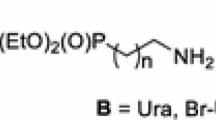
Abstract
A library of 18 structurally diverse semisynthetic lupane, oleanane, and ursane types triterpenoids, including C19- or C28-(1,2,3-triazolyl)- and aminomethylated derivatives obtained by the «click» reaction with various aromatic and sugar azides or by Mannich reaction with secondary amines, were tested for antiviral activity against HCMV, HSV-1, and HPV-11 types. C28-Triazolyl-derivative with a benzyl substituent of 2,3-indolo-oleanolic acid was the most active against the HCMV virus with EC50 < 0.05 (SI > 81). Lupane 3,28-diacetoxy-triazolyl derivatives with phenyl- and fluorophenyl-fragments possess the highest activity among all screened compounds toward HPV-11 type virus with EC50 values of 2.97 µM and 1.20 μM, SI90 values of 28 and >125, respectively. One can see that modification of triterpenic alkynes to Mannich bases was more efficient in increasing an activity against HSV-1 than their conversion to triazoles.
Similar content being viewed by others
Introduction
Natural products have played a leading role for many centuries as a rich source of biologically active compounds that can be employed in the development of new drugs [1,2,3,4,5]. Triterpenoids exhibit a variety of antiviral activities [6] mainly involving effects on DNA viruses [7,8,9]. Bevirimat [3-O-(3’,3’,-dimethylsuccinyl)-betulinic acid] has been shown to inhibit HIV-1 maturation by a previously described mechanism [7]. Betulin alone and in combination with acyclovir have been reported to inhibit HSV I and II [10]. Betulinic and betulonic acids are also active against HSV, as well as against influenza A and ECHO-6 picornavirus [11], and enveloped/non-enveloped viruses [12]. A series of triterpenoids were found to inhibit HPV-11 and HPV-16 [13,14,15]. The synergistic effect of rimantadine and betulin-derived compounds combinations against the reproduction of influenza virus types A (H1N1, H7N1, and H3N2) and B in vivo is established [16]. It was shown that betulin/betulinic acid and artesunic acid hybrids [17] and triazine derivatives of allobetulin and betulinic acid [18] were active against HCMV with an EC50 in the micromolar range. Recent data provide evidence for the sensitivity of RNA viruses, for example, the significant synergistic effects of betulin derivatives when combined with 3’-amino-3’-deoxy-adenosine against Semliki Forest virus were shown [19].
Triterpenoids with an alkynyl moiety at C2 [20,21,22], C19 [23], C3-, and C28 [24], have become one of the most actively developing areas of organic chemistry since they are used as key intermediates in the synthesis of biologically active compounds of Mannich and click reactions. 1,2,3-Triazole unit is known to decrease the overall lipophilicity of triterpenes, and to improve ADME parameters [24]. However, it was found that triterpenes with a triazole moiety had significantly lower cytotoxicity and, in some cases, even lower selectivity. In general, as summarized in the reference [25], the goals of becoming new promising anticancer leads have rarely been achieved, but there are also mentioned that in the field of antiviral-active compounds, triterpene-triazole hybrids showed slightly more potency. For example, oleanolic acid dimeric bis-triazole binds to the HCV envelope protein E2 and thus blocks the virus-host fusion with an IC50 10.3 nM [26]. Some anti-HCV activity was observed for triazole-speared ursolic acid cyclodextrin conjugates [27, 28]. Compounds of significantly higher anti-HIV activity were obtained from ursolic acid holding a propargyl moiety at position C-3 that were used for click reactions with analogs of the T20 peptide [29]. The two most important structural elements of bevirimat, AZT (an inhibitor of reverse transcriptase) and LH55 (an inhibitor of HIV fusion), were combined via triazole by classical click reactions, but no biological data have been published. However, most of the studies report on the biological activity of targeted compounds, and there is no information about the activity of alkynyl-derivatives. A large number of novel substrates have been synthesized using the aminomethylation Mannich reaction and evaluated as potential treatments for a multitude of diseases [30].
Here, we report the synthesis of new lupane, oleanane, and ursane types alkynyl-derivatives. Obtained compounds, as well as previously described, were evaluated for antiviral activity against human cytomegalovirus, herpes simplex virus, and human papillomavirus.
Results and discussion
Chemistry
The chemical synthesis and characterization of alkynes 1, 2, and 3 obtained from betulin by oxidation to methylketone fragment in cycle E with following dehydratation using POCl3, alkynes 10 and 12–14 synthesized via acyl chloride method from the corresponding acid, C19-(1,2,3-triazolyl)- 6–9, aminoalkyl- 4, 11, and 15–17 derivatives obtained using Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition or Mannich reaction screened in this study have been previously reported (chemical structures are presented in Fig. 1) [23, 31,32,33].
The 1,3-dipolar cycloaddition reaction of 3,28-diacetoxy-C19-alkynyl betulin 1 (for 5) or 2,3-indolo-olean-12-en-28-propargyl amide 13 (for 18 and 19) with 4-(azidomethyl)-2,3,5,6-tetramethylphenol, azidomethyl phenyl sulfide, or benzyl azide under standard conditions with CuSO4·5H2O and sodium ascorbate in CH2Cl2-H2O allowed us to obtain new C19- and C28-1,2,3-triazoles 5, 18, and 19 with yields of 45%, 62% and of 56%, correspondingly (Figs. 1 and 2). The structures of the compounds were ascertained by combined use of spectroscopy (1H NMR, 13C NMR and MS) and elemental analyses. Thus, in the spectra of compounds 18 and 19 disappearance of the acetylene fragment signals at δ 2.21–2.23 ppm (NMR 1H) and at δ 71.6–80.0 ppm (NMR 13C) and formation of 1,2,3-triazole ring with signal of methine carbon atom as singlets at δ 7.52 ppm, δ 7.59 ppm (NMR 1H) and at δ 122.1 ppm, δ 122.7 ppm (NMR 13C) were characteristic. The 1H NMR spectrum of compound 5 showed the characteristic signal of methylene group as multiplet at δ 5.59–5.69 ppm and of methine group of triazole fragment as singlet at δ 6.91 ppm, as well as the 13C NMR spectrum of 5 showed the signals of aromatic carbons at δ 120.6–153.0 ppm (NMR 13C) (the NMR spectra of compounds see in Supplementary Material Fig. S1–S6).
Antiviral activity
Our previous studies of triterpene oxidized indoles [15], azepanes [34], and l8αH,19βH-ursanes [35] have revealed their promising antiviral potency. For example, 19β,28-epoxy-18α-olean-28-oxo-2-nor-2,3-4’(1H)-quinolone was active against HPV-11 with EC50 0.45 μM and SI50 322 [15]. Azepanobetulin, azepanouvaol, and azepano-glycyrrhetol showed high potency toward HCMV (EC50 0.15, 0.11, 0.11 µM) with selectivity indexes SI50 115, 136, 172 respectively [34]. 3β-Acetoxy-21β-acetyl-20β,28-epoxy-18α,19βH-ursane showed moderate activity (EC50 4.87 µM) toward the HCMV-resistant isolate (GDGr K17) compared to standard drug Cidofovir and was four times more potent than Ganciclovir [35].
Taking into account these data, lupane and oleanane alkynyl-derivatives 1–19 were evaluated against DNA viruses (human herpes simplex virus 1, cytomegalovirus, and papillomavirus 11) using the possibilities of Division of Microbiology and Infectious Diseases of the National Institutes of Allergy and Infectious Diseases (http://www.niaid-aacf.org/) program for antiviral assays. The detailed information regarding antiviral screening and methods can be found at http://www.niaid-aacf.org/ and were described in the literature [36, 37].
The effects of compounds 1–19 on antiviral activity against a normal laboratory HCMV strain, AD-169, and their cytotoxicity were evaluated on HFF cells using CellTiter-Glo (cytopathic effect/toxicity) assay (Table 1). The compounds 1, 3, 5, 9, 10, 11, 15, 17, 18 and 19 demonstrated activity against HCMV with EC50 values > 6 µM, while compounds 2 and 8 showed a weak activity with EC50 values > 30 µM, and derivative 6 was turned out to be inactive (EC50 > 150 µM). The derivatives 4, 7, 12–14, and 16 have shown good viral inhibition toward HCMV (EC50 > 1.20 µM; EC90 > 1.20 µM) compared to standard drug Ganciclovir. The compound 19 was the most active with EC50 < 0.05 µM; EC90 < 0.05 µM, but at the same time it was cytotoxic with CC50 3.90 (SI > 81).
The antiviral activity of compounds 1–19 against HSV-1 was studied on the E-377 strain of HFF cell line using the CellTiter-Glo (cytopathic effect/toxicity) assay (Table 1). Compounds 5–9 were not active while derivatives 1, 2, 10, 13, and 18 showed a weak anti-herpes activity (EC50 > 30 µM). The compounds 3, 4, 11, 12, 15–17, and 19 showed a moderate potency (EC50 > 6 µM; EC90 > 6 µM). Compound 14 was the most active against HSV-1 with EC50 > 1.2 µM.
Compounds 1–19 were also evaluated against a HPV-11 strain HE611260.1 on C-33A cells using Nano-Glo Luciferase (NanoLuc)/CellTiter-Glo (toxicity) assay. Compounds 6, 13, 14, and 18 were not active against the studied virus strain, whereas compounds 1–3, 5, 8, 10–12, and 19 showed moderate or weak activity. The derivatives 4, 15, and 17 displayed good activity with EC50 > 1.20 µM and EC90 > 1.20 µM. Compounds 7 and 16 showed moderate activity against HPV-11 with EC50 2.97 µM and 4.25 µM, and low values of cytotoxicity (CC50 82.79 and 133.71 respectively) as well. Compound 9 showed activity toward HPV-11 (EC50 1.20 µM; EC90 20.07 µM) with a good selectivity index (SI50 > 125; SI90 > 7) compared to standard drug 9-[2-(phosphonomethoxy)ethyl]guanine (EC50 0.89 µM; EC90 102.22 µM; SI50 > 168; SI90 > 1). Generally it seems that modification of triterpenic alkynes such as 1, 13 or 14 to Mannich bases was more efficient in increasing an activity against HSV-1 than their conversion to triazoles, such as 5–8, 18.
Based on the differences in activity between the derivatives, the following structure-activity relationships could be observed. In the case of HCMV, C19-triazols with different phenyl substitutes 5, 7–9 were comparable with a starting alkyne 1, while the introduction of a sugar moiety 6 led to a complete loss of activity. The compound 7 with phenyl fragment was found to be more effective and at the same time more cytotoxic. The modification of C28-alkynyl amides had a positive influence on EC50 or CC50 value. The introduction of a benzyl fragment had the most favorable effect on activity (EC50 0.05 µM). The activity of Mannich bases 4, 11, 15–17 was comparable or better than an activity of parent compounds, but triterpenoids 4 and 16 were more cytotoxic.
In the case of HSV-1, modification of С19-alkyne 1 to triazoles 5–9 led to the loss of activity. For compounds 1–3 the beneficial effect of acetylene groups was observed. Modification of both C19- 1 and C28-alkynyl derivatives 13, 14 by Mannich reaction improved the values of activity, as well as against HPV-11. The exception was compound 18, which activity was comparable to the parent alkyne.
In addition, increased activity against HPV-11 was displayed by all triterpenic triazoles with aromatic fragment 5 and 7–9, except sugar triazole 6. Thus, compounds 7 and 9 with phenyl- and fluoro-phenyl-fragments possess the highest activity among all screened compounds with EC50 values of 2.97 µM and 1.20 μM, SI50 values of 28 and >125, respectively.
Experimental
General
The spectra were recorded at the Center for the Collective Use “Chemistry” of the Ufa Institute of Chemistry of the UFRC RAS and RCCU “Agidel” of the UFRC RAS. 1H and 13C NMR spectra were recorded on a “Bruker Avance-III” (Bruker, Billerica, MA, USA, 500 and 125.5 MHz respectively, δ, ppm, Hz) in CDCl3, internal standard—tetramethylsilane. Mass spectra were obtained on a liquid chromatograph–mass spectrometer LCMS-2010 EV (Shimadzu, Kyoto, Japan). Melting points were detected on a microtable «Rapido PHMK05» (Nagema, Dresden, Germany). Optical rotations were measured on a polarimeter Perkin-Elmer 241 MC (PerkinElmer, Waltham, MA, USA) in a tube length of 1 dm. Elemental analysis was performed on a Euro EA-3000 CHNS analyzer (Eurovector, Milan, Italy), the main standard is acetanilide. Thin-layer chromatography analyzes were performed on Sorbfil plates (Sorbpolimer, Krasnodar, Russia), using the solvent system chloroform–ethyl acetate, 40:1. Substances were detected by a 10% solution of sulfuric acid solution with subsequent heating at 100–120 °C for 2–3 min. All chemicals were of reagent grade (Sigma-Aldrich). Compounds 1, 2 and 3 [31], 4, 10–12 [32], 6–9 [23], 13–17 [33] were obtained according to the methods described previously.
Chemistry
Synthesis of compounds 5, 18, and 19
To a solution of compound 1 (0.51 g, 1 mmol) or 13 (0.56 g, 1 mmol), CuSO4·5H2O (0.04 g, 0.2 mmol) in СH2Cl2-H2O (1:1, 5 ml), 4-(azidomethyl)-2,3,5,6-tetramethylphenol (0.21 mg, 1 mmol), azidomethyl phenyl sulfide (0.14 ml, 1 mmol), or benzyl azide (0.13 ml, 1 mmol) were added. The reaction mixture was stirred for 1 h at 20 °С, then Na-l-Asc (2 mg, 0.01 mmol) was added and stirred at 50 °С for 24 h. The reaction mixture was poured into Н2О/Н+, the precipitate was filtered off, washed with water until neutral pH, dried in air. The resulting material was chromatographed on SiO2 using chloroform as an eluent.
3β,28-Diacetoxy-19-{1-(4-hydroxy-2,3,5,6-tetramethylbenzyl)-1H-1,2,3-triazol-4yl}-20,29,30-trinor-betulin (5)
Yield 0.32 g (45%). m.p. 192–194 °С; [α]D20 + 21.00 (c 0.1, CHCl3); δH (500.13 MHz, CDCl3) 0.81, 0.82, 0.83, 0.91, 0.98 (5 s, 15H, 5CH3), 1.01–1.99 (m, 25H, CH, CH2), 2.01 and 2.09 (2 s, 6H, 2COCH3), 2.19 and 2.21 (2 s, 12H, 4CH3-arom), 3.22 (m, 1H, H-19), 3.81 and 4.25 (both d, J = 11.0 Hz, H-28), 4.45 (dd, 1H, J = 10.8 Hz, J = 5.4 Hz, H-3), 5.59–5.69 (m, 2H, CH2), 6.91 (s, 1H, H-triazol); δC (125.76 MHz, CDCl3) 12.6, 12.6, 14.6, 16.0, 16.1, 16.5, 16.5, 18.1, 20.6, 21.1, 21.2, 21.3, 23.7, 26.8, 26.9, 27.9, 29.6, 32.1, 34.0, 35.4, 36.0, 37.0, 37.1, 37.8, 38.4, 40.8, 42.7, 46.6, 49.9, 50.6, 53.5, 55.3, 62.5 (C-3), 80.9 (C-28), 120.4 (CH-triazol), 120.6 (C-arom), 120.6 (C-arom), 121.1 (C-arom), 134.9 (C-arom), 134.9 (C-arom), 152.1 (C-triazol), 153.0 (C-arom), 171.0, 171.5. MS: m/z 716.49 [M + H]+; Anal. Calcd for C44H65N3O5: С, 73.81; H, 9.15; N, 5.87. Found: С, 73.80; H, 9.14; N, 5.86.
[3,2b]-Indolo-N-[1-((phenylthio)methyl)-1H-1,2,3-triazol-4-yl)methyl)]-olean-12(13)-en-28-carboxamide (18)
Yield 0.45 g (62%); m.p. 157 °С; [α]D20 + 29° (с 0.05, CHCl3); δH (500.13 MHz, CDCl3) 0.68, 0.92, 0.93, 0.94, 0.96, 1.22, 1.32 (7 s, 21H, 7CH3), 1.35–2.82 (m, 22H, CH, CH2), 4.34–4.57 (m, 2H, H-37), 5.51 (s, 1H, H-12), 5.58 (s, 1H, H-40), 6.68 (br. s., 1H, NH), 7.06–7.47 (m, 9H, H-arom), 7.59 (s, 1H, CH-triazol), 8.13 (br. s., 1H, NH); δC (125.76 MHz, CDCl3) 15.6, 16.4, 19.3, 23.3, 23.5, 23.6, 23.9, 25.6, 27.3, 30.7, 30.9, 31.9, 32.5, 33.0, 34.0, 34.1, 35.1, 36.8, 37.9, 39.5, 42.1, 46.2, 46.3, 46.6, 53.1, 53.8, 106.6 (C-2), 110.4 (C-arom), 117.9 (C-arom), 118.8 (C-arom), 120.9 (C-arom), 122.1 (CH- triazol), 123.5 (C-12), 128.2 (C-arom), 128.6 (C-triazol), 129.5 (2C, C-arom), 131.9 (2C, C-arom), 132.1 (C-arom), 136.2 (C-arom), 140.9 (C-arom), 144.1 (C-13), 145.3 (C-3), 178.4 (C-28); MS (APCI) m/z 730.50 [M]+, (calcd for C46H59N5OS, 730.07). Anal. Calcd for C46H59N5OS: C, 75.68; H, 8.15; N, 9.59; S, 4.39. Found: C, 75.75; H, 8.23; N, 9.46; S, 4.29.
[3,2b]-Indolo-N-[1-benzyl-1H-1,2,3-triazol-4-yl)methyl]-olean-12(13)-en-28-carboxamide (19)
Yield 0.39 g (56%); m.p. 164 °С; [α]D20 + 14° (с 0.05, CHCl3); δH (500.13 MHz, CDCl3) 0.62, 0.86, 0.87, 1.15, 1.17, 1.21, 1.30 (7s, 21H, 7CH3), 1.32-2.90 (m, 22H, CH, CH2), 5.13-5.20 (m, 2H, H-37), 5.34 (s, 1H, H-12), 5.46–5.51 (m, 1H, H-40), 7.03–7.44 (m, 9H, H-arom), 7.52 (s, 1H, H-triazol), 7.91 (br. s., 1H, NH); δC (125.76 MHz, CDCl3) 15.6, 16.6, 19.4, 23.1, 23.3, 23.4, 23.6, 25.7, 27.7, 30.7, 31.0, 32.2, 32.3, 33.1, 33.9, 34.0, 36.8, 38.1, 39.4, 41.5, 41.9, 45.9, 46.3, 46.8, 53.2, 54.2, 57.6 (C-40), 106.8 (C-2), 110.4 (C-arom), 117.9 (C-arom), 118.9 (C-arom), 120.9 (C-arom), 121.9 (C-arom), 122.7 (CH- triazol), 124.5 (C-12), 128.1 (2C, C-arom), 128.3 (C-arom), 128.9 (C-triazol), 129.2 (2C, C-arom), 134.4 (C-arom), 136.2 (C-arom), 140.9 (C-3), 143.4 (C13), 177.8 (C28); MS (APCI) m/z 699.5 [M + H]+ (calcd for C46H59N5O, 698.01). Anal. Calcd for C46H59N5O: C, 79.15; H, 8.52; N, 10.03. Found: C, 79.21; H, 8.45; N, 10.12.
Antiviral screening
All biology experimental procedures and molecular modeling methods are described in the Supplementary Materials.
Conclusions
By Cu(I)-catalyzed azide-alkyne cycloaddition and Mannich reaction 18 lupane, oleanane and ursane alkynyl-triterpenoids were synthesized and evaluated for antiviral activity against HCMV, HSV-1, and HPV-11. Among tested compounds, oleanane C28-1,2,3-triazole with a benzyl substituent 19 was active against HCMV with SI > 81, while lupane 3,28-diacetoxy-triazoles with phenyl-7 and fluorophenyl-9 fragments demonstrated strong HPV-11 antiviral activity. This approach was not effective toward HSV-1 type virus, while Mannich bases were more active than the parent alkyne. To sum up, of special interest are derivatives of 2,3-indolo-oleanolic acid and nor-lupane triazoles with promising potency against human cytomegalovirus and papillomavirus.
References
Dzubak P, Hajduch M, Vydra D, Hustova A, Kvasnica M, Biedermann D, Markova L, Urban M, Sarek J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat Prod Rep. 2006;23:394–411. https://doi.org/10.1039/b515312n.
Salvador JAR, Leal AS, Valdeira AS, Gonçalves BMF, Alho DPS, Figueiredo SAC, Silvestre SM, Mendes VIS. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: recent advances in cancer treatment. Eur J Med Chem. 2017;142:95–130. https://doi.org/10.1016/j.ejmech.2017.07.013.
Isah MB, Ibrahim MA, Mohammed A, Aliyu AB, Masola B, Coetzer TH. A systematic review of pentacyclic triterpenes and their derivatives as chemotherapeutic agents against tropical parasitic diseases. Parasitology. 2016;143:1219–31. https://doi.org/10.1017/S0031182016000718.
Martin DE, Salzwedel K, Allaway GP. Bevirimat: a novel maturation inhibitor for the treatment of HIV-1 infection. Antivir Chem Chemother. 2008;19:107–13. https://doi.org/10.1177/095632020801900301.
Frew Q, Rennekampff HO, Dziewulski P, Moiemen N. BBW-11 Study Group; Zahn T, Hartmann B. Betulin wound gel accelerated healing of superficial partial thickness burns: results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns. 2019;45:876–90. https://doi.org/10.1016/j.burns.2018.10.019.
Xiao S, Tian Z, Wang Y, Si L, Zhang L, Zhou D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med Res Rev. 2018;38:951–76. https://doi.org/10.1002/med.21484.
Lee KH. Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J Nat Prod. 2010;73:500–16. https://doi.org/10.1021/np900821e.
Kashiwada Y, Nagao T, Hashimoto A, Ikeshiro Y, Okabe H, Cosentino LM, Lee KH. Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. J Nat Prod. 2000;63:1619–22. https://doi.org/10.1021/np990633v.
Deng SL, Baglin I, Nour M, Flekhter O, Vita C, Cavé C. Synthesis of ursolic phosphonate derivatives as potential anti-HIV agents. Phosphorus Sulfur Silicon Relat Elem 2007;182:951–67. https://doi.org/10.1080/10426500601088838.
Gong Y, Raj KM, Luscombe CA, Gadawski I, Tam T, Chu J, Gibson D, Carlson R, Sacks SL. The synergistic effects of betulin with acyclovir against herpes simplex viruses. Antivir Res. 2004;64:127–30. https://doi.org/10.1016/j.antiviral.2004.05.006.
Baltina LA, Flekhter OB, Nigmatullina LR, Boreko EI, Pavlova NI, Nikolaeva SN, Savinova OV, Tolstikov GA. Lupane triterpenes and derivatives with antiviral activity. Bioorg Med Chem Lett. 2003;13:3549–52. https://doi.org/10.1016/s0960-894x(03)00714-5.
Pavlova NI, Savinova OV, Nikolaeva SN, Boreko EI, Flekhter OB. Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses. Fitoterapia. 2003;74:489–92. https://doi.org/10.1016/s0367-326x(03)00123-0.
Kazakova OB, Giniyatullina GV, Yamansarov EY, Tolstikov GA. Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorg Med Chem Lett. 2010;20:4088–90. https://doi.org/10.1016/j.bmcl.2010.05.083.
Kazakova OB, Medvedeva NI, Baikova IP, Tolstikov GA, Lopatina TV, Yunusov MS, Zaprutko L. Synthesis of triterpenoid acylates: effective reproduction inhibitors of influenza A (H1N1) and papilloma viruses. Russ J Bioorg Chem. 2010;36:771–8. https://doi.org/10.1134/S1068162010060142.
Khusnutdinova EF, Kazakova OB, Lobov AN, Kukovinets OS, Suponitsky KY, Meyers CB, Prichard MN. Synthesis of A-ring quinolones, nine-membered oxolactams and spiroindoles by oxidative transformations of 2,3-indolotriterpenoids. Org Biomol Chem. 2019;17:585–97. https://doi.org/10.1039/c8ob02624f.
Savinova OV, Pavlova NI, Boreko EI. [New betulin derivatives in combination with rimantadine for inhibition of influenza virus reproduction]. Antibiot Khimioter. 2009;54:16–20.
Karagöz AÇ, Leidenberger M, Hahn F, Hampel F, Friedrich O, Marschall M, Kappes B, Tsogoeva SB. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorg Med Chem. 2019;27:110–5. https://doi.org/10.1016/j.bmc.2018.11.018.
Dinh Ngoc T, Moons N, Kim Y, De Borggraeve W, Mashentseva A, Andrei G, Snoeck R, Balzarini J, Dehaen W. Synthesis of triterpenoid triazine derivatives from allobetulone and betulonic acid with biological activities. Bioorg Med Chem. 2014;22:3292–3300. https://doi.org/10.1016/j.bmc.2014.04.061.
Pohjala L, Alakurtti S, Ahola T, Yli-Kauhaluoma J, Tammela P. Betulin-derived compounds as inhibitors of alphavirus replication. J Nat Prod. 2009;72:1917–26. https://doi.org/10.1021/np9003245.
Spivak AY, Galimshina ZR, Nedopekina DA, Odinokov VN. Synthesis of new C-2 triazole-linked analogs of triterpenoid pentacyclic saponins. Chem Nat Compd. 2018;54:315–23. https://doi.org/10.1007/s10600-018-2331-1.
Spivak AY, Gubaidullin RR, Galimshina ZR, Nedopekina DA, Odinokov VN. Effective synthesis of novel C(2)-propargyl derivatives of betulinic and ursolic acids and their conjugation with β-D-glucopyranoside azides via click chemistry. Tetrahedron. 2016;72:1249–56. https://doi.org/10.1016/J.TET.2016.01.024.
Spivak AY, Nedopekina DA, Galimshina ZR, Khalitova RR, Sadretdinova ZR, Gubaidullin RR, Odinokov VN. Click chemistry-assisted synthesis of novel C-2 triazole-linked betulinic acid conjugates with azidothymidine as potential anti-HIV agents. Arkivoc. 2018;VII:1–19. https://doi.org/10.24820/ark.5550190.p010.632.
Khusnutdinova EF, Bremond P, Petrova AV, Kukovinets OS, Kazakova OB. Synthesis of lupane mono- and bis-C19-(1,2,3-triazolyl)-triterpenoids by “Click” reaction. Lett Org Chem. 2017;14:743–7.
Pokorny J, Borkova L, Urban M. Click reactions in chemistry of triterpenes—advances towards development of potential therapeutics. Curr Med Chem. 2018;25:636–58. https://doi.org/10.2174/0929867324666171009122612.
Csuk R, Deigner HP. The potential of click reactions for the synthesis of bioactive triterpenes. Bioorg Med Chem Lett. 2019;29:949–58. https://doi.org/10.1016/j.bmcl.2019.02.020.
Yu F, Wang Q, Zhang Z, Peng Y, Qiu Y, Shi Y, Zheng Y, Xiao S, Wang H, Huang X, Zhu L, Chen K, Zhao C, Zhang C, Yu M, Sun D, Zhang L, Zhou D. Development of oleanane-type triterpenes as a new class of HCV entry inhibitors. J Med Chem. 2013;56:4300–4019. https://doi.org/10.1021/jm301910a.
Xiao S, Wang Q, Si L, Shi Y, Wang H, Yu F, Zhang Y, Li Y, Zheng Y, Zhang C, Wang C, Zhang L, Zhou D. Synthesis and anti-HCV entry activity studies of β-cyclodextrin-pentacyclic triterpene conjugates. ChemMedChem. 2014;9:1060–70. https://doi.org/10.1002/cmdc.201300545.
Xiao S, Wang Q, Si L, Zhou X, Zhang Y, Zhang L, Zhou D. Synthesis and biological evaluation of novel pentacyclic triterpene α-cyclodextrin conjugates as HCV entry inhibitors. Eur J Med Chem. 2016;124:1–9. https://doi.org/10.1016/j.ejmech.2016.08.020.
Wang C, Lu L, Na H, Li X, Wang Q, Jiang X, Xu X, Yu F, Zhang T, Li J, Zhang Z, Zheng B, Liang G, Cai L, Jiang S, Liu K. Conjugation of a nonspecific antiviral sapogenin with a specific HIV fusion inhibitor: a promising strategy for discovering new antiviral therapeutics. J Med Chem. 2014;57:7342–54. https://doi.org/10.1021/jm500763m.
Roman G. Mannich bases in medicinal chemistry and drug design. Eur J Med Chem. 2015;89:743–816. https://doi.org/10.1016/j.ejmech.2014.10.076.
Kazakova OB, Medvedeva NI, Tolstikov GA, Kukovinets OS, Yamansarov EY, Spirikhin LV, Gubaidullin AT. Synthesis of terminal acetylenes using POCl3 in pyridine as applied to natural triterpenoids. Mendeleev Commun. 2010;20:234–6. https://doi.org/10.1016/j.mencom.2010.06.018.
Khusnutdinova EF, Apryshko GN, Petrova AV, Kukovinets OS, Kazakova OB. The synthesis and selective cytotoxicity of new Mannich bases, derivatives of 19- and 28-alkynyltriterpenoids. Russ J Bioorg Chem. 2018;44:123–7. https://doi.org/10.1134/S1068162018010090.
Khusnutdinova EF, Petrova AV, Kukovinets OS, Kazakova OB. Synthesis and cytotoxicity of 28-N-propargylaminoalkylated 2,3-indolotriterpenic acids. Nat Prod Commun. 2018;13:665–8. https://doi.org/10.1177/1934578X1801300603.
Kazakova O, Tret’yakova E, Baev D. Evaluation of A-azepano-triterpenoids and related derivatives as antimicrobial and antiviral agents. J Antibiot. 2021;74:559–73. https://doi.org/10.1038/s41429-021-00448-9.
Babaev M, Khusnutdinova E, Lobov A, Galimova Z, Petrova A, Rybalova T, Nguyen HTT, Meyers C, Prichard M, Kazakova O. Allobetulone rearrangement to l8αH,19βH-ursane triterpenoids with antiviral activity. Nat Prod Res. 2022;36:3286–96. https://doi.org/10.1080/14786419.2020.1855159.
Smee DF, Huffman JH, Morrison AC, Barnard DL, Sidwell RW. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activities. Antimicrob Agents Chemother. 2001;45:743–8. https://doi.org/10.1128/AAC.45.3.743-748.2001.
Prichard MN, Williams JD, Komazin-Meredith G, Khan AR, Price NB, Jefferson GM, Harden EA, Hartline CB, Peet NP, Bowlin TL. Synthesis and antiviral activities of methylenecyclopropane analogs with 6-alkoxy and 6-alkylthio substitutions that exhibit broad-spectrum antiviral activity against human herpesviruses. Antimicrob Agents Chemother. 2013;57:3518–27. https://doi.org/10.1128/AAC.00429-13.
Acknowledgements
This work was supported by the Federal program No. 1021062311392-9-1.4.1. The study of antiviral activity was funded in whole or in part with Federal funds from the National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract HHSN272201100016I (MNP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khusnutdinova, E.F., Petrova, A.V. & Kazakova, O.B. Antiviral potency of lupane and oleanane alkynyl-derivatives against human cytomegalovirus and papillomavirus. J Antibiot 77, 50–56 (2024). https://doi.org/10.1038/s41429-023-00672-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00672-5
- Springer Japan KK