Abstract
‘Microbial terroir’ relates to the influence of autochthonous yeasts associated with a grape cultivar on the resultant wine. Geographic region, vineyard site and topography, climate and vintage influence the biodiversity of these microbial communities. Current research focus attempts to correlate their ‘microbial fingerprint’ to the sensorial and chemical characteristics of varietal wines from distinct geographical wine regions. This study focuses on the minor red grape variety, Negro Saurí, which has seen a resurgence in the León Appellation of Origin in Spain as a varietal wine. An experimental vineyard at Melgarajo S.A. (42° 15′ 48.68_N 5° 9′ 56.66_W) was sampled over four consecutive vintages, with autochthonous yeasts being isolated from grapes, must and pilot-scale un-inoculated fermentations, and identified by ITS sequencing. Forty-nine isolates belonging to Metschnikowia pulcherrima, Lachancea thermotolerans, Hanseniaspora uvarum and Torulaspora delbrueckii were isolated from grapes and must, and early stages of fermentation dependent on seasonal variation. Saccharomyces cerevisiae predominated throughout fermentation, as a heterogeneous and dynamic population, with seven major biotypes identified amongst 110 isolates across four consecutive vintages. Twenty-four S. cerevisiae isolates representing five strains dominated in two or more vintages. Their persistence through fermentation warrants further validation of their oenological properties as starter cultures.
Similar content being viewed by others
Introduction
Autochthonous yeast, indigenous to a given wine region provide an opportunity to enhance wine, with the resulting style and organoleptic characteristics corresponding to a particular geographical 'terroir'1,2. These microbial communities are geographically distributed; each area having a characteristic population of microorganisms, which vary over time and have their own dynamics according to different aspects related to cultivar, vineyard management and climate3. Such populations are being defined as a ‘microbiological fingerprint’ of the region4, and are affected by the geographical location, grape variety, and vine development (reviewed in5).
Aureobasidium pullulans and Metschnikowia, Hanseniaspora (Kloeckera), Cryptococcus and Rhodotorula species predominate on healthy grape berries at different stages of maturity (reviewed by6). Saccharomyces cerevisiae is the principal yeast during fermentation3,7 and often is impossible to isolate from healthy, mature grapes8,9 indicating the winemaking environment as a reservoir. The vineyard and winery microbiota form the inoculum for ‘spontaneous’ wine fermentations. A specific succession of yeast communities10 occurs, with Hanseniaspora, Candida, Metschnikowia, Pichia, Issatchenkia and Kluyveromyces species often present in the initial stages11, Saccharomyces outcompete later in fermentation. Research has focused on the application of non-Saccharomyces yeasts to winemaking, given their ability to produce enzymes of biotechnological value12,13, and volatile and non-volatile constituents that contribute to the complexity of the final wine10,14,15. Often giving a lower ethanol yield and being unable to complete fermentation16, they are used in sequential inoculation with S. cerevisiae to obtain wines with lower alcohol content, diverse aromas17,18 and flavour6,19. As such, they provide a solution to the increasing trend of high alcohol wines, which are in part attributable to climate warming20,21,22,23. Whilst several commercial starters containing different strains of Torulaspora delbrueckii, Metschnikowia pulcherrima and Lachancea thermotolerans are now available24, there is a multitude of species that remain to be investigated, including those specific to geographical wine regions that can be directly linked to the wines’ organoleptic characteristics.
The biodiversity of Saccharomyces strains is proposed to be as important as that of non-Saccharomyces in the sensorial qualities of the final wine25. Numerous reports exist that examine the biodiversity and distribution of individual strains isolated from vineyards and spontaneous fermentations from different wine regions in Europe9,26,27,28,29,30,31,32,33,34,35 as well as developing wine regions including China36, Brazil37,38, India39 and Russia40. Correlations have been made between the genetic diversity and agricultural practices41, vineyard diffusion of commercial starters42,43, grape variety44,45 and geographical distances46.
The recovery of minority or local grape varieties throughout Spanish regions has been undertaken to rescue genetic resources at risk of extinction47,48,49,50. These grape varieties can (i) lead to specific sensory characteristics in the corresponding wine, (ii) be better adapted to local climatic conditions, pests and diseases51 and may harbour novel yeasts. Negro Saurí (synonym Merenzao), an early maturing red variety52, is part of the Spanish Grapevine Breeding and Recovery Program by the Instituto Tecnológico Agrario de Castilla y León (ITACYL)53 which aims to produce new certified clones for the industry suited to the climatic and topographical conditions of the wine region. Whilst considered a minor variety, Negro Saurí is authorised in various appellations of origin such as Merenzao (e.g., Canary Islands, Galicia and Rioja) and has undergone resurgence within the León region because of its elegant aroma/flavour qualities as a varietal wine54. The contribution of associated microflora to these characteristics is unreported.
This study reports on the yeast microbiota and genetic relationships among Saccharomyces isolates from Negro Saurí grapes and spontaneously fermenting must from an experimental vineyard at Melgarajo S.A. (ITACYL) within the León Appellation of Origin, Spain. The purpose was to preserve the microbial genetic pool associated with this grape variety and establish a strain collection, which may be useful as starter cultures that help contribute to the regional character of the wines.
Results
Isolation and identification of yeast species from grape, must and spontaneous fermentation
We looked at the biodiversity of yeasts isolated from Negro Saurí grapes selected from an experimental vineyard (42° 15′ 48.68_N 5° 9′ 56.66_W). A total of 159 indigenous yeasts were isolated over four vintages: 2014 to 2017 from hand-harvested grapes, must and spontaneous wine fermentations (Table 1). The yeasts were identified through colony morphology and PCR analysis of the internal transcribed spacer 1 (ITS1), ITS4 and 5.8S ITS ribosomal DNA (rDNA) regions of the fungal genome55. Species were identified through comparison of the DNA sequence data from the different sized PCR bands to the available sequences in the NCBI database (GenBank) using the standard nucleotide homology search program Basic Local Alignment Search Tool56 (BLAST, http://www.nbci.nlm.nih.gov/BLAST).
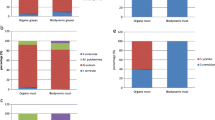
Saccharomyces cerevisiae and 4 non-Saccharomyces species (Metschnikowia pulcherrima, Lachancea thermotolerans, Hanseniaspora uvarum and Torulaspora delbrueckii) were identified during the 4 consecutive vintages (2014–2017). The number of each species identified was dependent upon the source material (i.e., grape, must, spontaneous ferment) and unique vintage (Table 1). Metschnikowia pulcherrima was the sole species isolated from grapes in all vintages except for 2014, where L. thermotolerans was also identified (Fig. 1). The predominance of M. pulcherrima in grape must was less, with other species being present in near equal proportions, with the exception of T. delbrueckii and S. cerevisiae. Hansenispora uvarum was present during the 2014 and 2017 vintages, whilst L. thermotolerans was only found in 2014. Saccharomyces cerevisiae was noted in must in equal proportions to M. pulcherrima in 2015.
Biodiversity of yeast species on grapes, in must and during fermentation. SF, Start of fermentation; MD, Mid fermentation; EF, End of fermentation. Saccharomyces cerevisiae (blue), Metschnikowia pulcherrima (brown), Hansenispora uvarum (green), Lachancea thermotolerans (purple), Torulaspora delbrueckii (yellow). The species distribution was calculated dividing the number of isolates for a particular species by the total number of isolates.
For each vintage, the spontaneous fermentations were sampled at the start, middle and end. Differences were noted between vintages in the species found (Fig. 1). Whilst Saccharomyces appear to outcompete the non-Saccharomyces species based on the number of isolates randomly picked and identified, differences were noted in how quickly this occurred (Table 1). In 2014, the yeasts identified at the start of fermentation were M. pulcherrima (40%), H. uvarum (40%) and L. thermotolerans (20%). Saccharomyces cerevisiae did not dominate (83%) until mid-fermentation, whilst in 2015 and 2016, its more rapid dominance alludes to spontaneous fermentation being initiated prior to crushing (in 2015) or shortly afterwards (as in 2016). In both cases, M. pulcherrima was the only other species. In 2017, microbial succession was more complex, with the non-Saccharomyces species being prevalent during the start and mid-fermentation, with S. cerevisiae only dominating at the end fermentation.
Temperature and precipitation data (Supplementary Table S1 online) were collected to check for extreme seasonal variations that might have affected grape ripening, disease and quality; parameters which could influence microbial dynamics. Seasonal temperatures were similar in the 4 consecutive vintages. Rainfall varied—2016 had 29.4% more and 2017, 62.2% less than the average rainfall (413.5 mm; 2014–2017). Both years were considerably drier at harvest time (September)—2016 (49.1%) and 2017 (11.7%) of the average (22.4 mm). In 2014 and 2015, there was 72.3% and 66.9% more rainfall, respectively. The small sample size in this study (number of yeast isolates) especially from grapes and must, makes it difficult to make any comparisons between the individual microbial populations per vintage and the weather data.
Genetic characterization of Saccharomyces cerevisiae strains
Saccharomyces cerevisiae represented the most abundant genus in the study. 110 indigenous isolates of S. cerevisiae were collected and analysed across four consecutive vintages (28 isolates (2014), 17 isolates (2015), 44 isolates (2016), 21 isolates (2017)). These were screened by inter-delta sequence analysis to evaluate genetic diversity and to determine their clonal relationships. Individual strains were identifiable through amplification of the delta sequences flanking the Ty1 retrotransposons within the yeast genome. The number and chromosomal position is strain-dependent57. Primers δ12 and δ21 were used since larger polymorphic differences can be identified, compared to the original δ1 and δ2 primers designed by Ness and co-workers58.
Typing analysis of the binary data from the amplified inter-delta sequence ‘fingerprint’ patterns was undertaken with Coheris Analytics SPAD software (2017), statistically analysed according to Lebart et al.59, and used the Euclidean distance and the Ward algorithm60. The number of partitions was based on the Davies-Bouldin and Calinski-Harabasz indicators61. To ensure accuracy, PCR analysis was repeated 4 times and in only one case was a strain discarded since results were not identical in at least 3 repetitions (data not shown). A total of 110 indigenous isolates were classified alongside a reference (Evo Cross (CROSS)) into seven clusters or biotypes, depending upon the presence or absence of 22 gel bands (Table 2). The genetic relationship of each of these groups is reported as a dendrogram (Fig. 2) with Multiple Correspondence Analysis (MCA) using the first factorial (F1) plane to display the (Euclidean) distances among clusters in the Ward’s dendrograms (data not shown). In order to describe each cluster quickly, value tests were calculated59 and the most significant modalities used. The distance of each individual isolate to the centroid of cluster allowing the identification of the homogeneity and similarity between these groups (data not shown).
Global Ward dendrogram showing the genetic relationship of the 110 Saccharomyces cerevisiae isolates. Seven biotypes (I and VII) were identified based on the highest Calinski-Harabasz criterion value (18.717) and smallest Davies-Bouldin index value (1.052). In instances of > 100 isolates, the SPAD software names the most similar ones using a C number code (e.g. C_100). The isolates repeated among the vintages are as follows: C_100 (2415, 3816, 3916), C_102 (1514, 1614, 1915, 2015, 4516), C_108 (3714, 1615) and C_109 (2014, 2315). Another group, named C_106 includes the isolates 4616, 4816 and 4916 from the same vintage. The isolates for each C number are identical and represent individual strains (Supplementary Table S2 online). Letters A to I indicate nine perennial strains isolated from two or more vintages. CROSS was used as a reference strain, and forms part of Biotype I (Euclidean distance of 1.373) with the closest related strain, 3614 (from 2014) having a distance of 1.145 (Supplementary Table S2 online).
Global dendrogram identifying strain diversity over four consecutive vintages
The study of 110 S. cerevisiae isolates from the four single spontaneous Negro Saurí fermentations undertaken identified not only strains specific to a single vintage, but those recoverable in successive vintages as well as clonal isolation of individual strains (Fig. 2, Table 3, and Supplementary Table S2 online). The 110 isolates represent 70 genetically distinct strains collected over the four vintages during different stages of fermentation. The percentage of genetically distinct strains amongst the total collected varied per vintage (85.7% (2014), 76.5% (2015), 63.6% (2016) and 80.9% (2017)). Seven major biotypes (Biotypes I–VII) were identified amongst the S. cerevisiae isolates (Fig. 2, Tables 2 and 3). 2014 showed the greatest variability with 25 strains identified amongst the 28 isolates, which were distributed between the 7 biotypes (I (41.6%), II (100%), III (6.4%), IV (11.1%), V (60%), VI (37.5%) and VII (100%); Fig. 2). 2016 exhibited the smallest genetic variability despite the number of isolates sampled with a high degree of clonality observed for this vintage, which is discussed below. The following biotypes lacked isolates from vintages 2015 (IV), 2016 (V) and 2017 (VI).
Biotypes II and VII were considered as “vintage medium” biotypes, defined by bands that did not identify the rest of the biotypes. In this case, the absence of bands 50, 1000, and 180 for Biotype VII and 50, 800 and 1000 for II, respectively (Table 2). These 2 biotypes had genetically distinct strains specific to 2014 (II; 2814, 3014 and VII; 3814). Biotype III (representing 42.7% of total) was largely represented by isolates from 2016 of which there was a high number of clonal isolates. This probably reflects the larger sample size (47) compared to the other vintages (Table 3). Three strains were specific to this vintage: 1 strain was present at the beginning and end of fermentation (SF: 0916, 1016; EF: 2016), whilst another 2 strains were confined to the EF ((2416, 3616, 4016, 4216, 4316) and (2216, 5216)). Biotype IV (representing 8.2% of total isolates) had 9 genetically similar but distinct strains from the first (2214), third (1116, 1216, 1316, 1516) and fourth vintage (4317, 4417, 4517, 4817). Whilst Biotype V (4.6% of total) consisted of 5 distinct strains; 1914 and 1815 isolated from mid-fermentation whilst 2314, 3514 and 4217 were found at the end of ferment (Table 3). In Biotype I (12.7% of total), 13 of the 14 isolates represented different strains, with only 2 isolates being clonal isolates (3317, 2417), which were from mid-ferment. The temporal distribution of these strains indicates a ‘flow’ of individual strains during the course of fermentation. For example, during vintage 2014, four strains belonging to Biotype I were found at EF (2414, 2614, 3614, 4314), whilst 1415 was at SF in 2015, and 4116 was at the EF (vintage 2016). In 2017, 4 strains (1617, 1817, 2117 and 2217) were from the start of fermentation, whilst 3 were from mid-fermentation (2417, 3217 and 3317).
Clonal enrichment of strains was observed for 3 biotypes (VI, III and I) and was typically observed at the end of fermentation (Fig. 2). The number of clonal isolates varied between 2 and 4 or 5, depending on the biotype and vintage (Table 3). Some strains were identified within a single vintage and are ‘annual’ strains, such as 4 strains belonging to Biotype III isolated only in 2016 (1816, 1716), (5216, 2216), (916, 1016, 2116) and (3416, 3616, 4016, 4216, 4316). Another 2 strains (Biotype III: 4717, 4617) and (Biotype I: 2417, 3317) were only observed in 2017. Other strains were ‘perennial’, being present in at least two vintages though not necessarily consecutive ones. For example, within biotype III, there were four different strains E (C_109: 2014, 2315, 3917), F (C_100: 2415, 3816, 3916), G (1714 and 1416), and H (2716, 2816, 3016, 3716, 5016, 3717, 3817, 4117) (Supplementary Table S3 online).
More importantly, in terms of useful starter cultures, a total of 9 ethanol tolerant strains (A to I) were found to dominate in two or more vintages (Fig. 2). For example, one strain (D) was identified amongst four isolates in Biotype VI (2714, 2914, 3314, 3414) at the end of fermentation in 2014, to be reisolated in 2015 in the must (M: 415) and at the start of fermentation (SF: 615, 1215, 1315). Strain D reoccurred again in 2016 at the end of fermentation (EF: 1916, 2316). Another strain (H) identified as clonal isolates in Biotype III in 2016 (EF: 2716, 2816, 3016, 3716) was similarly observed the following year (MF: 3717, EF: 3817, 4117). These findings suggest that these particular strains are endemic to the winery but do not identify as Evo Cross (CROSS), which is used as the reference strain and is part of Biotype I, with the closest related strain being 3614 (Fig. 2).
In conclusion, the S. cerevisiae population in Negro Saurí spontaneous fermentations appears to be both diverse and transient, with only a few strains being prominent over several vintages. Seventy distinct strains were identified over the four vintages, whilst the sum of strains per vintage was 82 (24 strains (2014), 13 strains (2015), 28 strains (2016), and 17 strains (2017); Fig. 2) as 12 strains were present in one or more vintages. Of the 9 ‘winery related’ strains, 7 were found across 2 vintages (A, B, E, F, G, H, I) and only 2 (C, D) persisted over 3 consecutive years. None were found to persist over the 4 years. Whilst the study revealed 61 ‘single vintage’ strains, which may be worthy of further investigation in terms of their oenological properties. More interesting are the 9 strains that were more persistent, as shown by their being identified in 2 or more vintages. Larger scale fermentations using these strains as starter cultures are required with an in-depth sensory and chemical analysis to determine whether they are useful to the local wine industry in terms of providing an aroma profile typical of this locality’s ‘microbial terroir’.
Discussion
We report for the first time on the selection and genetic characterization of autochthonous microorganisms from the minority variety, Negro Saurí. The goal was to preserve the genetic resources of the locality, whilst providing insights into the wine strain diversity and, in turn, their role in contributing to the aroma-flavour profile of regional wines. This study aligns with the increasing demand for indigenous S. cerevisiae isolates that are representative of a specific oenological area62, with particular wineries increasingly selecting yeasts within their vineyard for specific characteristics and suitability to local grape varieties63,64. Extensive studies of spontaneous fermentations in Italy65, Spain18,66, USA67 and Canada68 (including studies specifically of the genetic diversity of Saccharomyces cerevisiae in different wine-producing regions36,69,70) are validated or partly motivated by reports71 of a significant correlation between the region from where the strains were isolated and the aroma profile of the resulting wines.
In this study, four non-Saccharomyces yeasts (Metschnikowia pulcherrima, Lachancea thermotolerans, Hanseniaspora uvarum and Torulaspora delbrueckii) were isolated from Negro Saurí grapes and must, based on their colony morphology and subsequent ITS sequencing. This result is similar to earlier reports of the microbiota associated with Prieto Picudo, grown in the León Appellation of Origin72, and Carinyena and Garnacha grapes from the Priorat wine region of Tarragona, Spain73. The lack of diversity seen in all three studies most likely relates to the isolation procedure and the restricted number of isolates tested. Plating of dilutions of grape, must and ferment samples onto nutrient-rich media favouring the more numerous, and faster-growing species over minor, slower growing ones74. No interpretation could be made from the small numbers isolated in relation to grape variety, geographic location and climatic conditions and yeast diversity and number75. However, the reduced number of culturable species may relate to the use of organic (sulphur, copper) and inorganic fungicides (Supplementary Table S3 online), corroborating published data on the influence of fungicide spray regimes on yeast diversity and number76,77,78,79. Whilst these fungicides target Odium and other mildew fungi, they also inhibit other ascomycetes including wine non-Saccharomyces because of their broad mode of action. For example, ergosterol biosynthesis (triazoles—penconazole, cyproconazole, propiconazole), RNA synthesis (phenylamides—metalaxyl), mitochondrial respiration (synthetic strobilurins—trifloxystrobin), and arylaminopyridines (fluazinam), whilst others are non-specific (phthalimides—captan, folpet)80.
Aureobasidium pullulans is a well-documented species on grapes; its presence being independent of variety or viticultural practise77. The use of elemental sulphur and copper (as oxychloride and cuprocalcium sulphate) has resulted in its adaptation, with the fungus able to detoxify sulphur81 and copper82. In this study, fungal colonies were isolated, but not confirmed as Aureobasidium pullulans because of their low relative abundance compared to Metschnikowia sp., L. thermotolerans, H. uvarum and their inability to ferment, making them of limited oenological interest83,84. Metschnikowia pulcherrima was successively isolated from grapes and must and to a lesser extent, in the early stages of fermentation. L. thermotolerans and H. uvarum featured in 2014 and 2017, on grapes, in must and during early fermentation. In 2017, species diversity included T. delbrueckii, which was isolated from mid-fermentation samples. Metschnikowia spp have also been associated with Prieto Picudo, which is the main grape variety in the León Appellation of Origin72. Whilst the authors did not report on L. thermotolerans, this species is both genetically (and phenotypically) diverse85, and has a diverse geographical distribution86. Hanseniospora uvarum is well known to be abundant on grapes74 and can even predominate at the start of an un-inoculated (spontaneous) fermentation16,65 and in some instances in the final stages87. In our study, Hanseniospora could not be isolated from the aseptically sampled grape berries, but were present in the grape must and fermentation samples taken at the experimental winery. These findings, similar to those of Grangeteau and coworkers in France15, allude to implantation of the must from winery environment, with the possibility of some non-Saccharomyces species persisting from one year to another in the winery environment to later dominant during fermentation.
The non-Saccharomyces yeasts identified in our study and by others are of potential oenological interest. For instance Metschnikowia pulcherrima offers possibilities in biocontrol of spoilage yeasts88 whilst the use of M. pulcherrima, L. thermolerans and T. delbrueckii offer paradigms for reducing wine ethanol content89, in deacidification and improving aroma complexity90,91. Furthermore, the production of extracellular enzymes such as pectinases can aid anthocyanin extraction, clarification and filterability92. Likewise, H. uvarum can secrete β-glucosidases, which can release glycosidically bound grape-derived terpenes, thereby contributing to varietal aroma in wines14,93. ‘Tailored’ autochthonous starter cultures are of interest not only in terms of ‘terroir’ but provide safer alternatives to spontaneous fermentations in relation to human health, as microbial producers of contaminants (e.g., biogenic amines, ethyl carbamate, etc.) are screened for (reviewed in94). To date, there are only a small number of commercial starter cultures available for co- or mixed culture fermentations95. As such, there is a continued need for specific strains, with distinctive properties in terms of novel wine styles, and improving wine quality and process efficiency.
Whilst non-Saccharomyces yeasts are easily isolated from the grape surface16, the extremely low occurrence of S. cerevisiae on healthy, undamaged grapes, makes it largely undetectable96, and difficult to recover without enrichment through a fermentation97. In this study, spontaneous fermentation was conducted each vintage in sanitised 500 L steel tanks at the experimental winery. S. cerevisiae was typically isolated at the start of fermentation with the exception of 2014 when it was not evident until mid-fermentation, and 2015 when it was largely present in the grape must, alluding to possible fruit damage prior to harvesting, so promoting S. cerevisiae growth. In all cases, S cerevisiae typically dominated and completed the fermentation.
The 110 S. cerevisiae isolates were classified by their delta sequence patterns. Heterogenous populations were found both annually and over the four-year study period. In total, 70 strains were identified, of which the majority (61) were confined to a single vintage and most likely represent the indigenous population in the vineyard, which was dynamic and vintage dependent. Nine strains (Fig. 2A–I) were isolated in 2 or more consecutive vintages suggesting that they are part of the resident microbiota of the winery at Melgarajo S.A., which was 3 km from the vineyard and 17 km from the nearest neighbouring winery. Similar findings are reported by Clavijo et al.98, although the authors identified commercial strains in the laboratory fermentations, and concluded that the strains were transferred from the winery into nearby vineyards through insect transmission, the spreading of yeast lees in the vineyard as well water run-off from routine cellar operations. The yeasts persisted in only a single vintage, which is contrary to our ‘winery implanted’ strains. Whilst insect transfer from the winery or between neighbouring plots is possible, the strains are likely to be indigenous given that commercial strains were not used for experimental winemaking. There is still some debate as to whether grape variety or location may influence the indigenous Saccharomyces population. Clavijo et al.98 reported on separate populations in 3 grape varieties (Syrah, Merlot and Cabernet Sauvignon) whilst Capece’s research99 examining 11 varieties grown in a single vineyard, alluded to specific native S. cerevisiae strains being associated with a particular terroir. However, Santamaría and co-workers100 in a recent study of 11 wineries in the Rioja region showed that the strains varied with vintage and none were common to neighbouring wineries or the area. In a much larger study using delta PCR sequencing, Sun et al.36 concluded that not only do Saccharomyces populations differ between grape varieties, but also between wine and table grapes, as well as geographical location both on a local (region) and global (country) scale.
This is the first report of the microbiota associated with the minor red variety Negro Saurí. The objective of the study was to capture and preserve the yeast diversity, with a view to such strains being potentially used as starter cultures and/or contributing to a distinct terroir. The nine identified perennial Saccharomyces isolates require further characterisation to determine their suitability, a task beyond the scope of this initial ‘identify and survey’ study.
Material and methods
Experimental vineyard and management
The study was based at an experimental vineyard at Melgarajo S.A. (42° 15′ 48.68_N 5° 9′ 56.66_W), which is located in Melgar de Abajo within the León Appellation of Origin. Three rows of Negro Saurí grapes (15 cultivars) were cultivated as part of the ITACYL program to rescue this variety (Supplementary Fig. S1 online). The grapes used in the study were from these cultivars.
Viticultural management (fungicide, herbicide, vine nutrition) was recorded for each growing season (Supplementary Table S3 online). Glyphosate was used as a post-emergence herbicide each season. Sulfur (98%) was used as the organic fungicide and acricide in 2014 and 2017, whilst Caldo Bordeles (cuprocalcium sulphate) was used in 2015, and a combination of sulphur and Corbre Lainco (copper oxychloride) in 2016. Other organic sulphur fungicides used included dithiocarbamates such as mancazeb (Fantic-M). Inorganic fungicides were rotated annually, with several classes (differing in mode of action) used singularly and in combination to prevent fungal resistance (Supplementary Table S3 online).
The experimental facility (winery) used in the study was 3 km from the vineyard Winery equipment purchased for sole use with un-inoculated fermentations, with no commercial Saccharomyces used on the premises. The nearest winery was 17 km from Melgarajo S.A.
Sampling procedure
Samples were collected at maturity (~ 24°Brix) over 4 consecutive vintages: 2014, 2015, 2016 and 2017. Grape samples (1 kg) were aseptically collected, placed into sterile plastic bags and transported to the Microbiology Laboratory at the Veterinary Faculty of the University of León for analysis. Additional grapes (~ 500 kg) were hand-picked into 20 kg boxes, before being destemmed, pressed and the must transferred to potassium metabisulfite-treated steel tanks (500 L) to undergo un-inoculated (spontaneous) fermentation. Fermentation was conducted at 16–18 °C at the experimental facility within Melgarajo S.A., which is used only for research wine production of Negro Saurí. Wine samples (100 mL) were taken twice-weekly during the fermentation. For chemical data for Negro Saurí grapes and wine refer to Supplementary Table S4 online.
Yeast isolation from grapes and spontaneous fermentation
The 1 kg grape samples were aseptically homogenized using a Stomacher 400 laboratory paddle blender (Seward Ltd., England) for 1 min at normal intensity. Aliquots (0.1 mL) of homogenized grapes and wine samples were plated at different serial dilutions using 0.9% saline onto YED agar (1% yeast extract, 2% glucose and 2% agar; pH 4.5) supplemented with 150 µg mL−1 chloramphenicol (Sigma-Aldrich, St. Louis, Mo, USA) to inhibit bacterial growth. The plates were incubated at 30 °C for 2–3 days after which, the various colony types were isolated at random according to shape, colour, surface feature and frequency101. The yeast cultures were preserved at − 80 °C in liquid YED medium with glycerol 30% as a cryoprotectant ahead of identification.
S rRNA gene analysis
Genomic DNA was extracted according to Lõoke et al.102 using either single yeast colonies (or cells collected from 100 μL of liquid YPD culture (OD600 0.4)). The DNA pellet was dissolved in 100 μL water and the debris removed by centrifugation (15,000×g for 1 min) before one μL of supernatant was used for PCR. The 5.8S rRNA gene analysis was undertaken according to Esteve-Zarzoso et al.55, with the internal transcribed spacer regions (ITS1 and ITS4) used for yeast identification. PCR amplification was performed in a 50 µl reaction with 100 ng genomic DNA as template, 0.1 µM each primer, 0.4 mM dNTP and 0.5 unit of BiTOOLS DNA polymerase (ULTRATOOLS, Spain). The primers used for amplification were ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). PCR amplification was initiated with a 5 min denaturation at 95 °C, followed by 35 amplification cycles (94 °C, 1 min; 55.5 °C, 2 min; 72 °C, 2 min) and terminated with a 10 min extension at 72 °C. PCR products were separated on 0.5 and 3% agarose gels in 1× TAE buffer (40 mM Tris, 20 mM acetic acid, 1 mM EDTA (pH 8). Products were purified using Wizard SV Gel and PCR Clean-Up (Promega) and sent to the DNA Sequencing Service of the Laboratory of Instrumental Techniques of the University of León. Sequence comparisons were performed using the basic local alignment search tool (BLAST) program within the NCBI database56.
Genetic characterization of Saccharomyces cerevisiae strains using delta PCR analysis
The genetic diversity within 111 strains isolated from spontaneously fermented Negro Saurí juice and one commercial wine Saccharomyces cerevisiae (Cross Evolution, Lallemand) was evaluated by PCR amplification of inter-delta (δ) regions, which flank Ty elements in the yeast genome58. DNA isolation was performed according to Liu et al.103. PCR amplification of inter-delta sequences was with primers δ12 (5′-TCAACAATGGAATCCCAAC-3′) and δ21 (5′-CATCTTAACACCGTATATGA-3′)57. PCR amplification was performed in a 25 µl reaction using 30–100 ng template DNA, 0.8 µM each primer, 0.2 mM dNTP and 2.5 unit of Mango Taq (Bioline). PCR conditions were as follows: initial denaturation for 4 min at 95 °C followed by 35 cycles of 30 s at 95 °C; 30 s at 46 °C and 90 s at 72 °C, and a final extension step of 10 min at 72 °C. The PCR products were separated on 2% agarose gels and the size of the amplified DNA fragments estimated with 50 bp HyperLadder DNA markers (Bioline).
PCR amplification was repeated four times per isolate to ensure repeatability, with the results considered accurate when 3 or 4 replicates were equal; where this did not occur, the results were reviewed.
Statistical analysis
The interdelta sequence patterns were used to construct a presence/absence matrix (binary 0/169). All visible bands were assigned a number based upon relative position to the DNA ladder, with each position assigned a score to indicate the presence (1) or absence (0) of the band (Table 2).
The 110 isolates and Cross Evolution (referred to as CROSS) were classified on the basis of similarity among patterns of the 22 bands, depicted in a global dendrogram. For dendrogram construction, the statistical analysis was carried out according to Lebart et al.59. Firstly, due to the data described being 0 or 1, the strains were analysed by applying Multiple Correspondence Analysis (MCA104). For the construction of the global dendrogram, a hierarchical cluster analysis (Euclidean distance and Ward algorithm60) was applied, taking the components retained (Kaiser's Criterion) from the MCA analysis. Finally, the best partition of the dendrogram was based on the Davies-Bouldin and Calinski-Harabasz indicators61. The statistical software Coheris Analytics SPAD (2017) version 9.0.39 was used.
Change history
04 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-89721-3
References
Csoma, H., Zakany, N., Capece, A., Romano, P. & Sipiczki, M. Biological diversity of Saccharomyces yeasts of spontaneously fermenting wines in four wine regions: Comparative genotypic and phenotypic analysis. Int. J. Food Microbiol. 140, 239–248. https://doi.org/10.1016/j.ijfoodmicro.2010.03.024 (2010).
Di Maio, S. et al. Biodiversity of indigenous Saccharomyces populations from old wineries of South-Eastern Sicily (Italy): Preservation and economic potential. PLoS ONE 7, e30428. https://doi.org/10.1371/journal.pone.0030428 (2012).
Bokulich, N. A., Ohta, M., Richardson, P. M. & Mills, D. A. Monitoring seasonal changes in winery-resident microbiota. PLoS ONE 8, e66437. https://doi.org/10.1371/journal.pone.0066437 (2013).
Mas, A., Padilla, B., Esteve-Zarzoso, B. & Beltran, G. Utilización de inóculos mixtos de levaduras autóctonas como herramienta para reproducir la huella microbiológica de la zona. Acenologica. http://www.acenologia.com/cienciaytecnologia/inoculos_mixtos_levaduras_autoctonas_cienc0715.htm (2013).
Varela, C. & Borneman, A. R. Yeasts found in vineyards and wineries. Yeast 34, 111–128. https://doi.org/10.1002/yea.3219 (2017).
Fleet, G. H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 86, 11–22. https://doi.org/10.1016/S0168-1605(03)00245-9 (2003).
Mannazzu, I., Clementi, F. & Ciani, M. In Biodiversity and Biotechnology of Wine Yeasts 19–34 (2002).
Martini, A., Ciani, M. & Scorzetti, G. Direct enumeration and isolation of wine yeasts from grape surfaces. Am. J. Enol. Vit. 47, 435 (1996).
Mortimer, R. & Polsinelli, M. On the origins of wine yeast. Res. Microbiol. 150, 199–204. https://doi.org/10.1016/S0923-2508(99)80036-9 (1999).
Ciani, M., Comitini, F., Mannazzu, I. & Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 10, 123–133. https://doi.org/10.1111/j.1567-1364.2009.00579.x (2010).
Ribéreau-Gayon, P., Dubourdieu, D., Donéche, B. & Lonvaud, A. The Microbiology of Wine and Vinifications 2nd edn, Vol. 1, 512 (2006).
Charoenchai, C., Fleet, G. H., Henschke, P. A. & Todd, B. E. N. T. Screening of non-Saccharomyces wine yeasts for the presence of extracellular hydrolytic enzymes. Aus. J. Grape Wine Res. 3, 2–8. https://doi.org/10.1111/j.1755-0238.1997.tb00109.x (1997).
Fernández, M. T., Ubeda, J. F. & Briones, A. I. Comparative study of non-Saccharomyces microflora of musts in fermentation, by physiological and molecular methods. FEMS Microbiol. Lett. 173, 223–229. https://doi.org/10.1111/j.1574-6968.1999.tb13506.x (1999).
Zott, K., Miot-Sertier, C., Claisse, O., Lonvaud-Funel, A. & Masneuf-Pomarede, I. Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int. J. Food Microbiol. 125, 197–203. https://doi.org/10.1016/j.ijfoodmicro.2008.04.001 (2008).
Grangeteau, C. et al. Diversity of yeast strains of the genus Hanseniaspora in the winery environment: What is their involvement in grape must fermentation?. Food Microbiol. 50, 70–77. https://doi.org/10.1016/j.fm.2015.03.009 (2015).
Fleet, G. H. Wine yeasts for the future. FEMS Yeast Res. 8, 979–995. https://doi.org/10.1111/j.1567-1364.2008.00427.x (2008).
Canonico, L., Comitini, F., Oro, L. & Ciani, M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front. Microbiol. 7, 278–278. https://doi.org/10.3389/fmicb.2016.00278 (2016).
Padilla, B., Gil, J. V. & Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 7, 411–411. https://doi.org/10.3389/fmicb.2016.00411 (2016).
Esteve-Zarzoso, B., Manzanares, P., Ramön, D. & Quero, A. The role of non-Saccharomyces yeasts in industrial winemaking. Int. Microbiol. 1, 143–148 (1998).
Gonzalez, R., Quirós, M. & Morales, P. Yeast respiration of sugars by non-Saccharomyces yeast species: A promising and barely explored approach to lowering alcohol content of wines. Trends Food Sci. Techol. 29, 55–61. https://doi.org/10.1016/j.tifs.2012.06.015 (2013).
Quirós, M., Rojas, V., Gonzalez, R. & Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 181, 85–91. https://doi.org/10.1016/j.ijfoodmicro.2014.04.024 (2014).
Morales, P., Rojas, V., Quirós, M. & Gonzalez, R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 99, 3993–4003. https://doi.org/10.1007/s00253-014-6321-3 (2015).
Varela, C. et al. Strategies for reducing alcohol concentration in wine. Aus. J. Grape Wine Res. 21, 670–679. https://doi.org/10.1111/ajgw.12187 (2015).
Roudil, L. et al. Non-Saccharomyces commercial starter cultures: Scientific trends, recent patents and innovation in the wine sector. Recent Patents Food Nutr. Agric. https://doi.org/10.2174/2212798410666190131103713 (2019).
Le Jeune, C., Erny, C., Demuyter, C. & Lollier, M. Evolution of the population of Saccharomyces cerevisiae from grape to wine in a spontaneous fermentation. Food Microbiol. 23, 709–716. https://doi.org/10.1016/j.fm.2006.02.007 (2006).
Versavaud, A., Courcoux, P., Roulland, C., Dulau, L. & Hallet, J. N. Genetic diversity and geographical distribution of wild Saccharomyces cerevisiae strains from the wine-producing area of Charentes, France. Appl. Environ. Microbiol. 61, 3521 (1995).
Pérez-Coello, M. S., Briones Pérez, A. I., Ubeda Iranzo, J. F. & Martin Alvarez, P. J. Characteristics of wines fermented with different Saccharomyces cerevisiae strains isolated from the La Mancha region. Food Microbiol. 16, 563–573. https://doi.org/10.1006/fmic.1999.0272 (1999).
Torriani, S., Zapparoli, G. & Suzzi, G. Genetic and phenotypic diversity of Saccharomyces sensu stricto strains isolated from Amarone wine. Antonie Van Leeuwenhoek 75, 207–215. https://doi.org/10.1023/A:1001773916407 (1999).
Naumov, G. I., Masneuf, I., Naumova, E. S., Aigle, M. & Dubourdieu, D. Association of Saccharomyces bayanus var. uvarum with some French wines: Genetic analysis of yeast populations. Res. Microbiol. 151, 683–691. https://doi.org/10.1016/s0923-2508(00)90131-1 (2000).
Redžepović, S., Orlić, S., Sikora, S., Majdak, A. & Pretorius, I. S. Identification and characterization of Saccharomyces cerevisiae and Saccharomyces paradoxus strains isolated from Croatian vineyards. Letts. Appl. Microbiol. 35, 305–310. https://doi.org/10.1046/j.1472-765X.2002.01181.x (2002).
Rementeria, A. et al. Yeast associated with spontaneous fermentations of white wines from the “Txakoli de Bizkaia” region (Basque Country, North Spain). Int. J. Food Microbiol. 86, 201–207. https://doi.org/10.1016/S0168-1605(03)00289-7 (2003).
Cappello, M. S., Bleve, G., Grieco, F., Dellaglio, F. & Zacheo, G. Characterization of Saccharomyces cerevisiae strains isolated from must of grape grown in experimental vineyard. J. Appl. Microbiol. 97, 1274–1280. https://doi.org/10.1111/j.1365-2672.2004.02412.x (2004).
Fay, J. C. & Benavides, J. A. Evidence for domesticated and wild populations of Saccharomyces cerevisiae. PLoS Genet. 1, e5. https://doi.org/10.1371/journal.pgen.0010005 (2005).
Schuller, D., Alves, H., Dequin, S. & Casal, M. Ecological survey of Saccharomyces cerevisiae strains from vineyards in the Vinho Verde Region of Portugal. FEMS Microbiol. Ecol. 51, 167–177. https://doi.org/10.1016/j.femsec.2004.08.003 (2005).
Viel, A. et al. The geographic distribution of Saccharomyces cerevisiae isolates within three Italian neighboring winemaking regions reveals strong differences in yeast abundance, genetic diversity and industrial strain dissemination. Front. Microbiol. 8, 1595–1595. https://doi.org/10.3389/fmicb.2017.01595 (2017).
Sun, Y. et al. Evaluation of Chinese Saccharomyces cerevisiae wine strains from different geographical origins. Am. J. Enol. Vit. 68, 73. https://doi.org/10.5344/ajev.2016.16059 (2017).
da Silva, G. A. D., Agustini, B. C., de Mello, L. M. R. & Tonietto, J. Autochthonous yeast populations from different Brazilian geographic indications. BIO Web Conf. 7 (2016).
Crosato, G. et al. Genetic variability and physiological traits of Saccharomyces cerevisiae strains isolated from “Vale dos Vinhedos” vineyards reflect agricultural practices and history of this Brazilian wet subtropical area. World J. Microbiol. Biotechnol. 34, 105. https://doi.org/10.1007/s11274-018-2490-z (2018).
Chavan, P. et al. Natural yeast flora of different varieties of grapes used for wine making in India. Food Microbiol. 26, 801–808. https://doi.org/10.1016/j.fm.2009.05.005 (2009).
Kachalkin, A. V., Abdullabekova, D. A., Magomedova, E. S., Magomedov, G. G. & Chernov, I. Y. Yeasts of the vineyards in Dagestan and other regions. Microbiology 84, 425–432. https://doi.org/10.1134/S002626171503008X (2015).
Cordero-Bueso, G., Arroyo, T., Serrano, A. & Valero, E. Remanence and survival of commercial yeast in different ecological niches of the vineyard. FEMS Microbiol. Ecol. 77, 429–437. https://doi.org/10.1111/j.1574-6941.2011.01124.x (2011).
Valero, E., Schuller, D., Cambon, B., Casal, M. & Dequin, S. Dissemination and survival of commercial wine yeast in the vineyard: A large-scale, three-years study. FEMS Yeast Res. 5, 959–969. https://doi.org/10.1016/j.femsyr.2005.04.007 (2005).
Valero, E., Cambon, B., Schuller, D., Casal, M. & Dequin, S. Biodiversity of Saccharomyces yeast strains from grape berries of wine-producing areas using starter commercial yeasts. FEMS Yeast Res. 7, 317–329. https://doi.org/10.1111/j.1567-1364.2006.00161.x (2007).
Blanco, P., Mirás-Avalos, J. M. & Orriols, I. Effect of must characteristics on the diversity of Saccharomyces strains and their prevalence in spontaneous fermentations. J. Appl. Microbiol. 112, 936–944. https://doi.org/10.1111/j.1365-2672.2012.05278.x (2012).
Garofalo, C., Tristezza, M., Grieco, F., Spano, G. & Capozzi, V. From grape berries to wine: Population dynamics of cultivable yeasts associated to “Nero di Troia” autochthonous grape cultivar. World J. Microbiol. Biotechnol. 32, 59. https://doi.org/10.1007/s11274-016-2017-4 (2016).
Schuller, D. et al. Genetic diversity and population structure of Saccharomyces cerevisiae strains isolated from different grape varieties and winemaking regions. PLoS ONE 7, e32507. https://doi.org/10.1371/journal.pone.00325 (2012).
Martinez, M. C. & Perez, J. E. The forgotten vineyard of the Asturias Princedom (north of Spain) and ampelographic description of its grapevine cultivars (Vitis vinifera L.). Am. J. Enol. Vit. 51, 370–378 (2000).
Yuste, J. et al. Identification of autochthonous grapevine varieties in the germplasm collection at the ITA of “Castilla y León” in Zamadueñas Station, Valladolid. Spain. Spanish J. Agric. Res. https://doi.org/10.5424/sjar/2006041-175 (2006).
Cabello, F., Saiz, R. & Muñoz, G. Estudio de variedades españolas minoritarias de vid. Acenologica. http://www.acenologia.com/cienciaytecnologia/variedades_minoritarias_cienc0213.htm (2013).
Balda, P. & de Toda, F. M. Variedades minoritarias de vid en La Rioja. Consejería de Agricultura, Ganadería y Medio Ambiente. (2017).
Martínez de Toda, F. Veinte nuevas variedades de vid, rescatadas de la desaparición, en la viticultura española y nuevos vinos. Acenologica. http://www.acenologia.com/dossier/dossier135.htm (2013).
Arranz, C. et al. Variedades de vid cultivadas en la Sierra de Francia. Importancia, identificación, sinonimias y homonimias. La Semana Vitivinícola 3223, 1414–1420 (2008).
Ibáñez, J., Carreño, J., Yuste, J. & Martínez-Zapater, J. M. In Grapevine Breeding Programs for the Wine Industry (ed Reynolds, A.) 183–209 (Woodhead Publishing, 2015).
Arranz Hernández, C., Barajas Tola, E., Yuste Bombín, J. & Rubio Cano, J. A. 45–58 (Comunidad de Madrid (España): Ministerio de Agricultura, Alimentación y Medio Ambiente, 2016).
Esteve-Zarzoso, B., Belloch, C., Uruburu, F. & Querol, A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bact. 49, 329–337. https://doi.org/10.1099/00207713-49-1-329 (1999).
Madden, T. L., Tatusov, R. L. & Zhang, J. Methods in Enzymology Vol. 266, 131–141 (Academic Press, London, 1996).
Legras, J.-L. & Karst, F. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterisation. FEMS Microbiol. Lett. 221, 249–255. https://doi.org/10.1016/S0378-1097(03)00205-2 (2003).
Ness, F., Lavallée, F., Dubourdieu, D., Aigle, M. & Dulau, L. Identification of yeast strains using the polymerase chain reaction. J. Sci. Food Agric. 62, 89–94. https://doi.org/10.1002/jsfa.2740620113 (1993).
Lebart, L., Morineau, A. & Piron, M. Statistique Exploratoire Multidimensionnelle (Dunod Publishers, Paris, 1995).
Granato, D., Santos, J. S., Escher, G. B., Ferreira, B. L. & Maggio, R. M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 72, 83–90. https://doi.org/10.1016/j.tifs.2017.12.006 (2018).
Arbelaitz, O., Gurrutxaga, I., Muguerza, J., Pérez, J. M. & Perona, I. An extensive comparative study of cluster validity indices. Pattern Recogn. 46, 243–256. https://doi.org/10.1016/j.patcog.2012.07.021 (2013).
Orlić, S. et al. Diversity and oenological characterization of indigenous Saccharomyces cerevisiae associated with Žilavka grapes. World J. Microbiol. Biotechnol. 26, 1483–1489. https://doi.org/10.1007/s11274-010-0323-9 (2010).
Tristezza, M. et al. Molecular and technological characterization of Saccharomyces cerevisiae strains isolated from natural fermentation of Susumaniello grape must in Apulia, Southern Italy. Int. J. Microbiol. 897428–897428, 2014. https://doi.org/10.1155/2014/897428 (2014).
SchvarczovÁ, E. V. A., ŠtefáNiková, J., Jankura, E. & Kolek, E. Selection of autochthonous Saccharomyces cerevisiae strains for production of typical Pinot Gris wines. J. Food Nutr. Res. 56, 389–397 (2017).
Tristezza, M. et al. Biodiversity and safety aspects of yeast strains characterized from vineyards and spontaneous fermentations in the Apulia Region, Italy. Food Microbiol. 36, 335–342. https://doi.org/10.1016/j.fm.2013.07.001 (2013).
Sabate, J., Cano, J., Querol, A. & Guillamon, J. M. Diversity of Saccharomyces strains in wine fermentations: Analysis for two consecutive years. Lett. Appl. Microbiol. 26, 452–455. https://doi.org/10.1046/j.1472-765X.1998.00369.x (1998).
Bougreau, M., Ascencio, K., Bugarel, M., Nightingale, K. & Loneragan, G. Yeast species isolated from Texas High Plains vineyards and dynamics during spontaneous fermentations of Tempranillo grapes. PLoS ONE 14, e0216246–e0216246. https://doi.org/10.1371/journal.pone.0216246 (2019).
Martiniuk, J. T. et al. Impact of commercial strain use on Saccharomyces cerevisiae population structure and dynamics in Pinot Noir vineyards and spontaneous fermentations of a Canadian winery. PLoS ONE 11, e0160259. https://doi.org/10.1371/journal.pone.0160259 (2016).
Mercado, L., Jubany, S., Gaggero, C., Masuelli, R. W. & Combina, M. Molecular relationships between Saccharomyces cerevisiae strains involved in winemaking from Mendoza, Argentina. Curr. Microbiol. 61, 506–514. https://doi.org/10.1007/s00284-010-9645-y (2010).
de Celis, M. et al. Diversity of Saccharomyces cerevisiae yeasts associated to spontaneous and inoculated fermenting grapes from Spanish vineyards. Lett. Appl. Microbiol. 68, 580–588. https://doi.org/10.1111/lam.13155 (2019).
Knight, S., Klaere, S., Fedrizzi, B. & Goddard, M. R. Regional microbial signatures positively correlate with differential wine phenotypes: Evidence for a microbial aspect to terroir. Sci. Rep. 5, 14233. https://doi.org/10.1038/srep14233 (2015).
Álvarez-Pérez, J. M., Garzón-Jimeno, E. & Coque, J. J. R. Population of indigenous yeast strains from Prieto Picudo grapes in different growing areas of Denomination of Origin “Tierra de León”. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Hortic. 72, 17–26. https://doi.org/10.15835/buasvmcn-hort:11013 (2015).
Sabate, J., Cano, J., Esteve-Zarzoso, B. & Guillamón, J. M. Isolation and identification of yeasts associated with vineyard and winery by RFLP analysis of ribosomal genes and mitochondrial DNA. Microbiol. Res. 157, 267–274. https://doi.org/10.1078/0944-5013-00163 (2002).
Barata, A., Malfeito-Ferreira, M. & Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 153, 243–259. https://doi.org/10.1016/j.ijfoodmicro.2011.11.025 (2012).
Bokulich, N. A., Thorngate, J. H., Richardson, P. M. & Mills, D. A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. PNAS 111, E139–E148. https://doi.org/10.1073/pnas.1317377110 (2014).
Russo, P. et al. Pesticide residues and stuck fermentation in Wine: New evidences indicate the urgent need of tailored regulations. Fermentation 5, 23. https://doi.org/10.3390/fermentation5010023 (2019).
Agarbati, A., Canonico, L., Ciani, M. & Comitini, F. The impact of fungicide treatments on yeast biota of Verdicchio and Montepulciano grape varieties. PLoS ONE 14, e0217385. https://doi.org/10.1371/journal.pone.0217385 (2019).
Kosel, J., Raspor, P. & Čadež, N. Maximum residue limit of fungicides inhibits the viability and growth of desirable non-Saccharomyces wine yeasts. Aust. J. Grape Wine Res. 25, 43–52. https://doi.org/10.1111/ajgw.12364 (2019).
Čadež, N., Zupan, J. & Raspor, P. The effect of fungicides on yeast communities associated with grape berries. FEMS Yeast Res. 10, 619–630. https://doi.org/10.1111/j.1567-1364.2010.00635.x (2010).
Lewis, K. A., Tzilivakis, J., Warner, D. J. & Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 22, 1050–1064. https://doi.org/10.1080/10807039.2015.1133242 (2016).
Killham, K., Lindley, N. D. & Wainwright, M. Inorganic sulfur oxidation by Aureobasidium pullulans. Appl. Environ. Microbiol. 42, 629–631 (1981).
Gadd, G. M. & de Rome, L. Biosorption of copper by fungal melanin. Appl. Microbiol. Biotechnol. 29, 610–617. https://doi.org/10.1007/BF00260993 (1988).
Belda, I. et al. Unraveling the enzymatic basis of wine “flavorome”: A phylo-functional study of wine related yeast species. Front. Microbiol. 7, 12–12. https://doi.org/10.3389/fmicb.2016.00012 (2016).
Lin, M.M.-H. et al. Evaluation of indigenous non-Saccharomyces yeasts isolated from a South Australian vineyard for their potential as wine starter cultures. Int. J. Food Microbiol. 312, 108373. https://doi.org/10.1016/j.ijfoodmicro.2019.108373 (2020).
Hranilovic, A., Bely, M., Masneuf-Pomarede, I., Jiranek, V. & Albertin, W. The evolution of Lachancea thermotolerans is driven by geographical determination, anthropisation and flux between different ecosystems. PLoS ONE 12, e0184652. https://doi.org/10.1371/journal.pone.0184652 (2017).
Hranilovic, A. et al. Oenological traits of Lachancea thermotolerans show signs of domestication and allopatric differentiation. Sci. Rep. 8, 14812. https://doi.org/10.1038/s41598-018-33105-7 (2018).
Hu, K., Jin, G.-J., Mei, W.-C., Li, T. & Tao, Y.-S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 239, 495–501. https://doi.org/10.1016/j.foodchem.2017.06.151 (2018).
Oro, L., Ciani, M. & Comitini, F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 116, 1209–1217. https://doi.org/10.1111/jam.12446 (2014).
Contreras, A., Curtin, C. & Varela, C. Yeast population dynamics reveal a potential ‘collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 99, 1885–1895. https://doi.org/10.1007/s00253-014-6193-6 (2015).
Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 102, 6775–6790. https://doi.org/10.1007/s00253-018-9117-z (2018).
Morata, A. et al. Lachancea thermotolerans applications in wine technology. Fermentation https://doi.org/10.3390/fermentation4030053 (2018).
Belda, I. et al. Selection and use of pectinolytic yeasts for improving clarification and phenolic extraction in winemaking. Int. J. Food Microbiol. 223, 1–8. https://doi.org/10.1016/j.ijfoodmicro.2016.02.003 (2016).
Jolly, N., Augustyn, O. & Pretorius, I. The role and use of non-Saccharomyces yeasts in wine production. J. Enol. Vitic. 27. https://doi.org/10.21548/27-1-1475 (2006).
Capozzi, V., Fragasso, M. & Russo, P. Microbiological safety and the management of microbial resources in artisanal foods and beverages: The need for a transdisciplinary assessment to conciliate actual trends and risks avoidance. Microorganisms 8, 306. https://doi.org/10.3390/microorganisms (2020).
Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 102, 3081–3094. https://doi.org/10.1007/s00253-018-8849-0 (2018).
Attila, K., Ján, M., Eva, I., Margarita, T. & Miroslava, K. Microorganisms of grape berries. In Proc. Latvian Acad. Sciences. Section B. Natural, Exact & Appl. Sci. Vol. 71, 502–508, https://doi.org/10.1515/prolas-2017-0087 (2017).
Pretorius, I. S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 16, 675–729. https://doi.org/10.1002/1097-0061(20000615)16:8%3c675::AID-YEA585%3e3.0.CO;2-B (2000).
Clavijo, A., Calderón, I. L. & Paneque, P. Diversity of Saccharomyces and non-Saccharomyces yeasts in three red grape varieties cultured in the Serranía de Ronda (Spain) vine-growing region. Int. J. Food Microbiol. 143, 241–245. https://doi.org/10.1016/j.ijfoodmicro.2010.08.010 (2010).
Capece, A. et al. Diversity of Saccharomyces cerevisiae strains isolated from two Italian wine-producing regions. Front Microbiol. 7, 1018. https://doi.org/10.3389/fmicb (2016).
Santamaría, P. et al. Biodiversity of Saccharomyces cerevisiae yeasts in spontaneous alcoholic fermentations: Typical cellar or zone strains? Advances in Grape and Wine Biotechnology. (ed. Morata, A. & Loira, I.) 1–15 (Intech Open, 2019). https://doi.org/10.5772/intechopen.84870
Kurtzman, C., P., & Fell, J. W. The Yeasts, A Taxonomic Study. 4th edn, (Elsevier Science Publishers, 1998).
Lõoke, M., Kristjuhan, K. & Kristjuhan, A. Extraction of genomic DNA from yeasts for PCR-based applications. Biotechniques 50, 325–328. https://doi.org/10.2144/000113672 (2011).
Liu, Y., Wang, C., Joseph, C. M. L. & Bisson, L. F. Comparison of two PCR-based genetic fingerprinting methods for assessment of genetic diversity in Saccharomyces strains. Am. J. Enol. Vit. 65, 109. https://doi.org/10.5344/ajev.2013.13056 (2014).
Dazy, F. & Le Barzic, J.-F. L'analyse des donnees evolutives: Methodes et applications (Technip Publishers, 1996).
Acknowledgements
Funding was provided by the Confederation of Rural Development Centers/ “Valdecea” Center under the framework of the Ministry of Ecological Transition, Spain. We gratefully acknowledge Melgarajo S.A. for the use of their experimental vineyard containing Negro Saurí. Special thanks to Itacyl Woody and Horticultural Crop Unit for data on vineyard management, Dr Alejandro Vicente Castro for the winemaking of the experimental wine Negro Saurí, Dr José María Heras Manso (Lallemand Oenology) for providing Cross Evolution and Dr Pedro José Gómez de Nova and Sergio Llamazares for assistance with molecular methods. VJ was supported in part by ARC Project (IC170100008). MW and VJ were supported by Wine Australia projects (incl. UA 1803-2.1).
Author information
Authors and Affiliations
Contributions
All authors agree that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors, and all have contributed significantly to the paper. The order of authors listed in the manuscript has been approved by all of us. We understand that the Corresponding Author is the sole contact for the Editorial process and is responsible for communicating with the co-authors on the progress of the submission. I.G.A. and G.N.C conceived the study and initiated the experimental work. V.J. and M.E.W. advised on the analysis and interpretation. M.E.P.V. assisted with the sequence analysis. All contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
González-Alonso, I., Walker, M.E., Vallejo-Pascual, ME. et al. Capturing yeast associated with grapes and spontaneous fermentations of the Negro Saurí minority variety from an experimental vineyard near León. Sci Rep 11, 3748 (2021). https://doi.org/10.1038/s41598-021-83123-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83123-1
- Springer Nature Limited