5%) experienced mortality, with an additional 1433 individuals (10.9%) succumbing during their hospital stay. Cox regression analyses conducted in this study revealed a significant association between SHR and 1-year mortality in sepsis patients. In the regression models, none of the variables exhibited a VIF greater than 5. In the unadjusted model, we observed a U shaped relationship between 1-year mortality and SHR (Figure S2). This relationship persisted in the multivariable-adjusted model. Using Group 3 as the reference, groups 1, 2, 4, 5, 6, and 7 exhibited an elevated risk of 1-year mortality, with HRs of 1.64 (95% CI 1.40–1.91), 1.34 (95% CI 1.22–1.47), 1.18 (95% CI 1.09–1.29), 1.49 (95% CI 1.35–1.64), 1.46 (95% CI 1.30–1.65), and 1.70 (95% CI 1.53–1.89), respectively (Fig. 2). Furthermore, logistic regression was employed to examine the association between in-hospital mortality and SHR. Similar to the 1-year mortality findings, we identified a U-shaped association between in-hospital mortality and SHR in both unadjusted (Figure S3) and adjusted (Figure S4) models. After adjusting for potential covariates, the ORs for groups 1, 2, 4, 5, 6, and 7 were 1.83 (95% CI 1.32–2.54), 1.47 (95% CI 1.21–1.79), 1.28 (95% CI 1.09–1.50), 1.86 (95% CI 1.55–2.23), 1.93 (95% CI 1.53–2.42), and 2.73 (95% CI 2.26–3.31), respectively. The Kaplan–Meier curves depicted in Fig. 3 clearly demonstrate that patients falling within the SHR range of 0.75–0.99 exhibited the lowest one-year mortality rate (log-c p < 0.001).
Additionally, we employed the RCS curve to assess the continuous-scale relationship between all-cause mortality and SHR. As depicted in Fig. 4, the U shaped association between 1-year mortality and SHR was evident. Using an SHR of 0.99 as the reference point, the hazard ratio for predicted 1-year mortality increased by a factor of 1.17 (95% CI 1.08 to 1.27) per standard deviation above 0.99, whereas each standard deviation increase predicted the HR of 0.52 (95% CI 0.39 to 0.69) below 0.99. Similarly, this U shaped relationship was also observed in the prediction of in-hospital mortality (Figure S5). For SHR values below 0.99, an increase in standard deviation was associated with an OR of 0.48 (95% CI 0.26 to 0.91) for in-hospital mortality, whereas when SHR exceeded 0.99, the odds ratio per standard deviation increase was 1.37 (95% CI 1.16 to 1.61).
Moreover, there exist established scoring systems for the diagnosis and risk stratification of sepsis patients, including the SOFA and qSOFA scores, which demonstrate precision in predicting the prognosis of sepsis patients. To further substantiate the prognostic value of SHR in the assessment of sepsis patients, we evaluated whether the addition of SHR to conventional disease severity scoring systems could further enhance predictive efficacy. The findings indicate that the incorporation of the SHR index alongside SAPS II, SOFA, qSOFA, and LODS scores leads to a significant enhancement in the model’s predictive capacity for mortality (all DeLong test p values < 0.05) (Table 2). Furthermore, the predictive performances, both before and after the inclusion of SHR, for patients with and without DM are presented in Tables S5 and S6, respectively.
Subgroup analysis
In this study, we incorporated age, gender, and comorbidities, including DM, hypertension, pneumonia, AMI, stroke, and AKI, as stratification factors for conducting subgroup analyses. Across all subgroups, a consistent U-shaped association between SHR and one-year mortality was observed. Notably, significant interactions were identified, particularly between DM and non-DM patients. Patients without DM exhibited a comparatively higher risk of in-hospital mortality in contrast to patients with DM (p for interaction < 0.001). Additionally, similar noteworthy interactions were also observed with respect to pneumonia. The impact of SHR on one-year mortality was more pronounced among patients without pneumonia as compared to those with pneumonia (p for interaction < 0.001). The other detailed subgroup analyses are presented in Table 3. Furthermore, we conducted subgroup analyses for SHR and in-hospital mortality, yielding similar results (Table S3).
Discussion
In this study, we have reported a U-shaped relationship between SHR and all-cause mortality among sepsis patients. Specifically, the mortality rate reached its lowest point when the SHR value was approximately 0.99, with both lower and higher levels of SHR being linked to an increased risk of mortality. Additionally, our findings indicate that SHR could serve as an effective predictor for assessing poor prognosis and has the potential to augment the performance of traditional severity scoring systems.
Relative hyperglycemia rather than absolute hyperglycemia is identified as a better biomarker at risk of critical illness [13]. Zhou et al. conducted a study involving 1,904 patients with acute decompensated heart failure, examining the relationship between SHR and all-cause mortality over a 3-year follow-up period. Their findings indicated that SHR effectively predicted poor prognosis, revealing a U-shaped association between SHR and mortality [24]. In a separate study, Wei et al. enrolled a cohort of 1,099 patients diagnosed with ST-elevation myocardial infarction who underwent percutaneous coronary intervention. The primary outcomes of interest included in-hospital death and all-cause mortality. Their investigation demonstrated a significant association between SHR and an elevated risk of both in-hospital death and all-cause mortality in these patients [25]. Taking into account the potential impact of patients’ previous glycemic status, the SHR provides a more robust assessment of hyperglycemia and its prognostic value across a wide spectrum of patient populations.
The association between SHR and outcomes in patients with sepsis remains unclear. In 2020, Fabbri et al. conducted a retrospective observational study, a total of 915 patients with sepsis and DM were included for analysis. The authors divided patients into low SHR (< 1.14) and high SHR (≥ 1.14) groups and found that SHR ≥ 1.14 was highly associated with all-cause mortality (OR = 5.25, 95% CI 3.62–7.63) [26]. However, this binary classification approach implies an assumption of a linear relationship between SHR and outcome events. In fact, through the utilization of a multi-categorical approach and RCS analysis, we discovered that in sepsis patients, the correlation between SHR and mortality is U shaped, rather than linear. A U shaped relationship between SHR and prognosis has also been observed in patients with acute coronary syndrome (ACS). In a study conducted by Yang et al., which involved 5562 ACS patients, the relationship between SHR and major adverse cardiovascular events (MACE) over a 2-year follow-up period was investigated. The findings unveiled this U shaped association, indicating that when SHR deviated from the inflection point of 0.78, the risk of MACE significantly increased [27]. However, the underlying mechanisms of the U shaped association of SHR and prognosis remain unclear. Previous studies have suggested that mild-to-moderate stress-induced hyperglycemia can enhance cardiac output, optimize cellular glucose utilization, and diminish myocardial cell apoptosis. These physiological adaptations could form the basis for the observed protective effects in states of elevated stress hyperglycemia. Such adaptive responses are thought to confer a survival edge in the face of acute stress and critical illness, enabling the organism to more effectively meet the heightened metabolic demands posed by these strenuous conditions. [28,29,30]. Further studies are required to clarify the protective mechanisms.
Furthermore, we observed significant interactions between pneumonia and the association between SHR and mortality. While the U shaped association between SHR and prognosis was evident in both pneumonia and non-pneumonia patients, the relationship between SHR and all-cause mortality was more pronounced in patients without pneumonia. Similar interactions were also observed concerning glucose metabolic statuses, where patients without DM exhibited a higher risk of all-cause mortality compared to those with DM. Whereas the underlying mechanisms of these interactions were uncertain, some findings may help to explain these results. It is recognized that diabetic patients, who experience ongoing inflammation activation and oxidative stress, may demonstrate an adaptive response to the series of pathophysiological processes triggered by stress hyperglycemia [31,32,33]. Similarly, the acute aggregation of inflammatory and chemotactic factors and oxidative stress induced by pneumonia may also give rise to adaptive responses to stress hyperglycemia and the related pathophysiological processes [34].
In summary, we underscore that recognizing the U-shaped association between SHR and mortality invites a refined approach in sepsis management, highlighting the need for maintaining glucose levels within an optimal range to mitigate mortality risk and suggesting personalized treatment strategies based on individual patient profiles and risk factors.
Limitations
While this study is the first to unveil the U-shaped association between SHR and all-cause mortality in sepsis patients, it is important to acknowledge certain limitations. Firstly, this is a retrospective study conducted within a large cohort of critically ill patients, and thus, a causal relationship between SHR and prognosis cannot be established. Secondly, due to the unavailability of follow-up data beyond one year in this cohort, an assessment of the long-term association between SHR and adverse outcomes is precluded. Thirdly, the predictive value of SHR for mortality necessitates validation in external cohorts. Therefore, the findings of this study should be interpreted with caution.
Conclusion
In conclusion, this study has unveiled a U-shaped association between SHR and all-cause mortality in sepsis patients. The results underscore that mortality rates are elevated at both low and high SHR levels, with the inflection point for poor prognosis identified at 0.99. Additionally, SHR emerges as a novel and effective biomarker for mortality prediction, demonstrating the potential to augment the predictive precision of conventional severity scores. Future large-scale prospective studies are warranted to further corroborate these findings.
Data availability
The datasets used during the current study are available from the corresponding author upon reasonable request.
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801–10.
Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54.
Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of Global Incidence and Mortality of Hospital-treated. Sepsis Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259–72.
Xie J, Wang H, Kang Y, Zhou L, Liu Z, Qin B, et al. The Epidemiology of Sepsis in Chinese ICUs: A National Cross-Sectional Survey. Crit Care Med. 2020;48:e209–18.
Chiu C, Legrand M. Epidemiology of sepsis and septic shock. Curr Opin Anaesthesiol. 2021;34:71–6.
Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339–46.
van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA. 2016;315:1469–79.
Martin-Loeches I, Guia MC, Vallecoccia MS, Suarez D, Ibarz M, Irazabal M, et al. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis: a prospective, observational, multicenter cohort study. Ann Intensive Care. 2019;9:26.
Pandolfi F, Brun-Buisson C, Guillemot D, Watier L. One-year hospital readmission for recurrent sepsis: associated risk factors and impact on 1-year mortality-a French nationwide study. Crit Care. 2022;26:371.
Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013;17:305.
Zohar Y, Zilberman Itskovich S, Koren S, Zaidenstein R, Marchaim D, Koren R. The association of diabetes and hyperglycemia with sepsis outcomes: a population-based cohort analysis. Intern Emerg Med. 2021;16:719–28.
Lu Z, Tao G, Sun X, Zhang Y, Jiang M, Liu Y, et al. Association of Blood Glucose Level and Glycemic Variability With Mortality in Sepsis Patients During ICU Hospitalization. Front Public Health. 2022;10: 857368.
Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, et al. Relative Hyperglycemia, a Marker of Critical Illness: Introducing the Stress Hyperglycemia Ratio. J Clin Endocrinol Metab. 2015;100:4490–7.
Xu W, Song Q, Wang X, Zhao Z, Meng X, Xia C, et al. Association of stress hyperglycemia ratio and in-hospital mortality in patients with coronary artery disease: insights from a large cohort study. Cardiovasc Diabetol. 2022;21:217.
Cui K, Fu R, Yang J, Xu H, Yin D, Song W, et al. Stress hyperglycemia ratio and long-term mortality after acute myocardial infarction in patients with and without diabetes: A prospective, nationwide, and multicentre registry. Diabetes Metab Res Rev. 2022;38: e3562.
Li L, Ding L, Zheng L, Wu L, Hu Z, Liu L, et al. U-shaped association between stress hyperglycemia ratio and risk of all-cause mortality in cardiac ICU. Diabetes Metab Syndr. 2024;18: 102932.
Li L, Zhao M, Zhang Z, Zhou L, Zhang Z, Xiong Y, et al. Prognostic significance of the stress hyperglycemia ratio in critically ill patients. Cardiovasc Diabetol. 2023;22:275.
Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353: i1585.
Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30:748–56.
Tiruvoipati R, Chiezey B, Lewis D, Ong K, Villanueva E, Haji K, et al. Stress hyperglycemia may not be harmful in critically ill patients with sepsis. J Crit Care. 2012;27:153–8.
Leonidou L, Michalaki M, Leonardou A, Polyzogopoulou E, Fouka K, Gerolymos M, et al. Stress-induced hyperglycemia in patients with severe sepsis: a compromising factor for survival. Am J Med Sci. 2008;336:467–71.
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:775–87.
Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4:30.
Zhou Q, Yang J, Wang W, Shao C, Hua X, Tang YD. The impact of the stress hyperglycemia ratio on mortality and rehospitalization rate in patients with acute decompensated heart failure and diabetes. Cardiovasc Diabetol. 2023;22:189.
Wei QC, Chen YW, Gao QY, Ren KD, Liu YB, He F, et al. Association of stress hyperglycemia with clinical outcomes in patients with ST-elevation myocardial infarction undergoing percutaneous coronary intervention: a cohort study. Cardiovasc Diabetol. 2023;22:85.
Fabbri A, Marchesini G, Benazzi B, Morelli A, Montesi D, Bini C, et al. Stress Hyperglycemia and Mortality in Subjects With Diabetes and Sepsis. Crit Care Explor. 2020;2: e0152.
Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, et al. The Impact of the Stress Hyperglycemia Ratio on Short-term and Long-term Poor Prognosis in Patients With Acute Coronary Syndrome: Insight From a Large Cohort Study in Asia. Diabetes Care. 2022;45:947–56.
McNamara JJ, Mills D, Aaby GV. Effect of hypertonic glucose on hemorrhagic shock in rabbits. Ann Thorac Surg. 1970;9:116–21.
Losser MR, Damoisel C, Payen D. Bench-to-bedside review: Glucose and stress conditions in the intensive care unit. Crit Care. 2010;14:231.
Malfitano C, Alba Loureiro TC, Rodrigues B, Sirvente R, Salemi VM, Rabechi NB, et al. Hyperglycaemia protects the heart after myocardial infarction: aspects of programmed cell survival and cell death. Eur J Heart Fail. 2010;12:659–67.
Bahadoran Z, Mirmiran P, Ghasemi A. Role of Nitric Oxide in Insulin Secretion and Glucose Metabolism. Trends Endocrinol Metab. 2020;31:118–30.
Ling PR, Smith RJ, Bistrian BR. Hyperglycemia enhances the cytokine production and oxidative responses to a low but not high dose of endotoxin in rats. Crit Care Med. 2005;33:1084–9.
Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079–86.
Xu W, Zhao T, Xiao H. The Implication of Oxidative Stress and AMPK-Nrf2 Antioxidative Signaling in Pneumonia Pathogenesis. Front Endocrinol (Lausanne). 2020;11:400.
Acknowledgements
None.
Funding
This study was supported by the Medical and Health Technology Innovation Project of the Chinese Academy of Medical Sciences (2021-CXGC09-1).
Author information
Authors and Affiliations
Contributions
This study was designed by LL and LKZ. XP, ZXZ, LKZ, YLX, ZHZ, and ZH were responsible for data collation and statistical analysis. LL wrote the first draft. YY reviewed and checked the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Zhou, L., Peng, X. et al. Association of stress hyperglycemia ratio and mortality in patients with sepsis: results from 13,199 patients. Infection (2024). https://doi.org/10.1007/s15010-024-02264-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s15010-024-02264-3