Abstract
We assessed the predictive accuracy of the Warfarin Pharmacogenetics Consortium (IWPC) algorithm in a prospective cohort of 376 high-risk elderly patients (≥65 years) who required new treatment with warfarin for either medical (non valvular atrial fibrillation) or surgical conditions (heart valve replacement), had ≥1 comorbid conditions, and regularly used ≥2 other drugs. Follow-up visits were performed according to clinical practice and lasted for a maximum of 1 year. Two hundred and eighty-three (75%) patients achieved a stable maintenance dose. Warfarin maintenance doses were low on average (median 20.3 mg/week, interquartile range, 14.1–27.7 mg/week) and were substantially overestimated by the IWPC algorithm. Overall the percentage of patients whose predicted dose of warfarin was within 20% of the actual stable dose was equal to 37.5%, (95% CI 32.0–43.3%). IWPC algorithm explained only 31% of the actual warfarin dose variability. Modifications of the IWPC algorithm are needed in high-risk elderly people.
Similar content being viewed by others
Introduction
After several decades, warfarin continues to be the most prescribed oral anticoagulant worldwide [1, 2], notwithstanding the increasing use of new oral anticoagulants (DOAC) [3, 4]. However the clinical management of warfarin may be challenging because of its narrow therapeutic window along with within- and between- subject variability of dose required.
Individual warfarin response variability has been found to be associated with clinical factors (e.g., age, gender, compliance, nutritional status, presence of comorbidity, and concomitant medications), and polymorphisms involved in the metabolic route and warfarin effector/response pathway. Therefore, a number of models, which include both clinical and genetic factors, have been developed to predict warfarin dose requirements, but their usefulness is still under debate [5].
In 2009 the International Warfarin Pharmacogenetics Consortium (IWPC) proposed an algorithm that includes both clinical and genetic data [6]. The IWPC algorithm has been externally validated in other countries, mostly in small single-center studies that included adult patients irrespective of age [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. A recent meta-analysis showed that the proportion of warfarin doses that were systematically underpredicted by the IWPC algorithm in patients requiring higher than average doses was as high as 95.8% (95% confidence interval 92.4–97.9%) [35].
The predictive ability of the IWPC algorithm is particularly questionable in high-risk elderly people, who represent a highly vulnerable population because of increased risk of (i) bleeding during treatment with vitamin K antagonists, (ii) reduced metabolic clearance, and (iii) possible interactions with drugs used for comorbidities. In 2012 we planned the ‘Validation of IWPC algorithm in high-risk elderly patients (VIALE)’ study to validate the IWPC algorithm in a prospective cohort of high-risk elderly people (≥65 years) with at least one comorbid condition (clinicaltrials.gov identifier NCT02069132).
Materials and methods
Study design and participants
All patients aged 65 years or more, referring to six public hospitals, who first required treatment with warfarin because of either medical (non valvular atrial fibrillation) or surgical conditions (heart valve replacement), who had at least one comorbid condition, and regularly used two or more other drugs besides warfarin were prospectively recruited. Patients with systemic coagulopathies and malignancies needing chemotherapy were excluded.
Intervention
The investigators were allowed to adjust the dose of Warfarin or discontinue the treatment according to clinical practice, blind to genotype assessment. No additional interventional procedures were required.
IWPC algorithm
The IWPC pharmacogenetic algorithm [6] includes both clinical and genetic data: age, height, weight, race, VKORC1 genotype, CYP2C9 genotype, and use of drugs that are CYP2C9-inducer (rifampicin, phenytoin, and carbamazepine) or inhibitor (amiodarone). A different version of the algorithm that also included smoking and target INR was proposed in 2012 within the CoumaGen-II trial [36]. IWPC dosing algorithms 2009 and 2012 are reported in the Supplementary Table 1.
Variables
Baseline variables included demographic and clinical information, primary indication for warfarin treatment, risk factors such as smoking and drinking status, baseline and target INR, medical/surgical history, starting warfarin dose, comorbidity according to the Cumulative Index Rating Scale (CIRS) [37] and use of concomitant medications.
Follow-up (FU) visits were performed according to the clinical practice of each center and patients’ need. Maximum length of FU was set equal to 1 year. At each FU visit data on warfarin dose changes, INR levels, occurrence of cardiovascular, and cerebro-vascular (CCV) events and assumed drugs were recorded.
To assess the genetic variants included in the IWPC model (CYP2C9*2 [rs1799853], CYP2C9*3 [rs1057910] and VKORC1–1639G>A [rs9923231]), peripheral blood samples (4–5 ml) were collected in BD Vacutainers, containing EDTA as anticoagulant and stored at temperature of −80 °C, or −20 °C, for a period of maximum 3 months before shipping (in dry ice, to prevent defrost) to the central laboratory in Salerno. Genomic DNA was extracted using E.Z.N.A.® Blood DNA Kit (Omega Bio-Tek). Quantification and quality analysis of DNA was performed using a NanoDrop 2000c spettrophotometer. A BeadXpress Reader using Illumina VeraCode GoldenGate Assay Kit was used for genotyping. A total of 500 ng of DNA was used per assay.
Outcomes
Consistently with the 2009 IWPC paper [6], the primary outcome was the percentage of patients whose dose of warfarin, predicted by IWPC algorithm, was within 20% of the actual stable maintenance dose. The stable dose provided by centers was used for analyses, as defined in the Section 2 of Supplementary Appendix 1 of IWPC original paper [6]. All doses are reported as weekly doses.
Clinical and laboratory secondary endpoints were also evaluated:
-
overall incidence of CCV events either in the first year of warfarin treatment or in the first four weeks of warfarin treatment, defined as the occurrence of any one of death for any cause, hospitalization for CCV events, major bleeding, or thromboembolism;
-
overall incidence of major bleeding in the first year of treatment, defined as the occurrence of fatal bleeding, or symptomatic bleeding in a critical area or organ, or bleeding causing a fall in hemoglobin level of ≥2 g/dL or leading to transfusion of two or more units of blood or red cells [38];
-
overall incidence of thromboembolism in the first year of treatment, defined as the occurrence of cerebral infarction, or myocardial infarction, or peripheral arterial embolism;
-
time to therapeutic INR, defined as the time of first achieving INR measurement within the individual’s target range, providing that INR was also within the target range at the subsequent clinic visit;
-
percentage time (%TIR) in the therapeutic INR range during the first 3 months of treatment;
-
percentage time (%TIR) in the therapeutic INR range during the first four weeks of treatment.
As for patients lost at laboratory FU clinical information was retrieved through phone calls to patients’ home or from administrative registry offices of the patients’ towns of residence.
Sample size
Primary endpoint was the percentage of subjects who had a predicted dose within a range of ±20% of the stable dose [6], and sample size was calculated to achieve a predefined precision of the estimate. Initially a precision of ±3% of the 95% confidence interval (CI) was desired and 1067 subjects were required. Eventually the recruitment rate was much lower, and the actual sample size lead to a precision of CI of about ±5% of the estimate.
Statistical analysis
All statistical analyses were performed using both the 2009 [6] and the 2012 [36] version of IWPC algorithm in the whole sample and separately by primary indication (medical or surgical).
Warfarin dose distributions were graphically depicted using the box-and-whiskers plots. A scatter diagram showed the relationship between the actual and predicted warfarin therapeutic doses. Differences of percentages between groups were evaluated by means of chi-square test or Fisher’s exact test if needed. Mean differences between groups were tested with Student’s t test or Mann–Whitney test when normality assumption was unmet. The accuracy of the IWPC prediction algorithm was evaluated using linear regression models with the actual dose as dependent variable and the predicted dose as independent variable. Validity of the predictive algorithm was confirmed when the composite null hypothesis α = 0 (intercept) and β = 1 (slope) was not rejected at the two-tailed α level of 0.05. The determination coefficient (R2) measured the proportion of total variation of the actual therapeutic dose explained by the predicted dose. As measures of accuracy we also calculated the mean prediction error (MPE), defined as the average of the differences between the predicted and the actual dose, and the mean absolute error (MAE), defined as the average of the absolute value (in the mathematical sense) of the difference between the predicted and the actual doses. MAE is usually reported as a measure of predictive accuracy, but rather is a measure of variability of the difference distribution, with a similar interpretation of root mean square error [28].
The clinical usefulness of the IWPC algorithm was further quantified by the percentages of correct identification within subgroups of increasing levels of the warfarin actual dose.
Statistical analyses were performed with R software version 3.0.1 (R Foundation for Statistical Computing).
Results
Three hundred and seventy-six patients were enrolled between March 2013 and May 2016 (142 with medical and 234 with surgical indication). Baseline patients’ characteristics are shown in Table 1. All patients were Caucasian. Overall, most subjects (84%) had INR target between 2 and 3. However 55 patients (14%), almost exclusively with surgical indications, had target INR between 2.5 and 3.5. As expected most patients received a warfarin starting dose of 35 mg/week, but higher starting doses were also observed in medical patients. Relevant differences between medical and surgical subjects were found for age, NYHA class, comorbidity and INR target. The allelic frequencies of the CYP2C9*2, CYP2C9*3 and VKORC1-1639G>A SNPs were similar to those reported in other Caucasian populations [39, 40]. Comorbidity distribution as assessed by the CIRS index is reported in the Supplementary Table 2.
Stable maintenance dose was achieved in 283 (75%) patients without differences according to indication: 105/142 (74%) patients with medical indication and 178/234 (76%) with surgical indication. Overall characteristics of patients who achieved or did not achieve maintenance dose were similar (Supplementary Table 3), even though the latter ones were slightly older (76.5 vs 74.2 years on average) and had a higher prevalence of previous myocardial infarction (15.1% vs 8.1%). Twenty out of 93 (21.5%) patients, who did not achieve maintenance dose, discontinued warfarin treatment to move to DOAC or other anticoagulant, while 25/93 (26.9%) withdrew consent, possibly for the same reason. Consistently patients who did not achieve stable dose stayed less on warfarin therapy.
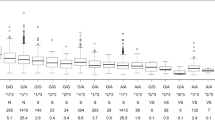
Distributions of warfarin maintenance doses reported by centers and predicted by the IWPC algorithm, using both the original version of 2009 [6] and the updated version of 2012 [36], are reported in Fig. 1. Median actual dose was equal to 20.3 mg/week (interquartile range, IQR, 14.1–27.7), much lower than the starting dose of 35 mg/week; median values in patients with medical and surgical indications were 17.5 mg/week (IQR 8.8–26.3) and 21.4 mg/week (IQR 15.9–28.9), respectively. On average, the IWPC 2009 predicted doses were higher than the actual therapeutic doses: overall median indeed was equal to 23.8 mg/week (IQR 19.0–28.7), and median values in patients with medical and surgical indication were 23.8 mg/week (IQR 17.8–28.2) and 23.9 mg/week (IQR 19.3–29.2), respectively. The analogous values predicted by IWPC 2012 algorithm were still higher.
The relationship between actual and predicted doses according to the 2009 (upper panel) and 2012 (lower panel) version of the algorithm is shown in Fig. 2. In the whole sample (Fig. 2, left panel) both intercept and regression coefficients were significantly far from the null values, and R2 was as small as 0.31. In medical patients (Fig. 2, middle panel) fitted values diverged strongly from the perfect prediction with very low R2 value (0.15 and 0.17), while a slightly better fitting was found in surgical patients (Fig. 2, right panel), with R2 values being equal to 0.41 and 0.39. In the whole sample the MPE was equal to 1.8 and 3.3 mg/week with the 2009 and 2012 version of the algorithm, respectively, slightly lower than corresponding median values (2.3 and 3.7 mg/week). Overprediction bias was greater in medical than in surgical subjects; MPE indeed was equal to 3.4 mg/week and 0.9 mg/week in medical and surgical patients, respectively, with IWPC 2009. Overprediction increased further with IWPC 2012, MPE being equal to 4.7 and 2.5 mg/week in medical and surgical patients, respectively. The MAE was equal to 7.64 mg/week in the whole sample and to 9.31 and 6.65 mg/week in medical and surgical patients, respectively, with IWPC 2009, and increased further with IWPC 2012.
The percentage of patients whose dose of warfarin, predicted by IWPC 2009 algorithm, was within the 20% of the actual dose was equal to 37.5%, (95% CI 32.0–43.3%), better in surgical (43.3%, 95% CI 36.1–50.7%) than in medical patients (27.6%, 95% CI 19.8–37.1%) (Table 2). Overprediction was always 2–3 times higher than underprediction. Similar results were found with IWPC 2012.
A deeper investigation of the ability of the pharmacogenetic algorithm to correctly identify patients requiring lower or higher therapeutic dose is reported in Table 3. The IWPC algorithm largely overestimated the dose in patients requiring very small doses, while underestimated the dose in the few patients requiring large doses. Results did not substantially change when analyses were repeated with the 2012 version of the IWPC algorithm (data not shown).
The occurrence of clinical and laboratory secondary outcomes in all patients is reported in Table 4. Forty-two (11.2%) cardio- and cerebro-vascular (CCV) events were observed in the first year of treatment, 14 (3.7%) of which in the first 4 weeks of treatment. The risk of bleeding was higher than the risk of thromboembolism (3.7% and 2.4%, respectively). Median time to first INR in target was nearly double for medical patients (12 days) with respect to surgical subjects (7 days). Overall, the percentage time in the therapeutic INR range was 52% in the first 3 months and 39% in the first 4 weeks of therapy, larger for medical than for surgical patients. About a third of subjects experienced at least one INR value ≥4.
Discussion
To our knowledge VIALE study is the first prospective study that assessed the predictive accuracy of the IWPC algorithm in a cohort of high-risk elderly people with comorbidity. In such a vulnerable population maintenance doses of warfarin were very low on average, particularly in medical subjects, and the IWPC algorithm substantially overestimated them. Only a third of patients had a predicted dose within a relative error of 20%. Further the pharmacogenetic algorithm only explained 30–40% of the warfarin dose variability (R2), and this value declined to only 15% in medical patients. Results did not change substantially when the 2012 version of the IWPC algorithm was used.
Anticoagulant doses were particularly low in medical subjects possibly as a consequence of the reduced metabolic clearance in elderly people [41] and prudence of doctors in the management of high-risk elderly patients. Conversely, higher doses were found in patients with surgical indications, leading to a slightly improved prediction. In these subjects higher doses might be expected as a consequence of higher target INR, but we cannot exclude that more accurate choice of surgical patients could have induced a selection of patients with less comorbidities.
In our study a quarter of subjects were unable to achieve a stable maintenance dose without differences between medical and surgical patients. As usually in the literature we did not take them into account when evaluating the predictive accuracy of the algorithm, but, from a clinical viewpoint they should contribute to get it worse. These percentages are lower than other figures previously reported in the literature [42,43,44]. Chappell et al. [42, 43] found that nearly 50% of subjects were unable to achieve the defined INR target during titration to a stable warfarin dose, whereas in Roberts et al. [44] about 55% of patients were ineligible due to maintenance INR outside the acceptable range. Different definitions of stable maintenance dose, characteristics of population, and setting of recruitment could explain discrepancies, that could heavily affect the clinical usefulness of pharmacogenetic algorithms.
In the only previous study that specifically addressed the predictive accuracy of the IWPC algorithm in elderly people, the algorithm also failed to identify older patients requiring low daily doses of warfarin; however, this study included a small number of subjects and in principle was prone to selection bias because of the retrospective design [12]. Most of the studies that validated the IWPC algorithm did not have age limits, and either excluded patients with comorbidity or did not report at all this information (Table 5). Moreover, almost all studies had a retrospective design and included only patients who achieved stable dose with possible selection biases.
Yan et al. [45] also report that more than 86% of patients receiving lower doses of warfarin (<13.16 mg/week) were overpredicted by the algorithm, while Saffian et al. [35] showed a systematic underprediction in patients requiring higher than average doses. The pharmacogenetic algorithm indeed predicts quite accurately warfarin dosing requirements on average, but possibly both higher- and lower-dose patients do not benefit substantially from this information. This is elucidated by the low percentage of warfarin dose variation explained by the model that in our study was as low as 31% and declined to a very low value of 15% in medical patients. Since published pharmacogenetic algorithms mainly include variables associated to a reduction in warfarin dose possible improvements of predictive accuracy in high-risk elderly people might be attained either encompassing comorbidity information into the algorithm or evaluating more complex non-linear models.
Our clinical findings were in line with literature results. Overall incidence of major bleedings was 3.7%, similar to the value of 3.5% reported by Burmester et al. [46] in relation to standard clinical warfarin therapy management. On the other hand, our incidence of thromboembolism was slightly higher than 2% reported by Verhoef et al. [47] in elderly subjects with atrial fibrillation. In purely surgical series, the rate of heart valve prosthesis-related thromboembolism ranged between 0 and 4 per 100 patients/year, whereas the rate of hemorrhagic events ranged between 0.2 and 7.2 per 100 patients/year [48].
Our study has several strengths. To our knowledge, it is the first prospective trial that attempted to externally validate the IWPC algorithm on vulnerable elderly people (≥65 years) only. Secondly, only incident cases were prospectively recruited, and we were able to identify patients unable to achieve dose stability, who are typically excluded from retrospective studies. Thirdly, we included patients with both medical and/or surgical indications in the attempt to provide a more complete picture of warfarin use in elderly population. Lastly, to our knowledge, this is the first study that assessed generalizability of the 2012 version of the IWPC algorithm.
Our study only included Caucasian subjects, although the predicted dose should not be affected, since the IWPC algorithm entails different coefficients for non Caucasian people. A further limitation of our study is sample size, that was smaller than the planned one; accordingly the precision of our estimates was reduced from the planned ±3% to about ±5%. However our study still remains larger than most previous trials, and our estimate was quite as precise as that observed in the original paper [6]. The reduced recruitment was mainly due to the increasing use of DOAC in clinical practice, which prevented the recruitment of elderly warfarin-naive patients with medical indication and favored withdrawal from the study. Sample size was further reduced because of subjects who did not achieve the maintenance dose. About half of them withdrew from the study, likely to move from warfarin to DOAC or other anticoagulant; another possible explanation could be the clinical complexity of the study population. Unfortunately, almost none of the studies reported the percentage of unstable patients thus interpretation is difficult.
The IWPC algorithm overpredicted warfarin maintenance doses in high-risk elderly people, mainly in patients with medical indication. More tailored models, possibly including comorbidity information, are to be evaluated in such a vulnerable population.
References
Friberg L, Rosenqvist M, Lip GYH. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298–307.
Franchini M, Mengoli C, Cruciani M, Bonfanti C, Mannucci PM. Effects on bleeding complications of pharmacogenetic testing for initial dosing of vitamin K antagonists: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:1480–7.
Mannucci PM, Franchini M. Old and new anticoagulant drugs: a minireview. Ann Med. 2011;43:116–23.
Franchini M, Mannucci PM. New anticoagulants for treatment of venous thromboembolism. Eur J Intern Med. 2012;23:692–5.
Johnson JJA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SSA, et al. Clinical Pharmacogenetics Implementation Consortium (Cpic) Guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharm Ther. 2017;102:1–30.
International Warfarin Pharmacogenetics Consortium, Klein TE, Altman RB, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–64.
Roper N, Storer B, Bona R, Fang M. Validation and comparison of pharmacogenetics-based warfarin dosing algorithms for application of pharmacogenetic testing. J Mol Diagn. 2010;12:283–91.
Sagreiya H, Sagrieya H, Berube C, Wen A, Ramakrishnan R, Mir A, et al. Extending and evaluating a warfarin dosing algorithm that includes CYP4F2 and pooled rare variants of CYP2C9. Pharmacogenet Genomics. 2010;20:407–13.
Shaw PB, Donovan JL, Tran MT, Lemon SC, Burgwinkle P, Gore J. Accuracy assessment of pharmacogenetically predictive warfarin dosing algorithms in patients of an academic medical center anticoagulation clinic. J Thromb Thrombolysis. 2010;30:220–5.
Takeuchi F, Kashida M, Okazaki O, Tanaka Y, Fukuda S, Kashima T, et al. Evaluation of pharmacogenetic algorithm for warfarin dose requirements in Japanese patients. Circ J. 2010;74:977–82.
Cho H-J, On Y-K, Bang OY, Kim J-W, Huh W, Ko J-W, et al. Development and comparison of a warfarin-dosing algorithm for Korean patients with atrial fibrillation. Clin Ther. 2011;33:1371–80.
Schwartz JB, Kane L, Moore K, Wu AHB. Failure of pharmacogenetic-based dosing algorithms to identify older patients requiring low daily doses of warfarin. J Am Med Dir Assoc. 2011;12:633–8.
Zambon C-F, Pengo V, Padrini R, Basso D, Schiavon S, Fogar P, et al. VKORC1, CYP2C9 and CYP4F2 genetic-based algorithm for warfarin dosing: an Italian retrospective study. Pharmacogenomics. 2011;12:15–25.
Bazan NS, Sabry NA, Rizk A, Mokhtar S, Badary O. Validation of pharmacogenetic algorithms and warfarin dosing table in Egyptian patients. Int J Clin Pharm. 2012;34:837–44.
Cini M, Legnani C, Cosmi B, Guazzaloca G, Valdrè L, Frascaro M, et al. A new warfarin dosing algorithm including VKORC1 3730 G>A polymorphism: comparison with results obtained by other published algorithms. Eur J Clin Pharm. 2012;68:1167–74.
Marin-Leblanc M, Perreault S, Bahroun I, Lapointe M, Mongrain I, Provost S, et al. Validation of warfarin pharmacogenetic algorithms in clinical practice. Pharmacogenomics. 2012;13:21–9.
Pathare A, Al Khabori M, Alkindi S, Al Zadjali S, Misquith R, Khan H, et al. Warfarin pharmacogenetics: development of a dosing algorithm for Omani patients. J Hum Genet. 2012;57:665–9.
Ramirez AH, Shi Y, Schildcrout JS, Delaney JT, Xu H, Oetjens MT, et al. Predicting warfarin dosage in European-Americans and African-Americans using DNA samples linked to an electronic health record. Pharmacogenomics. 2012;13:407–18.
Ramos AS, Seip RL, Rivera-Miranda G, Felici-Giovanini ME, Garcia-Berdecia R, Alejandro-Cowan Y, et al. Development of a pharmacogenetic-guided warfarin dosing algorithm for Puerto Rican patients. Pharmacogenomics. 2012;13:1937–50.
Tan SL, Li Z, Song GB, Liu LM, Zhang W, Peng J, et al. Development and comparison of a new personalized warfarin stable dose prediction algorithm in Chinese patients undergoing heart valve replacement. Pharmazie. 2012;67:930–7.
Ekladious SMM, Issac MSM, El-Atty Sharaf SA, Abou-Youssef HS. Validation of a proposed warfarin dosing algorithm based on the genetic make-up of Egyptian patients. Mol Diagn Ther. 2013;17:381–90.
Bosch LÁB. A proposal for an individualized pharmacogenetic-guided warfarin dosage regimen for Puerto Rican patients commencing anticoagulation therapy. J Pharmacogen Pharmacoproteom 2014; 5. https://doi.org/10.4172/2153-0645.T-001.
Hernandez W, Gamazon ER, Aquino-Michaels K, Patel S, O’Brien TJ, Harralson AF, et al. Ethnicity-specific pharmacogenetics: the case of warfarin in African Americans. Pharmacogenomics J. 2014;14:223–8.
Pavani A, Naushad SM, Uma A, Kutala VK. Methodological issues in the development of a pharmacogenomic algorithm for warfarin dosing: comparison of two regression approaches. Pharmacogenomics. 2014;15:1125–32.
Zhao L, Chen C, Li B, Dong L, Guo Y, Xiao X, et al. Verification of pharmacogenetics-based warfarin dosing algorithms in Han-Chinese patients undertaking mechanic heart valve replacement. PLoS ONE. 2014;9:1–8.
Karaca S, Bozkurt NC, Cesuroglu T, Karaca M, Bozkurt M, Eskioglu E, et al. International warfarin genotype-guided dosing algorithms in the Turkish population and their preventive effects on major and life-threatening hemorrhagic events. Pharmacogenomics. 2015;16:1109–18.
Peng Q, Huang S, Chen X, Yuan Y, Yu Y, Tao L, et al. Validation of warfarin pharmacogenetic algorithms in 586 Han Chinese patients. Pharmacogenomics. 2015;16:1465–74.
Saffian SM, Wright DFB, Roberts RL, Duffull SB. Methods for predicting warfarin dose requirements. Ther Drug Monit. 2015;37:531–8.
Santos PCJL, Marcatto LR, Duarte NE, Gadi Soares RA, et al. Development of a pharmacogenetic-based warfarin dosing algorithm and its performance in Brazilian patients: highlighting the importance of population-specific calibration. Pharmacogenomics. 2015;16:865–76.
Xu H, Su S, Tang W, Wei M, Wang T, Wang D, et al. Comparison of the performance of the warfarin pharmacogenetics algorithms in patients with surgery of heart valve replacement and heart valvuloplasty. Thromb Res. 2015;136:552–9.
Cho S-M, Lee K-Y, Choi JR, Lee K-A. Development and comparison of warfarin dosing algorithms in stroke patients. Yonsei Med J. 2016;57:635.
Duconge J, Ramos AS, Claudio-Campos K, Rivera-Miranda G, Bermúdez-Bosch L, Renta JY, et al. A novel admixture-based pharmacogenetic approach to refine warfarin dosing in Caribbean Hispanics. PLoS ONE. 2016;11:e0145480.
Lin M, Yu L, Qiu H, Wang Q, Zhang J, Song H. Verification of five pharmacogenomics-based warfarin administration models. Indian J Pharm. 2016;48:258–63.
Stack G, Maurice CB. Warfarin pharmacogenetics reevaluated: subgroup analysis reveals a likely underestimation of the maximum pharmacogenetic benefit by clinical trials. Am J Clin Pathol. 2016;145:671–86.
Saffian S, Duffull S, Wright D. Warfarin dosing algorithms underpredict dose requirements in patients requiring ≥7 mg daily: a systematic review and meta-analysis. Clin Pharm Ther. 2017;102:297–304.
Anderson JL, Horne BD, Stevens SM, Woller SC, Samuelson KM, Mansfield JW, et al. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II). Circulation. 2012;125:1997–2005.
Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–6.
Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4.
1000 Genomes Project Consortium RA, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74.
Mazzaccara C, Conti V, Liguori R, Simeon V, Toriello M, Severini A, et al. Warfarin anticoagulant therapy: a Southern Italy pharmacogenetics-based dosing model. PLoS ONE. 2013;8:e71505.
Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37:1075–80.
Chappell J, He J, Knadler MP, Mitchell M, Lee D, Lobo E. Effects of duloxetine on the pharmacodynamics and pharmacokinetics of warfarin at steady state in healthy subjects. J Clin Pharm. 2009;49:1456–66.
Chappell JC, Dickinson G, Mitchell MI, Haber H, Jin Y, Lobo ED. Evaluation of methods for achieving stable INR in healthy subjects during a multiple-dose warfarin study. Eur J Clin Pharm. 2012;68:239–47.
Roberts GW, Quinn S, Druskeit T, Helboe T, Jørgensen LE, Johansen C. Post-operative warfarin re-initiation—modelling loading dose strategy. J Pharm Pr Res. 2013;43:276–8.
Yan H, Yin JY, Zhang W, Li X. Possible strategies to make warfarin dosing algorithm prediction more accurately in patients with extreme dose. Clin. Pharmacol. Ther. 2018. https://doi.org/10.1002/cpt.800.
Burmester JK, Berg RL, Yale SH, Rottscheit CM, Glurich IE, Schmelzer JR, et al. A randomized controlled trial of genotype-based Coumadin initiation. Genet Med. 2011;13:509–18.
Verhoef TI, Ragia G, de Boer A, Barallon R, Kolovou G, Kolovou V, et al. A randomized trial of genotype-guided dosing of acenocoumarol and phenprocoumon. N. Engl J Med. 2013;369:2304–12.
Iung B, Rodés-cabau J. The optimal management of anti-thrombotic therapy after valve replacement: certainties and uncertainties. Eur Heart J. 2014;35:2942–9.
Acknowledgements
The study was fully supported by the Italian Drug Agency (Agenzia Italiana per il Farmaco) study code FARM9JNT9Y. The sponsor had no role in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. VS has been supported by Programma VALERE, University of Campania “Luigi Vanvitelli”. The authors thank Flavia Lo Passo for assistance with data management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the ethics committees of all participating centers. All participants gave written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Filippelli, A., Signoriello, S., Bancone, C. et al. Prospective validation of the International Warfarin Pharmacogenetics Consortium algorithm in high-risk elderly people (VIALE study). Pharmacogenomics J 20, 451–461 (2020). https://doi.org/10.1038/s41397-019-0129-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-019-0129-6
- Springer Nature Limited