Abstract
Wine quality is closely linked to the fermentation step, which is driven by the microbial ecology of grape and the use of selected microbial strains as well. The microbial species developing during fermentation determines the type and concentration of many substances, which contribute to the sensory properties of wine and its safety. In this view, the present work aims to characterise the yeast microbiota, chemical and sensory properties of Sangiovese red wines obtained from both biodynamic and organic agriculture. The natural yeast populations of grape musts and their evolution during spontaneous were monitored and investigated. In addition, the volatile composition, physico-chemical and safety features (ethyl-carbamate) and sensory properties of wines were evaluated. The results showed that the yeast population was mostly related to the grape management, i.e. organic or biodynamic, while the wine composition was mainly affected by the winemaking process, and then by the grape management.
Similar content being viewed by others
Introduction
The demand for wines derived from organic and biodynamic grapes has notably increased, as they are endowed with healthy properties and perceived as environmentally sustainable (Parpinello et al. 2015). In fact, one of the aims of organic viticulture is to dramatically reduce the use of synthetic chemical pesticides, replacing them with copper-based molecules (Martins et al. 2014), which can affect the size and structure of the grape microbial population (Berg et al. 2005; Ranjard et al. 2006; Martins et al. 2014). Biodynamic agriculture is a particular approach of organic farming that emphasises the interrelationship between soil, plants and animals as a self-nourishing system without external inputs and though the use of specific fermented chemical parameters of wine proposed by Rudolf Steiner (Reeve et al. 2005). Regarding wine production, it excludes the use of chemical agents and microbial starter cultures, in order to let the spontaneous microbiota drive the fermentations. Although some authors studied the effects of the organic and biodynamic grape practices on the final quality and sensory features of obtained wines (Parpinello et al. 2015; Tassoni et al. 2014; Laghi et al. 2014), little information is available on the evolution of the yeast population during biodynamic winemaking processes and their effects on wine quality and sensory features (Muñoz-Bernal et al. 2013).
Although a great number of yeast metabolites, e.g. ethanol, CO2, glycerol, acetic, succinic and lactic acid, and other volatile substances, including terpenes and sulphur volatile compounds, can affect the wine quality (Ugliano and Henschke 2009; Vernocchi et al. 2015), very little is known about the yeast characterisation and effect on biodynamic grape and wine. Moreover, the current interest in wines with features linked to production area has led to a rediscovery of fermentation performed by indigenous yeasts occurring on the grapes and/or in the winery (Francesca et al. 2012).
Non-Saccharomyces yeasts, and particularly Hanseniaspora and Candida genera, are predominant on grapes and in the early stages of winemaking (Pretorius 2000; Jolly et al. 2014). Other species belonging to Metschnikowia, Pichia, Zygoascus and Issatchenkia genera, but at lower cell load levels (Zott et al. 2008), were also described. Several ecology studies based on polymerase chain reaction (PCR) and denaturing gradient gel electrophoresis (DGGE) have demonstrated that indigenous non-Saccharomyces yeasts are not completely suppressed during the early stages of alcoholic fermentation and can survive during fermentation, even in the case of the addition of active dried starter yeasts affecting wine sensory features (Albertin et al. 2014). In fact, non-Saccharomyces yeasts can contribute to the wine’s flavour and taste by producing secondary metabolites, including esters, higher alcohols, acids, volatile thiols, extracellular enzymes like β-glucosidases and mannoproteins (Viana et al. 2008; Pérez-Nevado et al. 2006; Domizio et al. 2014). It is well known that the grape microbial community is influenced by several factors, including rainfall, temperature, maturity stage, health status, use of phytosanitary products and agronomic practices (Nisiotou and Nychas 2007; Martins et al. 2014). Tofalo et al. (2013), in a comprehensive review on the biogeographical characterisation of Saccharomyces cerevisiae wine yeast by molecular methods, showed that agricultural practices such as farming and winemaking management systems selected phylogenetically distinct populations within the main performer of wine production. Otherwise, it is well known the relationship between specific microbial strains and the vitivinicultural ‘terroir’ (Tofalo et al. 2007, 2013; Valero et al. 2007; Schuller et al. 2012).
In this perspective, the main aims of this work were to evaluate the yeast population deriving from organic and biodynamic grapes and to characterise the derived wines for chemical, safety (biogenic amines and ethyl carbamate) and sensory attributes. For this, guided and spontaneous fermentations, starting from organic and biodynamic grapes, were set up and the relationship between microbiota composition and wine features was investigated. To reach the last goal, the yeast isolates from either organic or biodynamic grapes or from musts during fermentations, also in relation to the starter addition, were identified though internal transcribed spacer (ITS) region sequencing.
Materials and methods
The Sangiovese grapes used in this experimentation were harvested in Tebano (Ravenna, Italy). In the vineyard (2 hectares), two different managements (organic and biodynamic) have been adopted since 2009. Differences between organic and biodynamic management rely on the use of preparations during the vegetative season such as 500 (cow manure), fladen (cow manure enriched with basalt powder and eggshell) and 501 (canopy-applied finely ground quartz powder) (Spaccini et al. 2012). On vintage 2012 from each management, two replicates were carried out collecting, for each of them, 200 kg of grapes from two adjacent rows, for a total of four grape trials (two managements × two replicates from independent rows). Once in the winery, each of the four grape trials (called parcels) was split into two and spontaneous (SV) and inoculated (GV) vinification were set up using a starting fermentation temperature of 23 °C, finally obtaining eight wines.
Briefly, grapes were destemmed and crushed on the day of harvest and the grape must was placed in 200-L stainless steel tanks. In the inoculated vinification, the grapes were treated with sulphur dioxide (as potassium metabisulphite: 5 g/hL, AEB, Italy), complex nutrients (30 g/hL, Nutristart, Laffort, France) and inoculated with an appropriate yeast strain (20 g/hl S. cerevisiae, F15, Laffort, France). In the spontaneous vinification, the grapes were not added to by either sulphur dioxide or nutrients. Before and during the winemaking process, samples of grapes from all the parcels (from biodynamic and organic management) and of musts vinificated in the spontaneous or guided methods were collected and analysed for yeast cell loads and composition.
Sugar consumption was monitored over time by means of a Babo densimeter throughout fermentation and the tank content was homogenised every day to dissolve the cap into the wine. At the end of fermentation and after stabilisation, the wines were bottled and stored at 15 °C prior to chemical and sensory analyses.
Microbiological analyses
For microbiology analyses, grapes, musts across fermentation and final wines (60 days from fermentation) were analysed. Yeast viable cell counts were evaluated by plate counting using YPD agar (Oxoid, Basingstoke, UK) and lysine medium (Oxoid, Basingstoke, UK), incubated at 28 °C for 72 h, for the recovery of Saccharomyces and non-Saccharomyces species, respectively. Three repetitions for each sample were considered.
Yeast molecular characterisation
The isolates were randomly picked and characterised by molecular tools. For each phase considered and fermenting time, 30 isolates were analysed. The molecular characterisation was achieved by sequencing the ITS polymorphic region comprising the sequence ITS1, the gene encoding ribosomal RNA 5.8S and ITS2 sequence. The DNA extraction was performed using the commercial kit Insta-Gene Matrix® (Bio-Rad, Hercules, CA, USA). The extracted DNA was quantified by using a spectrophotometry at 260 nm and then employed in the PCR reaction according to the method proposed by Gardes and Bruns (1993) for the amplification of the ITS region by using the universal primers ITS1 forward and ITS4 reverse (Eurofins, Genomics, Ebersberg, Germany). After the amplification, the amplicons were purified by using the QIAquick® PCR Purification Kit (Qiagen, Valencia, CA, USA). The samples sequencing was performed at the BMR Genomics Laboratories according to internal methods (Padova, Italy). The identification of the isolates was obtained comparing the obtained sequences with those of the database of the National Center for Biotechnology Information (NCBI) gene bank using the Basic Local Alignment Search Tool (BLAST).
Wine chemical analyses
Each wine was analysed for alcohol strength (%), pH, total acidity (g/L), dry matter (g/L), volatile acidity (g/L), optical density (AU) at 420, 520 and 620 nm, total colour intensity (420 + 520 + 620 nm AU), hue (420/520 nm AU) and total polyphenols at 280 nm (TP, g/L), according to European official methods (European Union 1990). Moreover, analyses of total and free sulphur dioxide (SO2, mg/L), (Ripper and Schmitt 1896) and reducing sugars (g/L) (Lane and Eynon 1923) were carried out. Data are presented as mean values obtained from two replicated analyses of each duplicated vinification.
Wine volatile molecule profiles
Wine volatile molecule profiles were analysed by headspace solid phase microextraction coupled with gas chromatography mass spectrometry (HS-SPME/GC-MS) according to the method proposed by Patrignani et al. (2016).
Wine biogenic amines and ethyl carbamate
The determination of biogenic amines (BA) was performed according to the methods proposed by Torrea and Ancín (2002) and Tabanelli et al. (2013). The determination of ethyl carbamate (EC) concentrations in wine samples was performed according to the method of Whiton and Zoecklein (2002), with some modifications proposed by Patrignani et al.(2012).
Wine sensory analysis
Twenty-four panellists (14 men and 10 women) were recruited among experienced students specialised in winemaking and sensory evaluation at the Department of Food Science and Agricultural Sciences of the University of Bologna (Cesena, Italy). They were requested to evaluate wines in terms of visual (colour intensity), olfactory (fruity, herbaceous, spicy, alcohol, overall aroma), gustatory (sourness, alcohol, bitterness, astringency, overall taste, persistence) and overall judgment characteristics. Assessors were presented with transparent glasses containing 30 mL of wine and asked to taste wines from left to right (International Organization for Standardization, ISO 1997). Samples were coded with three-digit numbers and distributed in a completely randomised order. Tasting was organised in four different sessions, in which assessors tasted four wines. The sensory characteristics had to be rated on an anchored not-structured rating scale of 10 cm from weak to intense as reference points. Data are presented using a spider plot as mean values obtained from two replicated analyses of each tasting session (Lawless and Heymann 2010).
Statistical analysis
Principal component analysis (PCA) was used as an unsupervised multivariate data tool to find hidden structure among the chemical parameters of wine and volatile molecule profile (XLSTAT version 2011.1.05; Addinsoft, Anglesey, UK; Statistica 8, Package for Windows). The one-way analysis of variance (ANOVA; significance p ≤0.05), Fisher’s least significant difference (LSD) post-hoc test and the spider plot representation of sensory profiles were performed using XLSTAT.
Results and discussion
Yeast cell loads and microbiota identification in grapes and in musts during fermentation in relation to the adopted winemaking conditions
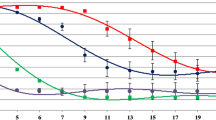
Eight different experimental wines were produced in relation to the farming system (two parcels from organic and two parcels from biodynamic management) and type of vinification (guided or spontaneous). Sugar consumption was monitored over time by means of a Babo densimeter throughout fermentation. As expected, the guided fermentations were faster than the spontaneous ones, independent of the farming system; the former ended after 13 days of fermentation while the spontaneous vinification required more than 20 days. The analyses of grapes showed that non-Saccharomyces yeasts had counts ranging between 1.0 and 4.2 log colony-forming units (CFU)/g, without any relationship to the farming system (Table 1). In fact, cell loads of about 4.0 log CFU/g were determined in parcels 2 and 3 belonging to the organic and biodynamic farming systems, respectively. In any case, the yeast population on the grape surface was in the range of cell loads reported by other authors (Francesca et al. 2010; Guzzon et al. 2011). After 3 days of fermentation, the level of non-Saccharomyces yeasts in guided fermentation ranged between 5.1 and 6.1 log CFU/mL for the parcels from organic grapes and 4.8 and 5.8 log CFU/mL for those from biodynamic ones. In the guided fermentations, independently of the grape agronomic practices, the level of yeasts detected on YPD ranged between 6.4 and 6.7 log CFU/mL after 3 days. The levels of non-Saccharomyces yeasts detected in the musts subjected to spontaneous fermentation ranged between 3.0 and 4.0 log CFU/mL. Yeasts detected on YPD medium reached levels higher than 7.0 log CFU/mL within 5 days, independently of starter addition. By contrast, the cell loads of Saccharomyces yeasts decreased more rapidly in guided fermentations compared to spontaneous ones, independently of grape management. The data recorded on lysine media showed that the levels of non-Saccharomyces yeasts remained high (levels ranging between 4.0 and 5.7 log CFU/mL) during the first 10 days of fermentation, independently of the farming system and starter supplementation. However, their disappearance was faster, particularly in organic samples, in the guided fermentation compared to the spontaneous one. The literature showed that non-Saccharomyces also persist in the fermentations that are inoculated with pure cultures of S. cerevisiae (Comitini et al. 2011). To identify a relationship between yeast microbiota and wine quality parameters, the yeast population was characterised on raw materials and through the fermentation processes in relation to farming and winemaking conditions adopted. In fact, it is well known that the yeast population present on grape plays a key role in the definition of quality features of wine independently of starter addition (Romano et al. 1997, 2003; Vernocchi et al. 2011). The yeast identification data obtained showed that, in spontaneous fermentations, Saccharomyces and non-Saccharomyces yeasts coexist (Romano et al. 2003; Ciani et al. 2006; Capece et al. 2011; Garofalo et al. 2016a, b; Nuñez-Guerrero et al. 2016) and confirm that grape berries are colonised by a complex, dynamic microbial ecosystem, in accordance with Barata et al. (2012). In fact, as shown by Fig. 1a, 90% and 84% of yeast isolates of biodynamic and organic grapes belonged to Kloeckera apiculata and Hanseniaspora uvarum, respectively. In biodynamic grapes, C. zemplinina (about 10%) was also found, while in organic grapes, in addition to H. uvarum, P. kluyveri, C. zemplinina and I. hanoinensis were identified for a proportion of 5.3% The different grape microbial communities can be influenced by several factors, such as the berry maturity stage, the use of phytosanitary products (Martins et al. 2012) or the organic or biodynamic management of farms based on copper-based fungicides (Berg et al. 2005; Ranjard et al. 2006; Verginer et al. 2010). However, the predominance of H. uvarum is in accordance with the literature data (Barata et al. 2012). According to Tristezza et al. (2016), H. uvarum can contribute to the production of volatile compounds of wine in spontaneous or scalar fermentation with S. cerevisiae, although some oenological features such as ethanol, volatile acidity or production of metabolites are strain-dependent. Also, C. zemplinina is considered to be of great oenological interest due to its ability to produce less ethanol from sugar consumed, tolerate high concentrations of ethanol present in wine and produce low levels of biogenic amines (Englezos et al. 2016). C. zemplinina and I. hanoinensis were also found by other authors to be minor species. For example, Drumonde-Neves et al. (2016) found their presence in yeasts collected from Azores Archipelago vineyards. None of the isolates from grapes belonged to S. cerevisiae. On the other hand, while the non-Saccharomyces species are dominant on raw grapes, reaching levels up to 5–6.0 log CFU/g, the presence of S. cerevisiae is very scarce on raw materials (Romano et al. 2003; Nisiotou and Nychas 2007). Due to its alcohol tolerance and oenological features, the incidence of S. cerevisiae increased during fermentation and, after 13 days, all the isolates belonged to this species, independently of the farming management (data not shown). However, the analyses of yeast identification performed in musts under spontaneous vinification after 5, 8, 10 and 11 days of fermentation showed a higher persistence of non-Saccharomyces species than in fermenting must from organic grapes. In particular, after 5 days of fermentation (Fig. 1b), the must from organic grapes was characterised by the presence of I. terricola (3.8%), H. uvarum (88.4%) and M. pulcherrima (7.8%), while in the must from biodynamic grapes, I. terricola was not found and S. cerevisiae (4.5%) was present. According to the literature data, the presence of I. terricola could be important since some strains are particularly gifted of an extracellular glucosidase to increase the amount of free monoterpenes and non-isoprenoids during vinification and, for this species, its application in wine fermentation was studied (Capozzi et al. 2015). Also, the presence of M. pulcherrima can contribute to increasing the aroma and flavour of wines thanks to a secreted arabino furanosidase. This can influence the amount of several varietal volatile compounds in the final products, such as volatile thiols and terpenes. Another important property of this species consists of the elevated production of esters and, in particular, of ethyl octanoate. Moreover, M. pulcherrima possesses antimicrobial activities against several spoilage yeasts (Capozzi et al. 2015). After 8 days of fermentation, the presence of S. cerevisiae in organic must was detected at higher levels with respect to the biodynamic must (Fig. 1c), although the presence of non-Saccharomyces yeast was still high. After 10 days of fermentation (Fig. 1d), S. cerevisiae represented the major species found in biodynamic must, while in organic must, S. pombe is at the highest level. This species is, in general, appreciated for its ability to completely transform malic acid into ethanol. Moreover, its ability to produce glycerol was recently described and it can be considered an interesting species to improve some sensory features of red wines (Loira et al. 2015). Its persistence continues after 11 days of fermentation in organic must, while S. cerevisiae is the only species found in biodynamic musts (Fig. 1e). However, also in organic must, after 20 days of fermentation, S. cerevisiae took over.
Chemical analyses
The chemical analysis of the experimental wines (Table 2) showed a satisfactory degree of grape ripeness consistent with the typical composition of Sangiovese wines (Parpinello et al. 2015). The alcohol strength was always higher than 14.0%, with the exception of the sample from biodynamic grapes of parcel 3 (13.2%), which was subjected to guided fermentation. Wines from spontaneous fermentations (SV) showed higher levels of reducing sugars (SV: 5.7 g/L; GV: 1.8 g/L), volatile acidity (SV: 0.64 g/L; GV: 0.41 g/L), as well as dry matter (SV: 31.8 g/L; GV: 27.5 g/L) compared to wines from guided fermentations (GV), regardless of the farming system. Total acidity, pH, total polyphenols, colour intensity and hue were not significant different. One of the reasons for the higher residual sugar content in spontaneous vinifications could be due to selection among the natural microbiota of wild S. cerevisiae strains not being endowed with a high alcohol tolerance. As mentioned, wines derived from spontaneous fermentations (SV) were characterised by higher volatile acidity (i.e. acetic acid) compared to those obtained with S. cerevisiae starter culture, independently of the farming system adopted. A wide variability of alcohol tolerance and acetic acid production, also in relation to the stress conditions during fermentation, is well known also in S. cerevisiae species (Rainieri and Pretorius 2000; Vernocchi et al. 2015). On the other hand, an efficient conversion of grape sugars to alcohol and low levels of acetic acid release in wine are the primary selection criteria of wine yeast strain selection (Rainieri and Pretorius 2000).
Wines from spontaneous fermentation showed lower concentrations of tartaric acid compared to those from guided fermentations, independently of the raw material farming system (Table 3). The ability of S. cerevisiae strains influence the tartaric stability throughout the release on mannoproteins during fermentation (Rosi et al. 2000), whereas the entity of the mannoprotein release, although influenced by physicochemical conditions, is strain-dependent (Vernocchi et al. 2015). So, it is probable that the spontaneous fermentation was dominated by strains endowed with a lower capability to release mannoproteins, although the literature data recognise non-S. cerevisiae strains as having a great role in mannoprotein release (Domizio et al. 2014). Moreover, citric and malic acids were under the detection limit in all the wines from spontaneous fermentation, as well as in wines obtained from grapes from biodynamic management processed by guided fermentation. The SV wines were also characterised by the presence of 1.2 g/L of lactic acid attributable to malo-lactic fermentation. This secondary fermentation performed by lactic acid bacteria also characterised wines obtained by guided fermentation of biodynamic musts, which showed an average lactic acid concentration of 0.7 g/L related to the absence of malic acid in GV of biodynamic grapes. In addition, the samples from spontaneous fermentations, although not supplemented with exogenous sulphite, showed higher levels of free SO2 and similar levels of total SO2 compared to those from guided fermentations. It is well known that wine yeast strains differ in their ability to produce sulphite, even if the medium composition and environmental conditions can affect its production (Vernocchi et al. 2015). With regard to vineyard management (organic and biodynamic) and regardless of the vinification undertaken, wines obtained from biodynamic grapes were characterised by higher volatile acidity (organic: 0.47 g/L; biodynamic: 0.57 g/L), whereas total acidity, total polyphenols and dry matter showed higher concentrations in wines obtained by organic farming. The pH colour intensity and hue were similar.
The chemical data were submitted to PCA in order to outline the differences among the samples in relation to farming and winemaking adopted conditions. All the samples were mapped in the spaces shared by the first two principal components PC1 and PC2, with an explained total variance of 64.4%. The score plots reported in Fig. 2 show that the wines would cluster in four groups. The first group included the wines from spontaneous fermentations (upper left side of the two PCAs space). The wines from guided fermentation grouped into three different clusters. Cluster 2 enclosed wines from guided fermentations of organic musts (lower left side of the two PCAs space), while wines from guided fermentation of biodynamic musts were quite widespread on the right side of the two PCAs space, in relation to the parcels considered, thus representing clusters 3 and 4. This result is not surprising, as grape composition variability due to a ‘parcel effect’ is a well-known phenomenon in viticulture.
Ethyl carbamate and biogenic amine content
The biogenic amine (BA) and ethyl carbamate (EC) contents detected in wines in relation to starter addition and farming managements were very low or under the detection limit (data not shown).
Volatile molecule profiles
In order to evaluate the effects of the farming and fermentation management on the wine volatile molecule profiles, at the end of the fermentations, the samples were analysed by means of HS-SPME/GC-MS. This technique allowed the identification of about 100 molecules belonging to different chemical classes, including alcohols, aldehydes, ketones, organic acids and esters. This technique has proven its potential to provide a volatile molecule fingerprinting of food and beverages in relation to their microbiota and/or production processes (Rocha et al. 2001; Patrignani et al. 2013). Due to the large dataset of information acquired, PCA was performed in order to pinpoint the differences among the samples in relation to fermentation management (spontaneous or guided) and farming practices (organic or biodynamic). In particular, the PCA loading plot of volatile molecules in relation to the variables taken into consideration showed that the samples were mapped in the space spanned by the first two principal components, PC1 and PC2, accounting for 41% and 16 % of the variability, respectively (Fig. 3). Four different clusters were evident. The wines obtained through guided fermentations grouped into two different clusters even if a relationship with the farming practices was not evident. In fact, the first cluster included the wines from guided fermentation of grapes of parcels 1 (ORG) and 3 (BIOD), while the second included those of parcels 2 (ORG) and 4 (BIOD). By contrast, the wines obtained though spontaneous fermentations were grouped in relation to organic or biodynamic farming systems. In general, the spontaneous fermentations give rise to wine significantly different from those from guided fermentations along PC1, explaining about 41% of the variability. Molecules such as hexanoic acid ethyl ester, nonanoic acid, ethyl acetate and 2,3-butanedione contributed to the clustering of biodynamic wines obtained without starter addition, while 3-methyl-propanol, decanoic acid ethyl ester and 5-phenethyl,2-pentanone grouped the organic wine obtained though spontaneous fermentations. It is well known that the formation of volatile compounds during grape must fermentation depends on several factors, including the nature and concentration of the compounds initially present in the must (their proportions differ from one grape variety to another), the capacity of the naturally occurring or inoculated yeast to transform them and the conditions used in winemaking. The impact of the yeasts upon wine flavour is largely determined by the array of volatile substances (e.g. higher alcohols, acids, esters, carbonyls, thiols) produced by the metabolism of grape juice components (Pretorius 2000). Therefore, the yeast microbiota of spontaneous and guided fermentations were completely different, affecting in a significant way the volatile molecule profiles of samples. In fact, the starter addition significantly reduced the differences induced by farming practices, and the former samples differed to each other only along PC2, explaining about 16% of the variability. In samples from guided fermentation, the contribution of raw material peculiarities, which differed also for naturally occurring yeasts, was less pronounced compared to wines from spontaneous fermentations.
The differences along PC2 of wines from different parcels of the same farming management indicated a high variability within both organic and biodynamic grapes.
Sensory analyses
Sensory evaluation was performed in order to assess differences among wines in relation to farming (organic and biodynamic) and winemaking (spontaneous or guided fermentations) managements. To better understand the sensory data, they were analysed by averaging the results obtained from the two replicated vinifications, thus considering the ‘vineyard management’ and ‘winemaking process’ variables only. The one-way ANOVA showed a significant difference among wines at p < 0.05 for astringency, taste complexity and overall judgment, whereas the level of p < 0.10 was found for odour complexity and persistence. The post-hoc test highlighted that the sensory profile was mainly affected by winemaking management rather than vineyard management (Fig. 4). As regards to significant differences, spontaneous fermentation (org SV: 5.4; biod SV: 5.5) and inoculated in organic grapes (org GV: 5.4) obtained higher scores than inoculated in biodynamic grapes (biod GV: 4.8) in terms of odour complexity. Comparable differences were obtained for taste complexity (org SV: 5.6; org GV: 5.2; biod SV: 5.7; biod GV: 4.7). The astringency was significantly higher in guided vinification of organic grapes when compared to biodynamic grape, regardless of the vinification adopted, whereas spontaneous vinification of organic grapes were not significantly different from all of the other vinifications (org SV: 5.6; org GV: 6.0; biod SV: 5.1; biod GV: 5.1). Biodynamic wines obtained lower scores in terms of persistence, regardless of the vinification (org SV: 5.6; org GV: 5.6; biod SV: 4.9; biod GV: 5.1). For overall judgment, spontaneous fermentation obtained significantly higher scores compared to the inoculated fermentation (org SV: 5.6; biod SV: 5.0; org GV: 4.6; biod GV: 4.4). All the other sensory descriptors gained similar scores; as a consequence, no significant differences in relation to starter addition and farming management were recorded. These wines were characterised by a minor persistence and heterogeneity of non-Saccharomyces species. Although non-Saccharomyces wine yeasts have some specific oenological characteristics that are absent in S. cerevisiae species having additive effects on wine flavour and aroma (Viana et al. 2008), these data suggest that the features of the S. cerevisiae strains dominating fermentation played a key role in the wine sensory profiles.
References
Albertin W, Miot-Sertier C, Bely M, Marullo P, Coulon J, Moine V, Colonna-Ceccaldi B, Masneuf-Pomarede I (2014) Oenological prefermentation practices strongly impact yeast population dynamics and alcoholic fermentation kinetics in Chardonnay grape must. Int J Food Microbiol 178:87–97. doi:10.1016/j.ijfoodmicro.2014.03.009
Barata A, Malfeito-Ferreira M, Loureiro V (2012) Changes in sour rotten grape berry microbiota during ripening and wine fermentation. Int J Food Microbiol 154:152–161. doi:10.1016/j.ijfoodmicro.2011.12.029
Berg J, Tom-Petersen A, Nybroe O (2005) Copper amendment of agricultural soil selects for bacterial antibiotic resistance in the field. Lett Appl Microbiol 40:146–151
Capece A, Pietrafesa R, Romano P (2011) Experimental approach for target selection of wild wine yeasts from spontaneous fermentation of “Inzolia” grapes. World J Microbiol Biotechnol 27:2775–2783. doi:10.1007/s11274-011-0753-z
Capozzi V, Garofalo C, Chiriatti MA, Grieco F, Spano G (2015) Microbial terroir and food innovation: the case of yeast biodiversity in wine. Microbiol Res 181:75–83. doi:10.1016/j.micres.2015.10.005
Ciani M, Beco L, Comitini F (2006) Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int J Food Microbiol 108:239–245
Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M (2011) Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol 28:873–882. doi:10.1016/j.fm.2010.12.001
Domizio P, Liu Y, Bisson LF, Barile D (2014) Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol 43:5–15. doi:10.1016/j.fm.2014.04.005
Drumonde-Neves J, Franco-Duarte R, Lima T, Schuller D, Pais C (2016) Yeast Biodiversity in Vineyard Environments Is Increased by Human Intervention. PLOSONE| 11:1--13
Englezos V, Rantsiou K, Cravero F, Torchio F, Ortiz-Julien A, Gerbi V, Rolle L, Cocolin L (2016) Starmerella bacillaris and Saccharomyces cerevisiae mixed fermentations to reduce ethanol content in wine. Appl Microbiol Biotechnol 100:5515–5526. doi:10.1007/s00253-016-7413-z
European Union (1990) Commission Regulation (EEC) No. 2676/90 of 17 September 1990 determining Community methods for the analysis of wines. Off J Eur Communities 272:64–73
Francesca N, Chiurazzi M, Romano R, Aponte M, Settanni L, Moschetti G (2010) Indigenous yeast communities in the environment of “Rovello bianco” grape variety and their use in commercial White wine fermentation. World J Microbiol Biotechnol 26:337–351. doi:10.1007/s11274-009-0181-5
Francesca N, Canale DE, Settanni L, Moschetti G (2012) Dissemination of wine-related yeasts by migratory birds. Environ Microbiol Rep 4:105–112. doi:10.1111/j.1758-2229.2011.00310.x
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Garofalo C, Russo P, Beneduce L, Massa S, Spano G, Capozzi V (2016a) Non-Saccharomyces biodiversity in wine and the ‘microbial terroir’: a survey on Nero di Troia wine from the Apulian region, Italy. Ann Microbiol 66:143–150. doi:10.1007/s13213-015-1090-5
Garofalo C, Tristezza M, Grieco F, Spano G, Capozzi V (2016b) From grape berries to wine: population dynamics of cultivable yeasts associated to “Nero di Troia” autochthonous grape cultivar. World J Microbiol Biotechnol 32:59. doi:10.1007/s11274-016-2017-4
Guzzon R, Widmann G, Settanni L, Malacarne M, Francesca N, Larcher R (2011) Evolution of yeast populations during different biodynamic winemaking processes. S Afr J Enol Vitic 32:242–250
International Organization for Standardization (ISO) (1997) ISO 3591:1977. Sensory analysis—Apparatus—Wine-tasting glass
Jolly NP, Varela C, Pretorius IS (2014) Not your ordinary yeast: non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res 14:215–237. doi:10.1111/1567-1364.12111
Laghi L, Versari A, Marcolini E, Parpinello GP (2014) Metabonomic investigation by 1H-NMR to discriminate between red wines from organic and biodynamic grapes. Food Nutr Sci 5:52–59. doi:10.4236/fns.2014.51007
Lane JH, Eynon L (1923) Determination of reducing sugars by Fehling’s solution with methylene blue indicator. J Soc Chem Ind 42:32–37
Lawless HT, Heymann H (2010) Sensory evaluation of food: principles and practices. Chapman and Hall, New York
Loira I, Morata A, Comuzzo P, Callejo MJ, González C, Calderón F, Suárez-Lepe JA (2015) Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res Int 76:325–333. doi:10.1016/j.foodres.2015.06.030
Martins G, Miot-Sertier C, Lauga B, Claisse O, Lonvaud-Funel A, Soulas G, Masneuf-Pomarède I (2012) Grape berry bacterial microbiota: Impact of the ripening process and the farming system. Int J Food Microbiol 158:93–100. doi:10.1016/j.ijfoodmicro.2012.06.013
Martins G, Vallance J, Mercier A, Albertin W, Stamatopoulos P, Rey P, Lonvaud A, Masneuf-Pomarède I (2014) Influence of the farming system on the epiphytic yeasts and yeast-like fungi colonizing grape berries during the ripening process. Int J Food Microbiol 177:21–28. doi:10.1016/j.ijfoodmicro.2014.02.002
Muñoz-Bernal E, Rodríguez ME, Benítez P, Fernández-Acero FJ, Rebordinos L, Cantoral JM (2013) Molecular analysis of red wine yeast diversity in the Ribera del Duero D.O. (Spain) area. Arch Microbiol 195:297–302. doi:10.1007/s00203-013-0872-z
Nisiotou AA, Nychas GJE (2007) Yeast populations residing on healthy or Botrytis-infected grapes from a vineyard in Attica, Greece. Appl Environ Microbiol 73:2765–2768. doi:10.1128/AEM.01864-06
Nuñez-Guerrero ME, Páez-Lerma JB, Rutiaga-Quiñones OM, González-Herrera SM, Soto-Cruz NO (2016) Performance of mixtures of Saccharomyces and non-Saccharomyces native yeasts during alcoholic fermentation of Agave duranguensis juice. Food Microbiol 54:91–97. doi:10.1016/j.fm.2015.10.011
Parpinello GP, Rombolà AD, Simoni M, Versari A (2015) Chemical and sensory characterisation of Sangiovese red wines: comparison between biodynamic and organic management. Food Chem 167:145–152. doi:10.1016/j.foodchem.2014.06.093
Patrignani F, Ndagijimana M, Belletti N, Gardini F, Vernocchi P, Lanciotti R (2012) Biogenic amines and ethyl carbamate in Primitivo wine: survey of their concentrations in commercial products and relationship with the use of malolactic starter. J Food Prot 75:591–596. doi:10.4315/0362-028X.JFP-11-311
Patrignani F, Tabanelli G, Siroli L, Gardini F, Lanciotti R (2013) Combined effects of high pressure homogenization treatment and citral on microbiological quality of apricot juice. Int J Food Microbiol 160:273–281. doi:10.1016/j.ijfoodmicro.2012.10.021
Patrignani F, Chinnici F, Serrazanetti DI, Vernocchi P, Ndagijimana M, Riponi C, Lanciotti R (2016) Production of volatile and sulfur compounds by 10 Saccharomyces cerevisiae strains inoculated in Trebbiano must. Front Microbiol 7:243. doi:10.3389/fmicb.2016.00243
Pérez-Nevado F, Albergaria H, Hogg T, Girio F (2006) Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int J Food Microbiol 108:336–345
Pretorius IS (2000) Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16:675–729
Rainieri S, Pretorius IS (2000) Selection and improvement of wine yeasts. Ann Microbiol 50:15–31
Ranjard L, Echairi A, Nowak V, Lejon DPH, Nouaïm R, Chaussod R (2006) Field and microcosm experiments to evaluate the effects of agricultural Cu treatment on the density and genetic structure of microbial communities in two different soils. FEMS Microbiol Ecol 58:303–315
Reeve JR, Carpenter-Boggs L, Reganold JP, York AL, McGourty G, McCloskey LP (2005) Soil and winegrape quality in biodynamically and organically managed vineyards. Am J Enol Vitic 56:367–376
Ripper M, Schmitt E (1896) Zeitschrift fach XXXV, 232
Rocha S, Ramalheira V, Barros A, Delgadillo I, Coimbra MA (2001) Headspace solid phase microextraction (SPME) analysis of flavor compounds in wines. Effect of the matrix volatile composition in the relative response factors in a wine model. J Agric Food Chem 49:5142–5151
Romano P, Suzzi G, Comi G, Zironi R, Maifreni M (1997) Glycerol and other fermentation products of apiculate wine yeasts. J Appl Microbiol 82:615–618
Romano P, Fiore C, Paraggio M, Caruso M, Capece A (2003) Function of yeast species and strains in wine flavour. Int J Food Microbiol 86:169–180. doi:10.1016/S0168-1605(03)00290-3
Rosi I, Domizio P, Fia G, Agnoletto R (2000) Biodiversità della popolazione di lieviti presente nel corso della fermentazione di mosti di uve Sangiovese. Dissertation, Simposio Internazionale Il Sangiovese
Schuller D, Cardoso F, Sousa S, Gomes P, Gomes AC, Santos MAS, Casal M (2012) Genetic diversity and population structure of Saccharomyces cerevisiae strains isolated from different grape varieties and winemaking regions. PLoS One 7:e32507. doi:10.1371/journal.pone.0032507
Spaccini R, Mazzei P, Squartini A, Giannattasio M, Piccolo A (2012) Molecular properties of a fermented manure preparation used as field spray in biodynamic agriculture. Environ Sci Pollut Res Int 19:4214–4225. doi:10.1007/s11356-012-1022-x
Tabanelli G, Montanari C, Grazia L, Lanciotti R, Gardini F (2013) Effects of aw at packaging time and atmosphere composition on aroma profile, biogenic amine content and microbiological features of dry fermented sausages. Meat Sci 94:177–186. doi:10.1016/j.meatsci.2013.01.018
Tassoni A, Tango N, Ferri M (2014) Polyphenol and biogenic amine profiles of Albana and Lambrusco grape berries and wines obtained following different agricultural and oenological practices. Food Nutr Sci 5:8–16. doi:10.4236/fns.2014.51002
Tofalo R, Torriani S, Chaves-López C, Martuscelli M, Paparella A, Suzzi G (2007) A survey of Saccharomyces populations associated with wine fermentations from the Apulia region (South Italy). Ann Microbiol 57:545–552
Tofalo R, Perpetuini G, Schirone M, Fasoli G, Aguzzi I, Corsetti A, Suzzi G (2013) Biogeographical characterization of Saccharomyces cerevisiae wine yeast by molecular methods. Front Microbiol 4:166–179. doi:10.3389/fmicb.2013.00166
Torrea D, Ancín A (2002) Content of biogenic amines in a Chardonnay wine obtained through spontaneous and inoculated fermentations. J Agric Food Chem 50:4895–4899
Tristezza M, Tufariello M, Capozzi V, Spano G, Mita G, Grieco F (2016) The oenological potential of Hanseniaspora uvarum in simultaneous and sequential co-fermentation with Saccharomyces cerevisiae for industrial wine production. Front Microbiol 7:1–14. doi:10.3389/fmicb.2016.00670
Ugliano M, Henschke PA (2009) Yeasts and wine flavour. In: Moreno-Arribas V, Polo MC (eds) Wine chemistry and biochemistry, chapter 8D. Springer, New York, pp 313–392
Valero E, Cambon B, Schuller D, Casal M, Dequin S (2007) Biodiversity of Saccharomyces yeast strains from grape berries of wine-producing areas using starter commercial yeasts. FEMS Yeast Res 7:317–329
Verginer M, Siegmund B, Cardinale M, Müller H, Choi Y, Míguez CB (2010) Monitoring the plant epiphyte Methylobacterium extorquens DSM 21961 by real-time PCR and its influence on the strawberry flavor. FEMS Microbiol Ecol 74:136–145. doi:10.1111/j.1574-6941.2010.00942.x
Vernocchi P, Ndagijimana M, Serrazanetti DI, López CC, Fabiani A, Gardini F, Guerzoni ME, Lanciotti R (2011) Use of Saccharomyces cerevisiae strains endowed with beta-glucosidase activity for the production of Sangiovese wine. World J Microbiol Biotechnol 27:1423–1433. doi:10.1007/s11274-010-0594-1
Vernocchi P, Patrignani F, Ndagijimana M, Chaves Lopez C, Suzzi G, Gardini F, Lanciotti R (2015) Trebbiano wine produced by using Saccharomyces cerevisiae strains endowed with β-glucosidase activity. Ann Microbiol 65:1565–1571. doi:10.1007/s13213-014-0995-8
Viana F, Gil JV, Genovés S, Vallés S, Manzanares P (2008) Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol 25:778–785. doi:10.1016/j.fm.2008.04.015
Whiton RS, Zoecklein BW (2002) Determination of ethyl carbamate in wine by solid-phase microextraction and gas chromatography/mass spectrometry. Am J Enol Vitic 53:60–63
Zott K, Miot-Sertier C, Claisse O, Lonvaud-Funel A, Masneuf-Pomarede I (2008) Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int J Food Microbiol 125:197–203. doi:10.1016/j.ijfoodmicro.2008.04.001
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• The vineyard management affected the grape yeast microbiota
• Wine volatile and sensory profile were affected by the winemaking management
• Wine from organic and biodynamic grapes were characterised by a low amount of biogenic amines
Rights and permissions
About this article
Cite this article
Patrignani, F., Montanari, C., Serrazanetti, D.I. et al. Characterisation of yeast microbiota, chemical and sensory properties of organic and biodynamic Sangiovese red wines. Ann Microbiol 67, 99–109 (2017). https://doi.org/10.1007/s13213-016-1241-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-016-1241-3