Abstract
Background
Numerous genotype-guided warfarin dosing algorithms have been developed to individualize warfarin doses, but they can only explain 47–52% of the variability.
Aim
This study aimed to develop new warfarin algorithms suitable to predict the stable warfarin dose for the Chinese population and to compare their prediction performance with those of the most commonly used algorithms.
Method
Multiple linear regression analysis with the warfarin optimal dose (WOD), logarithm (log) WOD, 1/WOD, and \(\sqrt {\text {WOD}}\), respectively, as the dependent variables were performed to deduce a new warfarin algorithm (NEW-Warfarin). WOD was the stable dose that maintained the international normalized ratio (INR) within the target range (2.0–3.0). Three major genotype-guided warfarin dosing algorithms were selected and compared against NEW-Warfarin predictive performance using the mean absolute error (MAE). Furthermore, patients were divided into five groups according to warfarin indications [atrial fibrillation (AF), pulmonary embolism (PE), cardiac-related disease (CRD), deep vein thrombosis (DVT), and other diseases (OD)]. Multiple linear regression analyses were also performed for each group.
Results
The regression equation with \(\sqrt {\text {WOD}}\) as the dependent variable had the highest coefficient of determination (R2 = 0.489). The NEW-Warfarin had the best predictive accuracy compared to the three algorithms selected. Group analysis, according to indications, showed that the R2 of the five groups were PE (0.902) > DVT (0.608) > CRD (0.569) > OD (0.436) > AF (0.424).
Conclusion
Dosing algorithms based on warfarin indications are more suitable for predicting warfarin doses. Our research provides a novel strategy to develop indication-specific warfarin dosing algorithms to improve the efficacy and safety of warfarin prescribing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
Individualized precise doses of warfarin are essential.
-
Developing genotype-guided indication-based warfarin algorithms is a novel strategy to improve the accuracy of warfarin dose prediction.
-
This study developed five indication-specific warfarin dosing algorithms suitable for the Chinese population.
Introduction
As the most common oral anticoagulant, warfarin is widely used to prevent and treat various thromboembolic conditions. These conditions include atrial fibrillation (AF), pulmonary embolism (PE), cardiac-related disease (CRD) (including rheumatic heart disease, myocardial infarction, dilated cardiomyopathy, cardiac failure, and valvular heart disease), deep vein thrombosis (DVT), and other conditions (OD) (including cerebral infarction, Budd-Chiari syndrome, and thrombophilia) [1]. Despite the emergence of new oral anticoagulants, these treatments have yet to replace warfarin due to the less available information on their safety and effectiveness. Warfarin is the only drug for patients with mechanical heart valve replacement [2]. However, warfarin has a narrow therapeutic index, a large individual variability, and many interactions with medicines and food [3]. Physicians and pharmacists must carefully monitor the international normalized ratio (INR) to avoid bleeding and loss of therapeutic efficacy [4]. Therefore, precise individual doses of warfarin are essential.
In recent years, investigators and clinicians have used pharmacogenetics to develop dosing algorithms that estimate the warfarin dose requirement based on genetic and clinical factors, among which regression analysis is commonly used [5,6,7]. Most of these algorithms typically incorporate demographic, genetic, and clinical data from patients, such as age, body weight, height, concomitant drug administration, and vitamin K epoxide reductase complex subunit 1 (VKORC1) and cytochrome P450 family 2 subfamily C member 9 (CYP2C9) genotypes [8, 9]. The genetic polymorphisms of VKORC1 and CYP2C9 are the most critical factors and can explain about 40% of the variation in inter-individual warfarin doses. Furthermore, non-genetic factors, such as age and sex, account for approximately 15% of the variation [10]. However, only around 50% of the variations in warfarin dosing can be explained by these algorithms that combine pharmacogenomic and demographic factors [11, 12]. More studies are needed to identify new factors related to warfarin to improve the precision of warfarin dose prediction.
The vast majority of the published literature on genotype-guided warfarin dosing has been derived from populations of European ancestry. Although it may be reasonable to use algorithms derived from Europe for some populations, this approach is unlikely to be valid in other races. Variants can have different effects depending on the patient’s race/ethnic group, resulting in incorrect effect sizes and dose estimates. As such, a fundamental question arose: Can algorithms developed in European populations be suitable for application in the Chinese population? Furthermore, although many studies have improved the precision of warfarin dose prediction algorithms by including as many influencing factors as possible, it remains challenging to get the precision of dose prediction to exceed 55% [13, 14].
Aim
This study aimed to develop new warfarin algorithms suitable to predict the stable warfarin dose for the Chinese population and to compare their prediction performance with those of the most commonly used algorithms.
Ethics approval
This study was approved by the Ethical Review Committee of Shanxi Bethune Hospital (approval date October 16, 2019, approval number YXLL-2019-128). Informed consent was obtained from patients or guardians of minors.
Method
Study population and genotyping
The same patient data from our previous study were used for the present investigation [15]. Patients who received warfarin treatment and underwent genotyping tests at Shanxi Bethune Hospital were enrolled. Genotyping included VKORC1 rs9923231 and CYP2C9 rs1057910. Patients with a critical condition, deep coma, lack of spontaneous breathing, severe organ dysfunction, or pregnancy were excluded.
For each patient, the following clinical data were collected: age (years), body weight (kg), height (cm), sex (male/female), smoking history (yes: daily smokers or occasional smokers; no: lifelong nonsmoker), drinking history (yes: if more than three times per week; no: if less than three times per week), hypertension (yes/no), diabetes (yes/no), thyroid function (normal, hyperthyroidism, hypothyroidism), concomitant drugs (amiodarone, omeprazole, aspirin, statins, or cephalosporins; yes/no), indication for treatment (AF, DVT, PE, CRD, and OD), and VKORC1 (rs9923231) and CYP2C9 (rs1057910) genotypes.
Data were collected during hospitalization when the INR range of a patient was maintained between 2.0 and 3.0 (target INR). Patients who did not achieve a target INR during hospitalization were followed for two months, and their INR was monitored once a week. During the follow-up phase, clinical data were recorded when the INR reached the target range. The warfarin optimal dose (WOD) was defined as the stable dose that kept the INR within the target range during hospitalization or the follow-up period.
Study design
The study consisted of two stages. The first stage was developing a new warfarin algorithm (NEW-Warfarin) suitable for application in Chinese populations using multiple linear regression. The predictive performance of the algorithm was compared with three published algorithms. The algorithm selection criteria were (1) designed to calculate the maintenance dose rather than the initial dose, (2) the minimum acceptable number of study subjects was ≥ 120, and (3) established for Asian populations. The selection resulted in three algorithms, the International Warfarin Pharmacogenetics Consortium (IWPC) algorithm [16], the HUANG algorithm [10], and the OHNO algorithm [17]. HUANG and OHNO were abbreviated according to the first author’s name (Huang and Ohno). We developed the indication-based warfarin algorithm (IND-Warfarin) in the second stage. Study participants were divided into five groups based on warfarin indication: AF, PE, CRD, DVT, or OD. Multiple linear regression analysis was performed for each group to develop the respective indication-based algorithm.
Statistical analyses
Univariate analysis was performed to evaluate the effects of certain clinical factors (i.e., sex, smoking history, and genotype) on WOD. Differences between dichotomous variables were established using an independent sample t-test, while differences between multiple categorical variables were examined using a one-way analysis of variance. Associations between WOD and continuous variables [e.g., body surface area (BSA)] were determined using a general linear model. Only variables with P < 0.05 were included in the multiple linear regression model. A stepwise regression method filtered the variables (αin = 0.10, αout = 0.15).
In the first stage, data conversions [WOD, logarithmic WOD (logWOD), 1/WOD, and] \(\sqrt {\text {WOD}}\) were performed for WOD. A multiple linear regression analysis was performed to develop NEW-Warfarin with these values acting as dependent variables The primary purposes were to evaluate which data conversion as the dependent variable yielded the highest coefficient of determination. Furthermore, the predictive accuracy among NEW-Warfarin, IWPC, HUANG, and OHNO was determined by calculating the mean absolute error (MAE;\(MAE = \frac{1}{n}\sum\nolimits_{{i = 1}}^{n} {|PDi - WODi|}\)) based on predicted dose (PD) and WOD values [18]. In the second stage, multiple linear regression analysis was performed for the five groups of patients based on different indications of warfarin, among which the optimal form of WOD conversion determined in the first stage was set as the dependent variable. Comparisons of prediction accuracy and correlations between NEW-Warfarin and IND-Warfarin in each group were conducted using MAE and Pearson’s correlation coefficients between the WOD of the two algorithms.
Two-sided or one-sided statistical tests were used when appropriate. All analyses were performed with SPSS version 25.0 (IBM Corporation, Armonk, NY, USA). Normally distributed variables were displayed as mean ± standard deviation (SD) values, with P < 0.05 deemed statistically significant.
Results
Patient characteristics
A total of 217 Shanxi Han Chinese patients who received therapeutic warfarin treatment were analyzed. The indications for warfarin treatment were AF (n = 47), PE (n = 13), CRD (n = 37), DVT (n = 73), and OD (n = 47). The mean age was 57.9 ± 15.3 years (range 14–84 years), the mean height was 164.1 ± 8.3 cm (range 145.0–190.0 cm), and the mean body weight was 67.0 ± 12.2 kg (range 43.0–111.0 kg). The frequency of the CYP2C9*1/*3 and CYP2C9*3/*3 genotypes was 7.4%, while the frequency of the VKORC1 A/A and A/G genotypes was 98.6%. The demographic and clinical characteristics of the study participants are summarized in Table 1.
A new warfarin dosing algorithm (NEW-Warfarin)
Multiple regression analysis showed that the regression equation taken \(\sqrt {\text {WOD}}\) as the dependent variable had the highest coefficient of determination (R2 = 0.489). The regression equation is shown as follows: \(\sqrt {\text {WOD}}\) = 1.479 + 0.391 × (VKORC1 A/G) + 0.782 × (VKORC1 G/G) + 0.006 × body weight (kg) − 0.091 × concurrent arrhythmia status − 0.254 × (CYP2C9*1/*3) − 0.508 × (CYP2C9*3/*3) − 0.002 × age (years) + 0.138 × aspirin status − 0.102 × AF − 0.125 × CRD + 0.072 × PE − 0.128 × OD. This algorithm explained 48.9% of the inter-individual variation in WOD: the VKORC1 genotype (28.9%), the body weight (5.8%), concurrent arrhythmia status (6.0%), CYP2C9 genotype (4.4%), warfarin indication (2.4%), age (0.7%), and aspirin status (0.7%). Where 1 = with a corresponding genotype, otherwise 0; 1 = with a concurrent arrhythmia status, otherwise 0; 1 = patient taking aspirin, otherwise 0; 1 = patient indication for warfarin was AF, CRD, PE, or OD, otherwise 0. The VKORC1 (rs9923231) genotype (A/A → A/G → G/G), body weight, concurrent application of aspirin (no → yes), and warfarin PE indication had a significantly positive correlation with \(\sqrt {\text {WOD}}\) In contrast, concurrent arrhythmia status (no → yes); CYP2C9 (rs1057910) genotype (*1/*1 → *1/*3 → *3/*3), age; and warfarin indication (AF, CRD, or OD) had a significantly negative correlation.
The warfarin PD algorithm is as follows: PD (mg/d) = [1.479 + 0.391 × (VKORC1 A/G) + 0.782 × (VKORC1 G/G) + 0.006 × body weight (kg) − 0.091 × concurrent arrhythmia status − 0.254 × (CYP2C9*1/*3) − 0.508 × (CYP2C9*3/*3) − 0.002 × age (years) + 0.138 × aspirin status − 0.102 × AF − 0.125 × CRD + 0.072 × PE − 0.128 × OD]2. The MAE analysis that ranked the predictive accuracy among the four algorithms in descending order is as follows: NEW-Warfarin > OHNO > IWPC > HUANG (Table 2).
Warfarin algorithms based on indications (IND-Warfarin)
According to warfarin indications, multiple linear regression showed that warfarin PE indication had the highest coefficient of determination (R2 = 0.902). The ranking of the coefficient of determination for the other four indication groups was DVT (R2 = 0.608) > CRD (R2 = 0.569) > OD (R2 = 0.436) > AF (R2 = 0.424). Each multiple linear regression equation contained different influencing factors: four variables (VKORC1, smoking history, drinking history, and sex) for PE, five variables (VKORC1, CYP2C9, body weight, concomitant aspirin, and hypertension) for DVT, five variables (CYP2C9, body height, age, VKORC1, and concomitant statins) for CRD, three variables (VKORC1, sex, and age) for OD, and two variables (VKORC1 and body weight) for AF. The respective warfarin PD algorithms based on indications are summarized in Table 3.
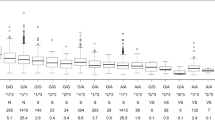
Statistical correlations and differences between WOD and PD derived from the NEW-Warfarin and IND-Warfarin algorithms are shown in Fig. 1. The IND-Warfarin algorithm had better accuracy and correlation between WOD and PD in the PE, CRD, OD, and AF groups than the NEW-Warfarin algorithm. In contrast, the NEW-Warfarin algorithm had better accuracy and correlation between the two values for the DVT group.
Statistical correlations and differences between WOD and PD derived from NEW-Warfarin and IND-Warfarin in the five indication groups (**P < 0.01). A Pulmonary embolism indication group; B Cardiac-related disease indication group; C Other diseases indication group; D Atrial fibrillation indication group; E Deep vein thrombosis indication group. WOD: warfarin optimal dose; PD: predicted dose; NEW-Warfarin: new warfarin algorithm; IND-Warfarin: indication-based warfarin algorithm; MAE: mean absolute error; (r): Pearson’s correlation coefficient
Discussion
Numerous genotype-guided warfarin dosing algorithms have been developed to reduce the adverse effects of warfarin [18, 19]. Approximately 50% of warfarin dose variations can be explained by these dosing models incorporating genetic and non-genetic factors [11]. Several studies evaluated the performance of genotype-guided warfarin dosing algorithms in populations of Asian ancestry. The performance of algorithms (R2) ranges from 34 to 67% [20,21,22,23]. VKORC1 (rs9923231), CYP2C9 (rs1057910), age, BMI, and concomitant amiodarone were associated with 37.0% of individual variations in the daily stable warfarin dose in older Han Chinese patients (≥ 65 years of age) [24]. A Chinese-specific pharmacogenetic-guided warfarin dosing algorithm that included VKORC1 (rs9923231), CYP2C19 (rs1057910), age, and BSA, could explain 54.1% of the variance in warfarin doses in Han Chinese patients [10]. We developed the NEW-Warfarin algorithm using multiple linear regression analysis. Seven factors [VKORC1 (rs9923231), weight, concurrent arrhythmia status, age, CYP2C9 (rs1057910), aspirin status, and indication] were included. The coefficient of determination (R2) was 0.489. VKORC1 (rs9923231) and the concurrent arrhythmia status were strongly associated with interindividual variability in the warfarin maintenance dose, accounting for 28.9% and 6.0%, respectively. Meanwhile, body weight and CYP2C9 (rs1057910) could explain approximately 5.8% and 4.4% of individual differences in daily stable dose, less than in previous studies [10, 24]. The type of warfarin indication contributed 2.4% to interindividual variability, while age or aspirin status could explain 0.7% of individual differences in the stable daily dose. Two new influencing factors (aspirin status and warfarin indication) were included in the NEW-Warfarin algorithm, which could contribute to the higher coefficient of determination (R2 = 0.489) compared to the previous study (R2 = 0.370) [24].
According to the guidelines, warfarin and aspirin are restricted to limited patients [25, 26]. However, 20% of patients taking warfarin for any indication receive aspirin inappropriately in daily practice [27]. These patients often have more comorbidities, shorter time in the warfarin therapeutic range, and higher bleeding rates. The use of combination therapy appears to occur due to multiple comorbidities. More studies are needed to fully explore whether increases in warfarin doses are due to various comorbidities. The NEW-Warfarin algorithm showed that indication was another factor significantly associated with warfarin dose. The result is similar to a previous study, which shows that, “warfarin dosing according to the genotype-guided algorithm is most beneficial in patients with atrial fibrillation” [28]. In addition, some researchers have established a warfarin dose prediction model for Chinese patients with venous thromboembolism using the VKORC1 and CYP2C9 genotypes and clinical variables [29]. Developing genotype-guided warfarin algorithms for each disease may be a novel strategy to improve dose prediction accuracy.
To improve the accuracy of prediction, we developed algorithms based on five common indications. The PE group (n = 13) had the highest coefficient of determination. However, the size of the PE group was relatively small, and the model equation was inevitably biased. Four variables (VKORC1, smoking history, drinking history, and sex) were included in the final regression model. VKORC1 and sex have been reported to be standard variables associated with warfarin doses in Chinese populations and could be used to predict warfarin dosing. However, smoking and drinking history are rarely present in genotype-guided warfarin algorithms. Although mechanistic research is understandably limited, these findings are consistent with previous studies that alcohol intake or smoking is known to increase or inhibit warfarin effects, respectively [30]. A larger group of independent patients is required to validate our algorithm in the future.
Five variables (VKORC1, CYP2C9, body weight, aspirin status, and hypertension status) were included in the final regression model of the DVT group. Concurrent application of aspirin (explaining 3.9% of individual differences) and hypertension status (explaining 4.1% of individual differences) were new factors significantly associated with warfarin dose in DVT patients. Meanwhile, statin status (explaining 4.5% of individual differences) was a new factor filtered into the regression equation in the CRD group. VKORC1, sex, and age were included in the regression model of the OD group, while VKORC1 and body weight were included in the regression model of the AF group. These factors are common variables associated with the warfarin dose requirements in Chinese populations. Our results support that the factors influencing warfarin doses change with warfarin indications. In addition, algorithms established based on warfarin indications have different degrees of prediction accuracy. Knowing these factors can help improve the quality and safety of warfarin treatment since prior identification of warfarin sensitivity is essential to avoid exposure to high doses and mitigate an excessive risk of anticoagulation.
The predictive accuracy between NEW-Warfarin, IWPC, HUANG, and OHNO was compared by calculating MAE based on PD and WOD values. NEW-Warfarin had the lowest MAE (0.68 mg/day). OHNO (0.72 mg/day) was slightly more accurate than IWPC (0.73 mg/day), which is consistent with a previous report that OHNO is close to IWPC and can be effectively implemented in Asian populations [18]. NEW-Warfarin showed better performance than the other algorithms because the frequency and effect of variants may differ based on race/ethnicity. The frequency of the A allele of VKORC1-1639 in Asian populations is substantial (90%) compared to other race/ethnic groups, which partially explains the reduced requirements for warfarin doses in Asian patients [31]. The distribution of the CYP2C9 genotype in the Han Chinese population is significantly different from that of Africans, Caucasians, and South and West Asians [32]. The NEW-Warfarin algorithm has been tested in the same population in which it was developed [15], probably increasing its validity.
Pearson’s correlation and MAE analyses showed that the IND-Warfarin algorithm had better precision and correlation between WOD and PD in the PE, CRD, OD, and AF groups. Theoretically, it should also be more predictive than TRA-Warfarin for patients with all indications. However, in the DVT group, NEW-Warfarin was more accurate in predicting WOD. DVT was the primary indication of warfarin among all thromboembolic events, with the highest prevalence among older and obese populations. The finding could explain why body weight was filtered into the regression model. The risk of DVT is known to increase dramatically above the age of 45 [30]. Age is an important variable, and older patients show increased sensitivity to warfarin [33]. The clinical conditions most closely associated with DVT are surgery, trauma, malignancy, prolonged immobility, pregnancy, congestive heart failure, varicose veins, obesity, advanced age, and a history of DVT. The complex and diverse pathogenic factors of DVT can make it challenging to capture all related factors that significantly affect the warfarin dose in our research.
This study has several limitations. First, some covariates associated with variation in warfarin dose (e.g., surgery or trauma, malignancy, or prolonged immobility) were not evaluated. Second, when performing a group analysis, this study was limited by its small size and relatively genetically homogeneous patient population. In particular, the size of the PE group was relatively small, and the model equation was inevitably biased. Therefore, a larger group of independent patients would be required to validate our algorithm, preferably recruited in multicenter studies, to minimize study bias. This may allow the model to be accepted more widely and used safely. However, the IND-Warfarin algorithm based on warfarin indications could constitute a new dose adjustment strategy for clinicians to prescribe warfarin.
Conclusion
Dosing algorithms based on warfarin indications are more suitable for predicting warfarin doses. Our research provides a novel strategy to develop indication-specific warfarin dosing algorithms to improve the efficacy and safety of warfarin prescribing.
References
Xie C, Xue L, Zhang Y, et al. Comparison of the prediction performance of different warfarin dosing algorithms based on Chinese patients. Pharmacogenomics. 2020;21:23–32.
Shaw K, Amstutz U, Kim RB, et al. Clinical practice recommendations on genetic testing of CYP2C9 and VKORC1 variants in warfarin therapy. Ther Drug Monit. 2015;37:428–36.
Johnson JA, Caudle KE, Gong L, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther. 2017;102:397–404.
Gustafsson D, Bylund R, Antonsson T, et al. A new oral anticoagulant: the 50-year challenge. Nat Rev Drug Discov. 2004;3:649–59.
Miao L, Yang J, Huang C, et al. Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients. Eur J Clin Pharmacol. 2007;63:1135–41.
Wei M, Ye F, Xie D, et al. A new algorithm to predict warfarin dose from polymorphisms of CYP4F2, CYP2C9 and VKORC1 and clinical variables: derivation in Han Chinese patients with non valvular atrial fibrillation. Thromb Haemost. 2012;107:1083–91.
Zhong SL, Yu XY, Liu Y, et al. Integrating interacting drugs and genetic variations to improve the predictability of warfarin maintenance dose in Chinese patients. Pharmacogenet Genomics. 2012;22:176–82.
Bader L, Mahfouz A. The effect of genetic and nongenetic factors on warfarin dose variability in Qatari population. Pharmacogenomics J. 2020;20:277–84.
Wu AH. Use of genetic and nongenetic factors in warfarin dosing algorithms. Pharmacogenomics. 2007;8:851–61.
Huang SW, Chen HS, Wang XQ, et al. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: a prospective study in Chinese patients. Pharmacogenet Genomics. 2009;19:226–34.
Li X, Liu R, Luo ZY, et al. Comparison of the predictive abilities of pharmacogenetics-based warfarin dosing algorithms using seven mathematical models in Chinese patients. Pharmacogenomics. 2015;16:583–90.
Wadelius M, Chen LY, Lindh JD, et al. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009;113:784–92.
Yang M, Choi R, Kim JS, et al. Evaluation of 16 genotype-guided warfarin dosing algorithms in 310 Korean patients receiving warfarin treatment: poor prediction performance in VKORC1 1173C carriers. Clin Ther. 2016;38:2666–74.
Zhu YB, Hong XH, Wei M, et al. Development of a novel individualized warfarin dose algorithm based on a population pharmacokinetic model with improved prediction accuracy for Chinese patients after heart valve replacement. Acta Pharmacol Sin. 2017;38:434–42.
Gao W, Li Z, Chen W, et al. Performance evaluation of warfarin dose prediction algorithms and effects of clinical factors on warfarin dose in Chinese patients. Ther Drug Monit. 2021;43(4):527–35.
Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–64.
Ohno M, Yamamoto A, Ono A, et al. Influence of clinical and genetic factors on warfarin dose requirements among Japanese patients. Eur J Clin Pharmacol. 2009;65:1097–103.
Sasano M, Ohno M, Fukuda Y, et al. Verification of pharmacogenomics-based algorithms to predict warfarin maintenance dose using registered data of Japanese patients. Eur J Clin Pharmacol. 2019;75:901–11.
Kaye JB, Schultz LE, Steiner HE, et al. Warfarin pharmacogenomics in diverse populations. Pharmacotherapy. 2017;37:1150–63.
Lin M, Yu L, Qiu H, et al. Verification of five pharmacogenomics-based warfarin administration models. Indian J Pharmacol. 2016;48:258–63.
Cho SM, Lee KY, Choi JR, et al. Development and comparison of warfarin dosing algorithms in stroke patients. Yonsei Med J. 2016;57:635–40.
Lei X, Guo Y, Sun J, et al. Accuracy assessment of pharmacogenetic algorithms for warfarin dose prediction in Chinese patients. Am J Hematol. 2012;87:541–4.
Cho HJ, On YK, Bang OY, et al. Development and comparison of a warfarin-dosing algorithm for Korean patients with atrial fibrillation. Clin Ther. 2011;33:1371–80.
Li W, Zhao P, Chen L, et al. Impact of CYP2C9, VKORC1, ApoE and ABCB1 polymorphisms on stable warfarin dose requirements in elderly Chinese patients. Pharmacogenomics. 2020;21:101–10.
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962.
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–95.
Kılıç S, Çelik A, Çekirdekçi E, et al. The prevalence and risks of inappropriate combination of aspirin and warfarin in clinical practice: results from WARFARIN-TR study. Balkan Med J. 2019;36:17–22.
Makar-Aušperger K, Krželj K, Lovrić Benčić M, et al. Warfarin dosing according to the genotype-guided algorithm is most beneficial in patients with atrial fibrillation: a randomized parallel group trial. Ther Drug Monit. 2018;40:362–8.
Zhang W, Zhang WJ, Zhu J, et al. Genetic polymorphisms are associated with variations in warfarin maintenance dose in Han Chinese patients with venous thromboembolism. Pharmacogenomics. 2012;13:309–21.
Maramba A, Ncube S, Mandisodza A, et al. An assessment of the effectiveness of warfarin therapy monitoring systems on thrombophilic patients in zimbabwe. TH Open. 2018;2:e325–8.
Dang MT, Hambleton J, Kayser SR. The influence of ethnicity on warfarin dosage requirement. Ann Pharmacother. 2005;39:1008–12.
Gaikwad T, Ghosh K, Shetty S. VKORC1 and CYP2C9 genotype distribution in Asian countries. Thromb Res. 2014;134:537–44.
Miura T, Nishinaka T, Terada T, et al. Relationship between aging and dosage of warfarin: the current status of warfarin anticoagulant therapy for Japanese outpatients in a department of cardiovascular medicine. J Cardiol. 2009;53:355–60.
Funding
This study was supported by the Fundamental Research Program of Shanxi Province (20210302124649) and WU JIEPING Medical Foundation (320.6750.2021-08-12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, W., Zhang, Z., Guan, Z. et al. Developing Chinese race-specific warfarin dose prediction algorithms. Int J Clin Pharm 45, 731–738 (2023). https://doi.org/10.1007/s11096-023-01565-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01565-1