Abstract
Tertiary lymphoid structures (TLSs) are defined as lymphoid aggregates formed in non-hematopoietic organs under pathological conditions. Similar to secondary lymphoid organs (SLOs), the formation of TLSs relies on the interaction between lymphoid tissue inducer (LTi) cells and lymphoid tissue organizer (LTo) cells, involving multiple cytokines. Heterogeneity is a distinguishing feature of TLSs, which may lead to differences in their functions. Growing evidence suggests that TLSs are associated with various diseases, such as cancers, autoimmune diseases, transplant rejection, chronic inflammation, infection, and even ageing. However, the detailed mechanisms behind these clinical associations are not yet fully understood. The mechanisms by which TLS maturation and localization affect immune function are also unclear. Therefore, it is necessary to enhance the understanding of TLS development and function at the cellular and molecular level, which may allow us to utilize them to improve the immune microenvironment. In this review, we delve into the composition, formation mechanism, associations with diseases, and potential therapeutic applications of TLSs. Furthermore, we discuss the therapeutic implications of TLSs, such as their role as markers of therapeutic response and prognosis. Finally, we summarize various methods for detecting and targeting TLSs. Overall, we provide a comprehensive understanding of TLSs and aim to develop more effective therapeutic strategies.
Similar content being viewed by others
Introduction
Tertiary lymphoid structures (TLSs) are organized, non-encapsulated aggregates of lymphoid cells that form in non-lymphoid tissues under pathological conditions after birth.1,2 TLSs mainly include germinal centers (GCs), surrounding T-cell areas, and distributed PANd+ high endothelial venules (HEVs).3,4,5 TLSs typically form in response to chronic inflammation, such as infections, autoimmune diseases, tissue transplants, and cancers.6,7,8,9,10,11,12 Recent studies have found a positive correlation between the presence of TLSs and the efficacy of immune checkpoint blockade (ICB) therapy. However, the underlying mechanism remains unclear, and only a minority of patients benefit from this therapy.6,13,14 Moreover, the prognostic value of TLSs has been widely discussed. In conditions that require an enhanced immune response, such as cancer and infectious diseases, the presence and maturation of TLSs generally indicate more favorable outcomes.6,15,16,17,18,19 Conversely, in autoimmune diseases and age-related chronic inflammatory conditions, which involve the immune system attacking self-tissues, the emergence and maturation of TLSs are often associated with poorer prognoses.20,21,22 Therefore, TLSs can be seen as a double-edged sword. To fully harness the potential of TLSs, a comprehensive understanding of TLSs is urgently needed.
In 1964, Ziff noticed similarities between inflamed rheumatoid synovial tissue and lymph nodes, where the primary immune response occurs.23,24 In the early 1970s, Söderström et al. found that the structure of thyroid tissue in patients with Hashimoto’s thyroiditis resembled that of lymph nodes.24,25 In 1992, Louis Picker and Eugene Butcher formally introduced the concept of “tertiary lymphoid organs” (TLOs) or “tertiary lymphoid tissues” (TLTs). At that time, it was believed that TLOs could occur in all tissues of the body, except primary lymphoid organs (bone marrow and thymus) and secondary lymphoid organs (lymph nodes, spleen, and gut-associated lymphoid tissue). TLOs were considered sites where memory lymphocytes and effector precursor cells could be re-stimulated by antigens, or where B cells and T cells could carry out terminal responses. However, they believed that TLOs are an unorganized structures composed of a small number of lymphocytes. If long-term stimulation leads to the formation of lymph node-like structures in TLOs, they have transformed into secondary lymphoid structures.9,26 In 1996, Kratz et al. demonstrated that lymphotoxin could induce the formation of lymphoid tissues in chronic inflammatory conditions. This is similar to the induction signals for lymphoid organogenesis during embryonic development. Thus, they termed the de novo formation process of organized lymphoid tissue “lymphoid neogenesis” in chronic inflammation.27,28 In 1998, Wagner et al. proposed the concept of “ectopic lymphoid tissues” (ELTs), because patients with rheumatoid synovitis had follicle-like structures in their synovial tissues that resembled secondary lymphoid follicles. They also clarified the cellular components required for GC formation within it.29 In 2001, Seisuke et al. discovered GCs in rheumatoid synovitis with the same morphology and function as lymph node GCs. Thus, they proposed the term “tertiary lymphoid structures” (TLSs).30 In 2015, Jin et al. described the characteristics of TLSs in breast cancer.31 Some studies also refer to TLSs as ectopic lymphoid organs or ectopic lymphoid structures.32,33 In the first decade of the 21st century, research on TLSs mainly focused on chronic inflammation, autoimmune diseases, and immune rejection after organ transplantation. In 2008, TLSs were reported to be associated with a better prognosis in non-small cell lung cancer, marking the first discovery of TLSs in tumors.34 ICB has been a focal point in tumor treatment, and in 2015, Giraldo et al. first linked the presence of TLSs with the efficacy of ICB therapy.35 Subsequent research further confirmed that the presence of TLSs can predict the positive effects of ICB treatment.36,37,38 Since 2020, this topic has received wider attention and has become a hot topic in the field of TLS research.6,12,39 However, our understanding of TLSs is still very limited. (Fig. 1).
Milestone events of the discovery and development of TLSs. Key milestones in tertiary lymphoid structures are indicated. In this figure, we summarize the concept of Tertiary Lymphoid Structures (TLSs), their discovery process, and the progress in research. TLSs are aggregates of lymphoid cells that form in non-lymphoid tissues under pathological conditions. The concept was formally introduced in 1992, and the term “TLSs” was coined in 2001. Over the past decade, TLSs have gained significant attention and have been widely studied. By Figdraw
In this review, we focused on the composition and cascade regulatory mechanisms of TLSs. Furthermore, we summarize the influence of TLSs on common diseases and ageing, especially primary and metastatic tumors. Based on these knowledge, we discuss the potential application of TLSs as predictors of prognosis and targets for immunetherapy. Finally, we explore the future research directions and challenges in the study of TLSs, aiming to provide a comprehensive and in-depth understanding of their scientific significance and clinical implications.
The cellular composition of TLS
Stromal cell
Stromal cells, including fibroblasts, endothelial cells, and follicular dendritic cells (FDCs), are vital for the formation of TLSs. They create a conducive microenvironment for TLS formation, promoting the accumulation and functional activation of immune cells. (Fig. 2).
The composition of TLS. TLSs mainly include T cells, B cells, fibroblasts, DCs, macrophages, FDCs, and HEV-EC. Stromal cells undergo phenotypic polarization in the TME, and they produce cytokines and chemokines, which induce aggregation and differentiation of immune cells and promote the formation of TLSs. TLSs have dual effects of pro-tumor and anti-tumor roles, which depend on the internal immune cell subtype
Fibroblast
Fibroblasts provide a space for TLS formation. They promote immune cell survival and aggregation in TLSs through inflammatory cytokines and chemokines, such as B cell activating factor (BAFF), transforming growth factor-β (TGF-β), IL-7, C-X-C motif ligand (CXCL) 13, CXCL12, C-C motif chemokine ligand (CCL) 19, and CCL21.9,40 In primary Sjӧgren’s syndrome, podoplanin+fibroblast activation protein 1+ (PDPN+FAP+) fibroblasts express platelet-derived growth factor receptor (PDGFR) α, PDGFRβ, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), mucosal vascular address-seeking protein cell adhesion molecule 1 (MadCAM), and receptor activator for NF-κB ligand (RANK-L) in TLSs.41 Chen et al. also identified two types of fibroblasts in bladder urothelial carcinoma: PDGFRα+ fibroblasts and regulator of G protein signaling 5+ (RGS5+) fibroblasts. The former express multiple cytokines and chemokines, including CXCL12, IL-6, CXCL14, CXCL1, and CXCL2, increasing immune cell infiltration.42 Additionally, Thy1+FAP+ fibroblasts significantly increased in melanoma, and their density in TLSs was higher than tumor parenchyma.43 Notably, fibroblasts from different sites or diseases appear to exhibit different functions in lymphocyte recruitment,10 probably reflecting differences in the activation signals or origination. Overall, the cross-talk between fibroblasts and immune cells leads to the production of cytokines and chemokines, creating favorable conditions for TLS formation.
Follicular dendritic cell
The presence of FDCs in GC is a marker of mature TLSs.44 They originate from local mesenchymal cells rather than hematopoietic cells, such as perivascular mural cell precursors and marginal reticular precursors.45 Additionally, FDCs seem to constitute a specialized subset of myofibroblasts, which derive from bone marrow stromal cell progenitor, expressing αSMA.46 FDCs interact with lymphoid tissue inducer (LTi) cells (especially B cells) and other stromal cells, promoting their early stages of development.47 The lymphotoxin β receptor (LTβR) initiates and maintains FDCs, and then tumor necrosis factor receptor (TNFR) 1 signaling further matures them. FDCs act as antigen-presenting cells (APCs) and are an essential component of the GC response.48 They express VCAM-1 and ICAM-1, complement and FcR (a receptor mediating antigen-antibody responses), delivering natural antigens to B cells by attaching antigens to the synapses (typically antigens captured by complement or antibodies).49,50 Moreover, FDCs promote GC formation and TLS mature by secreting cytokines and chemokines, such as CXCL12, CXCL13, IL-6, IL-7, BAFF, and TNF-α.45 In TLSs within giant cell arteritis, FDCs coexist with T cells and B cells, accompanied by high expression of CXCL13, CXCR5, CCL21, CCR7, LTβ, and BAFF.51 And deleting FDCs reduced B cells and impaired GC formation.52
B lymphocyte subsets
Different B cell subsets are associated with TLSs, including plasma cells (PCs), regulatory B (Breg) cells and memory B cells.53 They have been reported in many diseases, such as tumors,44,54 autoimmunity diseases,55,56 and organ transplantation. Thus, we will review these B cell subsets in TLSs and hope to further understand the function of TLSs. (Fig. 2).
Plasma cell
HPV-specific B cells and HPV-specific IgG antibodies were observed in HPV+ head and neck squamous cell carcinoma (HNSCC), especially in the B-cell-rich TLShigh HNSCC.57,58 Notably, peripheral blood lacks HPV-E antigen-specific IgG+CD27+CD38+ PCs, suggesting the humoral immune response is localized rather than systemic.58 Germain et al. also reported that PCs produced high levels of IgG and IgA, such as LAGE-1, MAGE family antigens, P53, and NY-ESO-1.59 Consistent with HPV+ HNSCC, immunoglobulin-producing PCs arise from B cells after being activated by antigen within TLSs in non-small cell lung cancer (NSCLC), rather than from peripheral lymphatic organs.59 Moreover, in high-grade serous ovarian cancer (HGSOC) patients, IgG recognizes tumor-associated antigens. The level of IgG3 significantly increases and the level of IgG4 decreases post-chemotherapy in stage 3/4 tumors.60 Additionally, IgM, IgE, and IgD deposit in TME.60,61 Besides, in the transplanted heart with TLSs, IgM- and IgG-producing PCs increase the possibility of coronary artery damage.62 It was also observed in Sjögren syndrome.63 Importantly, CD138+ PCs around the follicle produce immune reactivity to citrullinated antigens (an antigen determinant for rheumatoid arthritis) by secreting ACPA IgG. Human AID+GC+ synovial tissue generated ACPA IgG after it was transplanted into the RA/severe combined immunodeficiency chimera model,64 indicating that TLSs support antibody production independently of B cells in the peripheral lymph circulation.
Regulatory B cell
Breg cells produce TGF-β and/or IL-10, inhibiting the immune response.65 While there is currently insufficient evidence to support the existence of Breg cells within TLSs. Given the crosstalk between immune cells and the pro-tumor role of TLSs, we propose that Breg cells may not enter TLSs internal but rather surround them to regulate the formation and function of TLSs. In melanoma, they express pro-inflammatory cytokines, such as TNF-α and IL-6, which are linked to poor clinical outcomes in patients receiving Ipilimumab treatment.66 To support immunosuppressive TEM, Breg cells stimulate regulatory T (Treg) cell differentiation, leading to CD8+ T cell exhaustion.67 In ovarian cancer patients, IL-10+ Breg cells preferentially enrich in ascites, which are positively correlated with CD4+FOXP3+ T cell frequency and negatively correlated with IFN-γ+CD8+ T cell frequency.68 They also generated TGF-β polarizing CD4+ T cells into Treg cells.69 Additionally, Breg cells also secrete IL-35, directly suppressing CD8+ T cells.70 Notably, Breg cells regulate Treg cell function by intercellular contact, decreasing the proliferation of CD4+ T cells while reducing forkhead box protein P3 (FOXP3) and cytotoxic T lymphocyte-associated antigen 4 (CTLA4) expression in Treg cells.71 Moreover, TGF-β stimulates macrophages to shift towards the immunosuppressed M2 phenotype,72 or induces dendritic cells (DCs) to overexpress IL-4 and decrease IL-12, altering the Th1/Th2 balance.73 In non-cancerous diseases, such as organ transplantation, autoimmune diseases, and skin healing, Breg cells are often associated with immune tolerance by inflammatory molecules, such as IL-10, IL-35, and TGF-β.74 Immunotolerant patients typically exhibit a decrease in PCs and specific antibody titers, along with an increase in immature B cells and IL-10+ Breg cells.75 In allogeneic kidney grafts, the expression of Breg cell markers such as CD5, CD24a, CD38, Cr2, Fcer2a, IL-10, and Havcr1 increased.76 Nevertheless, since precise markers of Breg cells are still up for debate, their detection and role in TLSs remain to be further studied.
Memory B cell
Multiple memory B cell phenotypes have been detected in TLSs.6 Such as IgG1+ memory B lymphocytes were enriched in pancreatic cancer, showing a positive correlation with TLS density.77 Similarly, a group of memory B cells, including IgG+ switched memory B cells (IgG+CD38-IgD-CD27+), IgG- switched memory B cells (IgG-CD38-IgD-CD27+), and activated switched memory (CD38-IgD-CD27+CD21-), was identified in HNSCC.16 Notably, both unswitched and switched CD19+CD27+IgD+/- memory B cells were detected in TLSs and in peripheral blood,6 contradicting the previous report that B cell immune response is limited to TLSs. Additionally, it is reported that TLS density and CD27+CD19+ memory B cell numbers increase after ICB treatment. IgM+ memory B cells serve as indicators of patient response to Nivolumab monotherapy in melanoma.78 In breast cancer, the atlas of infiltrated B cells revealed a higher proportion of B cells within tumor-associated TLSs, predominantly memory B cells, compared to the naive B cells in peripheral blood. Additionally, TLS contains a greater proportion of B cell clones in TLSs, as well as somatic hypermutation (SHM). Moreover, memory B cells and PCs share VDJ sequences, indicating a common clonal origin.61
Although detecting memory B cells in TLSs remains challenging, Sdc1+Ighg+ memory B cells were also observed in kidney transplantation. They result in immune downregulation, possibly due to their failure to develop into plasma cells.76 Moreover, memory B cells were found in close proximity to CD8+ T cells in TLS adjacent tumors,79,80 which raises a question about the antigen-presenting ability of B cells. Wennhold et al. reported that CD21-CD86+ B cells accumulated in many tumors with TLSs, including HNSCC, NSCLC, hepatocellular carcinoma (HCC), renal cell carcinoma (RCC), esophagogastric adenocarcinoma, testicular germ cell carcinoma, ovarian cancer, urothelial carcinoma, and colorectal cancer, demonstrating a significant correlation between CD8+ T cells and CD21-CD86+ antigen-presenting B cells.81 CD21−CD86+ B cells trigger an IFN-γ response in CD3+ T cells in in vitro studies.81 Furthermore, non-typical CD27− memory B cells expressed APC markers, such as major histocompatibility complex (MHC) I, MHC-II, CD40, CD80, and CD86, and colocalized with CD8+ T cells in TLSs.82 Similarly, CD21lowCD86+IgD+CD27+ memory B cell subsets highly express MHC-I and MHC-II in rheumatoid arthritis, indicating a robust antigen presentation capacity.83
Naive B cell
Naive B cells are notably scarce in certain types of tumors, such as breast cancer,84 NSCLC,85 and melanoma.86 Nonetheless, the presence of CD38−IgD+CD27− naive B cells is related to the GC response within TLSs.16 Currently, it remains unclear why there is a difference in naive B cells within TLSs across various tumors, necessitating exploration of naive B cell function and development within TLSs. For example, IgD+ naive B cells are reduced in pancreatic cancer, with an increase in mature IgM+ B cells and IgG1+ memory B cells.77 This suggests that naive B cells in tumor sites undergo isotype class switching after antigenic stimulation.
T lymphocyte subsets
It has been discovered that T lymphocyte subsets correlate with TLSs in many diseases,87 such as tumors, autoimmune disorders, infections, and organ transplantation.17 However, the presence of T lymphocyte subset infiltration does not always indicate a good prognosis. Here, we will review the T cells within TLSs, hoping for a further understanding of the function of TLSs. (Fig. 2).
Tissue-resident memory T cell and exhausted T cell
Tissue-resident memory T (TRM) cells are a special T cell subset. They are perpetually present in peripheral immune tissue and do not participate in lymphatic circulation.88 TRM cells and TLSs are associated with a favorable prognosis for tumor patients.89 They express the typical marker CD103 and the activating marker CD69, but not CCR7, CD62L, or S1PR1, which are required for migration out of tissue.90 In stage III lung adenocarcinoma, the proportion of TRM cells was higher within TLSs, especially in mature TLSs, which is positively correlated with patient survival.91 CD103+ T cells penetrated the epithelial regions, tumor stroma, and TLSs in gastric cancer tissues. Even in patients with advanced gastric cancer, the infiltration of CD103+ TRM cells was also associated with a good prognosis.89 Moreover, tumors also contain non-tumor-specific TRM cells, which activate the immune system through the bystander effect.92 Nonetheless, TRM cells in progressive tumors frequently indicate immunosuppressive TME since there is an overlap between exhausted T cells and TRM cells in TLSs.93 TCR data analysis in NSCLC reveals that CD103+CD8+ TRM cells express exhausted markers, such as programmed death 1 (PD-1), T cell immunoglobulin domain and mucin domain-3 (TIM-3), and CD39.94,95 In addition to the inhibitory molecules, CD103+ T cells also express granzyme (GZM) B.96 This suggests that TRM cells serve as the source of exhausted T cells, transitioning from an activated state to terminally exhausted T cells without completely losing their function. Compared to CD103-CD8+ T cells, CD103+CD8+ T cells express higher levels of PD-1, GZMB, and IFN-γ in TLSs.89 Consequently, they are a predictive marker for the effectiveness of ICB treatment in gastric cancer.97
CD103+ exhausted T cells appear to be the source of CXCL13, a crucial chemokine in TLS formation.98 In epithelial ovarian cancer, CD8/CD103/TIM3-expressing lymphocytes secreted CXCL13,99 and the density of CD39+CD103+ T cells with high CXCL13 was linked to better relapse-free survival (RFS) at five years.100 CD103+ T cells mediate B cell recruitment and TLS formation by CXCL13 under the regulation of TGF-β.96 Transcription factor 7+ (TCF7+) T cells are located in TLSs, and their abundance correlates with a favorable prognosis in oral squamous cell carcinoma (OSCC).101,102 Recent studies show that over 80% of CD8+ T cells in mouse models of melanoma, lung cancer, colon cancer, and prostate adenocarcinoma express PD-1, which contains TCF1+ T cells.103 The transcription factor T cell factor 1 (TCF1, encoded by TCF7) contributes to memory T cell differentiation by the Wnt signaling pathway and response to ICB therapy,104 alleviating the exhausted state of T cells.105 Moreover, TCF1+ cells exhibit stem cell-like characteristics similar to memory T cells and are close to the blood vessel.106 In autoimmune vasculitis, TCF1+CD4+ T cell populations are present in perivascular TLSs and exhibit a high proliferative potential.107 In chronically lymphocytic choriomeningitis virus (LCMV)-infected mice, TCF1+PD-1+ T cells infiltrated more intensely and exhibited stem cell-like and CXCR5 expression.108 Siddiqui I et al. confirmed that tumor-specific TCF1+PD-1+CD8+ T cells promoted the ICB therapy after applying FTY720 to block the influx of peripheral T cells.109
Follicular helper T cell
Follicular helper T (Tfh) cells are situated in the B-cell region of TLSs.110 Differentiation of Tfh cell is distinct from other CD4+ T helper T subsets, which is initiated by the co-stimulatory molecules. Inducible co-stimulator (ICOS) inhibits Klf2 to increase BCL6 expression, promoting Tfh cell differentiation.111 CD28 upregulates extracellular regulated protein kinases 2 (ERK2) to suppress BCL6, but this suppression is counteracted by Zfp831, activated by ICOS.112 Moreover, TGF-β suppressed SATB1 expression through ICOS de-repression, reducing T follicular regulatory (Tfr) cells and triggering TLS formation.113 B lymphocyte-induced maturation protein-1 (Blimp1), an important transcriptional repressor, mediates the differentiation of immune cells.114 It drives naive CD4+ T cells to turn into CD4+ T help cells, excluding Tfh cells.115 BCL6 and Blimp1 are inter-antagonistic; the repressive activity of Blimp1 is antagonized by BCL6 (via repressing Prdm1, the gene encoding Blimp1), promoting the differentiation of Tfh cells.116,117 Additionally, ICOS promotes nuclear NFAT2 translocation and increases CXCR5 expression,118 while CXCL13 attracts CXCR5+ Tfh cells to migrate to GC.119 Overacre-Delgoffe et al. reported that intestinal microbes suppressed the progression of tumors through Tfh cells, driving TLS development. The colonization of Helicobacter hepaticus increased immune cell infiltration, especially CD4+ Tfh cells with TLS formation.120 Tfh cells produce cytokines, such as IL-4 and IL-21, promoting the GC response, immunoglobulin switch, and somatic hypermutation (SHM).121,122,123 Besides, cytotoxic Tfh cells express GZMK within TLSs in IgG-4-related diseases, indicating their direct involvement in anti-tumor effects.124 In contrast, a recent study showed that the number of TLSs in stage II colorectal cancer (CRC) was less than in stage III CRC. The density of Tfh cells was higher in patients with disease recurrence than in those without recurrence.125 Moreover, increased infiltration of Tfh cells after ICB therapy in tumors supports the notion that Tfh “dysfunction” may be attributed to PD-1 expression.14,126,127,128
Regulatory T cell
Treg cells are characterized by high expression of CD25 and the transcription factor FOXP3.129 They inhibit T cell activation by producing inhibitory cytokines, including IL-10, IL-35, and TGF-β, or by expressing CTLA4 that competes with CD28 to combine with CD80/86.130,131,132 In mouse lung cancer models, Treg cells increased in TLSs around blood vessels and are associated with TLSs in tumor stroma, leading to a poor prognosis for patients. In support of this, the proliferation of CD4+ T cells and CD8+ T cells was reactivated in TLSs after deleting Treg cells by diphtheria toxin, causing enhanced tumor destruction.133 In a mouse lung adenocarcinoma model, the depletion of Treg cells increases CD4+ T cell and CD8+ T cell infiltration, especially in TLSs. This also increases the co-stimulatory molecule levels of DCs in TLSs.134,135 Additionally, Treg cells appear to promote TLS formation, although the mechanism may be indirect.136 In the skin TLSs of patients with pemphigus, Treg cells directly contact Th1-like CD4+ T cells, which promotes CXCL13 production.137
Tfr cells, a unique subset of Treg, have a dual phenotype of Treg cells and Tfh cells. They express FOXP3 as well as BCL6 and the chemokine receptor CXCR5, allowing them to migrate to the GC.138 Based on TCR sequencing, Tfr cells and Treg cells are related in development, meaning that Tfr cells may originate from Treg cells.139 The differentiation process of Tfr cells highly overlaps with that of Tfh cells and also relies on BCL6 expression, which is regulated by CD28 and ICOS.111,140,141 However, compared to Tfh cells, Tfr cells express BCL6 and Blimp1 simultaneously. Hence, Tfr cell differentiation might result from a balance between two opposing-effect cytokines, as their quantity and function are influenced by BCL6 or Blimp1 ablation.142,143 In breast cancer, Tfr cells inhibit B cell proliferation and antibody generation by IL-10.144 The number of CXCR5+FOXP3+ Tfr cells in the peripheral blood was higher in early-stage BC patients than in advanced-stage, implicating their involvement in tumor progression and invasion.145 Moreover, Tfr cells exhibit a low response to anti-PD-1 therapy. Conversely, anti-CTLA4 therapy or anti-PD-1 therapy after treatment with anti-CTLA-4 achieves desirable therapeutic results,139 possibly due to their high expression of CTLA-4.146
Th17 cell
Th17 cells constitute a crucial component of the T-cell subset in TLSs, and their cytokines contribute to TLS development in chronic inflammatory tissues.147 Th17 cells express IL-17 in the nascent lymphoid tissues of renal grafts, promoting immune rejection.148 IL-17A also induces iBALT formation in mice with lipopolysaccharide-induced pneumonia.149 While IL-22, another effector cytokine expressed by Th17 cells, lacks direct evidence for TLS formation. In IL-23R-/- T cells and Rag1-/- mice, IL-23 drives T cell proliferation and Th17 cell accumulation.150 Notably, since naive CD4+ T cells lack the IL-23 receptor, IL-23 does not seem to drive Th17 development, while it promotes the stability and survival of Th17 cells.151 In esophageal squamous cell carcinoma (ESCC), patients with a promising prognosis had mature TLSs, which are characterized by a high ratio of proliferative B cells and CD4+ T cells, including memory B cells and Th17 cells.152 However, Th17 cells have also been implicated in facilitating breast cancer progression.153 Considering the diversity of Th17 cell functions, their differentiation requires further exploration in order to precisely characterize Th17 cell function. Retinoic acid receptor-related orphan receptor γt (RORγt) and signal transducer and activator of transcription-3 (STAT3) both promote the development of Th17 cells.154 The pro-inflammatory factors TGF-β and IL-6 activate RORγt, initiating a cascade of their differentiation.155,156 Th17 cells and Treg cells also maintain balance in the differentiation process. FOXP3 negatively regulates RORγt, inducing Treg cell differentiation, and down-regulates Th17 cells through STAT6.157 However, the role of IL-21 in Th17 cell differentiation remains controversial, as inconsistent effects of IL-21 on Th17 cells have been found in autoimmune encephalomyelitis and Crohn’s disease.158,159 We suggest that this is probably because Th17 cell differentiation is regulated by multiple cytokines. More importantly, IL-21 and IL-6 appear to rely on the same signaling pathway to regulate Th17 cell differentiation. Therefore, it is not sufficient to regulate downstream transcription factors within this pathway alone to demonstrate the role of IL-21 itself.
Macrophage
Macrophages, particularly pro-inflammatory M1 macrophages, promote TLS formation by producing inflammatory cytokines. (Fig. 2) In atherosclerosis, M1 macrophages act as LTi cells, secreting TNF-α and LTα, triggering the expression of the chemokines CCL19, CCL20, and CXCL16, which induce TLSs in the aortic wall.160 A week after infection in the mouse model of Chlamydia pneumoniae (CP), M1 macrophages predominated and contributed to the development of iBALT.161 And TLShigh tumors exhibit a higher prevalence of M1 macrophages, while TLSlow tumors tend to have more M0 and M2 macrophages.162 The infiltration of M1 macrophages suggested a favorable prognosis. However, M1 macrophages may gradually switch to the M2 macrophages in iBALT. Adoptively transferring M2 macrophages to CP-infected mice fails to observe iBALT.161 Moreover, CX3CR1+ macrophages were detected in TLSs, promoting B cell infiltration and IgA responses through TGFβ1, CXCL13, and BAFF, presenting antigen during infectious Salmonella colitis.163 Recently, Gunnarsdottir FB et al. have identified the CD169+ tissue-resident macrophages in TLSs and have also been proven to produce type I IFN in vitro. Type I IFN stimulates monocyte CD169 expression, which inhibits T cells and NK cells through M2 macrophage-like effects. They secrete prostaglandin E2 (PGE2), reactive oxygen species (ROS), and IL-10 and also contribute to the secretion of antibodies and IL-6 by activated B cells.164 In breast cancer, CD169, or TLS, is associated with decreased odds of surviving for 5 years.165 Furthermore, TIM4 expression in FOLR2+ resident macrophages was positively related to prognosis. Notably, TIM4+ macrophages in TLS− tumors express IL-10 and TGF-β, promoting immunosuppressive function, while those in TLS+ tumors enhance the prognosis of patients.166 In addition, the high expression of PD-L1 within TLSs is mainly from macrophages.167 Therefore, inhibition of macrophages or treatment with immune checkpoint blockers significantly enhances T cell and B cell infiltration and promotes tumor regression.168
Dendritic cell
DCs are leukocytes derived from bone marrow. They can uptake, process, and present antigens to T cells.169 The infiltration of mature dendritic cells (DCs), T cells, and B cells in TLSs correlates with long-term survival in various cancers, such as lung cancer,170 breast cancer,171 high-grade serous carcinoma (HGSC),172 and OSCC.173 Marinkovic T et al. revealed that T cells and CD11c+ DCs are recruited to the thyroid gland via CXCL21-CCR7, inducing TLSs in an LTβ signaling-dependent manner.174 In diffuse gastric cancer, DC-LAMP+ DCs are present in TLSs, and CCL19-CCR7 signaling is crucial for DC homeostasis.175 Mature DCs penetrated peribronchially and enhanced lymphocyte aggregation on day 4 after applying murine γ-herpesvirus MHV-68, with organized iBALT observed on day 8.176 Further study also confirms the role of DC in promoting TLS formation: bone marrow-derived cells were transferred to the lungs after co-cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF), leading to iBALT development;177 and deletion of CD11c+ DCs in the diphtheria toxin receptor (DTR) transgenic model results in the absence of iBALT.174 Furthermore, cDC2 expressed CXCR5 and co-located with Tfh cells in the T-cell zone of TLSs, polarizing naive CD4+ T cells to Tfh cells via ICOS signaling in GOLD stage IV chronic obstructive pulmonary disease (COPD).178 Meanwhile, DCs are recruited by respiratory bacteria via CCR2 into SIgA-deficient airways, leading to local disruption of the mucosal barrier and lymphocyte accumulation.179 Recently, Song-Yang Wu et al. reported that CCL19+ DCs acted as LTi cells in TLS formation and promoted ICB therapy in triple negative breast cancer. They were associated with high levels of CXCL13+ T cells and enhanced the efficacy of anti-PD-1 therapy.171 Furthermore, in HGSOC, DC-LAMP and CD20 displayed a significant correlation, increasing the levels of the immunocytotoxicity markers GZMA and perforin (PRF1) transcripts.60 It suggests that they modulate B cell responses. Additionally, plasmacytoid dendritic cells (pDCs) have been identified as a novel component of the T-cell zone of TLSs. In CRC, pDCs expressed IRF7 and were located close to CD8+ T cells, suggesting that they may enhance anti-tumor immunity through secretion of type I IFN and stimulation of CD8+ T cells.180 Interestingly, IRF7+ pDCs preferentially reside around CD4+ T cells during TLS development. In another study of breast cancer, pDCs inhibited the proliferation of FOXP3+ Treg cells by IFN-α, providing evidence for pDCs regular CD4+ T cell function.181 (Fig. 2).
Natural killer cell
NK cells do not require antigenic activation, and the receptors expressed on their surface often represent changes in their function. However, the relationship between NK cells and TLSs remains inadequately explored. A recent study on TLSs in OSCC showed that high-grade TLSs coincided with the infiltration of CD57+ NK cells.173 Furthermore, NKp30+ NK cells increased in Sjogren syndrome with TLSs, activating B cells to produce antibodies (IgG, IgM, and IgA).182 Circulating NK cells were recruited to salivary glands before TLS appearance, upregulating NKp46 expression and producing granzyme B and IFN-γ.183 Additionally, NK cells act as LTi cells and express IL-22 to induce chemokine production (CXCL12, CXCL13) in stromal cells.184
Innate lymphoid cell
Innate lymphoid cells (ILCs) include three main types: ILC1, ILC2, and ILC3. Among these, ILC3 contributes to the formation and/or maintenance of TLSs. The development of ILC3 depends on RORγt and can be considered as LTi cells. Ikeda A et al. noted a correlation between the abundance of NKp44+ ILC3 and the density of TLSs in CRC. Their numbers gradually decline as the cancer develops. However, NKp44+ ILC3 is reactivated in advanced CRC under exogenous stimulation, leading to the upregulation of LTα, LTβ, and TNF-α.185 This indicated that infiltration of NKp44+ ILC3 may contribute to a microenvironment rich in lymphocytes. In NSCLC, NCR+ ILC3 increases in proximity to TLSs, producing IL-22 in response to IL-23 stimulation.186 Another study reported that IL-21 increases IL-22 and Tbet production by ILC3 while decreasing IL-17 expression, thereby exerting a protective role in intestinal inflammation.187 Altogether, the available evidence suggests that ILC3 acts as LTi cells involved in TLS formation, which can be activated by diverse stimuli to produce multiple effectors.
Neutrophil
The ratio of peripheral blood neutrophils and lymphocytes, along with TLSs, was described as a tumor prognostic marker in patients with HCC and uterine leiomyosarcoma.188,189,190 Neutrophils can also be found within TLSs in omentum metastases of HGSOC and prostate cancer.60,191 However, their function has not been characterized. A recent study revealed that the number of CD15+ neutrophils correlates with TLS density. TLS- tumors exhibited better overall survival and disease-free survival compared to TLS+ tumors in peritumoral tissue.192 In fact, neutrophils express BAFF, IL-21, IL-17, and MHC, providing activation signals for B cells and T cells.193,194,195 Therefore, we believe that the role of neutrophils in TLSs deserves further investigation.
Cytokines involved in TLS formation
The cytokines and chemokines have been demonstrated to play a crucial role in the development of TLSs in mouse models. These molecules induce TLSs with distinct characteristics, consequently increasing the number and activity of lymphocytes within the TME (Table 1).
Interleukins
Interleukins are cytokines that mediate interactions between leukocytes and/or lymphocytes, activating and regulating immune cells, playing an important role in inflammatory responses. Furthermore, it acts as a signaling molecule to regulate TLS neogenesis.
IL-1 family
The IL-1 family includes 11 members of the cytokines and shares similar functions with Toll-like receptor (TLR) family. IL-1β accelerates the early inflammatory process and initiates kidney-specific TLS formation in lupus nephritis.196 In mice infected with the Influenza A virus (IAV), IL-1α administration increases the number of iBALT in the lung. However, IL-1r−/− mice, despite surviving long-term post-IAV infection, show no iBALT formation due to the IL-1 signaling pathway blockade, and CXCL13 expression is also reduced.197 Furthermore, an over-three-fold increase in IL-1β and IL-1α was observed on day 7 following intranasal instillation of cSiO2, leading to TLS development after 21 days in the lungs.198 Weinstein AM et al. reported that IL-36γ is predominantly expressed by M1 macrophages and endothelial cells (ECs) in colon cancer mouse models, including SMA+ smooth muscle cells and PNAd+ HEVs. Their expression increases the density of CD4+ T cells, CD20+ B cells, and PDPN+ fibroblasts in TLSs, supporting a favorable prognosis.199
IL-6 family
The IL-6 family includes IL-6, IL-11, oncostatin-M (OSM), and IL-27.200 OSM-expressing adenoviral vectors to activate B cells, leading to iBALT formation in mice’s lungs.201 In IL-6 and IL-6R double transgenic mice, peribronchial lymphocyte aggregation was observed, accompanied by the upregulation of CXCL13 and the GC response.202 However, IL-6 may indirectly induce TLS formation by promoting Th17 cell differentiation.203 In lupus nephritis, IL-6-producing mesenchymal stem cells promote CD4+ T cell proliferation and differentiate into Th17 cells in contact form.196 B cells also secreted IL-6, enhancing Th17 cell proliferation and polarizing Th17 cells to PDPN+ Th17 cells with the collaboration of CD4+ Tfh cells.204 Additionally, IL-6 was also reported to facilitate Tfh cell differentiation during chronic viral infection.205 IL-17 and STAT3 are highly expressed within TLSs in an experimental model of gastric cancer. However, it appears to promote gastric cancer progress.206 Notably, IL-27 is the only subtype of the IL-6 family that is reported to inhibit TLS formation by suppressing Th17 differentiation.207
IL-7
IL-7 plays a crucial role in all TLS development stages, including initiation, expansion, and maturation.208 Stromal cells secrete IL-7, which promotes the production of lymphotoxin α1β2 (LTα1β2) by lymphocytes. In viral-induced salivary gland inflammation, IL-7 and LTα1β2 synergistically promoted HEV establishment by inducing vascular endothelial growth factor C (VEGF-C) in stromal cells. IL-7 supports early lymph vascular remodeling, whereas LTα1β2 regulates the complex HEV networks.209,210 Notably, the expression of IL-7 significantly increases and precedes lymphatic vessel expansion in submandibular gland inflammation. Lymphatic endothelial cells (LECs) specifically express IL-7Ra, implying that fibroblast-derived IL-7 appears to support HEV development in a paracrine manner prior to LTα1β2.209,211 In addition, FDCs supported B cell survival, proliferation, and maturation by secreting IL-7 in GC.212 In the T-cell zone of TLSs, fibroblastic reticular cells (FRCs) secrete IL-7 to promote the survival of central memory T cells, thereby maintaining T cell homeostasis.213 Thy1 IL-7+ LECs generate IL-7, supporting the maintenance of memory Th2 cells in iBALT by inducing antiapoptotic protein BCl2 expression.214
IL-13 and IL-22
IL-13 is an indispensable cytokine for fibroblast activation, initiating TLS formation. The primary sources of IL-13 and IL-22 include T cells, ILCs, and NK cells.184,215,216 Upon stimulation by IL-13, fibroblasts switch to PDPN+FAP+ immunofibroblasts, accompanied by the upregulation of ICAM-1 and VCAM-1, which increase chemokine production.41 While direct evidence linking Th17-derived IL-22 to TLS formation is lacking, research suggests a positive correlation between IL-22+ ILC3 and TLSs.217 In primary Sjögren’s syndrome (pSS), the introduction of exogenous IL-22 resulted in PDPN+ FAP+ fibroblast expansion within the pathological parotid gland.41 And the function of FRCs obtained from IL-22−/− mice is impaired, affecting chemokine production and showing defects in TLS formation in vivo.184
IL-17
IL-17 plays a key role in the formation of TLSs.218 Th17 cells secrete IL-17, which promotes FRC proliferation, maintains the FRC network, and induces the secretion of CXCL13 and CCL19.219 In IgG nephropathy, blocking IL-17A significantly impaired TLS formation, which decreased kidney damage.219 It can also be observed that IL-17 promotes TLS formation in the inducible bronchus-associated lymphoid tissue (iBALT).220 However, Fleige H. and colleagues found that IL-17 is not a determinant of iBALT development. They successfully induced normal iBALT in IL-17A and IL-17F double-deficient mice with the cowpox virus.221 Similarly, cowpox virus-induced organized iBALT was also observed in IL-17-deficient mice.222 This suggests that IL-17 may have different functions in various organs and pathological conditions.
IL-21
IL-21, produced by Tfh cells, promotes cooperation between Tfh cells and B cells to maintain the GC response.96 A recent study demonstrated that IL-21 derived from CD4+ T cells directly enhances CXCL13 expression in myeloid cells in ICB therapy-induced immune-related adverse events.223 TGF-β mediated silencing of SATB1 resulted in Tfh cell differentiation and IL-21 expression, thereby driving TLS development, which is critical for ovarian tumor control.113 Within TLSs of transplanted kidneys, IL-21 is located in the GC, activating B cells and leading to graft failure.224 Moreover, Tfh cells secreting IL-21 in TLSs promote renal fibrosis in chronic kidney disease, and treatment with ICOS antibodies alleviates disease progression.225
Others
Apart from the interleukins mentioned previously, others also influence the formation of TLSs, although the specific mechanisms remain incompletely understood. For example, IL-4 from Tfh cells activates IL-4R on CD23+ light zone (LZ) GC-B cells, leading to STAT6 phosphorylation and inhibiting memory B cell development. Whereas IL-4R+ FDC binds IL-4 competitively to relieve its suppression of B cell differentiation.226 IL-21 appears to play a crucial role in this process.121 Additionally, IL-10 upregulates CXCL13 in lung macrophages and monocyte-derived macrophages through activation of the JAK/STAT pathway.227 However, whether it is associated with TLS development remains unclear and requires further investigation. Besides, IL-23 can induce IL-17 and IL-22 expression by Th17 cells,228 and promote the generation of IL-22 by ILCs and γδ-T cells.229
TNF-superfamily members
Tumor necrosis factors, a type of pro-inflammatory cytokine, can kill tumor cells or cause necrosis of tumor tissue through their cytotoxicity. TLS development has been shown to be dependent on cytokines from the TNF family, particularly lymphotoxin, TNF-α, and LIGHT.
Lymphotoxin
Lymphotoxin, a member of the TNF superfamily, is the key to lymphoid tissue development and exists in two main forms: lymphotoxin α (LTα) and lymphotoxin β (LTβ). Activated lymphocytes produce soluble homotrimers (lymphotoxin α3, LTα3) formed by LTα that are also known as TNF-β. LTα forms a transmembrane heterotrimer (lymphotoxin α1β2, LTα1β2) on the cell surface when it interacts with LTβ through their ectodomains. LTβR, also recognized as TNF receptor superfamily member 3, is expressed only by stromal cells and myeloid cells, not lymphocytes.230,231 This distribution is significant since it underscores the specificity of cellular interactions. The interaction between LTα1β2 and LTβR serves as a communication signal bridging lymphocytes, stromal cells, and myeloid cells through a non-classical NF-κB pathway dependent on the IKK complex.232 The LTα1β2 promotes stromal cells to express adhesion molecules and chemokines, such as CXCL12, CXCL13, CCL19, CCL21, VCAM-1, and ICAM-1. Additionally, it has also been reported to upregulate PANd expression in ECs and induce HEV transformation.233,234
LIGHT
The ligand for LTβR is not limited to LTα1β2; it is also activated by LIGHT, also known as the TNF superfamily 14. LIGHT is primarily expressed in activated T cells and DCs.235,236 Herpes virus entry mediator is an additional receptor for LIGHT; however, it appears to have no function in TLS development. The LIGHT-LTβR pathway in TLSs mainly induces HEV formation and promotes lymphocyte migration.237,238 Notably, many scholars have identified LIGHT as a potential modulator of tumor immunity. For example, He et al. developed a short peptide, CGKRK-LIGHT, to target tumor vasculature, successfully inducing HEV formation and lymphocyte infiltration in glioblastoma.239 CGKRK is a vascular targeting peptide (VTP) that targets specific receptors on tumor vessels. LIGHT-VTP normalizes tumor vasculature, enhances chemokine production by ECs, and promotes TLS formation.36 Surprisingly, combining LIGHT and ICB therapy improved the therapeutic efficacy against tumors.36
TNF-α
Although lymphotoxin signaling is indispensable for the development of TLSs, TNF-α also has a non-negligible role in the absence of lymphotoxin signaling. In particular, LTi cells are not necessary for TLS occurrence. For example, TNF-α activates stromal cells to produce chemokines, driving the formation of gut TLSs in an inflammatory environment lacking RORγt+ LTi cells.240 Furthermore, it also regulates HEV development independently of LTβ. In melanoma mice, blocking LTβR did not affect HEVs, whereas TNFR1/2−/− mice lacked PNAd+ HEVs.241 However, TNF-α does not appear to induce mature HEVs, and a more mature HEV phenotype still requires LTβR signaling.233,242
BAFF
B cell activating factor (BAFF), also known as TNFSF13B (CD257), belongs to the tumor necrosis factor ligand family.243 It binds to BAFFR, activating the non-classical NF-κB2 pathway, which increases the expression of the BCL family with anti-apoptotic activity. Moreover, BAFF enhances mitochondrial function and increases ATP production through the PI3K pathway. Both of these pathways improve the survival of B cells. The B cell activation marker CD83 and BAFF co-enriched in the B-cell zone of TLSs in encephalomyelitis.244 It suggests that elevated levels of BAFF contribute to B cell activation and PC differentiation.245 Conversely, blocking BAFF leads to a decrease in newly formed TLSs, reducing infiltration of B cells and T cells, preventing TLS formation, and mitigating lupus nephritis.246,247,248,249
Chemokines
Chemokines are secondary pro-inflammatory mediators induced by primary pro-inflammatory mediators, such as interleukins or TNF.250 It promotes immune cell migration and lymphoid tissue neogenesis by binding to specific receptors on the cell surface. Therefore, chemokines are critical for TLS formation and maintenance.
CXC subfamily
CXCL13 recruits B cells by selectively binding CXCR5. Initially, activated stromal cells, particularly FRCs, are the main source of CXCL13 during the early stages of TLS development.251 Subsequently, macrophages, DCs, FDCs, and T cells also act as lymphoid tissue organizer (LTo) cells and produce CXCL13.252,253,254,255 Moreover, before GC maturation, fibroblasts and lymphocytes are the primary producers of CXCL13. Notably, recruited B cells also generate CXCL13, which creates a positive feedback loop.43 After the constitution of the FDC network, FDCs become the main cells producing CXCL13 in GC.254 Tfh cells, in addition to B cells, express CXCR5 and migrate into the TLSs following the CXCL13 gradient, promoting GC maturation.119 Therefore, CXCL13 is often used as a marker for TLS formation and tumor prognosis.256 However, high levels of CXCL13 do not always indicate a favorable prognosis. Recent studies have shown that infiltration of CD8+ T cells with high CXCL13 expression in RCC results in an immunosuppressive microenvironment.257 Apart from CXCL13, stromal cells secrete CXCL12, which is also shown to promote B lymphoid follicle formation in the absence of FDCs.222 CXCL12/CXCR4 and CXCL13/CXCR5 mediate the migration of B cells between the dark zone (DZ) and light zone (LZ) in the GC. In the presence of CXCL13, GC B cells aggregate in the LZ for antigen selection, whereas CXCL12 attracts them to the DZ to undergo SHM.258 Furthermore, although CXCL9 and CXCL10 can attract T cells, their direct involvement in TLSs remains unclear. Nevertheless, they have been shown to enhance antitumor immunity in melanoma and are associated with the expression of TIS-related genes.259
CC subfamily
CCL19 and CCL21, which bind to CCR7, are primarily produced by stromal cells and ECs, attracting T cells and facilitating the development of a T-cell zone in TLSs.260,261 In triple-negative breast cancer, the presence of CCL19+ DCs has been linked to TLSs and T cell aggregates. Elevated levels of CCL19 improved ICB therapy outcomes and increased patient survival.171 The levels of CCL21 in peripheral blood also positively correlate with the efficacy of ICB therapy, with CCL21 expression observed in TLS-like areas.262 In lung cancer patients with a history of smoking, they have higher levels of TLSs and T cell infiltration, which is attributed to tobacco stimulation increasing CCL21 expression. However, CCL21 is not secreted by stromal cells but by lung adenocarcinoma (LUAD) tumor cells.263 Additionally, CCL20 has also been found to be upregulated in tumors containing TLSs,264 although direct evidence linking CCL20 to TLS formation is still lacking.
CX3CL1
CX3CL1 is the only member of the CX3C chemokine family associated with various diseases.265,266 Within TLSs, CX3CL1 expression was detected within the B-cell zone, while its receptor, CX3CR1, is predominantly found in the CD3+ T cell region.267
Interferons
Type I IFN stimulated PDGFRα+ fibroblasts to secrete CXCL13, which attracted naive B cells and initiated TLS development. It is worth noting that this process still required Tfh cell assistance.268 Additionally, type I IFN increased BAFF expression in neutrophils through the transcription factors IRF1 and IRF2,269 which might contribute to B cell proliferation and GC formation within TLSs. Andersson A et al. reported that the type I IFN pathway is enriched and co-localized with CXCL10+ macrophages in HER2-positive breast cancer. They also observed a connection between type I IFN-associated M2 macrophages and T cells in TLSs. However, they did not detect the GCs, implying TLSs may not be fully mature yet.270 Furthermore, type I IFN also directly induced STAT1 binding to BCL6, promoting CD4+ T cell differentiation into CXCR5+ PD-1+ Tfh cells.271 IFN-γ, a type II IFN, indirectly induces HEV formation by increasing lymphocyte infiltration.272 IFN-γ secreted by Th1 cells increases chemokine production in high-grade muscle-invasive bladder cancer, promoting TLS formation and enhancing the sensitivity to treatment.273 Additionally, it may also activate B cells, triggering TLS formation by upregulating BAFF and IL-6 expression.274
Transforming growth factor-β
TGF-β is an immunosuppressive factor that regulates immune homeostasis and tolerance, facilitating tumors in evading the immune system and resisting cancer immunotherapy.275 Several studies have shown an upregulation of TGF-β expression within TLSs. For instance, in CnAα+/- hybrid mice, LYVE-1+ ECs have been found to promote the spontaneous formation of TLSs. TGF-β could potentially create a conducive environment for the transformation of ECs into HEVs and the formation of TLSs.276 Additionally, TGF-β inhibits SATB1 activity, leading to the differentiation of CD4+ T cells into Tfh cells that express LIGHT, CXCL13, and IL-21.113 In pancreatic ductal adenocarcinoma (PDAC), TGF-β derived from fibroblasts activates T cells, stimulating them to secrete CXCL13.277 Moreover, another study has demonstrated that TGF-β activated T cells, resulting in their differentiation into CXCR5-BCL6- peripheral Th (Tph) cells that express CXCL13. It also reduces IL-2 production, driving CXCL13 upregulation in CD8+ T cells through the expansion of Treg cells.278 Moreover, in pemphigus chronic blisters, Treg cells have been shown to increase CXCL13 production in CD4+ T cells by mediating the attenuated stimulation of TGF-β in TLSs.137
Other
Vascular endothelial growth factor (VEGF) levels are elevated in various diseases, such as cancer and blinding eye diseases.279 It promotes tumor angiogenesis and regulates lymphatic vascularization.279 LTβ signaling stimulates FRCs to express VEGF, which enhances EC proliferation and expansion; notably, VEGF had a greater impact on PNAd− ECs.280 NRP1+ ILC3 located near the HEVs produce VEGF-A and act as LTi cells, promoting TLS formation in COPD.281 Additionally, anti-VEGF/VEGFR2 treatment has been found to convert tumor blood vessels into HEVs, enhancing T-cell permeation.282 B cell lymphoma 6 (BCL6) is essential for B cell maturation during the GC response, and BCL6 expression is specifically regulated.283 It targets Myc and Prdm1, inhibiting the processes of positive selection and differentiation of PCs, respectively.284 The mice lacking BCL6 fail to form GC.285 In addition, it also promotes Tfh cell differentiation.286 A recent study has shown that BCL6 regulates the interaction between Tfh cells and B cells to maintain the GC response.287 Notably, BCL6+ GC formation is a hallmark of mature TLSs.288,289 Activation-induced cytidine deaminase (AID) is associated with B-cell isotype switching and SHM, promoting the production of high-affinity antibodies in TLSs.290 In uveitis, Epps et al. observed that TLSs have distinct B cell and T cell zones, with expression of AID and BCL6 in the GC.291 Increased expression of AID leads to the differentiation of B cells in pemphigus skin lesions.292 And it is also often used as a marker for mature GCs. The intra-tumoral TLSs present in PDAC patients treated with the GM-CSF-secreting allogeneic PDAC vaccine (GVAX) led to the infiltration of CD83+DC-LAMP+ DC and the inhibition of Treg cell proliferation.293
Cascade regulatory mechanisms of TLS formation
Enlightenment of SLO development for TLS formation
TLSs lack some structures compared to secondary lymphoid organs (SLOs). The development of SLOs is a complex and ordered series of processes that occur in specific anatomical locations during the embryo, whereas the appearance of TLSs is a postnatal response to persistent inflammatory signals. Although TLSs are acquired and lack a specific site, their formation also involves interactions between lymphocytes and non-lymphoid stromal cells.234,235
TLSs are similar to SLOs in structure and function. Therefore, a thorough understanding of SLO development will enhance our comprehension of TLS formation. SLOs, encompassing lymph nodes, spleen, tonsils, Peyer’s patches, and mucosa-associated lymphoid tissues, are distributed throughout the body.294 The formation of SLOs is tissue-specific, involving a complex interplay between hematopoietic cells and non-lymphoid stromal cells in the embryonic stage.2 LTi cells, derived from fetal liver-generated IL-7Rα+Sca-1lowc-Kitlow progenitor cells, colonize around E12.5-E14.5 and express RORγt and ID2, representing ILCs. They migrate to lymph node progenitors through the chemokine receptors CXCR5 and CCR7.295 LTi cells express and deliver LTα1β2, which binds to the LTβR on LTo cells, promoting up-regulation of adhesion molecules such as VCAM-1, ICAM-1, and MADCAM-1. Additionally, this interaction increases the secretion of homeostatic chemokines, such as CCL19, CCL21, and CXCL13.294 Subsequently, these molecules collaborate to regulate immune cell recruitment to lymphoid niches, facilitating adult lymphoid structure development. Silencing LTα, LTβ, and LTβR resulted in lymphatic node (LN) defects.296 Furthermore, IL-7 triggers a positive feedback loop, inducing the secretion of LTα1β2 by LTi cells.294 Long-term interaction between LTi cells and LTo cells induces lymphoid tissue development, promoting the selective entry of naive T and B cells into lymph nodes.297
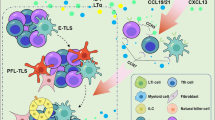
In general, the development of TLSs is divided into three stages. The first stage consists of dense lymphocyte aggregates with a large number of fibroblasts. However, there are no separate T-cell and B-cell zones, nor FDCs. It can be considered a “precursor” to TLSs. The second stage is referred to as “early” or “primary follicular-like” TLSs, in which T cells and B cells gradually form distinct areas, but there is a lack of GC response. The third stage is considered “mature” TLSs, which are characterized by a well-organized T-cell zone and B-cell zone and the presence of an FDC network in GCs (Fig. 3). In addition, HEV development occurs throughout TLS formation. Recently, Liu et al. reported the developmental process of TLSs in LUAD. They mapped the immune landscape during the development of LUAD by scRNA-seq on immune cells in the initiation, invasion, and progression stages of LUAD. In the early LUAD, CD4+ T cells prime aggregated around alveolar epithelial cells, and CD8+ T cells and B cells were scattered, constituting TLS precursors; with tumor invasion, Tfh cells appeared and were accompanied by B cell aggregation, forming early TLSs; finally, mature B cell zone and T cell zone was formed, which were defined as mature TLSs. In addition, the mature TLSs contained GC, PNAd+ HEVs, and CD21+ FDCs. More importantly, as the tumor progressed, the TLS density and the ratio of TLSs to tumor area increased. This may indicate that TLS mature with tumor progression and is positively correlated with better patient survival.298 Furthermore, it has also found three stages of TLS development in gastric cancer: secondary follicle-like TLSs, primary follicle-like TLSs, and early TLSs, which contained DCs and PNAd+ HEVs.299
The development stage and structure of TLS. The diagram illustrates the presence of TLSs in tissue. Firstly, dense lymphoid aggregates infiltrate the tumor tissue. Subsequently, primary follicular-like TLSs show B-cell zone and T-cell zone, but lack GCs. Mature TLSs are characterized by mature GCs and the presence of FDCs. In TLSs, CD8 + T cells have cytotoxic effects, and plasma cells produce antibodies, which promote tumor regression
Priming of stromal cells as a crucial step in the formation of TLSs
The origin of signals for activating stromal cells
As early as 2004, Cupedo et al. injected subcutaneously CD45+CD4+CD3− cells from LN and induced lymphoid neoorganogenesis in vivo after 2 weeks, emphasizing the critical role of stromal cells in TLS development.300 The priming of stromal cells, especially tissue fibroblasts, forms a fibrous reticulum scaffold, providing space for TLS formation.43 (Fig. 4) Despite controversies surrounding the cellular and molecular requirements for stromal cell differentiation and specialization during TLS establishment, IL-13, IL-22, and IL-17 are still acknowledged as pivotal factors. The activation of “immunofibroblasts” relies on IL-13 signaling, where IL-13 binds to IL-4R rather than IL-4.41 IL-17 and IL-22 promote their further proliferation.149,184 Furthermore, fibroblasts specifically bind to LTα1β2 and LIGHT by LTβR, stimulating their proliferation.251 LTα3 also binds to LTβR and mediates TLS development, which depends on the upregulation of ICOS-L by immune fibroblasts and other APCs.301 And LTβR deficiency impedes TLS formation.302 Furthermore, myeloid cells also induce stromal cells to secrete chemokines by TNF-superfamily members and TGF-β, which trigger lymphoid cell recruitment into TLSs.160,185,303
Stroma cells initiate the formation of TLS. The sketch displayed that activation of fibroblasts and vessel endothelial cells (VECs) initiates the formation of TLS, leading to the production of chemokines and promoting the migration of lymphocytes. During this process, IL-7 mediates the positive feedback loop between the stroma cells and lymphocytes. HEVs promote the transport of lymphocytes. Chemokines stimulate the expression of LFA-1 on lymphocytes. It binds to adhesion molecules (ICAM) and stops the rolling of lymphocytes. Subsequently, they cross over the HEVs and are recruited to specific regions formed by fibroblasts
In different organs, the stromal cells that trigger TLS formation appear tissue-specific. For example, TLSs always accumulate around alveolar epithelium cells or bronchi in lung cancer. More importantly, CD4+ T cells preferentially accumulate around the pulmonary alveolar epithelium cells in LUAD and subsequently initiate TLS development.298 Another study showed the activation of lung resident stromal cells in transplanted lung, including airway epithelial cells and type II pneumocyte-like cells. They produce CXCL12 and recruit lymphocytes.304 In addition, kidney-resident stromal cells promote TLS formation in lupus nephritis, such as podocytes, mesangial cells, and renal tubular epithelial cells.305 In lupus nephritis, nucleosome-containing immune complexes in the mesangial matrix lead to mesangial cell activation, inducing a high degree of chemokines, including CCL2, CCL7, CCL20, CXCL1, CXCL2, and CXCL5. This induces an infiltration of neutrophils, macrophages, T cells, and B cells in the renal stroma.306 Renal tubular epithelial cells express IL-23 receptors, increasing intracellular calcium flux, upregulating glycolysis, and enhancing L-arginine levels in response to IL-23, which regulate T cell proliferation and function.307 In addition, renal tubular epithelial cells also express BAFF and IL-6.308,309 These evidence suggest that renal stromal cells may play an important role in the development of TLSs in renal diseases
Activated stroma cells assumed the role of LTo cells
Activated stromal cells, acting as LTo cells, raise CD3+ T cells and B220+ B cells to TLSs by secreting chemokines such as CXCL12, CXCL13, CCL19, and CCL21, which bind to CXCR5 and CCR7, respectively.43,251 The transcription of CXCL13, CCL19, and CCL21 mRNA significantly increased prior to TLS development and peaked when TLSs were fully developed.310 In mice with melanoma, fibroblasts express high levels of CXCL12 and CXCL13, leading to the aggregation of T cells and B cells in the PDPN+ network and inducing follicle formation in TLSs.43 And CXCL13 inhibitors depress TLS formation.302 Notably, IL-17-induced CXCL12 recruits B cells and induces follicle formation in the absence of CXCL13+ FDCs.222
A positive feedback mechanism during TLS formation
Fibroblasts secrete chemokines to attract T cells and B cells for infiltration. In turn, T cells and B cells express several cytokines to promote fibroblast activation and proliferation. This positive feedback mechanism significantly improves the efficiency of TLS formation. (Fig. 4) Migrating lymphocytes accumulate in the early TLS stages and produce CXCL13. A recent study found that B cells recruited by fibroblasts produce more CXCL13 than fibroblasts themselves, facilitating the expansion of the lymphoid aggregates. This process appears to equally depend on LTβR signaling.302 In mice lacking lymphocytes or B cells, the CD45+PNPD+ network is reduced, along with poor TLS size and number.43 However, depleted B cells did not affect TLS function in multiple sclerosis.311 CXCL13+ T cells are pivotal in recruiting CXCR5+ B cells in TLSs and are associated with a favorable prognosis.312 TGF-β interacts with TCR in a chronic stimulation form, promoting CXCL13 expression by CD39+CD103+ TRM cells.257,313 In HGSCs with TLSs, CD4+ T cells and CD8+ T cells are the main sources of CXCL13, but as TLSs mature, CXCL13 is gradually expressed by CD21+ FDCs.254 Furthermore, fibroblasts produce IL-7, which supports LTi cells expressing LTα1β2 in TLSs, and IL-7-/- mice observe abnormal lymphoid organ development.208 Luther SA and colleagues reported that LTα1β2 expression in LTi cells depends on IL-7Rα, and their recruitment in vivo was mediated by CXCL13+ stromal cells.314 Furthermore, lymphocytes recruited by chemokines secrete LTα1β2, which binds to LTβR, inducing fibroblast proliferation and maturation.10
Critical role of HEVs in TLS formation
HEVs originate from postcapillary venules and express PNAd to bind L-selectin on lymphocytes, enhancing lymphocyte migration into TLSs. HEVs have been detected during the early stages of TLS development,315 and they are associated with survival.316 (Fig. 4)
The occurrence of HEV
To alleviate hypoxia during rapid tumor progression, tumor cells secrete hypoxia-inducible factor, VEGF, and platelet-derived growth factor to stimulate tumor endothelial cells (TECs), fostering angiogenesis. Tumor vessels are characterized by an incomplete wall, a lack of smooth muscle, and incomplete pericyte coverage. They restore integrity under the stimulation of inflammatory cytokines and convert to HEVs in TLS formation. Vascular remodeling recruits lymphocytes to inflammatory tissues and coordinates TLS formation in chronic inflammatory conditions.317 TLS-HEVs exhibit a hybrid phenotype of TECs and LN-HEVs, suggesting that they may originate from TECs and share similar functions to LN-HEVs.13 HEVs, function as lymphatic vessels, are specialized post capillary microvessels, serving as the primary channel for lymphocyte trafficking within TLSs,242 with their formation reliant on persistent LTβR signaling.13,318 In Sjogren’s syndrome, lymphatic aggregates are characterized by a gp38+/pdpn+ fibroblast network, accompanied by CD31+LYVE-1+ HEVs.41 Meanwhile, LTβR is also detected on ECs, and the absence of its ligand, LTα1β2, attenuates lymphatic vessel formation in TLSs.319 LIGHT also binds to LTβR on ECs, causing them to convert to HEVs. LIGHT-VTP normalizes tumor vasculature and triggers lymphoid aggregate.36 The AAV-LIGHT therapy modulates the vascular phenotype, eliciting the formation of HEVs and TLSs, which prolong the survival of mice with gliomas resistant to anti-PD-1 treatment.320 Furthermore, HEV neogenesis also relies on TNFR signaling. Blockade of TNFR by TNFRII.FC reduces the expression of PNAd and MAdCAM-1 in ECs, leading to HEV destruction. Notably, Fleig et al. found that conditional deletion of Rbpj, a key mediator of Notch signaling, led to a significant downregulation of the Notch pathway in vascular endothelial cells, inducing arterial endothelial cells to transform into HEV. In addition, they also observed the spontaneous formation of mature TLSs in the kidney, liver, and lung. This suggests that HEVs originate from vascular endothelial cells rather than lymphatic endothelial cells and are independent of SLOs.321
Several studies have shown a positive association between HEVs and prognosis in various tumors, such as colon carcinoma,316 oral cancer,322 carcinoma of the lungs,323 and breast cancer.324 For example, in a study of 203 samples with colon carcinoma, HEVs express sulfated L-selectin ligand, and patients with higher HEV/TLS ratios have longer overall and disease-free survival.316 Increased HEV frequency and maturation were associated with T-cell infiltration in tumors. They significantly improved ICB efficacy, especially survival after anti-PD-1/anti-CTLA-4 combination therapy.325 In addition, it has been reported that ICB treatment also promotes HEV formation, especially combined with antiangiogenic treatment, which may be due to the infiltration of immune cells.326 Allen E. et al. reported that anti-VEGF/VEGFR2 treatment induces HEVs and increases PD-L1+ T cells in MC38-bearing or glioma mice. Therefore, an anti-angiogenic combination with anti-PD-L1 induces a plump morphology of ECs and typical characteristics of MECA79+ HEVs.282 In human breast cancer, DC-LAMP+ DCs are found close to HEVs,327 and depletion of DCs attenuates HEV development,328 suggesting that DCs may also regulate HEVs through LTα1β2. Moreover, CD8+ T cells are correlated with the HEVs in TLSs, as evidenced by the absence of HEVs in anti-CD8-treated mice.13 T cells express TNF-α and LTα3 in FOXP3+ Treg deficiency mice,329 indicating that increased activated CD8+ T cells promote intertumoral HEV formation in a TNFR and LTβR-dependent manner.136
HEVs promote lymphocyte transport
Naive lymphocytes migrate into lymphoid organs through HEVs and undergo four key processes: rolling, adhesion to HEVs, intraluminal crawling, and transendothelial migration.242,330,331 HEVs express PNAd, a highly sulfated glycoprotein expressed on HEV-ECs.332,333 This protein translational modification enhances the combination of L-selectin+ lymphocytes with HEV-ECs. Chemokines induce the expression of integrin on lymphocytes, stopping lymphocyte rolling when they bind with an adhesion molecule (ICAM-1, ICAM-2) on ECs. Subsequently, these cells cross the HEVs and are recruited to a specified area formed by FRCs.334 More importantly, HEVs express CCL19 and CCL21, which bind to CCR7 on the surface of T cells and mediate T cell entry into lymphoid tissues.335 In addition, they also express CXCL13, which activates α4 integrins to induce B cell adhesion to MAdCAM-1+ HEV.336 Further study into HEVs revealed differences between MECA79+ HEVs within TLS+ and TLS− regions in tumors. The HEVs upregulated TSPAN7 and MEOX2 gene expression in TLSs, which increased T cell and B cell infiltration and survival rates.334
Differentiation of B cells and GC maturation
Human B cells are derived from hematopoietic stem cells and differentiate from pro-B cells to pre-B cells in the bone marrow, eventually becoming immature B cells. The early development of B cells involves the functional rearrangement of immunoglobulin gene fragments. Initially, the Ig heavy chain is constructed on the surface of pro-B cells, and the recombination-activating gene facilitates the rearrangement of V (VH), D (DH), and J (JH) gene segments in the variable region. Subsequently, the λ or κ light chain is rearranged and pairs with the heavy chain to form the B cell receptor (BCR) complex. The complete IgM molecules are expressed on the cell surface, indicating the cells have developed into immature B cells. Upon encountering specific antigens, B cells undergo affinity maturation and isotype switching, developing into memory B cells or antibody-producing PCs. This process occurs in the germinal centers of peripheral lymphoid tissue.337
Nascent GCs in the follicles were first detected a few days after immunization and were fully established within 1 week.338 Mature GC exhibits a distinct structure, comprising the peripheral light zone (LZ) and the inner dark zone (DZ). GC mediates humoral immunity by somatic hypermutation (SHM) and isotype switching, enabling B cells to generate high-affinity antibodies. Stromal cells and immune cells produce the chemokines CXCL12 and CXCL13, mediating the migration and localization of B cells.339,340 (Fig. 5) Pre-B cells are recruited into the DZ by HEVs after initially developing in the bone marrow.284 DZ-B cells undergo clonal expansion in the immunoglobulin variable zone.341 Negative selection eliminates self-reactive GC-B cells through SHM under the stimulation of antigen.342,343 Those processes increase their affinity for antigen and favor GC-B cell differentiation into proliferative PCs, promoting antibody production.344 After SHM and proliferation, B cells shift chemokine receptor expression: down-regulating CXCR4 and upregulating CXCR5, which directs them towards the LZ in response to CXCL13.345 The maturation of LZ-B cells increases antigenic affinity through positive selection, and lower-affinity B cells are deleted.346,347 Subsequently, CXCL12 promotes high-affinity B cells to reenter the DZ for clonal proliferation.258,348 During migration from the LZ to the DZ, partial B cells are eliminated.349,350 Mayer et al. reported that B cells express c-Myc, a positive selection marker, which helps them to evade death during migration.351 Additionally, BCR signaling triggers positive selection and enhances B cell survival in GC.352 However, this process requires antigen presentation by FDCs and CD40 signaling from Tfh cells, as BCR signaling alone causes B cell death.353,354,355 Tfh cells are crucial in driving B cells toward PC differentiation by costimulating factors. In vitro studies demonstrate that anti-CD40 induces the nuclear translocation of the NF-κB subunit p65/RelA in B cells.356 Interestingly, blockade of CD40L does not significantly affect Blimp1 expression in GC-B cells,357 suggesting that B cell differentiation may be regulated by multiple mechanisms. Besides, GC-B cells are characterized by the expression of B cell lymphoma 6 (BCL6) and Ki67.58,358 The absence of BCL6 leads to the failure of GC formation and high-affinity antibody production.285,359 FRCs are located at the boundary of the T-cell zone and B-cell zone, producing BAFF to maintain B-cell survival and GC structure.360 AID, a mutant enzyme, is critical to B cell maturation in DZ-B cells.361,362 Meanwhile, SEMA4A appears in TLS+GC+ HNSCC, suggesting that SEMA4A is correlated with B cell differentiation and TLS formation.16 Interestingly, the receptor for SEMA4A is TIM2, which is expressed on the surface of activated T cells.363 This seems to suggest that GC-B cells enhance T-cell function in TLSs.
Differentiation of B cells, and GCs response. The diagram illustrates that GC is divided into two distinct compartments, with B cells entering the DZ under the influence of the CXCL12-CXCR4 axis, where GC-B cells proliferate and SHM occurs. Subsequently, B cells migrate along the CXCL13 gradient and enter the LZ. B cells capture antigens presented on FDCs for selection and immunoglobulin isotype switching. This process is also regulated by Tfh cells. B cells further proliferate, and SHM occurs through multiple migrations between the DZ and LZ. Eventually, they exit the GCs as memory B cells or high-affinity antibody-secreting plasma cells
The GC-B cells in TLSs overlap with those from healthy tonsils,16 indicating GCs are sites of B-cell expansion, maturation, and isotype switching in the TLSs. And GC formation is one of the indicators of TLS maturity.364,365 Analytical studies of B cell profiles within TLSs provide direct evidence of intact B cell responses, particularly in tumors. It has been shown that B cell density is higher in tumors with TLSs, and B cell density and function are also close to TLS presence.59 In breast cancer, Wang et al. analyzed B cell differentiation trajectories in the TME. They found that B cells transitioned from the transitional state of naive B cells and CXCR4+ B cells into follicular B cells and PCs, which are key components of GC-B cells. Notably, IgHG1 levels increased in the later stages of the pseudo-time trajectory, indicating that B cells underwent immunoglobulin conversion to become plasma cells.366 B cell immunity can arise from reactivating memory B cells or stimulating naive B cells. Naïve B cells and memory B cells are transported to the TME via HEV. A study indicated that naive B cells are primarily detected within TLSs, suggesting TLSs as the source of intratumoral B cells.170 However, another study noted memory B cell concentrations in the GC for in situ tumor activation, contrasting with scarce naive B cells.367 We think that elevated memory B cell levels may reflect the in situ maturation of naive B cells. This perspective is also supported in NSCLC.59 Spatial transcriptomics revealed that genes involved in all B cell maturation processes, from naive B cells to PCs, are present in TLS+ tumors. Notably, these gene signatures are preferentially expressed in TLSs.367 In TLShigh tumors, SHM levels, including BCR IgVL and IgVH, were significantly elevated compared to TLSlow tumors. Furthermore, TLS+ tumors exhibited a high density of Ig-producing PCs and Ig antibodies, especially IgG. In fact, the IgH clonotype was found both near and distant from the TLSs within tumor nests. This suggests that B cells undergo hypermutation in TLSs and that MZB1+ PCs migrate into the tumor nest along with CXCL12+ fibroblast networks after maturation.367 In multiple sclerosis, IgG-VH transcripts exhibit a high frequency of somatic hypermutation (SHM). SHM is a necessary stage for B cell affinity maturation, indicating the occurrence of GC responses within meningeal TLSs.53
The role of TLS in the disease
TLSs represent crucial immune tissues capable of eliciting antigen-specific immune responses at sites of chronic inflammation. Their roles across different disease contexts and their implications for patient outcomes are diverse. In the realm of autoimmune diseases and age-related chronic inflammatory conditions, which are typically characterized by the immune system’s attack on self-tissues, the emergence and maturation of TLSs are often associated with poorer prognoses. Conversely, in scenarios necessitating an enhanced immune response, such as in cancer and infectious diseases, the presence and maturation of TLSs generally signify more favorable outcomes.368,369,370 Additionally, ageing serves as a critical influencing factor in the formation of TLSs induced by diseases. Various studies have demonstrated an age-dependent probability of TLSs formation across multiple diseases, including bladder cancer and acute kidney injury. This trend underscores the significant role of ageing in modulating the immune landscape and disease pathology through the promotion of TLS development371,372,373,374 (Table 2).
Tumor
TLSs are associated with tumor progression, immune responses, and patient prognosis. They might contribute to understanding cancer and developing new treatment strategies.375,376,377
TLSs are intricately linked to the prognosis of tumors. The location (intra-tumor or peri-tumor) and the maturation of TLSs can greatly influence the prognosis of cancer.378,379,380,381 In the majority of investigations, when TLSs are located intra-tumorally, patients with cancer often exhibit an improved prognosis.380,382 However, when TLSs are located peri-tumor, the prognosis of cancer patients shows strong heterogeneity. For instance, in cancers such as esophageal squamous cell carcinoma and colorectal cancer, patients with peri-tumor TLSs exhibited better outcomes,383,384 but in cancers such as hepatocellular carcinoma, cholangio carcinoma, and breast cancer, the outcomes were worse.385,386,387 Additionally, cancer patients with mature TLSs often exhibit a favorable prognosis.277,367
TLSs play a crucial role in enhancing adaptive immune responses. B cells in TLSs can produce specific antibodies and promote phagocytosis, complement-mediated cytotoxicity, or antibody-dependent cellular cytotoxicity, which may contribute to the adaptive immune defense against tumors.59,388 Hans A. Schlößer et al. demonstrated that in cancer, specific T-cell responses were induced by the accumulation of CD86+ antigen-presenting B cells within GCs in TLSs. TLSs may facilitate adaptive immunity in cancer by providing an organized space that promotes interactions between immunity.389 Willard-Gallo et al. further demonstrated the interactions of immune cells within TLSs in the breast cancer immune microenvironment.390
In this section, we delineate the associations between TLSs and tumor prognosis, highlighting their heterogeneity across various cancer types.
Head and neck cancer
Head and neck cancer is a group of malignancies originating from the oral cavity, nasal cavity, pharynx, larynx, and other structures in the neck.391 Head and neck squamous cell carcinoma (HNSCC), the most predominant subtype among head and neck tumors, has shown a better prognosis and a greater response to immunotherapy when associated with TLSs. In patients with HPV-positive (HPV+) or HPV-negative (HPV−) HNSCC, an increased presence of TLSs has been observed.16 Regardless of HPV status, the presence of TLSs with GCs correlates with favorable prognoses, indicating a potential role in immune-mediated tumor control. Furthermore, the enrichment of TLSs in HPV− HNSCC patients is associated with extended overall survival and heightened responsiveness to PD-1 inhibitory treatment.392 These findings underscore TLSs’ significance in enhancing antitumor immunity and improving patient outcomes in the context of HNSCC, particularly in the HPV− subgroup.368 Additionally, a specific subtype of TLSs, known as follicular-like tertiary lymphoid structures (FL-TLSs), serves as an independent prognostic marker in laryngeal carcinoma. Patients with FL-TLSs exhibit increased immune cell infiltration and demonstrate markedly improved responses to immunotherapeutic approaches, further highlighting the potential of TLSs as a biomarker for predicting treatment outcomes and guiding therapeutic strategies in HNSCC.393 Overall, these studies emphasize the crucial role of TLSs in modulating the immune landscape within HNSCC tumors and suggest their utility as prognostic indicators and therapeutic targets for improving patient outcomes.
Esophageal cancer
Originating from the mucosal lining of the esophagus, it falls under the category of cancers of the digestive system.394 Furthermore, current research indicates that the presence of TLSs both within the tumor itself and in the surrounding tissue is associated with a more favorable prognosis. Recent studies have consistently demonstrated that the presence of TLSs, particularly mature TLSs, within ESCC is closely associated with improved prognostic outcomes for patients. Mature TLSs are characterized by a rich composition of proliferating B cells, plasma cells, CD4+ helper T cells, B memory cells, and Th17 cells, and are correlated with an increased infiltration rate of CD8+ T cells.152 This heightened infiltration is intimately linked to enhanced survival rates. Furthermore, cases featuring mature TLSs significantly outperform others in terms of recurrence-free survival (RFS) and overall survival (OS).395,396 Additionally, a high density of peri-tumoral TLSs is associated with earlier tumor stages, the absence of lymphovascular invasion, improved serum nutritional parameters, and extended survival periods. Remarkably, the density of TLSs can also serve as a predictive marker for the response to PD-1 inhibitor therapy and survival outcomes.383 The convergence of findings across multiple studies underscores the critical clinical relevance of considering the tumor microenvironment, especially the assessment of TLSs, in the treatment and prognostic evaluation of ESCC. This emphasizes the pivotal role of TLSs in cancer progression and treatment response.
Non-small cell lung cancer
Lung cancer, one of the most prevalent cancers worldwide, originates from the cells within the lungs and stands as a leading cause of cancer-related mortality globally. It is broadly categorized into two main types based on its origin and cellular characteristics: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC accounts for approximately 85% of all lung cancer cases and is characterized by a relatively slower progression. It primarily encompasses three subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Multiple studies underscore the pivotal role of TLSs in enhancing prognoses and therapeutic outcomes in NSCLC patients. It has been demonstrated that in NSCLC, the maturity and abundance of TLSs significantly influence the efficacy of neoadjuvant chemotherapy, with patients exhibiting higher TLS maturity and quantity experiencing improved DFS.397 This suggests that fostering the maturation of TLSs could be a novel approach to boosting the effectiveness of neoadjuvant chemotherapy.
Similarly, analysis of data from 891 NSCLC patients treated with atezolizumab revealed increased activity of B cells and plasma cells, indicating their crucial role in extending overall survival, regardless of CD8 T cell characteristics. This was further confirmed through scRNA-seq, highlighting the significant impact of the specific organization of B cells and plasma cells within TLSs on treatment outcomes.369 In lung adenocarcinoma, an automated workflow for quantifying TLS density in whole-slide images established TLS density as an independent prognostic marker for patients with resectable disease.369 Furthermore, patients with mature TLSs exhibited significantly lower rates of lymph node metastasis, earlier cancer staging, and higher numbers of CD8+ and FOXP3+ cells in TILs, with mature TLSs being independently associated with better DFS and OS. In lung squamous carcinoma (LSCC), specific niches consisting of CXCL13+ perivascular cells and CXCL12+LTB+ and PD-L1+ epithelial cells were identified as facilitators for TLS formation, with TLS density serving as the strongest independent prognostic marker.398,399 However, the prognostic value of TLS density was compromised in patients receiving neoadjuvant chemotherapy, attributed to impaired GC formation.5 Additionally, preoperative corticosteroid treatment, regardless of chemotherapy, was found to reduce TLS density and GC formation, highlighting the complex interplay between treatment modalities and TLS dynamics in influencing cancer outcomes.61
Ovarian cancer
Ovarian cancer, a malignant tumor originating from ovarian cells, can be classified into three primary types based on the cell of origin: epithelial ovarian cancer, stromal tumors, and germ cell tumors. Of these, epithelial ovarian cancer is the most prevalent, representing approximately 90% of all ovarian cancer cases. Furthermore, epithelial ovarian cancer can be subdivided into several histological subtypes, with high-grade serous ovarian cancer being the most common.400 Research on TLSs in ovarian cancer indicates their association with favorable prognoses. It has been discovered that CDK4/6 inhibitors (CDK4/6i) can promote the formation of TLSs and enhance the efficacy of anti-PD-1 antibody immunotherapy, potentially through the regulation of SCD1 and its modulatory molecules ATF3 and CCL4.376 Additionally, another study investigated the distribution and gene expression of TLSs in high-grade serous ovarian cancer (HGSOC) samples, finding that the expression of the CXCL13 gene is not only closely related to the presence of TLSs but also associated with the infiltration of T and B cells, suggesting that CXCL13 is a marker of favorable prognosis in HGSOC patients. These studies reveal that the coexistence of CD8 T cells and B cells in the tumor microenvironment (TME) significantly improves the prognosis of HGSOC, which is associated with the presence of TLSs.254 The expression of CXCL13 transitions to CD21+ follicular DCs as TLS matures, initially emanating from CD4 T cells. Furthermore, patients with HGSOC showing high expression of TLS exhibit significantly better progression-free survival (PFS), linked to the maturation of B cells and the activation and proliferation of tumor-specific T cells.401 These findings underscore the critical role of TLSs in the treatment and prognostic assessment of ovarian cancer, offering potential targets for future immunotherapy strategies.
Colorectal cancer
Colorectal Cancer (CRC), originating from the glandular tissues of the colon’s mucosa, ranks third globally in both incidence and mortality rates among cancers. Preventative measures and early screening are crucial in mitigating its epidemiological impact.402 Several studies in CRC and colorectal liver metastases (CRCLM) have underscored the role of TLSs and their association with patient prognosis. One study observed significantly higher densities of Tregs, M2 macrophages, and Tfh cells within intra-tumoral TLSs compared to those at the tumor periphery. Intra-tumoral TLSs were positively associated with RFS and OS, whereas peritumoral TLSs correlated negatively with RFS and OS.403 Another study identified that a high density of peri-tumoral TLSs, combined with a low tumor stroma percentage, serve as an independent and favorable prognostic factor in non-metastatic colorectal cancer (nmCRC) patients. This finding emphasizes the significance of tumor microenvironment characteristics in influencing the prognosis of nmCRC patients.384 Furthermore, the presence of mature TLSs is linked to a favorable prognosis in CRC patients, particularly those with nmCRC.404 Additional research highlighted that CRC tissues with high levels of HEVs within TLSs demonstrated enhanced recruitment capabilities for CD3 T cells, CD8 T cells, and macrophages. This was positively associated with improved OS, DFS, and lower TNM staging, suggesting that high HEV/TLSs could serve as potential biomarkers for CRC patients.316 In summary, the density and location of TLSs, along with the cellular composition within these structures, significantly impact the prognosis of CRC and CRCLM patients. This highlights the critical prognostic value of TLSs within the tumor microenvironment, underscoring their potential as therapeutic targets and biomarkers in CRC management.
Hepatocellular carcinoma
HCC represents the most prevalent form of liver cancer globally, commonly associated with chronic liver diseases and cirrhosis, constituting a significant oncological challenge.405 In the study of HCC patients undergoing surgery, an increased number of TLSs in non-tumor liver tissues was observed to correlate with a higher likelihood of late recurrence and a shorter overall survival period.385 Contrasting this, research into the presence of TLSs within tumors found that patients harboring these structures demonstrated a significantly reduced risk of early postoperative recurrence, suggesting that intra-tumoral TLSs may serve as indicators of effective anti-tumor immune responses.19 Furthermore, analysis revealed that HCC patients with high intra-tumoral TLS content exhibited significantly improved RFS and DFS and identified characteristic genes associated with TLS positivity. This provides potential predictive markers for prognosis.406 These findings collectively underscore the pivotal role of TLSs in hepatocellular carcinoma, revealing that the location of TLS presence (intra-tumoral versus peri-tumoral tissue) has a significant impact on patient outcomes. These discoveries not only enhance our understanding of the immune composition within the tumor microenvironment but also offer new avenues for future therapeutic strategies, especially in designing treatments aimed at activating or enhancing intra-tumoral immune responses. Moreover, the identification of genes associated with TLS positivity opens avenues for personalized medicine and precision treatment, aiding in the identification of HCC patients who may benefit from specific immunotherapies.
Cholangiocarcinoma
Cholangiocarcinoma (CCA) represents a relatively rare yet severe form of cancer originating from the bile duct cells. The bile ducts are a series of small tubes tasked with the transport of bile, produced by the liver, to the small intestine, facilitating the digestion process. Based on the location of the cancer’s onset, CCA can be categorized into two main types: intrahepatic and extrahepatic CCA, with the latter further subdivided into proximal and distal extrahepatic CCA. In the investigation of extrahepatic CCA, this study delineated and categorized TLSs into lymphoid aggregates, primary, and secondary follicle-like configurations. Notably, the presence of intra-tumoral secondary follicle-like TLSs was significantly associated with enhanced overall survival and improved recurrence-free survival rates.407 Key markers, including PAX5, TCL1A, TNFRSF13C, and CD79A, were identified as positive indicators for the presence of TLSs within CCA, suggesting that intra-tumoral TLSs portend a favorable prognostic outlook. In contrast, TLSs situated in the peri-tumoral region were linked to negative clinical outcomes, marked by an increased frequency of satellite lesions, lymph node metastasis, and a higher incidence of fatty liver disease.386 Extending the scope to intrahepatic CCA (iCCA), a multicenter analysis elucidated that an augmented presence of TLSs within the tumor is correlated with superior overall survival, whereas proliferation of TLSs in the peri-tumoral area is associated with diminished overall survival.382
The abundance of TLS within tumors is identified as an effective prognostic marker for favorable outcomes in iCCA, while the presence of peritumoral TLS significantly correlates with adverse outcomes. This dual functionality of spatially distinct TLS, also recognized in hepatocellular carcinoma and breast cancer, remains to be fully understood. Our findings reveal a marked increase in the frequency of CD4+BCL6+ T follicular helper (Tfh) cells within tumor-infiltrating TLS compared to those surrounding the tumor, underscoring the pivotal role of Tfh cells in the maturation and maintenance of germinal centers, thereby shaping the antitumor immune milieu.408,409 High levels of Tfh cells within tumor-associated TLS could be foundational to favorable prognostic outcomes. In contrast, a significant enrichment of CD4+FOXP3+ regulatory T (Treg) cells is observed within tumor-associated TLS compared to peritumoral ones, suggesting a potential for immune suppression. Moreover, as peritumoral TLS abundance increases, there’s a significant rise in the frequency of Treg cells within tumor-associated TLS, hinting at a potential linkage between peritumoral TLS and the tumor’s immunosuppressive environment.134,385 Given the previous insights that peritumoral TLS may reflect an inflammatory backdrop fostering tumor growth and serving as a niche for malignant cell migration, we postulate that the scarcity of Tfh cells, coupled with morphological observations, suggests peritumoral TLS might be in an immature and dysfunctional state, potentially compromising the antitumor immunity facilitated by tumor-infiltrating TLS. This investigation into the contrasting roles of TLS within and around tumors offers a deeper insight into the intricate dynamics of the tumor microenvironment, highlighting the sophisticated interplay between Tfh and Treg cells that could pave the way for novel therapeutic interventions targeting the immune landscape of tumors.382 The investigation further illuminated a notable elevation in T follicular helper cells and regulatory T cells within tumor-associated TLSs, revealing a sophisticated immune milieu that markedly influences patient prognosis. Additionally, the detection of HEVs in TLSs was significantly correlated with extended recurrence-free survival, thereby emphasizing the prognostic relevance of these immune structures within the tumor microenvironment.410 These collective insights accentuate the pivotal role of TLSs as prognostic indicators and potential therapeutic targets in CCA, underscoring the intricate nexus between tumor biology and immune system dynamics in shaping patient outcomes.
Renal cancer
Renal cancer ranks as the thirteenth most common cancer worldwide. Clear cell renal cell carcinoma (ccRCC) is the most common subtype of RCC, accounting for 70–80% of all RCC cases. Typically originating from the epithelial cells of the renal tubules, ccRCC holds a central position in the research, diagnosis, and treatment of renal cell carcinoma due to its high incidence and distinct pathological features.411 Research has unveiled that the presence of TLSs within the tumor microenvironment is linked to adverse post-surgical outcomes in ccRCC, as well as resistance to anti-angiogenic treatments upon disease recurrence. Comparative analyses between renal and bladder cancer samples have highlighted a persistent immaturity of TLSs in renal tumors, with mature follicle-like TLSs absent in the germinal centers of ccRCC. This discrepancy in TLS maturation may contribute to the observed variances in patient prognoses.377 Further investigations have elucidated the dual roles of TLSs in tumor progression, revealing that TLSs in ccRCC predominantly localize to distal tumor regions, exhibiting immature and immunosuppressive characteristics. Such localization is correlated with poorer PFS and OS.
Conversely, TLSs situated in proximal tumor regions are significantly associated with enhanced PFS and OS. Notably, the presence of TLSs bearing mature characteristics, particularly those containing CD23+ GCs, is closely linked to more favorable clinical outcomes in ccRCC patients.412 Analyses of TME in ccRCC and adjacent non-cancerous tissues have demonstrated significantly higher levels of CD8 T cells, CD163+ macrophages, regulatory T cells, endothelial cells, and fibroblasts in ccRCC tissues. The immune infiltration patterns within the TME exhibited clustered and dispersed configurations. Tissues harboring TLSs showcased interactions between CD8 T cells and B cells, including GZMB+ cells, indicative of antitumor properties and improved prognosis. On the contrary, the macrophage/T cell cluster phenotype was associated with poorer outcomes. For patients with dispersed immune structures, further classification based on immune core numbers (CN) identified dispersed CN-hot and CN-cold phenotypes, both of which displayed better prognoses than the macrophage/T cell cluster phenotype, albeit with distinct immune characteristics and potential treatment responses.135 Moreover, leveraging spatial transcriptomics, the critical role of TLSs within the context of RCC has been underscored, highlighting their significance in promoting antitumor immune responses. The study meticulously detailed the transformation process of B cells within TLSs into plasma cells, a maturation process culminating in the production of specific antibodies targeting tumor cells, thereby triggering apoptosis. Furthermore, these findings emphasize the indispensable role of intra-tumoral TLSs in amplifying anti-tumor immune mechanisms, offering profound insights for the development of future therapeutic strategies.367
Bladder cancer
Bladder cancer is a prevalent malignancy of the urinary system, posing a significant challenge to public health worldwide. Originating from the epithelial cells of the bladder, the most common type is urothelial carcinoma. In the domain of bladder cancer research, a recent discovery has unveiled that the expression of CXCL13 is significantly associated with both PFS and OS in patients undergoing immune checkpoint blockade (ICB) therapy. This association, however, does not extend to patients who have not received ICB therapy, underscoring the potential role of CXCL13 in predicting the efficacy of ICB treatments. Moreover, the expression of CXCL13 is closely linked to the presence of TLSs within tumors, further affirming its importance as a marker of TLSs in bladder cancer.256 Analysis also revealed that bladder cancer patients with high levels of TLSs exhibit better prognoses and higher immune cell infiltration compared to those with low TLSs. Nonetheless, patients characterized by low TLSs demonstrated superior responses to ICB therapy and conventional chemotherapy. These findings highlight the pivotal role of TLSs in determining therapeutic responses and prognoses in bladder cancer patients.413 Further investigations have shown that the presence of TLSs in patients with muscle-invasive bladder cancer (MIBC) correlates with higher survival rates and increased responsiveness to PD-1 blockade therapy.414 TLSs are significantly more prevalent in MIBC patients compared to those with non-muscle invasive bladder cancer (NMIBC). The presence of TLSs is closely associated with the formation of GCs and an increased density of TILs. Furthermore, analyses indicate that TLS presence is associated with enhanced DFS in both NMIBC and MIBC patients.288 Additionally, another study has shown that, in comparison to untreated tumors, the fifth category (low macrophage) of TLSs was significantly higher following pre-operative immunotherapy. These critical findings underscore the key role of TLSs in the treatment and prognosis of bladder cancer patients, offering invaluable insights for future research and therapeutic strategies.415
Gastric cancer
Gastric cancer ranks among the most prevalent cancer types globally and is characterized by a diversity of biological properties, histological classifications, and clinical manifestations. Adenocarcinoma emerges as the most common form among these variants.416 In a study focusing on patients with gastric cancer, it was discovered that the presence of mature TLSs within tumor tissues is significantly positively correlated with the overall survival rates of the patients. Further analyses revealed a close association between high levels of TLSs and several clinicopathological characteristics of the tumor, including tumor size, histological grade, and disease staging. These associative findings underscore the potential role of TLSs in the progression of gastric cancer and suggest their value as a biomarker for assessing patient prognosis.417 Subsequent research indicated that in patients with specific stages of gastric cancer, a high density of TLSs is closely linked to better survival outcomes, a finding that was confirmed through various statistical analysis methods. These results not only reinforce the importance of TLSs within the tumor immune microenvironment but also provide vital reference information for future clinical practice, highlighting the potential to improve prognosis in gastric cancer patients by enhancing TLS levels.418 These research findings enrich our understanding of the role of TLSs in gastric cancer, offering significant scientific evidence for the development of new treatment strategies and the improvement of patient management.
Breast cancer
Breast cancer, a malignant tumor originating from breast tissue, stands as the most prevalent cancer among women worldwide.419 A series of studies have elucidated the pivotal role of TLSs in breast cancer. It has been observed that there is a positive correlation between tumor-associated neutrophils and tumor-infiltrating B cells, with this correlation being more pronounced in patients with low TLS levels compared to those with high TLS levels. Furthermore, patients exhibiting mature TLSs demonstrated superior outcomes in both chemotherapy and immunotherapy.366 Analysis of the Cancer Genome Atlas breast cancer cohort revealed that the presence of TLSs is associated with improved DFS and OS. This association positively correlates with early tumor TNM staging, a high quantity of TILs, and the expression levels of Human Epidermal Growth Factor Receptor 2 and Ki-67, while exhibiting a negative correlation with the status of estrogen and progesterone receptors.420 This indicates that a high TLS signature is indicative of a more favorable TEM, leading to better survival outcomes. In triple-negative breast cancer, combining the assessment of TLSs with tumor budding (TB) showed a positive correlation with OS and RFS, suggesting that the TLS/TB model may surpass the classical tumor-lymph node metastasis staging system in predicting patient outcomes.421 Moreover, in the breast cancer tumor microenvironment, TLS presence is positively associated with high tumor grade, phenotype, necrosis, extensive in situ components, lympho-vascular invasion, and high levels of TILs, as well as with hormone receptor negativity, HER2 positivity, and c-kit expression. Notably, in HER2-positive breast cancer, the presence of TLSs significantly correlates with better DFS, independently of high TIL expression, indicating that the combined status of TLSs and TILs represents an independent favorable factor related to DFS.422
Uterine cancer
Uterine cancer, a malignancy originating from the cervix of the female uterus, represents a significant category of gynecological cancers that can be effectively managed through regular screening and timely intervention.423 Within the context of endometrial cancer patients, the presence of TLSs has been demonstrated to be a favorable prognostic marker, significantly impacting both recurrence timelines and cancer-related mortality rates. Specifically, patients with TLS exhibit a markedly reduced risk of recurrence within a 5-year period compared to those devoid of TLSs. Moreover, research indicates that mature TLSs serve as the optimal predictive markers when integrated with clinicopathological factors and molecular classifications, offering superior predictive value over densities of CD8 or CD20. Intriguingly, mature TLSs may express L1 Cell Adhesion Molecule (L1CAM), a feature not observed in immature TLSs, and the employment of L1CAM as a marker for mature TLSs achieves a high level of observer agreement.424 Further investigations have elucidated a negative correlation between the presence of TLSs and patient tumor progression, particularly noting a significant extension in progression-free survival when CD23+ GCs are contained within TLSs. Additionally, the quantity of CD20+ B cells within TLSs significantly surpasses that in patients without TLSs, with these B cell numbers positively correlating with other TLS components and associated with improved progression-free survival outcomes.425 Overall, the presence of TLSs and the infiltration of B cells within them are closely linked to more favorable survival outcomes in patients with endometrial cancer.
Pancreatic cancer
Pancreatic cancer, characterized by its highly lethal nature, originates from the cells within the pancreas and is frequently diagnosed at an advanced stage, presenting significant challenges for effective treatment.426 The presence of TLSs, whether nascent or mature, is associated with higher survival rates and favorable prognoses in pancreatic ductal adenocarcinoma (PDAC). Studies suggest that TLSs enhance adaptive immune responses within TEM. PDAC with TLSs is enriched with memory lymphocytes and IgG-switched B cells, which correlates with prolonged survival.77 Moreover, a subset of PDAC exhibits TLSs that support the proliferation and differentiation of B cells into plasma cells, and these mature TLSs also bolster T cell activity, being rich in tumor-reactive T cells. Chronic activation of tumor-reactive T cells may organize TLSs through the production of the B cell chemoattractant CXCL13.277 In PDAC patients undergoing chemoimmunotherapy, a gene signature reflective of mature TLSs in pretreatment biopsy samples correlates with extended survival. The presence of TLSs in patients receiving surgical treatment significantly correlates with improved PFS and OS, associated with increased infiltration levels of CD8+ T cells, CD4+ T cells, B cells, and activated immune cells.427 However, the prognostic value of TLSs is not significant in patients receiving neoadjuvant therapy. TLSs are associated with high levels of CD4+ TILs, CD8+ TILs, and CD45RO+ TILs but not with high levels of FOXP3+ TILs, correlating with longer pancreas-specific survival and favorable outcomes with adjuvant S-1 therapy.428 These findings highlight the potential of TLSs as prognostic markers and therapeutic targets in PDAC, revealing complex interactions between the tumor microenvironment and the immune system. They suggest that enhancing or mimicking the functions of TLSs could improve therapeutic outcomes for PDAC patients.
Skin cancer
Skin cancer is primarily categorized into three major types based on the cell of origin: basal cell carcinoma, squamous cell carcinoma, and malignant melanoma.429 Additionally, there exist rare yet highly aggressive forms of skin cancer, such as Merkel cell carcinoma. Current research on TLSs in skin cancer has predominantly focused on melanoma, squamous cell carcinoma of the skin, and Merkel cell carcinoma, reflecting a concentrated effort to understand the immunological landscape within these malignancies. In studies of cutaneous malignant melanoma, it has been found that the presence of TLSs in 47% of cases was associated with an increase in TILs, although the proliferation of lymphocytes within the TLSs did not correlate with TIL proliferation.388 The presence of TLSs was linked to a reduced risk of tumor recurrence and an improvement in OS, particularly in cases with a lower proportion of CD21+ B cells in TLSs.
Further research has shown that the presence of tumor-associated CD8+ T cells and CD20+ B cells correlate with better survival outcomes, a correlation that is independent of other clinical factors. Tumors with a high concentration of B cells also exhibited increased levels of TCF7, indicative of naive and/or memory T cells. In melanoma lacking TLSs, T cells displayed a dysfunctional molecular profile, underscoring the importance of TLSs in influencing the immune microenvironment and T cell phenotypes for an enhanced immune response.378 In Merkel Cell Carcinoma (MCC), analysis indicated that samples with TLSs had a significantly better prognosis compared to those without. Merkel cell polyomavirus (MCPyV) is a major causative agent of MCC. It contributes to the malignant transformation and oncogenesis of host cells through the integration of its viral genome into the host cell DNA and the action of viral proteins. Notably, the prognosis of MCPyV-positive samples was favorable regardless of TLS presence, but MCPyV-negative samples with TLSs had a prognosis similar to that of MCPyV-positive samples, highlighting the complex interplay between viral factors and immune structures in tumor environments.430 For Cutaneous Squamous Cell Carcinoma (cSCC), the presence of TLSs was significantly associated with better histopathological grades and higher levels of sun exposure. Additionally, the presence of intra-tumoral TLSs was linked to lower lympho-vascular invasion, suggesting that TLSs may play a protective role in the progression of cSCC.379 Collectively, these findings emphasize the critical role of TLSs in modulating the immune landscape across various skin cancers, potentially guiding more effective therapeutic strategies.
Other tumors
In studies of other tumor types, such as gliomas and epithelioid mesotheliomas, the presence of TLSs within the tumor microenvironment has been significantly correlated with a more favorable prognosis and a better response to therapy. These findings underscore the critical role of TLSs in the tumor immune microenvironment, suggesting that they may serve as key modulators of tumor biological behavior, influencing patient survival and response to treatment. Therefore, a deeper exploration of the functions and mechanisms of TLSs across different tumor types not only aids in our understanding of the complexities of tumor immune evasion but also lays the theoretical groundwork for developing novel immune therapeutic strategies based on TLSs. Furthermore, employing TLSs as biomarkers for clinical prognostication and personalized treatment decisions may pave a new pathway for enhancing the efficacy of cancer therapies.381,431,432
Recent studies have shown that not only the heterogeneity of TLS itself but also changes in cellular metabolic levels and the acidity of TME can affect TLS formation and function, thereby altering the immune niche. Hu et al. recently reported that T-cell glucose metabolism promotes TLS formation. In TLSs within advanced-stage gastric cancer, B cells produce LTα binding to TNFR2 on the surface of CXCL13+CD103+T cells, which activates tumor necrosis factor receptor-associated factor 2 (TRAF2), enhancing CXCL13 expression through the photoshatidylinositol-3 kinase (PI3K)/protein kinase B (AKT)/mTOR pathway. And mTOR is an essential glycolysis-regulating pathway.299 However, there appears to be a negative feedback regulatory function that prevents immune over-activation. Because an acidic environment inhibits T cell function. A study suggested that the pH of the T-cell area in the LN (6.3) was significantly lower than other sites (LN peripheral pH 7.1 and peripheral vessel pH 7.1).433 Due to aerobic glycolysis occurring in T cell activity leading to lactate accumulation.434 However, decreased pH inhibits T-cell lactic acid efflux and glycolysis, and suppresses cytokine release, such as IL-2 and IFN-γ, but not T-cell activation.433 Tumor cells are characterized by aerobic glycolysis, which suppresses T cell function. Additionally, T cells produce lactic acid, leading to a decrease in pH in TLS, which suppresses T cell-dependent immune function and even inhibits TLS development. More importantly, this probably explains the absence of mature TLS in some tumors. We speculate that, in the early stages of TLS development, tumors with high levels of glycolysis produce acid accumulating in the TME and decreasing in pH, which inhibits the LTo cell-like function of T cells and impairs the efficiency of TLS formation. Therefore, selectively manipulating the pH of tumor-associated TLSs may enhance T cell function, which is beneficial for immunotherapy. However, the effect of acidic TME on B cell function in TLS remains unknown, which also requires further study.
Autoimmune diseases and immunoinflammatory
Rheumatoid arthritis
Rheumatoid arthritis (RA) is among the most prevalent autoimmune diseases, characterized pathologically by synovitis, marked by the infiltration of immune cells, proliferation of synovial fibroblasts, and neovascularization.435 RA can be categorized into three distinct pathological types: the lympho-myeloid type, featuring aggregates of B cells; the myeloid type, characterized by infiltration of macrophages in the synovial lining; and the pauci-immune fibroid, notable for its lack or minimal inflammatory cell infiltration.55 In the assessment of untreated RA cases, it was discovered that high synovial mast cell counts correlate with local and systemic inflammation, autoantibody positivity, and increased disease activity. These mast cells, situated on the periphery of lymphoid aggregates, were shown to play a significant role in promoting the activation and differentiation of naive B cells, as well as inducing the production of anti-citrullinated protein antibodies (ACPAs), primarily through contact-dependent interactions.436 Additionally, the presence of TLSs was associated with a more severe disease course in RA. In a separate evaluation, it was found that endothelial cells can produce NF-κB-inducing kinase, a key player in non-classical NF-κB signaling triggered by LTβ receptor signaling, which contributes to the formation of TLSs in RA.437 This highlights the complexity of cellular interactions and signaling pathways involved in the progression and severity of RA, underscoring the importance of TLSs and mast cells in the disease’s pathology.
Systemic lupus erythematosus and lupus nephritis
Systemic lupus erythematosus (SLE) is a chronic, multisystem, autoimmune disease characterized by clinical heterogeneity. Lupus nephritis is a form of glomerulonephritis that can occur in patients with SLE and is one of the most common and severe organ manifestations of the disease. Lupus nephritis is characterized by the deposition of immune complexes and inflammation of the renal tubulointerstitial area.438 A study found that in patients with lupus nephritis or SLE, the development of TLSs in the kidneys is a significant phenomenon. It was observed that under conditions of chronic inflammation, mesenchymal stem cells express various inflammatory markers and play a crucial role as lymphoid tissue organizers in accelerating the inflammatory process and initiating the formation of kidney-specific TLSs.196 Additionally, an analysis of SLE patients revealed that those with lupus nephritis exhibited different classes of disease severity based on the expression of CD20, CD3, and CD21 in the kidneys. The presence of CD20 and the formation of TLSs were associated with a longer disease course, a poorer response to therapy, and higher levels of CXCL13, a chemokine linked to B cell recruitment and renal function impairment. Importantly, CXCL13 levels were found to be positively correlated with the severity of tissue inflammation and the number of B cells in the renal tissue, highlighting its potential role as a marker for disease progression and treatment response in lupus nephritis patients.439
Sjögren syndrome
Sjögren’s syndrome (SS) is a chronic, systemic autoimmune disorder characterized by lymphocytic infiltration of the exocrine glands, primarily the salivary and lacrimal glands, and notable B-cell hyperactivity.440 Numerous studies have found the presence of TLSs in patients with Sjögren’s syndrome, which contribute to the progression of the disease. It was discovered that in salivary glands with TLSs, the expression of NCR3 (NKp30, CD337) was upregulated and showed a strong positive correlation with genes encoding granzyme B, perforin, and IFN-γ. This suggests an enhanced immune response in these regions. Furthermore, treatment with rituximab was found to prevent the reduction of NKp30 expression on NK cells, thus diminishing B cells, FDC networks, and ectopic germinal centers.182 In a separate evaluation focusing on the serum levels of CX3CL1 in primary Sjögren’s syndrome (pSS) patients, a significant elevation compared to controls was observed, indicating an inflammatory response. This elevation was also noted in patients with rheumatoid arthritis but not in those with Sicca syndrome, highlighting a potential marker for distinguishing between these conditions. Histological analysis further revealed the presence of CX3CL1 and its receptor within the salivary glands of pSS patients, specifically localized within tertiary lymphoid structures, reinforcing the role of this chemokine in the pathogenesis of pSS.267
Pemphigus
Pemphigus constitutes a group of rare autoimmune skin disorders characterized by the emergence of blisters and erosions on the skin and mucous membranes, resulting in significant pain and potential infections.441 In the study of patients with Pemphigus Vulgaris (PV) and Pemphigus Foliaceus (PF), it was found that CD3 T cells were present in all samples, and a high percentage contained CD20 B cells, CD138 plasma cells, HEVs positive for peripheral node addressin, and CD21+ monocytic cells, indicating that TLSs are prevalent in most pemphigus lesions.292 Similarly, another investigation into the skin of pemphigus patients revealed the presence of skin TLSs and desmoglein-specific B cells in chronic blisters. Within these TLSs, CD4 T cells, predominantly producing CXCL13, exhibited characteristics of activated Th1-like cells with downregulated genes related to T cell receptor-mediated signaling. Regulatory T cells (Tregs) were found to interact directly with CXCL13CD4 memory T cells, enhancing CXCL13 production through mechanisms involving IL-2 consumption and TGF-β stimulation. Additionally, the application of intralesional corticosteroids showed improvement in chronic blisters by reducing skin TLSs, suggesting a potential therapeutic strategy for pemphigus.137
Autoimmune encephalomyelitis
Autoimmune encephalomyelitis refers to a group of diseases caused by an autoimmune response, in which the body’s immune system mistakenly attacks and destroys tissues of the central nervous system (CNS), including the brain, spinal cord, and optic nerve. This type of inflammation is typically associated with the presence of specific autoantibodies that target particular antigens in the CNS.442 Research has illuminated the presence of TLSs within the meninges of multiple sclerosis (MS) patients, particularly during the progressive phase, which is tightly linked to cortical lesions and disability.443 Further investigations have revealed TLSs in the subarachnoid space, associated with extensive periventricular infiltration predominantly composed of CD20+ B cells and FDCs in some instances.444 Additionally, analyses of post-mortem brain tissues from MS cases have shown an upregulation in the expression of pro-inflammatory cytokines, such as tumor necrosis factor and interferon-gamma, within the meninges of patients with secondary progressive MS, characterized by the manifestation of TLSs. The severity of meningeal inflammation and the presence of TLSs in the MS brain is directly correlated with increased levels of TNF and IFN genes, along with their protein expressions in the meninges and cerebrospinal fluid compartments.445 This body of evidence underscores the significant role of TLSs in the pathogenesis and progression of MS, highlighting their potential as targets for therapeutic intervention.
Diabetes mellitus
Diabetes mellitus is a chronic metabolic disorder characterized by elevated blood sugar levels, primarily caused by insufficient insulin secretion or impaired insulin action. In a study involving 24 patients with type 1 diabetes, it was found that 21 of them exhibited insulitis in the pancreas, with 12 showing the presence of TLSs. The frequency of TLSs in the pancreas significantly diminished with increasing age. The occurrence of TLSs was closely associated with the severity of the disease, with the highest frequency observed in islets exhibiting insulitis (stages 1 and 2 islets). Mature TLSs, characterized by segregated T and B cell regions, were identified in only four patients with type 1 diabetes. Compared to patients with immature TLSs or without TLSs, those with mature TLSs had a significantly shorter duration of disease, with mean durations of 0.03 ± 0.035 years, 2.909 ± 2.709 years, and 2.948 ± 2.564 years, respectively. These findings suggest that the presence of TLSs in the pancreas of patients with type 1 diabetes is intricately linked to the progression and severity of the disease, and that mature TLSs may be associated with a shorter disease course.446
Uveitis
Uveitis is an inflammatory condition affecting the uvea, the middle layer of the eye, encompassing the iris, ciliary body, and choroid. It can lead to symptoms such as blurred vision, eye redness, pain, and photosensitivity, with severe cases potentially resulting in vision loss.447 The presence of TLSs was identified within the retinas of mice with uveitis, indicating a complex immunological involvement. In a related study on human samples, TLSs were found in 20% of the cases, particularly in those with chronic, persistent uveitis. This suggests that the condition leads to a significant dysregulation of ocular immune surveillance, marked by the emergence of TLSs.291,448 These findings collectively underscore the importance of TLSs in the pathophysiology of uveitis and highlight the critical role of immune system dysregulation in its progression.
Giant cell arteritis
Giant Cell Arteritis (GCA) is an autoimmune condition predominantly affecting individuals over the age of 50, characterized by chronic inflammation of large and medium-sized arteries, especially those in the head and neck region, potentially leading to vision loss and other severe complications.449 Recent investigations into GCA have revealed the pivotal role of TLSs in the disease’s pathology. In aortic tissues from patients with GCA, TLSs were identified in a significant majority, with a notable portion containing GCs, a feature less commonly observed in atherosclerotic tissues.450 Similar findings were echoed in temporal artery samples from GCA patients, where TLSs were frequently detected within the media layer, closely associated with high endothelial venules. These structures’ formation appeared to be linked to the expression of critical molecules and cells such as CXCL13, BAFF, APRIL, lymphotoxin β, and interleukins IL-17 and IL-7, among others. This association suggests a complex interplay of immune responses in GCA, further underscored by the observation that stimulating myofibroblasts with specific agonists and cytokines enhances the expression of BAFF and CXCL13.51 These insights underscore the intricate immunological landscape of GCA, highlighting the significance of TLSs in its pathogenesis.
Dermatomyositis
Dermatomyositis (DM) is an immune-mediated inflammatory disease characterized by specific lesions in the skin and skeletal muscles.451 It was observed that the lesions were infiltrated by a diverse immune cell population, including CD8+ cytotoxic T cells, macrophages, CD4+ cells, and B cells. Notably, these immune cells were arranged in a manner reminiscent of lymphoid follicles, with CD21+ follicular dendritic cells within these structures. Additionally, the vasculature associated with these lesions displayed features of HEVs, indicating a complex immune microenvironment within the affected muscle tissue.452,453 This indicates the presence of TLSs in DM, which influences the disease progression.
Transplant
The history of solid organ and skin transplantation in experimental and clinical practice is marked by early attempts that were often thwarted by immune rejection.454 However, with advancements in more precise tissue matching and immunosuppressive techniques, the success rates of organ transplants have improved, although rejection remains a potential issue. Notably, the occurrence of rejection can lead to the formation of TLSs.
In the majority of current research on transplant-related TLSs, it was found that TLSs can enhance immune rejection, leading to a poorer prognosis.455 However, some studies have discovered that certain components within TLSs inhibit immune functions, facilitating immune tolerance, which may result in a reduction of immune rejection responses.456
Heart transplant
An analysis of 319 murine allograft heart transplants revealed the presence of TLSs in 78 cases, with a significant observation that 78% of allografts undergoing chronic rejection showed TLSs.455 Further validation highlighted the association of TLSs with immune rejection responses in allogeneic heart transplants. In another study, epicardial coronary arteries from 59 transplant patients were examined, showing a significant correlation between the presence and size of TLSs surrounding the coronary vessels and the time post-transplantation. Patients were divided into groups based on the average size of TLSs, revealing that those with larger TLSs (classified as TLS-2) had consistently shorter post-transplantation survival times compared to those with no or smaller TLSs (classified as TLS-0 and TLS-1).62 This suggests a strong link between the presence and size of TLSs and the progression of cardiac allograft vasculopathy.
Kidney transplant
In kidney transplantation, it has been discovered that although B cell subpopulations within TLSs include naive, plasma, and memory B cells predominantly localized within or near these organs, TLSs are associated with immune tolerance in the renal allograft model.456 The presence of small B cell clusters within the first two months post-transplantation is not linked to early rejection episodes. TLS-like structures can be present even in animals without rejected allografts and may disappear over time. While TLSs are more commonly found in samples with interstitial fibrosis and tubular atrophy (IFTA), their presence is also noted in samples without IFTA, indicating that the presence and density of clusters resembling TLSs reflect immune responses within the graft and do not necessarily predict poor graft outcomes or IFTA. TLSs are less common shortly after transplantation but become denser and significantly increase in rejected grafts over time. TLSs with GCs can be observed after 6 to 10 weeks post-transplantation, even in the absence of acute rejection episodes, and in acutely rejected grafts, the formation of TLSs can be detected as early as 12 days post-transplantation, suggesting a dynamic response in the graft immune environment.457
Lung transplant
IL-2 pretreatment of donors was found to effectively prevent acute immune rejection, as evidenced by macroscopic and histological examinations on day 5 post-transplantation. This intervention substantially enhanced graft survival, preserving lung functionality beyond 90 days. Moreover, the targeted deletion of FOXP3+ Tregs via diphtheria toxin receptor-mediated treatment disrupted the formation of TLSs, consequently eliciting a pronounced immune rejection.458
Infection
During the transition to the chronic phase of inflammation, there is a gradual increase in the numbers of plasma cells and lymphocytes, which induces the formation of lymphatic vessels. Concurrently, blood vessels acquire the characteristics of
HEVs, specifically tailored to facilitate the extravasation of lymphocytes. In certain instances, chronic infiltration organizes into structured lesions, such as the granulomatous inflammation observed in tuberculosis, Crohn’s disease, and sarcoidosis. These chronic infiltrates form distinct aggregates rich in T and B cells, known as TLSs.459,460
Colitis
Large amounts of TLS are found in colitis lesions, which are formed by inflammation induction. Its formation process is closely related to the regulation of nerve secretion. In a colitis mouse model, the vagus nerve stimulates intestinal neurons, causing stromal cells to produce CXCL13, which in turn induces the formation of TLSs. However, cutting off the vagus nerve eliminates the formation of TLSs without affecting the progression of colitis, possibly due to the limitations of the model. This phenomenon is worth further study to clarify the functional relevance of TLSs in intestinal immune responses.459
Crohn’s disease is a specific inflammation of the intestines. Tissue imaging results show that the lymphatic structures of the patients with CD were dilated, and their permeability was altered by using Patent Blue Tracing technology. Compared to non-inflammatory bowel disease samples, there are more TLSs in Crohn’s disease samples. These TLSs reshape the structure of lymphatic pathways, altering lymph fluid flow and potentially affecting immune function and disease development.370 Guedj et al. found that the inflammatory cytokines TNF-α and LPS stimulate adipocytes around CD, making them organizers of TLS and thus coordinating TLS formation. They believe that in CD, TLSs are harmful because TLSs are associated with a more severe phenotype of CD.460
Chronic obstructive pulmonary disease (COPD)
New research evidence supports a key role for TLSs in exacerbating lung damage and promoting the exacerbation of COPD. Ladjemi et al. revealed that T cells activated B cells to secrete IgA by secreting IL-21, triggering an adaptive immune response. In COPD patients, increased pulmonary TLSs correlate with disease severity, but they lack direct evidence to show that TLSs will lead to the deterioration of COPD.461 Further research discovered that DCs were attracted by damaged airway mucosal SIgA immune barriers through the CCR2 pathway in COPD patients. This led to local lymphocyte aggregation, TLS formation, and T cell activation, thereby exacerbating lung damage.179 This study further reveals the association between the presence of TLSs and the pathological deterioration of COPD. And another study confirmed that IL-18+ myeloid cells in COPD lungs’ TLSs stimulate responsive lymphocytes to release IFN-γ, promoting tissue damage. IL-18+ macrophages and DCs are first located in pulmonary TLSs, making increased TLSs a marker of severe COPD.462 Thus, TLSs may exacerbate COPD tissue damage.
Nasal Polyps
In patients with chronic rhinosinusitis with nasal polyps (CRSwNP), TLSs are prevalent in the nasal mucosa. The TLSs contribute to the inflammatory burden in nasal polyps by synthesizing immunoglobulins, such as IgE and IgA, as well as proteins such as IL-1β and IL-33, thereby precipitating chronic conditions.302,463,464,465 More eosinophilic (accounting for 20.69%) and noneosinophilic (accounting for 17.31%) CRSwNP patients have TLSs, with higher levels of IgE, IgG, and IgA in eosinophilic polyps with TLSs, indicating increased inflammation. The study confirmed the presence of numerous plasma cells in TLSs that produce these immunoglobulins. Patients with TLSs have a longer disease course, more severe nasal congestion, and a higher frequency of past surgeries compared to those without TLSs.464
Bacterial infectious disease
TB is the most common bacterial infectious disease globally, and clinical results show that gender is an influential factor in the progression of TB. Further studies found that male patients may experience aggravated disease progression due to TLS impairment during Mycobacterium tuberculosis (Mtb) infection. By experimenting with Mtb infection in mice of both sexes, it was found that male mice had smaller B-cell follicles in the TLSs of the lungs, as well as lower expression of the chemokines CXCL13 and CCL19 in vivo, compared to female mice. This could be due to less IL-23 expression in males, impairing TLS formation in the lungs after Mtb infection, making males more susceptible to Mtb, which in turn promotes the progression of TB.466 In pneumococcal pneumonia, TLS’s role differs. In a model of continuous Staphylococcus aureus (S. aureus) infection in mouse lungs, B cells and T cells are key in TLS formation. Blocking TLS formation doesn’t cause death or increase bacterial load in the lungs, and the absence of B and/or T cells doesn’t exacerbate lung infection or reduce survival rate, indicating TLSs aren’t necessary in controlling S. aureus infection.467
Previous studies demonstrated that TLSs have a positive therapeutic role in viral infections. In models of pulmonary infection, TLSs rapidly develop following a viral or bacterial challenge, orchestrating a potent immune response. This response encompasses antigen-specific T and B cells, offering protection against harmful pathogens. By blocking the IL-1 signaling pathway, Neyt et al. found that iBALT formation was impaired, along with delayed viral clearance from the lungs, prolonged viral replication, and an increase in the severity of disease.197 Therefore, we can speculate that the formation of TLSs may be related to a good prognosis for viral infection. DCs are crucial for iBALT formation and maintenance in the lungs. The disintegration of iBALT by depleting DCs in the lungs confirmed that iBALT produces protective antiviral serum immunoglobulin antibodies through GC-dependent Ig class conversion.177 They play an important role in the removal of the virus. Therefore, iBALT plays a positive immune response role in viral infections.
Myocarditis
The formation of TLSs in recurrent severe myocarditis, potentially a case of autoimmune myocarditis, was first reported in 2020.468 The specific role of TLSs in this condition is unclear. However, in viral myocarditis, viral infection induces TLS formation in myocardial tissue, the amount of which positively correlates with the severity of the disease. Zhu et al. found the GC response of TLSs promotes local anti-heart autoantibodies (AHA) production, exacerbating heart inflammation. Blocking IL-17 or PDPN to reduce TLS formation can alleviate myocarditis symptoms.469 Therefore, TLS may become a new marker to predict the development and prognosis of viral myocarditis.
Ageing
The frequency of TLS occurrence in aged individuals receiving stimuli significantly increased compared to younger individuals, which markedly influenced disease progression and prognosis. Yuki Sato et al. have established a potential new model for inducible sterile TLS formation, demonstrating the destruction of the adjacent renal parenchyma around the kidney TLS, a close association between TLS size and renal dysfunction, and the renal expression of the pro-inflammatory cytokine AID. In aged damaged kidneys, TLSs exhibit different stages of maturation. In the early stages of TLS formation, perivascular fibroblasts receive retinoic acid via paracrine signaling from Retinaldehyde Dehydrogenase fibroblasts and dedifferentiate into p75NTR fibroblasts, some of which acquire the ability to produce homeostatic chemokines (CXCL13/CCL19). As the TLT grows and expands, the number of retinoic acid-producing fibroblasts around the TLS gradually decreases. During this stage, CD21+ p75NTR− FDCs (follicular dendritic cells) emerge as part of the stromal network, and PNAd+ HEVs (peripheral node addressin-positive high endothelial venules) develop within the TLS. Notably, TLSs are observed in aged, but not young, human kidneys, with cellular and molecular components highly similar to those in mouse TLSs. Therefore, age-dependent kidney TLS formation appears to be conserved across species, and the mechanisms underlying the progression of renal diseases in elderly humans might be similar to those in aged mice. Although renal TLSs have only been reported in patients with immune-mediated diseases such as autoimmune disorders, chronic rejection, glomerulonephritis, and infections, we found that age-dependent kidney TLSs are also present in various types of renal diseases, including diabetic nephropathy and benign nephrosclerosis. This suggests the universality of TLS formation with ageing.371
Studies have shown that as mice age, there are significant changes in the expression of immune-related genes in bladder tissue, particularly an increase in B cell-related gene expression, which is especially pronounced in female mice.470 The increased density of B cells in the bladder is closely associated with the formation of TLS, which are typically induced by exposure to commensal or pathogenic microorganisms, mucosal administration of IFN-1 activating vaccines, or chronic local inflammation.471 The formation of TLS in the bladder is not only linked to the elevated systemic levels of TNF-α observed during normal ageing but also to chronic inflammatory conditions such as interstitial cystitis and bladder cancer.472,473Mechanistically, the study found that with advancing age, there is a significant upregulation of B cell-related genes such as Cd19, Cd79a, Pax5, Mzb1, and Ms4a1 in the bladders of aged female mice. This suggests that the diversity and distribution of B cells in the bladder mucosa may be related to the formation of TLS. Additionally, the increased expression of CXCR5 and its ligand CXCL13 in the bladders of aged female mice further supports the recruitment of B cells to the bladder mucosa and the formation of TLS.474 The research also revealed an increased expression of immune checkpoint genes such as PD-L1 in the bladders of aged female mice, which may reflect a state of exhaustion in these immune cells. Furthermore, the expression of other immune checkpoint genes like Lag3 and Btla was significantly elevated in aged female mice, suggesting that these genes may play important roles in regulating immune responses. Since large-scale sequencing methods cannot identify the specific cell types exhibiting the exhaustion phenotype, further identification and functional analysis of these cells using single-cell sequencing and other methods will be crucial for comprehensively understanding these changes and developing precise therapeutic targets. Moreover, the study found that exposure to the carcinogen BBN led to increased PD-L1 protein expression in the bladders of aged female mice, particularly in urothelial and endothelial cells. This may be due to N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) -induced DNA damage and activation of the cellular IFN pathway.475,476 High expression of PD-L1 on endothelial cells is known to inhibit T cell activation, indicating that future research is needed to elucidate the mechanisms underlying this checkpoint to define its dynamic role in disease progression and potential immune evasion.372
The relationship between TLSs and current anti-tumor treatment
Research has demonstrated that TLSs are associated with the prognosis and responsiveness of various cancer treatments, such as surgical resection, immune checkpoint blockade (ICB), chemotherapy, antiangiogenic therapy, radiotherapy, and vaccines (Fig. 6).
Therapies related to TLS formation and function. Several methods have been designed to induce TLS formation. Stromal cells injected alone or co-injected with artificial scaffolds, or DCs, provide a favorable space for TLSs. Chemokines and cytokines involved in TLS formation and B cell maturation, such as IL-13, CXCL13, LIGHT, LTα1β2, and CCL21, are introduced through engineering DCs and biomaterials. In addition, some receptor agonists also promote TLS formation. Other therapy strategies, including chemotherapy, vaccines, agonists, and ICB therapies, can also modulate TLS function
In certain cancers, such as ESCC396 and colon cancer,477 TLSs have been identified as being related to the prognosis following resection. In a study involving 650 cases of surgically resected ESCC, it was demonstrated that ESCCs with mature TLSs exhibited significantly improved survival compared to both TLS-immature and TLS-negative ESCCs (p < 0.05). Specifically, the 5-year OS rates were 66.5%, 50.0%, and 50.2% (p = 0.001), while the disease-free survival (DFS) rates were 55.6%, 39.9%, and 46.3% (p = 0.003) for patients with TLS-mature, TLS-immature, and TLS-negative ESCCs, respectively.396 In the research findings on colorectal cancer, an analysis of the prognosis following resection of left and right colon cancers revealed heterogeneity in the presence of TLS. Specifically, the density of TLS serves as a prognostic marker for survival post-resection in patients with right-sided colon cancer. However, this marker does not offer a reliable prediction for patients with left-sided colon cancer.477
Neoadjuvant chemotherapy, a type of neoadjuvant therapy, was originally used as a preoperative treatment for tumors. However, in recent years, a new method of neoadjuvant chemotherapy has often been proposed: combination with other drugs. With the prevalence of immunotherapeutic agents, they are often combined with ICB in neoadjuvant chemotherapy, namely neoadjuvant chemoimmunotherapy. Many reports have described increased immune cell infiltration in tumors locally after treatment with neoadjuvant chemotherapeutic agents.478 In hepatoblastoma, pathological analysis revealed extensive TLSs in the tumor after neoadjuvant cisplatin chemotherapy, which were associated with a favorable prognosis.479 In the NEOSTAR trial, the combination of ipilimumab and nivolumab resulted in higher levels of intra-tumoral immune cell infiltration and more mature TLS characteristics.480 Additionally, the combination of nivolumab and cabozantinib increased the infiltration of effector T cells and memory T cells in hepatocellular carcinoma. And compared to those surgically resected, responders for combination treatment exhibit a highly immune-infiltrated focus, i.e., TLSs.481 Moreover, TLSs also play a prognostic role in neoadjuvant chemoimmunotherapy, such as NSCLC. Patients with mature or high-density TLSs show better prognoses in cancers after neoadjuvant chemoimmunotherapy.397 Sun and colleagues conducted a comparative analysis of tumor tissues with untreated, neoadjuvant chemoimmunotherapy, and neoadjuvant chemotherapy. Their investigation revealed increased CD8+ T cells and mature TLSs after neoadjuvant chemoimmunotherapy. Besides, the neoadjuvant chemoimmunotherapy group showed a higher major pathological response rate and pathological complete response rate.397 In another clinical study of NSCLC, preoperative application of 2-4 cycles of nivolumab combined with chemotherapy improved long-term survival, significantly increasing 3-year DFS from 50% to 63%. Responders also showed higher levels of mature TLSs.482 Xu et al. also reported that TLSs were more readily observed in patients responsive to neoadjuvant chemoimmunotherapy and validated the advantageous prognostic value of TLSs in NSCLC patients.483 These findings suggest that immunotherapy may induce TLS formation, and the maturity of TLSs could serve as a biomarker for better prognoses in patients with neoadjuvant chemoimmunotherapy. However, neoadjuvant chemotherapy treatment with combined corticosteroids impairs the formation of GCs in LSCC, negatively affects the development of TLS, and eliminates its prognostic value in patients with lung cancer.5
The presence of intra-tumoral TLS is associated with positive clinical outcomes and responses to immunotherapy in cancer. In multiple types of cancer, such as NSCLC,398 HNSCC,484 and ESCC,383 it has been discovered that TLSs enhance the efficacy of anti-PD-L1 therapy, increasing their responsiveness to ICB. In ESCC patients, mature TLSs serve as an independent prognostic factor. They show increased proliferative B cells, PCs, and memory B cells. Additionally, patients with mature TLSs and high T-cell infiltration (including CD8+ T cells, Th cells, and Th17 cells) exhibited higher survival rates. Notably, immunotherapy-treated patients had a higher proportion of mature TLSs compared to untreated patients (50.7% vs. 41.3%).152 Data from a study involving patients with triple-negative breast cancer suggests that TME with high levels of CCL19+ DC infiltration is characterized by immunogenic structures. After anti-PD-1 immunotherapy, CCL19+ DCs improved T-cell responses.171 Furthermore, high levels of CXCL13+ T cells and pro-inflammatory macrophages were associated with paclitaxel or its combination with anti-PD-L1 atezolizumab. And CXCL13+ T cells co-localized with B cells in TLSs.485 In uveal melanoma, pembrolizumab and entinostat treatment increased activated T cells, monocytes, and chemokines. And the presence of TLS prolonged PFS and OS in patients.262 Another study on metastatic melanoma demonstrated a significant improvement in survival trends for patients with tumor-infiltrating TLSs. In tumors lacking TLSs, T cells exhibited a dysfunctional molecular phenotype. Additionally, TLShigh tumors were linked to increased survival after treatment with CTLA4 antibody and anti-PD-1 antibody, either alone or in combination. Thus, TLS-associated genes can predict treatment responses to ICB therapy.486 The PANDORE clinical trial further supports the role of TLS in enhancing immunotherapy efficacy, showing that pembrolizumab preferentially targets Tfh cells in muscle-invasive bladder cancer, increasing the number of Tfh cells in TLSs. This promoted CD8+ T cell infiltration, thereby enhancing anti-tumor ability, which benefits patients.487 We speculate that if high-density TLSs persist in the tumor tissues, it may indicate that the patient’s immune system is still actively fighting against the remaining tumor cells. This could potentially decrease the risk of recurrence and extend both disease-free survival and overall survival. Conversely, low-density TLSs might indicate a poorer prognosis, as this suggests a weaker immune response to the tumor. Therefore, TLSs could serve as a potential prognostic biomarker for selecting individuals suitable for ICB treatment.
Furthermore, B cells might be essential for the antitumor effect. Generally, based on pan-B-cell phenotyping, B cells are associated with favorable clinical outcomes in many cancers. TLS+ tumors exhibited high frequencies of IgG-producing PCs and IgG-stained and apoptotic malignant cells, suggesting antitumor effector activity. Importantly, therapeutic responses and progression-free survival were correlated with IgG-stained tumor cells in RCC patients treated with immune checkpoint inhibitors. Thus, intra-tumoral TLSs sustain B cell maturation and antibody production, which is associated with response to immunotherapy, potentially via direct anti-tumor effects.367 In lung cancer, a cluster of genetic features, including CD20+ B cells, GC-B cells, memory B cells, and PCs, is highly expressed. Their density correlates with long-term survival.59 In ESCC, CD23 aggregates in TLSs. Compared to the low GC-TLS group, patients with high GC-TLS had a higher 3-year survival rate, especially after neoadjuvant chemotherapy.488 In patients with endometrial cancer, three major B-cell clusters in TLSs, including naive B-cell genes, GC-like genes, and plasma cell genes, were identified by scRNA-seq. Intrastromal CD20 cell density is associated with a lower risk of recurrence.424 The value of B cells and TLSs in the response to ICB has been demonstrated in several cancer types. Based on immune cell analysis, B cells were located in the tumor-stromal margins, which significantly enhanced the effect of anti-PD-1 therapy in melanoma patients.86 In another analysis of melanoma patients receiving nivolumab with or without ipilimumab as neoadjuvant therapy, responsive patients had higher densities of B cells and TLSs, as well as a higher ratio of TLSs to tumor area.6 Notably, prior to treatment, responding patients also exhibited higher B-cell and TLS densities. According to transcriptomics studies, responders exhibited greater intratumor memory B cells and fewer naive B cells, along with higher BCR diversity (both IgH and IgL).6
Radiotherapy triggers NK cell migration to the irradiated tumor by NF-κB and mTOR-coordinated irradiated tumor cells to secrete chemokines.489 In addition, local radiation therapy induces increased expression of MHC-II molecules as well as death receptors FAS/CD95 and DR5 on tumor cell surfaces, and synergies with antigen vaccines.490 A study examined tissue samples from 198 patients who had undergone surgery and found that TLSs are associated with a favorable prognosis for lung adenocarcinoma (LUAD). The research also revealed that low-dose radiation therapy (LDRT) promoted the early formation of TLSs in a Kras-LSL-G12D mouse model of lung cancer. Furthermore, the combination of LDRT and anti-PD-1 therapy significantly enhanced the maturation of TLSs in mice with LUAD, resulting in a more robust anti-tumor response. This antitumor effect was closely correlated with the number of CD8+ T cells within the TLSs.491
Human tumor antigens have low immunogenicity, and now the research and development of cancer vaccines have been explored by many scholars. The formation of TLS was also observed when therapeutic vaccination was administered to patients with immunocompromised tumors. This indicates that TLS in the tumor can be induced by the cancer vaccine and is associated with increased immune function and beneficial clinical outcomes.492,493,494 Therefore, adjuvants can be added to support the formation of TLS induced by cancer vaccines. However, most tumor vaccines are still experimental, and only a few are approved.
The detection of TLS
TLSs are associated with disease development and prognosis. Given their potential as prognostic markers, efficient detection methods are needed. We will summarize some techniques for detecting TLSs and aim to provide valuable insights for research and clinical application (Fig. 6).
Hematoxylin and Eosin staining
Hematoxylin and eosin (H&E) staining of tissue sections allows for the evaluation of TLS size, density, and maturity, including the formation of lymphocyte clusters and GC.5,19,495 HEV-like features can also be observed in TLSs.496 Currently, H&E staining is widely used for detecting TLSs due to its simplicity and cost-effectiveness. However, it provides limited information and allows only for rough quantification.497,498 At the same time, because of objective and subjective differences between observers, there may be inconsistencies in the results.497
Immunohistochemistry (IHC)/immunofluorescence (IF) techniques
Immunohistochemistry/immunofluorescence (IHC/IF) techniques can label 3–4 markers in tissue slices, providing a basic cell phenotype analysis of TLSs. In contrast, the H&E staining technique can typically label 1–2 markers.378 These techniques can clearly identify the CD20+ GC and determine its positional relationship with TLSs in tumor tissue.404 Additionally, they can reveal the PDPN+FAP+DAPI+ fibroblast network and CD3+CD26L+ T cells in TLSs.41,170 Cell markers and chemokines, such as CD3, CD19, CXCL13, and CXCL21, were detected in TLSs through ICH/IF.41 Although this technique has advanced in the number of markers, the precise and detailed visualization of TLS structure and comprehensive research still require the combined use of multiple tissue slices.41,378
Multiplex immunohistochemistry (mIHC)
To overcome the limitations of IHC/IF, mIHC technology allows for the labeling of more than 5 markers to comprehensively analyze the structure of TLSs in a single tissue section. Compared to IHC/IF with multiple slices, mIHC increases the credibility of the results. With the support of mIHC, researchers inferred the composition and function of TLSs more accurately.378,499 Helmink et al. used mIHC to analyze the TLS structure, clarifying CD20+ B cells co-localized with CD21+ FDCs, CD4+ T cells, CD8+ T cells, and FOXP3+ Treg cells in GC, in proximity to MECA79+ HEVs.6 Stowman et al. tilized six antigens—CD20, CD8, PNAd, Ki67, CD83, and FOXP3—to detect TLS structure. They found that CD20+ B cell follicles are located in the center, surrounded by CD8+ T cells and PNAd+ vascular systems, and CD83+ DCs, FoxP3+ Treg cells, and Ki67+ proliferating cells.500 The OpalTM-TSA mIHC system provides a standardized experimental protocol for detecting TLS characterization, capable of simultaneously label 7 markers, including CD4, CD8, CD19, CD21, DC-LAMP, PNAd, and DAPI.501
Medical imaging technigue
While H&E staining, IF, IHC, and mIHC are invasive methods for detecting TLSs, medical imaging technologies, such as computed tomography (CT), positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI), provide non-invasive and real-time alternatives. For instance, CT images of lung adenocarcinoma patients revealed part-solid nodules (PSNs). Comparing with the pathological results, the punctate solid changes in PSN might be TLSs.502 However, CT alone only proves the aggregation of lymphocytes, lacking specific evidence to support the existence of TLSs. Dorraji et al. injected 99mTC-labeled albumin Nanocoll (99mTC-Nanocoll), showing TLSs in the kidneys and pancreas during the progression of systemic lupus erythematosus via SPECT. They found that TLSs were located near the islets in the pancreas.503 However, 99mTC-Nanocoll is taken up by cells through the phagocytosis receptor rather than specific binding with TLS-related markers. Although lymphocytes show a high signal of 99mTC-Nanocoll, it still lacks TLS specificity.503 Due to limitations of accuracy and specificity, few imaging applications are used for direct TLS detection. Researchers have developed materials for imaging single cells in TLSs. For example, a zirconium-89 labeled deferoxamine-modified anti-CD3 mAb detected CD3+ T cell infiltration in tumors via PET.504 Similarly, 89Zr-labeled PEGylated single-domain antibody fragments were used for PET imaging of CD8+ T cells in and around tumors.505 Thorek et al. monitored the distribution and consumption of B cells labeled with superparamagnetic iron oxide nanoparticles and near-infrared fluorescent dyes through MRI in the spleen of mice.505 However, we believe that the imaging of a single lymphocyte cannot effectively indicate TLS presence.
Machine learning model
Machine learning techniques are being used to improve the efficiency and accuracy of TLS evaluation in histopathological sections. Researchers have successfully achieved automatic TLS identification in H&E sections by combing the DeepLab v3+ network, active contour models, and lymphocyte segmentation methods. This method shows a specificity of 92.87% at a sensitivity of 95% and a specificity of 84.32% at a sensitivity of 99% in the detection of TLSs.506 The HookNet model has been proposed for the semantic segmentation of whole slide images. Its further version, the HookNet-TLS model, allows for TLS quantification in H&E digital pathology slides, and the accuracy is comparable to that of senior pathologists.507,508 Additionally, an interpretable machine learning model can automatically detect and quantify TLSs, classify them based on their status, and evaluate their prognostic value.497 These advances are crucial to enhancing our understanding and utilization of TLSs.
Strategies for targeted regulation of TLS
Inducing the formation of TLS
Engineered DCs
The coexistence of mature DCs and CD8+ T cells is a key prognostic factor for overall survival, and DCs are usually associated with TLSs.509,510 Thus, DCs are potential targets for inducing TLS formation (Fig. 6). By genetically engineering DCs, such as creating DC-Ad-CCL21 via adenovirus vectors, it is possible to increase CD8+ T cell infiltration at tumor sites, enhance T cell immunity and elevate PD-L1 mRNA expression in mouse bronchoalveolar cell carcinoma.511 This complements ICB therapies to ensure the effectiveness of anti-tumor immunotherapy. In a phase I trial on NSCLC patients, Ad-CCL21-DC increased the number of CD8+ T cells by an average of 3.4 times by intra-tumoral injection in over half of the patients.512 Tumor immunosuppression, particularly immune resistance against tumor-specific T cells, is associated with dysregulated expression of tumor immune checkpoint proteins. The above two studies targeting engineered DCs enhanced antigen presentation and promoted tumor T-cell infiltration and T-cell reactivity. It can increase PD-L1 expression while upregulating tumor co-stimulatory molecules. This further enhanced the anti-tumor effect. However, the role relationship between TLSs induced by DC and anti-tumor immune checkpoint inhibition needs to be further explored.511,512,513 In MCA205 sarcoma and MC38 colon cancer, T-bet gene-transduced DCs (mDC.T-bet) slow tumor growth, increase lymphocyte infiltration, and promote TLS development.514 Overexpression of IL-36γ in DCs also promotes rapid lymphocyte infiltration and TLS formation in MCA205 tumors in mice. The co-injection with rmIL-1F5, an IL-36R antagonist, suppresses the anti-tumor response.515 Furthermore, a CD1c+ DC vaccine increased CD8+ T cell activity, showing promising immune responses in metastatic melanoma patients.513 Ad-IL-15 vaccine induces DC maturation and activates the STING pathway, enhancing CD8+ T cell function against tumors.516 Here, we further summarize and discuss how engineered DCs as vaccines can effectively induce TLSs and suggest their role in upregulating PD-L1 expression. However, there is no clear experimental data to confirm the relationship and regulatory mechanism between them.
Stromal cell transplantation
The activation of fibroblasts initiates TLS formation by providing space and cytokines.517 Researchers have extensively studied stromal cells for their potential to induce TLSs. (Fig. 6) Zhu et al. demonstrated that PDPN+CD31− FRCs extracted from lymph nodes were transplanted into the subcutaneous. After 3–4 months, significant swelling was observed at the injection site, with an increase in CD69+ PD-1+ T cells, indicating TLS formation.518 The expression of IFN-γ was detected in TLSs, which inhibit tumor growth and reduce PD-1 and TIM-3 expression on T cells.518 This suggests that transplanting stromal cells can induce the formation of functional TLSs. However, direct injection of stromal cells may not form sufficient or functional TLSs in a short time, as they must provide space for TLS formation in advance.519 The interaction between local fibroblasts and DCs can modify the matrix characteristics, supporting TLS formation.520 Lee et al. constituted a 3D fibrous spheroidal scaffold loading stromal vascular fraction cells from adipose tissue and/or mature DCs. This scaffold provides a specialized niche for DCs, promoting T cell recruitment and T cell lymph cluster formation in situ, thereby enhancing antitumor effects. Therefore, co-injecting DCs and stromal cells significantly improves TLS formation efficiency and success rate.521 In a previous study, researchers used TEL-2 thymic stromal cells with normal immune function as a substitute for lymphoid stromal cells. They engineered TEL-2 cells to express LTα (TEL-2-LTa) and devised a sponge-like 3D scaffold made of bovine collagen to load these cells. After transplanting the mixture into the subcapsular space of the mice’s kidneys, they observed B-cell clusters, T-cell clusters, and HEVs by immunostaining, with the formation of FDC networks and GC, which are highly similar to SLO structures.522,523
Recombinant cytokines
As previously discussed, numerous cytokines are involved in TLS neogenesis, and their injection has been used to modulate TLSs (Fig. 6). For example, CXCL13 and CCL21 recruit lymphocytes by binding to CXCR5 and CCR7 respectively, which is related to TLS formation.524 In a mouse ovarian cancer model, intraperitoneal injection of recombinant CXCL13 increased intra-tumor TLSs. Concurrently, the infiltration of CD8+ T cells around TLSs increased, aiding in TLS formation and improving survival rates.254 Intra-tumoral injection of CXCL13/CCL21 promoted lymphatic infiltration and TLS formation in a mouse model of PDAC. Notably, co-administration of CXCL13/CCL21 with gemcitabine into orthotopic tumors significantly increased the efficiency of TLS formation, resulting in tumor reduction.525 Moreover, Treg cells, a type of T cell subtype, are closely related to immunosuppression in TLSs. FOXP3+ Treg cells increased after treatment with IL-2/anti-IL-2 mAb complexes (IL-2cx) within TLS in allogeneic lung transplants of mice, facilitating allograft acceptance and increasing the survival time over 90 days.458 In contrast, the absence of IL-2cx or the depletion of FOXP3+ Treg cells inhibited TLS development.
LIGHT normalizes intra-tumoral blood vessels, promoting TLS formation. Yukun Huang et al. co-loaded α-mangostin and plasmid encoding LIGHT into Nano-sapper, which reverses the abnormally activated fibroblasts, normalizes tumor vessels, promotes T cell infiltration, and ultimately induces TLS formation.526 CGKRK is a short peptide that targets adhesion molecules on the tumor vessel in glioblastoma. The LIGHT-CGKRK protein, which consists of CGKRK and LIGHT, is produced by transfecting E. coli with the pET-44a plasmid. In a glioblastoma model, it induces HEV formation in the tumor and enhances cytotoxic T cell entry following intravenous injection.239 Researchers have also engineered chimeric antigen receptor (CAR) T cells (Pbbz-LV CAR-T) to express PSMA and LIGHT, and OT-1 T cells (LIGHT-OT-1) to overexpress LIGHT. In a mouse tumor model injected with Pbbz-LV CAR-T, the antitumor effect was enhanced. In the B16F10-OVA melanoma tumor model injected with LIGHT-OT-1 cells, normalized tumor blood vessels and intra-tumoral lymphatic structures were observed.238 While none of the studies directly detected TLSs, vascular normalization and HEV formation are conducive to TLSs, suggesting that LIGHT is an effective target in regulating TLS formation and anti-tumor effects.
Toll-like receptors agonists
TLRs, part of the pattern recognition receptor family, regulate immune responses and are linked to autoimmune diseases and TLS formation.527 (Fig. 6) Li et al. found that monocytes activated by LPS (a TLR4 agonist) generated TGF-β1 stimulation, inducing the activation of Sox4 in CD4+ T cells in nasopharyngeal carcinoma. This led to the maturation and proliferation of PD-1+CXCR5-CD4+ Th-CXCL13 cell, which secrete CXCL13 and facilitate TLS occurrence.255 In a myasthenia gravis mouse model, TLR4 activation maintained high CXCL13 mRNA expression induced by Poly(I:C) in the thymus, leading to B cell recruitment related to GC development.528 Another study on PEMBROSARC found that intra-tumoral administration of G100, a TLR4 agonist, increased CD4+ T cell and CD8+ T cell infiltration in soft tissue sarcoma patients.529 However, its effect on TLS formation is unclear due to the limitations of objective factors in this study. TLR7 is also associated with TLS formation. In a chronic hepatitis B chimpanzee model, Li et al. observed that lymphoid aggregates formed in the portal areas of the liver after administration of GS-9620 (a TLR7 agonist). These aggregates, composed of CD3+ T cells, CD8+ T cells, and B cells, appeared to be immature TLSs, as they had not formed GCs.530 Despite these findings, the effect of TLR agonists on TLS formation requires further confirmation.
Lymphotoxin β receptor agonists
Stimulation of LTβR with polyclonal antibodies enhanced the construction of FRC networks, which is crucial for TLS531 (Fig. 6). In CT26 colon cancer mice, an agonistic anti-murine LTβR monoclonal antibody ACH6 increased the infiltration of T cells and B cells, inhibiting tumor growth. However, they observed only an increase in T cells in the tumor, which could be a precursor to the development of TLS.532 Since the initial stage of TLS formation is characterized by preferential infiltration of T cells, followed by the accumulation of B cells. Another study confirmed that LTβR agonists increased HEV density in PyMT-BC and RT2-PNET models by 1-fold and 4-fold, respectively, with lymphocyte infiltration.282 Furthermore, anti-LTβR monoclonal antibody agonists can also reverse the disorder of DC subsets in LTβ/LIGHT-deficient mice and promote DC cell proliferation, differentiation, and migration.533 While none of these studies directly detected TLS formation, they provide ideas for future exploration.
STING agonists
Stimulator of interferon genes (STING), a pattern recognition receptor, recognizes the non-self DNA from viruses or tumor cells and activates DCs or ECs to secrete cytokines, promoting tumor blood vessel normalization (VN) and lymphocyte infiltration. (Fig. 6) Studies have confirmed that low-dose STING agonists, such as cGAMP and ADU-S100, can be used as a method to mediate tumor immunity.534 In a Lewis lung cancer model, intra-tumoral injection of ADU-S100 promoted VN, modulating the tumor immune microenvironment, and increasing CD8+ T cell infiltration, controlling tumor growth.535 Additionally, ADU-S100 administration induced the secretion of cytokines and chemokines, including LTα, LTβ, LIGHT, CCL19, and CCL21, as well as the anti-angiogenic factors Tnfsf15 and Cxcl10, in a mouse melanoma model. This induced tumor blood VN, enhanced CD8+ T cell and DC infiltration, and non-classical TLS neogenesis.536 Demaria et al. found that using the dinucleotide cGAMP to activate the STING pathway increased the number of CD8+ T cells and inhibited tumor growth, with endothelial cells identified as the main responders to STING activation.537 Additionally, in Influenza A virus-infected IFN-αr1−/− mice, CXCL13 mRNA decreased on the third-day post-infection, but its expression increased after injecting IFN-β in the lungs of wild-type mice. And co-administration of IFN-β and cGAMP can induce the formation of GC in the lungs.268 However, STING agonists alone cannot recruit B cells, suggesting the need for combination therapies to enhance the antitumor effect of TLSs.
Nanoparticles
Nanoparticles commonly used as transport carriers for small-molecule drugs targeting tumors, have shown promise in inducing TLS formation. (Fig. 6) In the B16F10 melanoma model in mice, researchers synthesized a novel type of lipid nanoparticle (LNPs), characterized by diamino-ionizable lipid materials with various head groups (DAL). These nanoparticles encapsulated mRNA-encoding cytokines, including IL-12 and IL-27. Following intra-tumoral delivery, robust immune effector cell infiltration was observed in the tumor tissue. Notably, they also found that DAL4-LNP selectively delivers mRNA to CD19+ B cells rather than T cells. The combination of DAL4-LNP-IL-12 mRNA and DAL4-LNP-IL-27 mRNA worked synergistically to inhibit tumor growth without obvious toxicity.538 However, the formation of TLSs was not assessed, making it challenging to ascertain the efficacy of this approach in promoting TLS development. Wiley et al. developed the protein cage nanoparticle (PCN). This PCN is a hollow spherical protein cage structure with a diameter of 12 nm prepared from the small heat-shock protein (sHsp 16.5) of the hyperthermophilic archaeon Methanococcus jannaschii. Treatment with PCNs successfully induces the formation of iBALT structures in the submucosa of the lung, comprising CD20+ B cells, CD4+ T cells, FDCs, and CD8+ T cells after treatment. Mice infected with the influenza virus showed higher survival rates, faster virus clearance, quicker virus-specific antibody production, and reduced lung injury.539 Furthermore, FHK-pLIGHT@CaMP, known as Nano-sapper, composed of antifibrotic phosphates modified by α-mangostin and plasmids encoding LIGHT, was administered in a mouse PDAC model. It targets tumor tissue with the ECM glycoprotein (tenascin C) targeting peptide (FHK); subsequently, MP and LIGHT cooperatively improved vascular structure and promoted CLT cell infiltration. The histological results showed a clear TLS structure.526 Although these methods are still limited in regulating TLSs, they provide valuable insights into the rational utilization of nanoparticles for potential TLS induction and modulation.
Hydrogel
Hydrogel, a multifunctional platform, can bind and retain water, forming a viscoelastic gel polymer network in an aqueous solution. Due to these properties, they are frequently utilized in various biomedical applications, such as carriers for the localized release of antitumor drugs.540 (Fig. 6) Kobayashi et al. created a collagen sponge scaffold containing slow-release hydrogel beads that load LTα1β2, CCL19, CCL21, CXCL12, CXCL13, and soluble RANK ligand. When transplanted into the subrenal capsule space of Balb/c mice, it induced a TLS-like structure surrounded by lymphatic capillaries.541 Another study developed a STING-activated hydrogel (ZCCG), composed of Zn2+ and 4,5-imidazole dicarboxylic acid complexes, integrating chitosan nanoparticles and CpG. When administered intra-tumorally in melanoma mice, ZCCG activated the cGAS-STING and TLR9 pathways, inducing the secretion of chemokines CXCL13, CCL19, and CCL21, promoting DC maturation, enhancing lymphocyte infiltration, producing pro-inflammatory cytokines, and inducing TLS formation in the tumor, ultimately inhibiting tumor growth.542 Additionally, a potent anti-tumor nanoscale peptide hydrogel (MRM-coated spores) was reported to increase CXCL9 expression, promote CD8+ T cell responses, and induce M1 macrophage polarization. This hydrogel coated C-novyispores with a melittin-RADA32 (MR) hybrid peptide and loaded metformin (MET). It enhances the antitumor immune activation of C-novyi-NT spores, remodeling the immune system by slowly releasing MET after intra-tumoral injection.543 However, its effect on inducing the formation of TLSs needs further study.
Biological scaffold
Synthetic scaffolds, with their porous structure, can induce the formation of TLSs by providing necessary space and releasing encapsulated cells or cytokines.544 (Fig. 6) Tomei et al. created a macroporous polyurethane scaffold using type I collagen and Matrigel. In vitro, they cultured FRCs within the synthetic scaffold, and finally constructed a 3D lymph node T-zone stromal cell network tissue model. This 3D scaffold with FRCs not only provides a space for lymphocyte aggregation but also secretes the chemokines CCL19 and CCL21 to enhance lymphocyte migration and localization.545 Stachowiak et al. developed a composite material that mixed macroporous polyethylene glycol (PEG) hydrogel and collagen matrix. The PEG hydrogels can stably retain CCL21, while the collagen matrix loads T cells and DCs.546 Recently, a 3D-printed scaffold loaded with immunomodulators was developed as a vaccine. Its porous structure, similar to lymph node structure, facilitated immune cell infiltration and TLS formation. The scaffold recruited APCs and T cells in vivo and trained DCs and naive cells to mature. This 3D scaffold inhibits metastatic tumor development and prolongs survival time in tumor resection and metastasis models.547 However, the exploration of synthetic scaffolds to induce TLS formation remains challenging, with safety and feasibility issues limiting their development.
Exosomes
Apoptotic extracellular vesicle-like exosomes (ApoExo) are a special type of extracellular vesicle with a diameter of 30–100 nm, belonging to the structure of nanoscale lipid encapsulation. These vesicles are released by endothelial cells activated by caspase-3. ApoExo contains an active 20S proteasome core, basement membrane protein glycan LG3 C-terminal fragment, and long non-coding RNA. Among them, the active 20S proteasome core can regulate immunogenic activity.548,549,550 The secretion of ApoExo increased the number of GC-B cells and Tfh cells and promoted the generation of LG3 IgG in mice, exacerbating the rejection reaction.550 In aortic transplanted mice, the formation of TLSs was observed after ApoExo injection into the graft, accompanied by massive γδT cell infiltration, promoting IL-17 expression, resulting in complement deposition, and autoantibody production.549 This suggests that exosomes are a feasible approach to regulating TLSs. (Fig. 6).
Inhibiting the formation of TLS
Antibody-based blockade
Th17 cells play a crucial role in the development of TLSs by secreting cytokines, such as IL-17 and IL-22.147,551 IL-17A+ PDPN+ T cells are present within TLSs in the myocardial tissue of viral myocarditis. Targeting Th17 cells by using IL-17 mAb and PDPN mAb inhibits their proliferation and differentiation, therapy decreasing pro-inflammatory factor secretion. This reduction lowers reduces the number of active B cells and the formation of TLSs.469 In ischemic reperfusion injury (IRI) kidney injury models, the absence of IL-17A results in reduced TLS volume in IL-17A−/− mice. Although IL-17A−/− mice exhibit similar cellular components in TLSs as seen in normal mouse IRI models, the proportions of B220+ B cells, CD3+ T cells, CD4+ T cells, CD8+ T cells, and FRCs are significantly reduced in IL-17A-/- mice, leading to impaired TLS function.219
In a Sjögren’s syndrome model, Th17 cells expanded in Il-27ra−/− mice. Using anti-IL-17A antibodies reduces infiltration of T cells and B cells in salivary glands, along with impaired GC function.207 Additionally, the IL-27 antibodies increase CD21+ FDC networks and GL7+ GC B cells in IL-27ra−/− mice. This suggests that IL-27 negatively regulates the GC response in TLSs. Notably, IL-17 inhibitors can reverse the abnormal activation of TLSs caused by the lack of the IL-27 signal.207 Liu et al. found that delivering IL-12 and IL-27 mRNA promoted immune effector cell infiltration, mainly CD8+ T cells and NK cells, and significantly inhibited tumor growth.538 This result is the synergy of IL-12 and IL-27 and does not appear to conflict with the role of IL-27 in inhibiting the GC response in TLSs.
Tfh cells secrete CXCL13 to recruit and activate B cells, mediating TLS formation.390,408,409 In chronic kidney disease, targeting Tfh cells reduces IL-21 expression by blocking the ICOS signal, which inhibits TLS formation and alleviates renal fibrosis.225 Additionally, Treg cells appear to promote TLS formation indirectly. In a mouse model of fibrosarcoma, after Treg cell depletion, activated CD8+ T cells promote the formation of HEVs by injecting anti-CD4 and anti-CD8 antibodies. This promotes T-cell infiltration and tumor destruction.136 Moreover, in SS-NOD mice, the B-cell zone and T-cell zone decrease, the FDC network is lost, and HEVs are reduced after blocking LTβR by LTβR-Ig in the salivary glands. Meanwhile, the function of salivary glands, which was lost due to TLS, has been partially restored.552 In a rheumatoid arthritis mouse model, knocking out CXCR5 and CCR7 reduced mature TLSs and joint destruction.553 Notably, blocking CXCL13 in NOD mice destroyed TLSs, but it didn’t decrease B cell infiltration and inflammation554 (Fig. 6).
Inhibitors
AY61-3606, an inhibitor targeting spleen tyrosine kinase, reduces B cell vitality in tonsils, impairing the GC response.554 However, there is no research to prove that BAY61-3606 can affect the formation of TLSs. In contrast, FTY720, a sphingosine-1-phosphate receptor regulator, decreases B220+ B cells and CD4+ T cells in the kidney, thereby reducing TLS volume.219 Interestingly, FTY720 can prevent diabetes by maintaining TLS integrity in the pancreas of NOD mice555 (Fig. 6).
Perspective and future challenges
Tertiary lymphoid structures (TLSs) are observed in various diseases, and with a complex association with prognosis. Some studies suggest TLSs may promote tumor progression,495,556 which contradicts findings that associate TLSs with improved patient survival. This discrepancy is likely due to the heterogeneity of TLSs, which complicates their clinical application. Recent reports highlight significant differences in the morphology, composition, and GC responses within TLSs.44,557 These differences can affect the TME through various inflammatory cytokines, leading to a switch in humoral immune type in TLSs, which will severely promote tumor progression.557 Additionally, the characteristics of TLSs are different between the early and late stages of disease.44,234 And the maturity of TLSs also influences the risk of tumor recurrence.19 Therefore, given the “double-edged sword” nature of TLSs, it is important to consider the proportion of various immune cell subpopulations within TLSs, especially the diversity of immune cell phenotypes. The TME regulates different immune cell subtypes and their phenotypes, and a detailed characterization of these subtypes in TLSs will help understand their impact on disease and their contribution to TLS formation and function. Moreover, the specific aspects of TLS formation, particularly the involved cell signaling pathways, need further exploration.
Currently, tumor immunity research focuses primarily on T cells, with B-cell immunity being less understood. Since TLSs are characterized by lymphocyte aggregates centered on B cells, future research should shift towards understanding B-cell involvement in tumor immunity. The activation of GC-B cells and antibody secretion in TLSs emphasize the importance of B cells in the immune response. In addition, it remains unclear whether the varying locations of TLSs lead to differences in their composition, immune responses, or tumor progression. For example, recent studies have shown that intra-tumoral TLSs are associated with prolonged patient survival in HCC, whereas peritumoral TLSs have no impact on prognosis and may even promote tumor progression.19,385 Notably, TLSs that negatively regulate disease often appear in the peritumoral region, regardless of their maturity. However, peritumoral TLSs are not always associated with a poor prognosis, as they can still support patient survival.558 The various prognostic implications of TLSs are due to discrepancies in detection methodologies in cancer. Considering that the density, localization, and maturation stage of TLSs are pivotal determinants of their prognostic value, it is crucial to stratify data collection and analysis according to these criteria. This approach will significantly improve the precision of prognostic estimations.
Given the diversity and complexity of TLS structures, various methods have been developed for detecting them, including IHC/IF, H&E staining, and transcriptional analysis.559 However, these traditional methods are invasive, and limited by subjective or objective factors, such as standardized differences in evaluators and information loss during sample processing, which can distort the real data of TLSs. This limits their application in disease prediction and treatment. Therefore, the development of a reliable method for detecting TLS formation, maturation, and density is essential for maximizing the clinical benefits of TLSs. While computerized scanning techniques and magnetic resonance imaging (MRI) have been used for TLS detection, they lack the specificity and accuracy to distinguish between TLSs and high-density lymphocyte aggregates.503 Biomaterials provide a more accurate and sensitive way to detect TLS components. They are typically designed as fluorescent imaging probes that chelate antibodies and facilitate radiolabeling for targeted biomarkers.135 However, current nanofluorescent probes are insufficient for simultaneously detecting more than two components of TLSs, let alone displaying their structures, which is important for defining TLSs.560 Accordingly, imaging techniques have significant limitations in monitoring TLSs. Furthermore, a big data learning model has been proposed for accurate digital quantitative analysis, which translates TLS histopathological features into a set of universal and reproducible mathematical values to standardize TLSs.497 However, the method requires a large number of identified TLS-containing tumor sections for algorithmic practice, which is a lengthy process, and it also relies on the previously accurate identification of TLSs. And it can only identify TLSs in tissue sections and cannot monitor TLS formation in real time, providing limited value for disease treatment. There is still a lack of a standard method for detecting TLSs, which has led to a significant reduction in confidence in the results across different studies. Merely identifying the cellular components of TLSs is not sufficient to accurately determine their presence, localization, and maturation stage. Given the significant role of TLSs in various diseases, particularly their potential to enhance immunotherapy, there is an urgent need to develop a minimally invasive or non-invasive method for monitoring TLSs. This will create new possibilities for the development of future patient treatment programs. Until now, only techniques such as multiplex immunohistochemistry (mIHC) and multiplex immunofluorescence could elucidate the spatial structure of TLSs. And mIHC is still the most accurate way to identify TLSs, as it labels the major cellular components in TLSs and reveals their structure. Importantly, because the heterogeneity and maturity of TLSs have different effects on disease, it is necessary to mark CD21+ FDCs and CD23+ GCs as TLS indicators. This will provide a detailed delineation of the potential relationship between TLS and disease, particularly the differences in maturity and components. At the same time, it will greatly enhance the clinical guidance value of TLSs and provide a measure for the extent of targeted regulation of TLSs in the future.
TLSs have shown great potential as a new field of immunotherapy. Future studies should focus on the relationship between TLSs, disease risks, and clinical outcomes. Recent articles have demonstrated the value of TLSs as a prognostic indicator of disease, particularly cancer.561 This may identify TLSs as a new biomarker for clinical intervention, contributing to the development of more effective disease prevention and diagnostic methods. Furthermore, TLSs have attracted interest as potential anti-tumor immune mediators. Therefore, it is a promising research direction that explores a combined therapeutic strategy based on TLS modulation to improve clinical outcomes. Modulating TLS immune capacity through targeted delivery of specific agonists, antibodies, or therapeutic agents holds immense promise for enhancing the efficacy of biotherapeutics. However, given their complexity and time-consuming features, it is difficult to obtain the desired results with a single approach. The lack of appropriate induction protocols, imaging methods, and research models has also hindered the scientific research and clinical application of TLSs. Studies have shown that successful induction of TLSs can take at least one week, and even then, the formed TLSs may not be ideal, with low success rates. This means that TLSs remain extremely challenging to apply in clinical settings. Recent studies have shown promise in utilizing biomaterials in disease, suggesting that leveraging biomaterial versatility to create an environment conducive to TLS formation deserves further investigation. This also forces us to consider ethical issues during the research process. Although our aim is to improve the prognosis of patients by modulating TLSs as a therapeutic tool, their formation is uncontrollable and may cause unpredictable damage to patients during the treatment. Therefore, patients should be fully informed.
Conclusion
Over the past two decades, the understanding of TLSs has significantly increased. Similar to SLOs, the formation of TLSs is driven by the interaction between LTi cells and LTo cells, with multiple cytokines involved. Among them, mesenchymal cells obtaining LTo features are a prerequisite for triggering TLS formation. Subsequently, chemokines, as the most critical factors for immune cell aggregation, promote lymphocyte recruitment and segregation of B/T cell compartments in TLSs. TLSs are classically defined as lymphoid aggregates formed in non-hematopoietic organs, allowing the generation of effector T cells and effector B cells. We hypothesize that TLSs initiate a faster immune response in the lymphocyte niche. They avoid DCs transporting antigens over long distances and increase contact with T cells to improve antigen presentation efficiency. Meanwhile, TLSs facilitate the penetration of immune cells and cytokines. Consistent with this, “newborn” T cells and B cells in TLSs are present in the local tissue, reducing dependence on naive lymphocytes transported distantly through HEVs. In recent years, the link between TLSs and immunotherapy has gained great attention, and many researchers have realized the broad application of TLSs in immunotherapy, especially anti-tumor immunotherapy. A series of clinical studies have demonstrated that TLSs can significantly enhance the survival of tumor patients, especially as markers of immunotherapy. And many immunotherapies can also promote the formation of TLSs. Therefore, targeting and modulating TLSs will help establish a new immunotherapeutic pathway. However, TLSs are still poorly understood, and their composition, formation, and regulatory pathways need further exploration. In addition, it is still poorly understood whether direct modulation of TLS formation or function by biomaterials or drugs contributes to the clinical prognosis of patients, necessitating enhanced clinical studies.
References
Gago da Graça, C., van Baarsen, L. G. M. & Mebius, R. E. Tertiary lymphoid structures: diversity in their development, composition, and role. J. Immunol. 206, 273–281 (2021).
Schumacher, T. N. & Thommen, D. S. Tertiary lymphoid structures in cancer. Science 375, eabf9419 (2022).
Hsiao, H. M. et al. The role of lymphoid neogenesis in allografts. Am. J. Transplant. 16, 1079–1085 (2016).
Salomonsson, S. et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren’s syndrome. Arthritis Rheum. 48, 3187–3201 (2003).
Siliņa, K. et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 78, 1308–1320 (2018).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Pitzalis, C., Jones, G. W., Bombardieri, M. & Jones, S. A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 14, 447–462, (2014).
Koenig, A. & Thaunat, O. Lymphoid neogenesis and tertiary lymphoid organs in transplanted organs. Front Immunol. 7, 646 (2016).
Asam, S., Nayar, S., Gardner, D. & Barone, F. Stromal cells in tertiary lymphoid structures: architects of autoimmunity. Immunol. Rev. 302, 184–195 (2021).
Barone, F. et al. Stromal fibroblasts in tertiary lymphoid structures: a novel target in chronic inflammation. Front Immunol. 7, 477 (2016).
Neyt, K. et al. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 33, 297–305 (2012).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Hua, Y. et al. Cancer immunotherapies transition endothelial cells into HEVs that generate TCF1(+) T lymphocyte niches through a feed-forward loop. Cancer Cell. 40, 1600–1618.e1610 (2022).
Thommen, D. S. et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 24, 994–1004 (2018).
Garaud, S. et al. Antigen specificity and clinical significance of IgG and IgA autoantibodies produced in situ by tumor-infiltrating B cells in breast cancer. Front Immunol. 9, 2660 (2018).
Ruffin, A. T. et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat. Commun. 12, 3349 (2021).
Dieu-Nosjean, M. C. et al. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 35, 571–580 (2014).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Calderaro, J. et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J. Hepatol. 70, 58–65 (2019).
Serafini, B. et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 204, 2899–2912 (2007).
Manzo, A., Bombardieri, M., Humby, F. & Pitzalis, C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling. Immunol. Rev. 233, 267–285 (2010).
Thaunat, O. Pathophysiologic significance of B-cell clusters in chronically rejected grafts. Transplantation 92, 121–126 (2011).
Ziff, M. Heberden Oration, 1964. some immunologic aspects of the connective tissue diseases. Ann. Rheum. Dis. 24, 103–115 (1965).
Hjelmström, P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J. Leukoc. Biol. 69, 331–339, (2001).
Söderström, N., & Blörklund, A. Organization of the invading lymphoid tissue in human lymphoid thyroiditis. Scand. J. Immunol. 3, 295–301 (1974).
Picker, L. J. & Butcher, E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu. Rev. Immunol. 10, 561–591 (1992).
Kratz, A., Campos-Neto, A., Hanson, M. S. & Ruddle, N. H. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J. Exp. Med. 183, 1461–1472 (1996).
Tang, H., Zhu, M., Qiao, J. & Fu, Y. X. Lymphotoxin signalling in tertiary lymphoid structures and immunotherapy. Cell Mol. Immunol. 14, 809–818 (2017).
Wagner, U. G. et al. The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis. J. Immunol. 161, 6390–6397 (1998).
Takemura, S. et al. T cell activation in rheumatoid synovium is B cell dependent. J. Immunol. 167, 4710–4718 (2001).
Figenschau, S. L. et al. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer 15, 101 (2015).
Liao, S. et al. Transgenic LacZ under control of Hec-6st regulatory sequences recapitulates endogenous gene expression on high endothelial venules. Proc. Natl Acad. Sci. USA 104, 4577–4582 (2007).
Weyand, C. M., Kang, Y. M., Kurtin, P. J. & Goronzy, J. J. The power of the third dimension: tissue architecture and autoimmunity in rheumatoid arthritis. Curr. Opin. Rheumatol. 15, 259–266 (2003).
Dieu-Nosjean, M. C. et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 26, 4410–4417 (2008).
Giraldo, N. A. et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin. Cancer Res. 21, 3031–3040 (2015).
Johansson-Percival, A. et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat. Immunol. 18, 1207–1217 (2017).
Savas, P. et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 24, 986–993 (2018).
Vanhersecke, L. et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer 2, 794–802 (2021).
Wang, Y. et al. Tumor mutational burden related classifier is predictive of response to PD-L1 blockade in locally advanced and metastatic urothelial carcinoma. Int Immunopharmacol. 87, 106818 (2020).
Rossi, A. et al. Stromal and immune cell dynamics in tumor associated tertiary lymphoid structures and anti-tumor immune responses. Front. Cell Dev. Biol. 10, 933113 (2022).
Nayar, S. et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc. Natl Acad. Sci. USA 116, 13490–13497 (2019).
Chen, Z. et al. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 11, 5077 (2020).
Rodriguez, A. B. et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep. 36, 109422 (2021).
Lauss, M., Donia, M., Svane, I. M. & Jönsson, G. B cells and tertiary lymphoid structures: friends or foes in cancer immunotherapy? Clin. Cancer Res. 28, 1751–1758 (2022).
Aguzzi, A., Kranich, J. & Krautler, N. J. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol. 35, 105–113, (2014).
Muñoz-Fernández, R. et al. Follicular dendritic cells are related to bone marrow stromal cell progenitors and to myofibroblasts. J. Immunol. 177, 280–289 (2006).
Krautler, N. J. et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell 150, 194–206 (2012).
Heesters, B. A., Myers, R. C. & Carroll, M. C. Follicular dendritic cells: dynamic antigen libraries. Nat. Rev. Immunol. 14, 495–504 (2014).
Batista, F. D. & Harwood, N. E. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 9, 15–27 (2009).
Van den Berg, T. K. et al. Selective inhibition of immune complex trapping by follicular dendritic cells with monoclonal antibodies against rat C3. Eur. J. Immunol. 22, 957–962 (1992).
Ciccia, F. et al. Ectopic expression of CXCL13, BAFF, APRIL and LT-β is associated with artery tertiary lymphoid organs in giant cell arteritis. Ann. Rheum. Dis. 76, 235–243 (2017).
El Shikh, M. E. et al. Activation of B cells by antigens on follicular dendritic cells. Trends Immunol. 31, 205–211 (2010).
Lehmann-Horn, K. et al. B cell repertoire expansion occurs in meningeal ectopic lymphoid tissue. JCI Insight 1, e87234 (2016).
Fridman, W. H. et al. Tertiary lymphoid structures and B cells: an intratumoral immunity cycle. Immunity 56, 2254–2269 (2023).
Bombardieri, M., Lewis, M. & Pitzalis, C. Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat. Rev. Rheumatol. 13, 141–154 (2017).
Sato, Y. et al. The roles of tertiary lymphoid structures in chronic diseases. Nat. Rev. Nephrol. 19, 525–537 (2023).
Kim, S. S. et al. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin. Cancer Res. 26, 3345–3359 (2020).
Wieland, A. et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 597, 274–278 (2021).
Germain, C. et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 189, 832–844 (2014).
Montfort, A. et al. A strong B-cell response is part of the immune landscape in human high-grade serous ovarian metastases. Clin. Cancer Res. 23, 250–262 (2017).
Hu, Q. et al. Atlas of breast cancer infiltrated B-lymphocytes revealed by paired single-cell RNA-sequencing and antigen receptor profiling. Nat. Commun. 12, 2186 (2021).
Huibers, M. M. et al. The composition of ectopic lymphoid structures suggests involvement of a local immune response in cardiac allograft vasculopathy. J. Heart Lung Transplant. 34, 734–745 (2015).
Lucchesi, D. & Bombardieri, M. The role of viruses in autoreactive B cell activation within tertiary lymphoid structures in autoimmune diseases. J. Leukoc. Biol. 94, 1191–1199, (2013).
Humby, F. et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 6, e1 (2009).
Rosser, E. C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015).
de Jonge, K. et al. Inflammatory B cells correlate with failure to checkpoint blockade in melanoma patients. Oncoimmunology 10, 1873585 (2021).
Bao, J., Betzler, A. C., Hess, J. & Brunner, C. Exploring the dual role of B cells in solid tumors: implications for head and neck squamous cell carcinoma. Front. Immunol. 14, 1233085 (2023).
Wei, X. et al. Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumour Biol. 37, 6581–6588 (2016).
Olkhanud, P. B. et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 71, 3505–3515 (2011).
Mirlekar, B. et al. B cell-derived IL35 drives STAT3-dependent CD8(+) T-cell exclusion in pancreatic cancer. Cancer Immunol. Res. 8, 292–308 (2020).
Kessel, A. et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun. Rev. 11, 670–677 (2012).
Wu, H. et al. PD-L1(+) regulatory B cells act as a T cell suppressor in a PD-L1-dependent manner in melanoma patients with bone metastasis. Mol. Immunol. 119, 83–91 (2020).
Moulin, V. et al. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J. Exp. Med. 192, 475–482 (2000).
Ohm, B. & Jungraithmayr, W. B cell immunity in lung transplant rejection - effector mechanisms and therapeutic implications. Front Immunol. 13, 845867 (2022).
Newell, K. A., Adams, A. B. & Turka, L. A. Biomarkers of operational tolerance following kidney transplantation—the immune tolerance network studies of spontaneously tolerant kidney transplant recipients. Hum. Immunol. 79, 380–387 (2018).
Guinn, M. T. et al. Intragraft B cell differentiation during the development of tolerance to kidney allografts is associated with a regulatory B cell signature revealed by single cell transcriptomics. Am. J. Transplant. 23, 1319–1330 (2023).
Gunderson, A. J. et al. Germinal center reactions in tertiary lymphoid structures associate with neoantigen burden, humoral immunity and long-term survivorship in pancreatic cancer. Oncoimmunology 10, 1900635 (2021).
Amaria, R. N. et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 24, 1649–1654 (2018).
Becht, E. et al. Immune contexture, immunoscore, and malignant cell molecular subgroups for prognostic and theranostic classifications of cancers. Adv. Immunol. 130, 95–190 (2016).
Nielsen, J. S. & Nelson, B. H. Tumor-infiltrating B cells and T cells: Working together to promote patient survival. Oncoimmunology 1, 1623–1625 (2012).
Wennhold, K. et al. CD86(+) antigen-presenting B cells are increased in cancer, localize in tertiary lymphoid structures, and induce specific T-cell responses. Cancer Immunol. Res. 9, 1098–1108 (2021).
Kinker, G. S. et al. B cell orchestration of anti-tumor immune responses: a matter of cell localization and communication. Front. Cell Dev. Biol. 9, 678127 (2021).
Shimabukuro-Vornhagen, A. et al. Antigen-presenting human B cells are expanded in inflammatory conditions. J. Leukoc. Biol. 101, 577–587 (2017).
Jiang, J. et al. Tumour-infiltrating immune cell-based subtyping and signature gene analysis in breast cancer based on gene expression profiles. J. Cancer 11, 1568–1583 (2020).
Chen, J. et al. Single-cell transcriptome and antigen-immunoglobin analysis reveals the diversity of B cells in non-small cell lung cancer. Genome Biol. 21, 152 (2020).
Griss, J. et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 10, 4186 (2019).
Welshman, M. D. Doped dobermann. Vet. Rec. 119, 512 (1986).
Parga-Vidal, L., van Aalderen, M. C., Stark, R. & van Gisbergen, K. Tissue-resident memory T cells in the urogenital tract. Nat. Rev. Nephrol. 18, 209–223 (2022).
Mori, T. et al. Tertiary lymphoid structures show infiltration of effective tumor-resident T cells in gastric cancer. Cancer Sci. 112, 1746–1757 (2021).
Mackay, L. K. et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14, 1294–1301 (2013).
Zhao, H. et al. Tumor-resident T cells, associated with tertiary lymphoid structure maturity, improve survival in patients with stage III lung adenocarcinoma. Front Immunol. 13, 877689 (2022).
Simoni, Y. et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018).
Paijens, S. T., Vledder, A., de Bruyn, M. & Nijman, H. W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol. Immunol. 18, 842–859 (2021).
Corgnac, S. et al. Cancer stem-like cells evade CD8(+)CD103(+) tumor-resident memory T (T(RM)) lymphocytes by initiating an epithelial-to-mesenchymal transition program in a human lung tumor model. J. Immunother Cancer 10, e004527 (2022).
Oja, A. E. et al. Functional heterogeneity of CD4(+) tumor-infiltrating lymphocytes with a resident memory phenotype in NSCLC. Front. Immunol. 9, 2654 (2018).
Workel, H. H. et al. A transcriptionally distinct CXCL13(+)CD103(+)CD8(+) T-cell population is associated with B-cell recruitment and neoantigen load in human cancer. Cancer Immunol. Res. 7, 784–796 (2019).
Djenidi, F. et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 194, 3475–3486 (2015).
Tokunaga, R. et al. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat. Rev. 73, 10–19 (2019).
Vlaming, M. et al. Tumor infiltrating CD8/CD103/TIM-3-expressing lymphocytes in epithelial ovarian cancer co-express CXCL13 and associate with improved survival. Front. Immunol. 13, 1031746 (2022).
Koppensteiner, L. et al. Location of CD39(+) T cell subpopulations within tumors predict differential outcomes in non-small cell lung cancer. J. Immunother. Cancer 11, e006770 (2023).
Peng, Y. et al. Single-cell profiling of tumor-infiltrating TCF1/TCF7(+) T cells reveals a T lymphocyte subset associated with tertiary lymphoid structures/organs and a superior prognosis in oral cancer. Oral. Oncol. 119, 105348 (2021).
Rong, H. et al. Correlation between TCF7(+) T cells and prognosis of patients with oral squamous cell carcinoma. Front. Oncol. 12, 782058 (2022).
Im, S. J. et al. Characteristics and anatomic location of PD-1(+)TCF1(+) stem-like CD8 T cells in chronic viral infection and cancer. Proc. Natl Acad. Sci. USA 120, e2221985120 (2023).
Zhang, J., Lyu, T., Cao, Y. & Feng, H. Role of TCF-1 in differentiation, exhaustion, and memory of CD8(+) T cells: a review. FASEB j. 35, e21549 (2021).
Raghu, D., Xue, H. H. & Mielke, L. A. Control of Lymphocyte Fate, Infection, and Tumor Immunity by TCF-1. Trends Immunol. 40, 1149–1162 (2019).
Robinson, M. H. et al. Subtype and grade-dependent spatial heterogeneity of T-cell infiltration in pediatric glioma. J. Immunother. Cancer 8, e001066 (2020).
Sato, Y. et al. Stem-like CD4(+) T cells in perivascular tertiary lymphoid structures sustain autoimmune vasculitis. Sci. Transl. Med. 15, eadh0380 (2023).
Hudson, W. H. et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1(+) stem-like CD8(+) T cells during chronic infection. Immunity 51, 1043–1058.e1044 (2019).
Siddiqui, I. et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e110 (2019).
Couillault, C., Germain, C., Dubois, B. & Kaplon, H. Identification of tertiary lymphoid structure-associated follicular helper T cells in human tumors and tissues. Methods Mol. Biol. 1845, 205–222 (2018).
Panneton, V. et al. ICOS costimulation is indispensable for the differentiation of T follicular regulatory cells. Life Sci Alliance. 6, e202201615 (2023).
Wan, S. et al. Costimulation molecules differentially regulate the ERK-Zfp831 axis to shape T follicular helper cell differentiation. Immunity 54, 2740–2755.e2746 (2021).
Chaurio, R. A. et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity 55, 115–128.e119 (2022).
Nadeau, S. & Martins, G. A. Conserved and unique functions of blimp1 in immune cells. Front. Immunol. 12, 805260 (2021).
Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Choi, J. & Crotty, S. Bcl6-mediated transcriptional regulation of follicular helper T cells (T(FH)). Trends Immunol. 42, 336–349 (2021).
Nurieva, R. I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Seth, A. et al. AP-1-independent NFAT signaling maintains follicular T cell function in infection and autoimmunity. J. Exp. Med. 220, e20211110 (2023).
Deng, J. et al. T follicular helper cells and T follicular regulatory cells in rheumatic diseases. Nat. Rev. Rheumatol. 15, 475–490 (2019).
Overacre-Delgoffe, A. E. et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity 54, 2812–2824.e2814 (2021).
Gonzalez, D. G. et al. Nonredundant roles of IL-21 and IL-4 in the phased initiation of germinal center B cells and subsequent self-renewal transitions. J. Immunol. 201, 3569–3579 (2018).
Linterman, M. A. et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Aoyagi, R. et al. Single-cell transcriptomics reveals granzyme K-expressing cytotoxic Tfh cells in tertiary lymphoid structures in IgG4-RD. J. Allergy Clin. Immunol. 153, 513–520.e510 (2024).
Yamaguchi, K. et al. Helper T cell-dominant tertiary lymphoid structures are associated with disease relapse of advanced colorectal cancer. Oncoimmunology 9, 1724763 (2020).
Hollern, D. P. et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell 179, 1191–1206.e1121 (2019).
Kawamoto, S. et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science 336, 485–489 (2012).
Sánchez-Alonso, S. et al. A new role for circulating T follicular helper cells in humoral response to anti-PD-1 therapy. J. Immunother Cancer 8, e001187 (2020).
Schmetterer, K. G., Neunkirchner, A. & Pickl, W. F. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J. 26, 2253–2276 (2012).
Burzyn, D., Benoist, C. & Mathis, D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 14, 1007–1013 (2013).
Collison, L. W. et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–569 (2007).
Woo, E. Y. et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J. Immunol. 168, 4272–4276 (2002).
Devi-Marulkar, P. et al. Regulatory T cells infiltrate the tumor-induced tertiary lymphoïd structures and are associated with poor clinical outcome in NSCLC. Commun. Biol. 5, 1416 (2022).
Joshi, N. S. et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity 43, 579–590 (2015).
Li, H., Lin, W. P., Zhang, Z. N. & Sun, Z. J. Tailoring biomaterials for monitoring and evoking tertiary lymphoid structures. Acta Biomater. 172, 1–15 (2023).
Colbeck, E. J. et al. Treg depletion licenses T cell-driven HEV neogenesis and promotes tumor destruction. Cancer Immunol. Res. 5, 1005–1015 (2017).
Han, D. et al. Microenvironmental network of clonal CXCL13+CD4+ T cells and Tregs in pemphigus chronic blisters. J. Clin. Investig. 133, e166357 (2023).
Lu, Y. & Craft, J. T follicular regulatory cells: choreographers of productive germinal center responses. Front. Immunol. 12, 679909 (2021).
Eschweiler, S. et al. Intratumoral follicular regulatory T cells curtail anti-PD-1 treatment efficacy. Nat. Immunol. 22, 1052–1063 (2021).
Gonzalez-Figueroa, P. et al. Follicular regulatory T cells produce neuritin to regulate B cells. Cell 184, 1775–1789.e1719 (2021).
Sage, P. T., Francisco, L. M., Carman, C. V. & Sharpe, A. H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 14, 152–161 (2013).
Linterman, M. A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Yang, G. et al. Transcriptional repressor Blimp1 regulates follicular regulatory T-cell homeostasis and function. Immunology 153, 105–117 (2018).
Song, H. et al. T follicular regulatory cells suppress Tfh-mediated B cell help and synergistically increase IL-10-producing B cells in breast carcinoma. Immunol. Res. 67, 416–423 (2019).
Miao, X. et al. The characteristics and novel clinical implications of CD4+CXCR5+Foxp3+ follicular regulatory T cells in breast cancer. Ann. Transl. Med. 9, 1332 (2021).
Sage, P. T., Paterson, A. M., Lovitch, S. B. & Sharpe, A. H. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 41, 1026–1039 (2014).
Grogan, J. L. & Ouyang, W. A role for Th17 cells in the regulation of tertiary lymphoid follicles. Eur. J. Immunol. 42, 2255–2262, (2012).
Deteix, C. et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J. Immunol. 184, 5344–5351 (2010).
Rangel-Moreno, J. et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat. Immunol. 12, 639–646 (2011).
Ahern, P. P. et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33, 279–288 (2010).
Hall, G. F. & Cohen, M. J. The pattern of dendritic sprouting and retraction induced by axotomy of lamprey central neurons. J. Neurosci. 8, 3584–3597, (1988).
Ling, Y. et al. The prognostic value and molecular properties of tertiary lymphoid structures in oesophageal squamous cell carcinoma. Clin. Transl. Med. 12, e1074 (2022).
Zhang, Y. et al. IL-22 promotes tumor growth of breast cancer cells in mice. Aging 12, 13354–13364 (2020).
Yang, J., Sundrud, M. S., Skepner, J. & Yamagata, T. Targeting Th17 cells in autoimmune diseases. Trends Pharm. Sci. 35, 493–500 (2014).
Knochelmann, H. M. et al. IL6 fuels durable memory for Th17 cell-mediated responses to tumors. Cancer Res. 80, 3920–3932 (2020).
Wang, J., Zhao, X. & Wan, Y. Y. Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell Mol. Immunol. 20, 1002–1022 (2023).
Fasching, P. et al. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules 22, 134 (2017).
Coquet, J. M., Chakravarti, S., Smyth, M. J. & Godfrey, D. I. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J. Immunol. 180, 7097–7101 (2008).
Zhao, X. et al. Th17 cell-derived amphiregulin promotes colitis-associated intestinal fibrosis through activation of mTOR and MEK in intestinal myofibroblasts. Gastroenterology 164, 89–102 (2023).
Guedj, K. et al. M1 macrophages act as LTβR-independent lymphoid tissue inducer cells during atherosclerosis-related lymphoid neogenesis. Cardiovasc. Res. 101, 434–443 (2014).
Jupelli, M. et al. Chlamydia pneumoniae infection in mice induces chronic lung inflammation, iBALT formation, and fibrosis. PLoS One 8, e77447 (2013).
Wang, L. et al. Genomic properties and clinical outcomes associated with tertiary lymphoid structures in patients with breast cancer. Sci. Rep. 13, 13542 (2023).
Koscsó, B. et al. Gut-resident CX3CR1(hi) macrophages induce tertiary lymphoid structures and IgA response in situ. Sci. Immunol. 5, eaax0062 (2020).
Gunnarsdottir, F. B. et al. Breast cancer associated CD169(+) macrophages possess broad immunosuppressive functions but enhance antibody secretion by activated B cells. Front. Immunol. 14, 1180209 (2023).
Briem, O. et al. CD169(+) Macrophages in primary breast tumors associate with tertiary lymphoid structures, T(regs) and a worse prognosis for patients with advanced breast cancer. Cancers 15, 1262 (2023).
Bugatti, M. et al. A population of TIM4+FOLR2+ macrophages localized in tertiary lymphoid structures correlates to an active immune infiltrate across several cancer types. Cancer Immunol. Res. 10, 1340–1353 (2022).
Chen, L. et al. The immunosuppressive niche of soft-tissue sarcomas is sustained by tumor-associated macrophages and characterized by intratumoral tertiary lymphoid structures. Clin. Cancer Res. 26, 4018–4030 (2020).
Singh, S. et al. Chemotherapy coupled to macrophage inhibition induces T-cell and b-cell infiltration and durable regression in triple-negative breast cancer. Cancer Res. 82, 2281–2297 (2022).
Banchereau, J. & Palucka, A. K. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 5, 296–306 (2005).
de Chaisemartin, L. et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 71, 6391–6399 (2011).
Wu, S. Y. et al. CCL19(+) dendritic cells potentiate clinical benefit of anti-PD-(L)1 immunotherapy in triple-negative breast cancer. Medicines 4, 373–393.e378 (2023).
Truxova, I. et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J. Immunother. Cancer 6, 139 (2018).
Li, Q. et al. Prognostic value of tertiary lymphoid structure and tumour infiltrating lymphocytes in oral squamous cell carcinoma. Int. J. Oral. Sci. 12, 24 (2020).
Marinkovic, T. et al. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J. Clin. Investig. 116, 2622–2632 (2006).
Derks, S. et al. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann. Oncol. 31, 1011–1020 (2020).
Halle, S. et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J. Exp. Med. 206, 2593–2601 (2009).
GeurtsvanKessel, C. H. et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J. Exp. Med. 206, 2339–2349 (2009).
Naessens, T. et al. Human lung conventional dendritic cells orchestrate lymphoid neogenesis during chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 202, 535–548 (2020).
Richmond, B. W. et al. Monocyte-derived dendritic cells link localized secretory IgA deficiency to adaptive immune activation in COPD. Mucosal Immunol. 14, 431–442 (2021).
Kießler, M. et al. Tumor-infiltrating plasmacytoid dendritic cells are associated with survival in human colon cancer. J. Immunother Cancer 9, e001813 (2021).
Sisirak, V. et al. Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 72, 5188–5197 (2012).
Pontarini, E. et al. NKp30 receptor upregulation in salivary glands of Sjögren’s syndrome characterizes ectopic lymphoid structures and is restricted by rituximab treatment. Front. Immunol. 12, 706737 (2021).
Pontarini, E. et al. NK cell recruitment in salivary glands provides early viral control but is dispensable for tertiary lymphoid structure formation. J. Leukoc. Biol. 105, 589–602 (2019).
Barone, F. et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc. Natl Acad. Sci. USA 112, 11024–11029 (2015).
Ikeda, A. et al. Human NKp44(+) group 3 innate lymphoid cells associate with tumor-associated tertiary lymphoid structures in colorectal cancer. Cancer Immunol. Res. 8, 724–731 (2020).
Carrega, P. et al. NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat. Commun. 6, 8280 (2015).
Poholek, C. H., Dulson, S. J., Zajac, A. J. & Harrington, L. E. IL-21 controls ILC3 cytokine production and promotes a protective phenotype in a mouse model of colitis. Immunohorizons 3, 194–202 (2019).
Komura, K. et al. Tertiary lymphoid structure and neutrophil-lymphocyte ratio coordinately predict outcome of pembrolizumab. Cancer Sci. 114, 4622–4631 (2023).
Matsuda, N. et al. Prognostic impact of tumor-infiltrating lymphocytes, tertiary lymphoid structures, and neutrophil-to-lymphocyte ratio in pulmonary metastases from uterine leiomyosarcoma. Ann. Surg. Oncol. 30, 8727–8734 (2023).
Yamakoshi, Y. et al. Association between the preoperative neutrophil-to-lymphocyte ratio and tertiary lymphoid structures surrounding tumor in gastric cancer. Mol. Clin. Oncol. 14, 76 (2021).
García-Hernández, M. L. et al. A unique cellular and molecular microenvironment is present in tertiary lymphoid organs of patients with spontaneous prostate cancer regression. Front. Immunol. 8, 563 (2017).
Zhang, T. et al. Peritumor tertiary lymphoid structures are associated with infiltrating neutrophils and inferior prognosis in hepatocellular carcinoma. Cancer Med. 12, 3068–3078 (2023).
Li, S. et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J. Exp. Clin. Cancer Res. 38, 6 (2019).
Lok, L. S. C. & Clatworthy, M. R. Neutrophils in secondary lymphoid organs. Immunology 164, 677–688 (2021).
Lok, L. S. C. et al. Phenotypically distinct neutrophils patrol uninfected human and mouse lymph nodes. Proc. Natl Acad. Sci. USA 116, 19083–19089 (2019).
Dorraji, S. E. et al. Mesenchymal stem cells and T cells in the formation of tertiary lymphoid structures in lupus nephritis. Sci. Rep. 8, 7861 (2018).
Neyt, K. et al. Early IL-1 signaling promotes iBALT induction after influenza virus infection. Front. Immunol. 7, 312 (2016).
Chauhan, P. S. et al. Rapid induction of pulmonary inflammation, autoimmune gene expression, and ectopic lymphoid neogenesis following acute silica exposure in lupus-prone mice. Front. Immunol. 12, 635138 (2021).
Weinstein, A. M. et al. Association of IL-36γ with tertiary lymphoid structures and inflammatory immune infiltrates in human colorectal cancer. Cancer Immunol. Immunother. 68, 109–120 (2019).
Hill, D. G., Ward, A., Nicholson, L. B. & Jones, G. W. Emerging roles for IL-6 family cytokines as positive and negative regulators of ectopic lymphoid structures. Cytokine 146, 155650 (2021).
Botelho, F. M. et al. Pulmonary expression of oncostatin M (OSM) promotes inducible BALT formation independently of IL-6, despite a role for IL-6 in OSM-driven pulmonary inflammation. J. Immunol. 191, 1453–1464 (2013).
Goya, S. et al. Sustained interleukin-6 signalling leads to the development of lymphoid organ-like structures in the lung. J. Pathol. 200, 82–87 (2003).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Barr, T. A. et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 209, 1001–1010 (2012).
Harker, J. A., Lewis, G. M., Mack, L. & Zuniga, E. I. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 334, 825–829 (2011).
Hill, D. G. et al. Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes submucosal tertiary lymphoid structure development. Int. J. Cancer 143, 167–178 (2018).
Lucchesi, D. et al. Impaired interleukin-27-mediated control of CD4+ T cell function impact on ectopic lymphoid structure formation in patients with Sjögren’s syndrome. Arthritis Rheumatol. 72, 1559–1570 (2020).
Meier, D. et al. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 26, 643–654 (2007).
Nayar, S. et al. Bimodal expansion of the lymphatic vessels is regulated by the sequential expression of IL-7 and lymphotoxin α1β2 in newly formed tertiary lymphoid structures. J. Immunol. 197, 1957–1967 (2016).
Vondenhoff, M. F. et al. LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J. Immunol. 182, 5439–5445 (2009).
Iolyeva, M. et al. Interleukin-7 is produced by afferent lymphatic vessels and supports lymphatic drainage. Blood 122, 2271–2281 (2013).
Kröncke, R., Loppnow, H., Flad, H. D. & Gerdes, J. Human follicular dendritic cells and vascular cells produce interleukin-7: a potential role for interleukin-7 in the germinal center reaction. Eur. J. Immunol. 26, 2541–2544 (1996).
Knop, L. et al. IL-7 derived from lymph node fibroblastic reticular cells is dispensable for naive T cell homeostasis but crucial for central memory T cell survival. Eur. J. Immunol. 50, 846–857 (2020).
Shinoda, K. et al. Thy1+IL-7+ lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation. Proc. Natl Acad. Sci. USA 113, E2842–E2851 (2016).
Kabata, H. et al. ILCs and allergy. Adv. Exp. Med. Biol. 1365, 75–95 (2022).
St Paul, M. et al. IL6 Induces an IL22(+) CD8(+) T-cell subset with potent antitumor function. Cancer Immunol. Res. 8, 321–333 (2020).
Bruchard, M. & Ghiringhelli, F. Deciphering the roles of innate lymphoid cells in cancer. Front. Immunol. 10, 656 (2019).
Ware, M. B. et al. The role of interleukin-7 in the formation of tertiary lymphoid structures and their prognostic value in gastrointestinal cancers. J. Immunother. Precis. Oncol. 5, 105–117 (2022).
Luo, R. et al. Tertiary lymphoid organs are associated with the progression of kidney damage and regulated by interleukin-17A. Theranostics 11, 117–131 (2021).
Zhu, M. & Fu, Y. Proinflammatory IL-17 induces iBALT development. Cell Mol. Immunol. 9, 101–102, (2012).
Fleige, H. et al. Induction of BALT in the absence of IL-17. Nat. Immunol. 13, 1 (2011).
Fleige, H. et al. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J. Exp. Med. 211, 643–651 (2014).
Tsukamoto, H. et al. Aging-associated and CD4 T-cell-dependent ectopic CXCL13 activation predisposes to anti-PD-1 therapy-induced adverse events. Proc. Natl Acad. Sci. USA 119, e2205378119 (2022).
de Leur, K. et al. Characterization of ectopic lymphoid structures in different types of acute renal allograft rejection. Clin. Exp. Immunol. 192, 224–232 (2018).
Luo, R. et al. T Follicular helper cells in tertiary lymphoid structure contribute to renal fibrosis by IL-21. Int. J. Mol. Sci. 24, 12535 (2023).
Duan, L. et al. Follicular dendritic cells restrict interleukin-4 availability in germinal centers and foster memory B cell generation. Immunity 54, 2256–2272.e2256 (2021).
Bellamri, N. et al. TNF-α and IL-10 Control CXCL13 Expression in Human Macrophages. J. Immunol. 204, 2492–2502 (2020).
Mills, K. H. G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 23, 38–54 (2023).
Gomez-Nguyen, A. et al. Chronic stress induces colonic tertiary lymphoid organ formation and protection against secondary injury through IL-23/IL-22 signaling. Proc. Natl Acad. Sci. USA 119, e2208160119 (2022).
Borelli, A. & Irla, M. Lymphotoxin: from the physiology to the regeneration of the thymic function. Cell Death Differ. 28, 2305–2314 (2021).
Kabashima, K. et al. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22, 439–450 (2005).
Kucharzewska, P. et al. NIK-IKK complex interaction controls NF-κB-dependent inflammatory activation of endothelium in response to LTβR ligation. J. Cell Sci. 132, jcs225615 (2019).
Browning, J. L. et al. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 23, 539–550 (2005).
Sautès-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Schneider, K., Potter, K. G. & Ware, C. F. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol. Rev. 202, 49–66 (2004).
Remouchamps, C., Boutaffala, L., Ganeff, C. & Dejardin, E. Biology and signal transduction pathways of the Lymphotoxin-αβ/LTβR system. Cytokine Growth Factor Rev. 22, 301–310, (2011).
Gantsev, S. K. et al. The role of inflammatory chemokines in lymphoid neoorganogenesis in breast cancer. Biomed. Pharmacother. 67, 363–366 (2013).
Zhang, N. et al. LIGHT/TNFSF14 promotes CAR-T cell trafficking and cytotoxicity through reversing immunosuppressive tumor microenvironment. Mol. Ther. 31, 2575–2590 (2023).
He, B. et al. Vascular targeting of LIGHT normalizes blood vessels in primary brain cancer and induces intratumoural high endothelial venules. J. Pathol. 245, 209–221 (2018).
Furtado, G. C. et al. TNFα-dependent development of lymphoid tissue in the absence of RORγt+ lymphoid tissue inducer cells. Mucosal Immunol. 7, 602–614 (2014).
Rodriguez, A. B., Parriott, G. & Engelhard, V. H. Tumor necrosis factor receptor regulation of peripheral node addressin biosynthetic components in tumor endothelial cells. Front. Immunol. 13, 1009306 (2022).
Blanchard, L. & Girard, J. P. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis 24, 719–753 (2021).
Möckel, T., Basta, F., Weinmann-Menke, J. & Schwarting, A. B cell activating factor (BAFF): structure, functions, autoimmunity and clinical implications in Systemic Lupus Erythematosus (SLE). Autoimmun. Rev. 20, 102736 (2021).
Diddens, J. et al. Single-cell profiling indicates a proinflammatory role of meningeal ectopic lymphoid tissue in experimental autoimmune encephalomyelitis. Neurol. Neuroimmunol. Neuroinflamm. 11, e200185 (2024).
Sabat, R. et al. Neutrophilic granulocyte-derived B-cell activating factor supports B cells in skin lesions in hidradenitis suppurativa. J. Allergy Clin. Immunol. 151, 1015–1026 (2023).
Morissette, M. C. et al. Role of BAFF in pulmonary autoantibody responses induced by chronic cigarette smoke exposure in mice. Physiol. Rep. 4, e13057 (2016).
Collison, J. Lupus nephritis: novel role for BAFF in tertiary lymphoid neogenesis. Nat. Rev. Rheumatol. 13, 260 (2017).
Kang, S. et al. BAFF induces tertiary lymphoid structures and positions T cells within the glomeruli during lupus nephritis. J. Immunol. 198, 2602–2611 (2017).
Steines, L. et al. B Cell activating factor (BAFF) is required for the development of intra-renal tertiary lymphoid organs in experimental kidney transplantation in rats. Int. J. Mol. Sci. 21, 8045 (2020).
Graves, D. T. & Jiang, Y. Chemokines, a family of chemotactic cytokines. Crit. Rev. Oral. Biol. Med. 6, 109–118, (1995).
Rouanne, M., Arpaia, N. & Marabelle, A. CXCL13 shapes tertiary lymphoid structures and promotes response to immunotherapy in bladder cancer. Eur. J. Cancer 151, 245–248 (2021).
Carlsen, H. S. et al. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood 104, 3021–3027 (2004).
McDonald, K. G., McDonough, J. S., Dieckgraefe, B. K. & Newberry, R. D. Dendritic cells produce CXCL13 and participate in the development of murine small intestine lymphoid tissues. Am. J. Pathol. 176, 2367–2377, (2010).
Ukita, M. et al. CXCL13-producing CD4+ T cells accumulate in the early phase of tertiary lymphoid structures in ovarian cancer. JCI Insight 7, e157215 (2022).
Li, J. P. et al. PD-1(+)CXCR5(-)CD4(+) Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J. Immunother. Cancer 9, e002101 (2021).
Groeneveld, C. S. et al. Tertiary lymphoid structures marker CXCL13 is associated with better survival for patients with advanced-stage bladder cancer treated with immunotherapy. Eur. J. Cancer 148, 181–189 (2021).
Dai, S. et al. Intratumoral CXCL13(+)CD8(+)T cell infiltration determines poor clinical outcomes and immunoevasive contexture in patients with clear cell renal cell carcinoma. J. Immunother. Cancer 9, e001823 (2021).
Allen, C. D. et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 5, 943–952 (2004).
Reschke, R. et al. Immune cell and tumor cell-derived CXCL10 is indicative of immunotherapy response in metastatic melanoma. J. Immunother Cancer 9, e003521 (2021).
Hauser, M. A. & Legler, D. F. Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J. Leukoc. Biol. 99, 869–882 (2016).
Han, L. & Zhang, L. CCL21/CCR7 axis as a therapeutic target for autoimmune diseases. Int. Immunopharmacol. 121, 110431 (2023).
Sah, V. R. et al. Chemokine analysis in patients with metastatic uveal melanoma suggests a role for CCL21 signaling in combined epigenetic therapy and checkpoint immunotherapy. Cancer Res. Commun. 3, 884–895 (2023).
Yin, X. et al. Tobacco exposure primes the secretion of CCL21 positively associated with tertiary lymphoid structure and response to immunotherapy. Immunother. Cancer 11, e006939 (2023).
De Silva, P. et al. FOXP1 negatively regulates tumor infiltrating lymphocyte migration in human breast cancer. EBioMedicine 39, 226–238 (2019).
Korbecki, J. et al. Fractalkine/CX3CL1 in neoplastic processes. Int. J. Mol. Sci. 21, 3723 (2020).
Murphy, G., Caplice, N. & Molloy, M. Fractalkine in rheumatoid arthritis: a review to date. Rheumatology 47, 1446–1451 (2008).
Astorri, E. et al. CX3CL1 and CX3CR1 expression in tertiary lymphoid structures in salivary gland infiltrates: fractalkine contribution to lymphoid neogenesis in Sjogren’s syndrome. Rheumatology 53, 611–620 (2014).
Denton, A. E. et al. Type I interferon induces CXCL13 to support ectopic germinal center formation. J. Exp. Med. 216, 621–637 (2019).
Sjöstrand, M. et al. The expression of BAFF is controlled by IRF transcription factors. J. Immunol. 196, 91–96 (2016).
Andersson, A. et al. Spatial deconvolution of HER2-positive breast cancer delineates tumor-associated cell type interactions. Nat. Commun. 12, 6012 (2021).
Nakayamada, S. et al. Type I IFN induces binding of STAT1 to Bcl6: divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. J. Immunol. 192, 2156–2166 (2014).
Tian, L. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 (2017).
Olkhov-Mitsel, E. et al. Upregulation of IFNɣ-mediated chemokines dominate the immune transcriptome of muscle-invasive urothelial carcinoma. Sci. Rep. 12, 716 (2022).
Yoshimoto, K. et al. Regulatory mechanisms for the production of BAFF and IL-6 are impaired in monocytes of patients of primary Sjögren’s syndrome. Arthritis. Res. Ther. 13, R170 (2011).
Batlle, E. & Massagué, J. Transforming growth factor-β signaling in Immunity and Cancer. Immunity 50, 924–940 (2019).
Kelly, F. M. et al. TGF-beta upregulation drives tertiary lymphoid organ formation and kidney dysfunction in calcineurin A-alpha heterozygous mice. Am. J. Physiol. Ren. Physiol. 296, F512–F520 (2009).
Kinker, G. S. et al. Mature tertiary lymphoid structures are key niches of tumour-specific immune responses in pancreatic ductal adenocarcinomas. Gut 72, 1927–1941 (2023).
O’Connor, R. A. et al. Cancer-associated fibroblasts drive CXCL13 production in activated T cells via TGF-beta. Front. Immunol. 14, 1221532 (2023).
Lin, Q. Y. et al. VEGF-C/VEGFR-3 axis protects against pressure-overload induced cardiac dysfunction through regulation of lymphangiogenesis. Clin. Transl. Med. 11, e374 (2021).
Chyou, S. et al. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J. Immunol. 181, 3887–3896 (2008).
Shikhagaie, M. M. et al. Neuropilin-1 is expressed on lymphoid tissue residing LTi-like group 3 innate lymphoid cells and associated with ectopic lymphoid aggregates. Cell Rep. 18, 1761–1773 (2017).
Allen, E. et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 9, eaak9679 (2017).
Cattoretti, G. et al. BCL-6 protein is expressed in germinal-center B cells. Blood 86, 45–53 (1995).
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu Rev. Immunol. 40, 413–442 (2022).
Fukuda, T. et al. Disruption of the Bcl6 gene results in an impaired germinal center formation. J. Exp. Med. 186, 439–448 (1997).
Hatzi, K. et al. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J. Exp. Med. 212, 539–553 (2015).
Liu, D. et al. BCL6 controls contact-dependent help delivery during follicular T-B cell interactions. Immunity 54, 2245–2255.e2244 (2021).
Ma, G. et al. Presence, subtypes, and prognostic significance of tertiary lymphoid structures in urothelial carcinoma of the bladder. Oncologist 29, e248–e258 (2024).
Werner, F. et al. A standardized analysis of tertiary lymphoid structures in human melanoma: disease progression- and tumor site-associated changes with germinal center alteration. Front. Immunol. 12, 675146 (2021).
Çakan, E. & Gunaydin, G. Activation-induced cytidine deaminase: an old friend with new faces. Front. Immunol. 13, 965312 (2022).
Epps, S. J. et al. Features of ectopic lymphoid-like structures in human uveitis. Exp. Eye Res. 191, 107901 (2020).
Zhou, S. et al. Autoreactive B cell differentiation in diffuse ectopic lymphoid-like structures of inflamed pemphigus lesions. J. Invest Dermatol. 140, 309–318.e308 (2020).
Lutz, E. R. et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol. Res. 2, 616–631 (2014).
Blum, K. S. & Pabst, R. Keystones in lymph node development. J. Anat. 209, 585–595 (2006).
van de Pavert, S. A. & Mebius, R. E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 10, 664–674 (2010).
Fütterer, A. et al. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9, 59–70 (1998).
Drayton, D. L., Liao, S., Mounzer, R. H. & Ruddle, N. H. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 7, 344–353 (2006).
Liu, W. et al. An immune cell map of human lung adenocarcinoma development reveals an anti-tumoral role of the Tfh-dependent tertiary lymphoid structure. Cell Rep. Med. 5, 101448 (2024).
Hu, C. et al. Tertiary lymphoid structure-associated B cells enhance CXCL13(+)CD103(+)CD8(+) tissue-resident memory T-cell response to programmed cell death protein 1 blockade in cancer immunotherapy. Gastroenterology 166, 1069–1084 (2024).
Cupedo, T., Jansen, W., Kraal, G. & Mebius, R. E. Induction of secondary and tertiary lymphoid structures in the skin. Immunity 21, 655–667 (2004).
Nayar, S. et al. Immunofibroblasts regulate LTα3 expression in tertiary lymphoid structures in a pathway dependent on ICOS/ICOSL interaction. Commun. Biol. 5, 413 (2022).
Wang, Z. Z. et al. Stromal cells and B cells orchestrate ectopic lymphoid tissue formation in nasal polyps. Allergy 76, 1416–1431 (2021).
Witherel, C. E. et al. Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Biomaterials 269, 120667 (2021).
Sato, M. et al. Stromal activation and formation of lymphoid-like stroma in chronic lung allograft dysfunction. Transplantation 91, 1398–1405 (2011).
Jamaly, S. et al. Interplay of immune and kidney resident cells in the formation of tertiary lymphoid structures in lupus nephritis. Autoimmun. Rev. 20, 102980 (2021).
Kanapathippillai, P., Hedberg, A., Fenton, C. G. & Fenton, K. A. Nucleosomes contribute to increase mesangial cell chemokine expression during the development of lupus nephritis. Cytokine 62, 244–252 (2013).
Li, H. et al. IL-23 reshapes kidney resident cell metabolism and promotes local kidney inflammation. J. Clin. Investig. 131, e142428 (2021).
Schwarting, A. et al. Renal tubular epithelial cell-derived BAFF expression mediates kidney damage and correlates with activity of proliferative lupus nephritis in mouse and men. Lupus 27, 243–256 (2018).
Yung, S., Cheung, K. F., Zhang, Q. & Chan, T. M. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J. Am. Soc. Nephrol. 21, 1912–1927 (2010).
Bombardieri, M. et al. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J. Immunol. 189, 3767–3776 (2012).
Brand, R. M. et al. Anti-CD20 depletes meningeal B cells but does not halt the formation of meningeal ectopic lymphoid tissue. Neurol Neuroimmunol. Neuroinflamm. 8, e1012 (2021).
Yuan, H. et al. Single-cell sequencing reveals the heterogeneity of B cells and tertiary lymphoid structures in muscle-invasive bladder cancer. J. Transl. Med. 22, 48 (2024).
Zhang, S. et al. Characteristics of B lymphocyte infiltration in HPV(+) head and neck squamous cell carcinoma. Cancer Sci. 112, 1402–1416 (2021).
Luther, S. A., Ansel, K. M. & Cyster, J. G. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J. Exp. Med. 197, 1191–1198 (2003).
Martinet, L. et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 71, 5678–5687 (2011).
Zhan, Z. et al. High endothelial venules proportion in tertiary lymphoid structure is a prognostic marker and correlated with anti-tumor immune microenvironment in colorectal cancer. Ann. Med. 55, 114–126 (2023).
Jeucken, K. C. M., Koning, J. J., Mebius, R. E. & Tas, S. W. The role of endothelial cells and TNF-receptor superfamily members in lymphoid organogenesis and function during health and inflammation. Front Immunol. 10, 2700 (2019).
Onder, L. et al. Endothelial cell-specific lymphotoxin-β receptor signaling is critical for lymph node and high endothelial venule formation. J. Exp. Med. 210, 465–473 (2013).
Luther, S. A. et al. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 12, 471–481 (2000).
Ramachandran, M. et al. Tailoring vascular phenotype through AAV therapy promotes anti-tumor immunity in glioma. Cancer Cell. 41, 1134–1151.e1110 (2023).
Fleig, S. et al. Loss of vascular endothelial notch signaling promotes spontaneous formation of tertiary lymphoid structures. Nat. Commun. 13, 2022 (2022).
Yoshida, H. et al. Role of sialyl 6-sulfo Lewis X in antitumor immunity against oral squamous cell carcinoma. J. Oral. Pathol. Med. 46, 759–765 (2017).
He, M. et al. Intratumoral tertiary lymphoid structure (TLS) maturation is influenced by draining lymph nodes of lung cancer. J. Immunother Cancer 11, e005539 (2023).
Sawada, J. et al. Molecular signature of tumor-associated high endothelial venules that can predict breast cancer survival. Cancer Immunol. Res. 10, 468–481 (2022).
Asrir, A. et al. Tumor-associated high endothelial venules mediate lymphocyte entry into tumors and predict response to PD-1 plus CTLA-4 combination immunotherapy. Cancer Cell. 40, 318–334.e319 (2022).
Zhu, C., Kros, J. M., Cheng, C. & Mustafa, D. The contribution of tumor-associated macrophages in glioma neo-angiogenesis and implications for anti-angiogenic strategies. Neuro Oncol. 19, 1435–1446 (2017).
Martinet, L. et al. High endothelial venule blood vessels for tumor-infiltrating lymphocytes are associated with lymphotoxin β-producing dendritic cells in human breast cancer. J. Immunol. 191, 2001–2008 (2013).
Moussion, C. & Girard, J. P. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 479, 542–546 (2011).
Hindley, J. P. et al. T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res. 72, 5473–5482 (2012).
Choe, K. et al. Stepwise transmigration of T- and B cells through a perivascular channel in high endothelial venules. Life Sci Alliance 4, e202101086 (2021).
Vella, G., Guelfi, S. & Bergers, G. High endothelial venules: a vascular perspective on tertiary lymphoid structures in cancer. Front. Immunol. 12, 736670 (2021).
Hemmerich, S. et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity 15, 237–247 (2001).
Kawashima, H. et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat. Immunol. 6, 1096–1104 (2005).
Vella, G., Hua, Y. & Bergers, G. High endothelial venules in cancer: regulation, function, and therapeutic implication. Cancer Cell. 41, 527–545 (2023).
Girard, J. P., Moussion, C. & Förster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 12, 762–773 (2012).
Kanemitsu, N. et al. CXCL13 is an arrest chemokine for B cells in high endothelial venules. Blood 106, 2613–2618 (2005).
Wang, Y., Liu, J., Burrows, P. D. & Wang, J. Y. B Cell Development and Maturation. Adv. Exp. Med. Biol. 1254, 1–22 (2020).
Huang, C. Germinal center reaction. Adv. Exp. Med. Biol. 1254, 47–53 (2020).
Schulz, O., Hammerschmidt, S. I., Moschovakis, G. L. & Förster, R. Chemokines and chemokine receptors in lymphoid tissue dynamics. Annu. Rev. Immunol. 34, 203–242 (2016).
Stebegg, M. et al. Regulation of the germinal center response. Front. Immunol. 9, 2469 (2018).
Huang, C. & Melnick, A. Mechanisms of action of BCL6 during germinal center B cell development. Sci. China Life Sci. 58, 1226–1232 (2015).
Shokat, K. M. & Goodnow, C. C. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature 375, 334–338 (1995).
Pulendran, B. et al. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature 375, 331–334 (1995).
Paus, D. et al. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J. Exp. Med. 203, 1081–1091 (2006).
Bannard, O. et al. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity 39, 912–924 (2013).
Dal Porto, J. M., Haberman, A. M., Kelsoe, G. & Shlomchik, M. J. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J. Exp. Med. 195, 1215–1221 (2002).
Schwickert, T. A. et al. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J. Exp. Med. 208, 1243–1252 (2011).
Amitai, A. et al. A population dynamics model for clonal diversity in a germinal center. Front Microbiol. 8, 1693 (2017).
Meyer-Hermann, M. et al. A theory of germinal center B cell selection, division, and exit. Cell Rep. 2, 162–174 (2012).
Victora, G. D. et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143, 592–605 (2010).
Mayer, C. T. et al. The microanatomic segregation of selection by apoptosis in the germinal center. Science 358, eaao2602 (2017).
Liu, Y. J. et al. Mechanism of antigen-driven selection in germinal centres. Nature 342, 929–931 (1989).
Turner, J. S., Marthi, M., Benet, Z. L. & Grigorova, I. Transiently antigen-primed B cells return to naive-like state in absence of T-cell help. Nat. Commun. 8, 15072 (2017).
Turner, J. S., Ke, F. & Grigorova, I. L. B cell receptor crosslinking augments germinal center B cell selection when T cell help is limiting. Cell Rep. 25, 1395–1403.e1394 (2018).
Luo, W., Weisel, F. & Shlomchik, M. J. B cell receptor and CD40 signaling are rewired for synergistic induction of the c-Myc transcription factor in germinal center B cells. Immunity 48, 313–326.e315 (2018).
Heise, N. et al. Germinal center B cell maintenance and differentiation are controlled by distinct NF-κB transcription factor subunits. J. Exp. Med. 211, 2103–2118 (2014).
Radtke, D. & Bannard, O. Expression of the plasma cell transcriptional regulator Blimp-1 by dark zone germinal center B cells during periods of proliferation. Front. Immunol. 9, 3106 (2018).
Fridman, W. H. et al. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol. 19, 441–457 (2022).
Cattoretti, G. et al. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell. 7, 445–455 (2005).
Cremasco, V. et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat. Immunol. 15, 973–981 (2014).
Yu, K. AID function in somatic hypermutation and class switch recombination. Acta Biochim Biophys. Sin. 54, 759–766 (2022).
Wang, Y. et al. Mesoscale DNA feature in antibody-coding sequence facilitates somatic hypermutation. Cell 186, 2193–2207.e2119 (2023).
Kumanogoh, A. et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 419, 629–633 (2002).
Fridman, W. H. et al. Activation of B cells in tertiary lymphoid structures in cancer: anti-tumor or anti-self? Semin. Immunol. 65, 101703 (2023).
Wang, M. et al. Tertiary lymphoid structures as local perpetuators of organ-specific immune injury: implication for lupus nephritis. Front. Immunol. 14, 1204777 (2023).
Wang, Q. et al. Single-cell transcriptome sequencing of B-cell heterogeneity and tertiary lymphoid structure predicts breast cancer prognosis and neoadjuvant therapy efficacy. Clin. Transl. Med. 13, e1346 (2023).
Meylan, M. et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541.e525 (2022).
Zhang, B. et al. Single-cell chemokine receptor profiles delineate the immune contexture of tertiary lymphoid structures in head and neck squamous cell carcinoma. Cancer Lett. 558, 216105 (2023).
Wang, Y. et al. Computerized tertiary lymphoid structures density on H&E-images is a prognostic biomarker in resectable lung adenocarcinoma. iScience 26, 107635 (2023).
Randolph, G. J. et al. Lymphoid aggregates remodel lymphatic collecting vessels that serve mesenteric lymph nodes in Crohn disease. Am. J. Pathol. 186, 3066–3073 (2016).
Sato, Y. et al. Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tissues in the kidney. JCI Insight 1, e87680 (2016).
Hamade, A. et al. Sex differences in the aging murine urinary bladder and influence on the tumor immune microenvironment of a carcinogen-induced model of bladder cancer. Biol. Sex. Differ. 13, 19 (2022).
Camell, C. D. et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 30, 1024–1039.e1026 (2019).
Grubb, B. R. et al. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am. J. Physiol. Lung Cell Mol. Physiol. 310, L860–L867 (2016).
Tertiary lymphoid structures validated as biomarker. Cancer Discov. 14, Of2, (2024).
Feng, W. et al. CDK4/6i enhances the antitumor effect of PD1 antibody by promoting TLS formation in ovarian cancer. Heliyon 9, e19760 (2023).
Masuda, T. et al. Unique characteristics of tertiary lymphoid structures in kidney clear cell carcinoma: prognostic outcome and comparison with bladder cancer. J. Immunother Cancer 10, e003883 (2022).
Munoz-Erazo, L., Rhodes, J. L., Marion, V. C. & Kemp, R. A. Tertiary lymphoid structures in cancer—considerations for patient prognosis. Cell Mol. Immunol. 17, 570–575 (2020).
Wu, Y. H. et al. Features and clinical significance of tertiary lymphoid structure in cutaneous squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 36, 2043–2050 (2022).
Liu, Z. et al. Intratumoral tertiary lymphoid structures promote patient survival and immunotherapy response in head neck squamous cell carcinoma. Cancer Immunol. Immunother. 72, 1505–1521 (2023).
Zhou, X. et al. Tertiary lymphoid structure stratifies glioma into three distinct tumor subtypes. Aging 13, 26063–26094 (2021).
Ding, G. Y. et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. J. Hepatol. 76, 608–618 (2022).
Hayashi, Y. et al. Density and maturity of peritumoral tertiary lymphoid structures in oesophageal squamous cell carcinoma predicts patient survival and response to immune checkpoint inhibitors. Br. J. Cancer 128, 2175–2185 (2023).
Wang, Q. et al. Peritumoral tertiary lymphoid structure and tumor stroma percentage predict the prognosis of patients with non-metastatic colorectal cancer. Front Immunol. 13, 962056 (2022).
Finkin, S. et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat. Immunol. 16, 1235–1244 (2015).
Shang, T. et al. Tertiary lymphoid structures predict the prognosis and immunotherapy response of cholangiocarcinoma. Front. Immunol. 14, 1166497 (2023).
Sofopoulos, M. et al. The prognostic significance of peritumoral tertiary lymphoid structures in breast cancer. Cancer Immunol. Immunother. 68, 1733–1745 (2019).
Lynch, K. T. et al. Heterogeneity in tertiary lymphoid structure B-cells correlates with patient survival in metastatic melanoma. J. Immunother. Cancer 9, e002273 (2021).
Datta, R. R. et al. Post-transplant malignancies show reduced t-cell abundance and tertiary lymphoid structures as correlates of impaired cancer immunosurveillance. Clin. Cancer Res. 28, 1712–1723 (2022).
Noël, G. et al. Functional Th1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J Clin Invest 131, e139905 (2021).
Johnson, D. E. et al. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 6, 92 (2020).
Li, H. et al. Tertiary lymphoid structure raises survival and immunotherapy in HPV(-) HNSCC. J. Dent. Res. 102, 678–688 (2023).
Liang, H. et al. Follicle-like tertiary lymphoid structures: a potential biomarker for prognosis and immunotherapy response in patients with laryngeal squamous cell carcinoma. Front. Immunol. 14, 1096220 (2023).
Lagergren, J. & Lagergren, P. Oesophageal cancer. BMJ 341, c6280 (2010).
Nakamura, S. et al. Tertiary lymphoid structures correlate with enhancement of antitumor immunity in esophageal squamous cell carcinoma. Br. J. Cancer 129, 1314–1326 (2023).
Li, R. et al. Tertiary lymphoid structures favor outcome in resected esophageal squamous cell carcinoma. J. Pathol. Clin. Res. 8, 422–435 (2022).
Sun, X. et al. Maturation and abundance of tertiary lymphoid structures are associated with the efficacy of neoadjuvant chemoimmunotherapy in resectable non-small cell lung cancer. J. Immunother. Cancer 10, e005531 (2022).
Patil, N. S. et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell 40, 289–300.e284 (2022).
Wakasu, S. et al. Preventive effect of tertiary lymphoid structures on lymph node metastasis of lung adenocarcinoma. Cancer Immunol. Immunother. 72, 1823–1834 (2023).
Barnett, R. Ovarian cancer. Lancet 387, 1265 (2016).
Lu, H. et al. Tumor and local lymphoid tissue interaction determines prognosis in high-grade serous ovarian cancer. Cell Rep. Med. 4, 101092 (2023).
Dekker, E. et al. Colorectal cancer. Lancet 394, 1467–1480 (2019).
Zhang, C. et al. Localization and density of tertiary lymphoid structures associate with molecular subtype and clinical outcome in colorectal cancer liver metastases. J. Immunother. Cancer 11, e006425 (2023).
Posch, F. et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology 7, e1378844 (2018).
Forner, A., Reig, M. & Bruix, J. Hepatocellular carcinoma. Lancet 391, 1301–1314 (2018).
Jia, W. et al. Protective effect of tertiary lymphoid structures against hepatocellular carcinoma: New findings from a genetic perspective. Front. Immunol. 13, 1007426 (2022).
Zhang, F. P. et al. Intra-tumoral secondary follicle-like tertiary lymphoid structures are associated with a superior prognosis of overall survival of perihilar cholangiocarcinoma. Cancers 14, 6107 (2022).
Gu-Trantien, C. et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Invest. 123, 2873–2892 (2013).
Gu-Trantien, C. et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI insight 2, e91487 (2017).
Zhou, Y. et al. High endothelial venule is a prognostic immune-related biomarker in patients with resected intrahepatic cholangiocarcinoma. Cell Prolif. 56, e13513 (2023).
Capitanio, U. & Montorsi, F. Renal cancer. Lancet 387, 894–906 (2016).
Xu, W. et al. Heterogeneity in tertiary lymphoid structures predicts distinct prognosis and immune microenvironment characterizations of clear cell renal cell carcinoma. J. Immunother Cancer 11, e006667 (2023).
An, Y. et al. Tertiary lymphoid structure patterns aid in identification of tumor microenvironment infiltration and selection of therapeutic agents in bladder cancer. Front. Immunol. 13, 1049884 (2022).
Zhou, L., Xu, B., Liu, Y. & Wang, Z. Tertiary lymphoid structure signatures are associated with survival and immunotherapy response in muscle-invasive bladder cancer. Oncoimmunology 10, 1915574 (2021).
van Dijk, N. et al. The tumor immune landscape and architecture of tertiary lymphoid structures in urothelial cancer. Front. Immunol. 12, 793964 (2021).
Smyth, E. C. et al. Gastric cancer. Lancet 396, 635–648 (2020).
Yin, Y. X. et al. Impact of mature tertiary lymphoid structures on prognosis and therapeutic response of Epstein-Barr virus-associated gastric cancer patients. Front Immunol. 13, 973085 (2022).
He, W. et al. The high level of tertiary lymphoid structure is correlated with superior survival in patients with advanced gastric cancer. Front. Oncol. 10, 980 (2020).
Harbeck, N. & Gnant, M. Breast cancer. Lancet 389, 1134–1150 (2017).
Wang, B. et al. The presence of tertiary lymphoid structures provides new insight into the clinicopathological features and prognosis of patients with breast cancer. Front. Immunol. 13, 868155 (2022).
Hou, X. et al. Triple-negative breast cancer survival prediction using artificial intelligence through integrated analysis of tertiary lymphoid structures and tumor budding. Cancer 130, 1499–1512 (2024).
Liu, X. et al. Distinct tertiary lymphoid structure associations and their prognostic relevance in HER2 positive and negative breast cancers. Oncologist 22, 1316–1324 (2017).
Saso, S. et al. Endometrial cancer. BMJ 343, d3954 (2011).
Horeweg, N. et al. Tertiary lymphoid structures critical for prognosis in endometrial cancer patients. Nat. Commun. 13, 1373 (2022).
Qin, M. et al. Tertiary lymphoid structures are associated with favorable survival outcomes in patients with endometrial cancer. Cancer Immunol. Immunother. 71, 1431–1442 (2022).
Vincent, A. et al. Pancreatic cancer. Lancet 378, 607–620 (2011).
Zou, X. et al. Characterization of intratumoral tertiary lymphoid structures in pancreatic ductal adenocarcinoma: cellular properties and prognostic significance. J. Immunother. Cancer 11, e006698 (2023).
Tanaka, T. et al. Integrated analysis of tertiary lymphoid structures in relation to tumor-infiltrating lymphocytes and patient survival in pancreatic ductal adenocarcinoma. J. Gastroenterol. 58, 277–291 (2023).
Wolff, T., Tai, E. & Miller, T. Screening for skin cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 150, 194–198 (2009).
Nakamura, M. et al. Tertiary lymphoid structures and chemokine landscape in virus-positive and virus-negative Merkel cell carcinoma. Front. Oncol. 12, 811586 (2022).
Mannarino, L. et al. Epithelioid pleural mesothelioma is characterized by tertiary lymphoid structures in long survivors: results from the MATCH Study. Int. J. Mol. Sci. 23, 5786 (2022).
Benzerdjeb, N. et al. Tertiary lymphoid structures in epithelioid malignant peritoneal mesothelioma are associated with neoadjuvant chemotherapy, but not with prognosis. Virchows Arch. 479, 765–772 (2021).
Wu, H. et al. T-cells produce acidic niches in lymph nodes to suppress their own effector functions. Nat. Commun. 11, 4113 (2020).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Smolen, J. S., Aletaha, D. & McInnes, I. B. Rheumatoid arthritis. Lancet 388, 2023–2038 (2016).
Rivellese, F. et al. Mast cells in early rheumatoid arthritis associate with disease severity and support B cell autoantibody production. Ann. Rheum. Dis. 77, 1773–1781 (2018).
Noort, A. R. et al. Tertiary lymphoid structures in rheumatoid arthritis: NF-κB-inducing kinase-positive endothelial cells as central players. Am. J. Pathol. 185, 1935–1943 (2015).
Anders, H. J. et al. Lupus nephritis. Nat. Rev. Dis. Prim. 6, 7 (2020).
He, N. et al. Association of serum CXCL13 with intrarenal ectopic lymphoid tissue formation in lupus nephritis. J. Immunol. Res. 2016, 4832543 (2016).
Fox, R. I. Sjögren’s syndrome. Lancet 366, 321–331 (2005).
Bystryn, J. C. & Rudolph, J. L. Pemphigus. Lancet 366, 61–73 (2005).
Baxter, A. G. The origin and application of experimental autoimmune encephalomyelitis. Nat. Rev. Immunol. 7, 904–912 (2007).
Morille, J. et al. Multiple sclerosis CSF is enriched with follicular T cells displaying a Th1/eomes signature. Neurol Neuroimmunol. Neuroinflamm. 9, e200033 (2022).
Magliozzi, R. et al. “Ependymal-in” gradient of thalamic damage in progressive multiple sclerosis. Ann. Neurol. 92, 670–685 (2022).
Gardner, C. et al. Cortical grey matter demyelination can be induced by elevated pro-inflammatory cytokines in the subarachnoid space of MOG-immunized rats. Brain 136, 3596–3608 (2013).
Korpos, É. et al. Identification and characterisation of tertiary lymphoid organs in human type 1 diabetes. Diabetologia 64, 1626–1641 (2021).
Burkholder, B. M. & Jabs, D. A. Uveitis for the non-ophthalmologist. BMJ 372, m4979 (2021).
Heng, J. S. et al. Comprehensive analysis of a mouse model of spontaneous uveoretinitis using single-cell RNA sequencing. Proc. Natl Acad. Sci. USA 116, 26734–26744 (2019).
Rebello, A. & Joshi, P. Giant-cell arteritis. N. Engl. J. Med. 387, e36 (2022).
Graver, J. C. et al. Massive B-cell infiltration and organization into artery tertiary lymphoid organs in the aorta of large vessel giant cell arteritis. Front. Immunol. 10, 83 (2019).
Callen, J. P. Dermatomyositis. Lancet 355, 53–57 (2000).
Maghrabi, Y. et al. Adult-type dermatomyositis with secondary lymphoid follicles harbouring reactive B-cells component. Neuromuscul. Disord. 31, 881–885 (2021).
Matsubara, S. et al. Tertiary lymphoid organs in the inflammatory myopathy associated with PD-1 inhibitors. J. Immunother. Cancer 7, 256 (2019).
Chu, Z. et al. Primed macrophages directly and specifically reject allografts. Cell Mol. Immunol. 17, 237–246 (2020).
Baddoura, F. K. et al. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am. J. Transpl. 5, 510–516 (2005).
Nowocin, A. K. et al. Characterizing the B-cell and humoral response in tertiary lymphoid organs in kidney allografts. Exp. Clin. Transpl. 17, 330–338 (2019).
Jonker, M., Wubben, J. A., t Hart, B. A. & Haanstra, K. G. Lymphoid-like structures with distinct B cell areas in kidney allografts are not predictive for graft rejection. a non-human primate study. Inflammation 38, 2191–2202 (2015).
Yamada, Y. et al. Biased IL-2 signals induce Foxp3-rich pulmonary lymphoid structures and facilitate long-term lung allograft acceptance in mice. Nat. Commun. 14, 1383 (2023).
Olivier, B. J. et al. Vagal innervation is required for the formation of tertiary lymphoid tissue in colitis. Eur. J. Immunol. 46, 2467–2480 (2016).
Guedj, K. et al. Adipocytes orchestrate the formation of tertiary lymphoid organs in the creeping fat of Crohn’s disease affected mesentery. J. Autoimmun. 103, 102281 (2019).
Ladjemi, M. Z. et al. Increased IgA expression in lung lymphoid follicles in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 199, 592–602 (2019).
Briend, E. et al. IL-18 associated with lung lymphoid aggregates drives IFNγ production in severe COPD. Respir. Res. 18, 159 (2017).
Paramasivan, S. et al. Tertiary lymphoid organs: A novel target in patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 142, 1673–1676 (2018).
Song, J. et al. Ectopic lymphoid tissues support local immunoglobulin production in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 141, 927–937 (2018).
Wang, Z. Z. et al. B cell-activating factor promotes B cell survival in ectopic lymphoid tissues in nasal Polyps. Front Immunol. 11, 625630 (2020).
Hertz, D. et al. Increased male susceptibility to Mycobacterium tuberculosis infection is associated with smaller B cell follicles in the lungs. Sci. Rep. 10, 5142 (2020).
Regard, L. et al. Effective control of Staphylococcus aureus lung infection despite tertiary lymphoid structure disorganisation. Eur. Respir. J. 57, 2000768 (2021).
Omatsu, T. et al. Recurrent fulminant myocarditis accompanied by lymphoid follicle formation in myocardium. Intern. Med. 59, 3045–3049 (2020).
Zhu, M. et al. Cardiac ectopic lymphoid follicle formation in viral myocarditis involving the regulation of podoplanin in Th17 cell differentiation. FASEB j. 35, e21975 (2021).
Ligon, M. M. et al. Single cell and tissue-transcriptomic analysis of murine bladders reveals age- and TNFα-dependent but microbiota-independent tertiary lymphoid tissue formation. Mucosal Immunol. 13, 908–918 (2020).
Fantini, D. et al. A Carcinogen-induced mouse model recapitulates the molecular alterations of human muscle invasive bladder cancer. Oncogene 37, 1911–1925 (2018).
Menees, K. B. et al. Sex- and age-dependent alterations of splenic immune cell profile and NK cell phenotypes and function in C57BL/6J mice. Immun. Ageing 18, 3 (2021).
Miyamoto, H. et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl Cancer Inst. 99, 558–568 (2007).
Goswami, S. et al. ARID1A mutation plus CXCL13 expression act as combinatorial biomarkers to predict responses to immune checkpoint therapy in mUCC. Sci. Transl. Med. 12, eabc4220 (2020).
Degoricija, M. et al. The dynamics of the inflammatory response during BBN-induced bladder carcinogenesis in mice. J. Transl. Med. 17, 394 (2019).
Rodig, N. et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33, 3117–3126 (2003).
Mao, Y. et al. Prediction values of tertiary lymphoid structures in the prognosis of patients with left- and right-sided colon cancer: a multicenter propensity score-matched study. Int. J. Surg. 109, 2344–2358 (2023).
Galluzzi, L. et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 17, 725–741 (2020).
Morcrette, G. et al. APC germline hepatoblastomas demonstrate cisplatin-induced intratumor tertiary lymphoid structures. Oncoimmunology 8, e1583547 (2019).
Cascone, T. et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat. Med 29, 593–604 (2023).
Ho, W. J. et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced HCC into resectable disease with enhanced antitumor immunity. Nat. Cancer 2, 891–903 (2021).
Xu, F. et al. Tertiary lymphoid structures combined with biomarkers of inflammation are associated with the efficacy of neoadjuvant immunochemotherapy in resectable non-small cell lung cancer: a retrospective study. Thorac. Cancer 15, 172–181 (2024).
Xu, F. et al. Combined inflammatory parameters and tertiary lymphoid structure predict prognosis in patients with resectable non-small cell lung cancer treated with neoadjuvant chemoimmunotherapy. Front Immunol. 14, 1244256 (2023).
Gavrielatou, N. et al. B-cell infiltration is associated with survival outcomes following programmed cell death protein 1 inhibition in head and neck squamous cell carcinoma. Ann. Oncol. 35, 340–350 (2023).
Zhang, Y. et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell 39, 1578–1593.e1578 (2021).
Cabrita, R. et al. Author Correction: Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 580, E1 (2020).
Goubet, A. G. et al. Escherichia coli-specific CXCL13-producing TFH are associated with clinical efficacy of neoadjuvant PD-1 blockade against muscle-invasive bladder cancer. Cancer Discov. 12, 2280–2307 (2022).
Deguchi, S. et al. Clinical relevance of tertiary lymphoid structures in esophageal squamous cell carcinoma. BMC Cancer 22, 699 (2022).
Walle, T. et al. Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through CXCL8. Sci. Adv. 8, eabh4050 (2022).
Lhuillier, C. et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J. Clin. Investig. 131, e138740 (2021).
Wang, D. et al. Low-dose radiotherapy promotes the formation of tertiary lymphoid structures in lung adenocarcinoma. Front. Immunol. 14, 1334408 (2023).
Chen, J. et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8(+) T cell response. Proc. Natl Acad. Sci. USA 119, e2207841119 (2022).
Alameh, M. G. et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54, 2877–2892.e2877 (2021).
Zheng, L. et al. Vaccine-induced intratumoral lymphoid aggregates correlate with survival following treatment with a neoadjuvant and adjuvant vaccine in patients with resectable pancreatic adenocarcinoma. Clin. Cancer Res. 27, 1278–1286 (2021).
You, X., Koop, K. & Weigert, A. Heterogeneity of tertiary lymphoid structures in cancer. Front. Immunol. 14, 1286850 (2023).
Lee, M. et al. Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis. Mod. Pathol. 32, 70–80 (2019).
Li, Z. et al. Development and validation of a machine learning model for detection and classification of tertiary lymphoid structures in gastrointestinal cancers. JAMA Netw. Open 6, e2252553 (2023).
Yang, M. et al. Detection and quantitative analysis of tumor-associated tertiary lymphoid structures. J. Zhejiang Univ. Sci. B 24, 779–795 (2023).
Boisson, A. et al. Fluorescent multiplex immunohistochemistry coupled with other state-of-the-art techniques to systematically characterize the tumor immune microenvironment. Front. Mol. Biosci. 8, 673042 (2021).
Stowman, A. M. et al. Lymphoid aggregates in desmoplastic melanoma have features of tertiary lymphoid structures. Melanoma Res. 28, 237–245 (2018).
Quigley, L. T. et al. Protocol for investigating tertiary lymphoid structures in human and murine fixed tissue sections using Opal™-TSA multiplex immunohistochemistry. STAR Protoc. 4, 101961 (2023).
Xie, M. et al. Consolidation radiographic morphology can be an indicator of the pathological basis and prognosis of partially solid nodules. BMC Pulm. Med. 22, 369 (2022).
Dorraji, E. S. et al. Positron emission tomography and single photon emission computed tomography imaging of tertiary lymphoid structures during the development of lupus nephritis. Int. J. Immunopathol. Pharm. 35, 20587384211033683 (2021).
Beckford Vera, D. R. et al. Immuno-PET imaging of tumor-infiltrating lymphocytes using zirconium-89 radiolabeled anti-CD3 antibody in immune-competent mice bearing syngeneic tumors. PLoS One 13, e0193832 (2018).
Rashidian, M. et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J. Exp. Med. 214, 2243–2255 (2017).
Barmpoutis, P. et al. Tertiary lymphoid structures (TLS) identification and density assessment on H&E-stained digital slides of lung cancer. PLoS One 16, e0256907 (2021).
van Rijthoven, M. et al. HookNet: multi-resolution convolutional neural networks for semantic segmentation in histopathology whole-slide images. Med. Image Anal. 68, 101890 (2021).
van Rijthoven, M. et al. Multi-resolution deep learning characterizes tertiary lymphoid structures and their prognostic relevance in solid tumors. Commun. Med. 4, 5 (2024).
Federico, L. et al. Distinct tumor-infiltrating lymphocyte landscapes are associated with clinical outcomes in localized non-small-cell lung cancer. Ann. Oncol. 33, 42–56 (2022).
Ahluwalia, P. et al. Natural killer cells and dendritic cells: expanding clinical relevance in the non-small cell lung cancer (NSCLC) tumor microenvironment. Cancers. 13, 4037 (2021).
Yang, S. C. et al. Intrapulmonary administration of CCL21 gene-modified dendritic cells reduces tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res. 66, 3205–3213 (2006).
Lee, J. M. et al. Phase I trial of intratumoral injection of CCL21 gene-modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8(+) T-cell infiltration. Clin. Cancer Res. 23, 4556–4568 (2017).
Schreibelt, G. et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin. Cancer Res. 22, 2155–2166 (2016).
Chen, L. et al. Extranodal induction of therapeutic immunity in the tumor microenvironment after intratumoral delivery of Tbet gene-modified dendritic cells. Cancer Gene Ther. 20, 469–477 (2013).
Weinstein, A. M. et al. Tbet and IL-36γ cooperate in therapeutic DC-mediated promotion of ectopic lymphoid organogenesis in the tumor microenvironment. Oncoimmunology 6, e1322238 (2017).
He, T. et al. Oncolytic adenovirus promotes vascular normalization and nonclassical tertiary lymphoid structure formation through STING-mediated DC activation. Oncoimmunology 11, 2093054 (2022).
Buckley, C. D. et al. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu. Rev. Immunol. 33, 715–745 (2015).
Zhu, G. et al. Induction of tertiary lymphoid structures with antitumor function by a lymph node-derived stromal cell line. Front. Immunol. 9, 1609 (2018).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016).
Davidson, S. et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 21, 704–717 (2021).
Lee, J. W. et al. Inducing ectopic T cell clusters using stromal vascular fraction spheroid-based immunotherapy to enhance anti-tumor immunity. Adv. Sci. 9, e2203842 (2022).
Suematsu, S. & Watanabe, T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat. Biotechnol. 22, 1539–1545 (2004).
Okamoto, N. et al. Artificial lymph nodes induce potent secondary immune responses in naive and immunodeficient mice. J. Clin. Investig. 117, 997–1007 (2007).
Hsieh, C. H. et al. Potential role of CXCL13/CXCR5 signaling in immune checkpoint inhibitor treatment in cancer. Cancers 14, 294 (2022).
Delvecchio, F. R. et al. Pancreatic cancer chemotherapy is potentiated by induction of tertiary lymphoid structures in mice. Cell Mol. Gastroenterol. Hepatol. 12, 1543–1565 (2021).
Huang, Y. et al. Dual-mechanism-based CTLs infiltration enhancement initiated by Nano-sapper potentiates immunotherapy against immune-excluded tumors. Nat. Commun. 11, 622 (2020).
Lim, K. H. & Staudt, L. M. Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 5, a011247 (2013).
Robinet, M. et al. Use of toll-like receptor agonists to induce ectopic lymphoid structures in myasthenia gravis mouse models. Front. Immunol. 8, 1029 (2017).
Spalato-Ceruso, M. et al. Pembrolizumab combined with low-dose cyclophosphamide and intra-tumoral injection of the toll-like receptor 4 agonist G100 in patients with advanced pretreated soft tissue sarcoma: results from the PEMBROSARC basket study. J. Hematol. Oncol. 15, 157 (2022).
Li, L. et al. Anti-HBV response to toll-like receptor 7 agonist GS-9620 is associated with intrahepatic aggregates of T cells and B cells. J. Hepatol. 68, 912–921 (2018).
Rennert, P. D. et al. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity 9, 71–79 (1998).
Lukashev, M. et al. Targeting the lymphotoxin-beta receptor with agonist antibodies as a potential cancer therapy. Cancer Res. 66, 9617–9624 (2006).
De Trez, C. et al. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J. Immunol. 180, 238–248 (2008).
Amouzegar, A. et al. STING agonists as cancer therapeutics. Cancers 13, 2695 (2021).
Yang, H. et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J. Clin. Investig. 129, 4350–4364 (2019).
Chelvanambi, M., Fecek, R. J., Taylor, J. L. & Storkus, W. J. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J. Immunother Cancer 9, e001906 (2021).
Demaria, O. et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl Acad. Sci. USA 112, 15408–15413 (2015).
Liu, J. Q. et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control Release 345, 306–313 (2022).
Wiley, J. A. et al. Inducible bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS One 4, e7142 (2009).
Cao, H. et al. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target Ther. 6, 426 (2021).
Kobayashi, Y. & Watanabe, T. Gel-trapped lymphorganogenic chemokines trigger artificial tertiary lymphoid organs and mount adaptive immune responses in vivo. Front. Immunol. 7, 316 (2016).
Jin, X. K. et al. Engineering metal-based hydrogel-mediated tertiary lymphoid structure formation via activation of the STING pathway for enhanced immunotherapy. Mater. Horiz. 10, 4365–4379 (2023).
Zhu, L. et al. Bacteria-mediated metformin-loaded peptide hydrogel reprograms the tumor immune microenvironment in glioblastoma. Biomaterials 288, 121711 (2022).
Zhu, G. et al. Tumor-associated tertiary lymphoid structures: gene-expression profiling and their bioengineering. Front. Immunol. 8, 767 (2017).
Tomei, A. A. et al. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 183, 4273–4283 (2009).
Stachowiak, A. N. & Irvine, D. J. Inverse opal hydrogel-collagen composite scaffolds as a supportive microenvironment for immune cell migration. J. Biomed. Mater. Res. A 85, 815–828, (2008).
Zhang, Y. et al. 3D printing scaffold vaccine for antitumor immunity. Adv. Mater. 33, e2106768 (2021).
Dieudé, M., Kaci, I. & Hébert, M. J. The impact of programmed cell death on the formation of tertiary lymphoid structures. Front. Immunol. 12, 696311 (2021).
Dieudé, M. et al. Extracellular vesicles derived from injured vascular tissue promote the formation of tertiary lymphoid structures in vascular allografts. Am. J. Transpl. 20, 726–738 (2020).
Dieudé, M. et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci. Transl. Med. 7, 318ra200 (2015).
Mourik, B. C. et al. Interactions between type 1 interferons and the Th17 response in tuberculosis: lessons learned from autoimmune diseases. Front. Immunol. 8, 294 (2017).
Gatumu, M. K. et al. Blockade of lymphotoxin-beta receptor signaling reduces aspects of Sjögren’s syndrome in salivary glands of non-obese diabetic mice. Arthritis. Res. Ther. 11, R24 (2009).
Wengner, A. M. et al. CXCR5- and CCR7-dependent lymphoid neogenesis in a murine model of chronic antigen-induced arthritis. Arthritis Rheum. 56, 3271–3283 (2007).
Roders, N. et al. SYK inhibition induces apoptosis in germinal center-like B cells by modulating the antiapoptotic protein myeloid cell leukemia-1, affecting B-cell activation and antibody production. Front. Immunol. 9, 787 (2018).
Penaranda, C., Tang, Q., Ruddle, N. H. & Bluestone, J. A. Prevention of diabetes by FTY720-mediated stabilization of peri-islet tertiary lymphoid organs. Diabetes 59, 1461–1468 (2010).
Zhang, N. N. et al. Prognostic impact of tertiary lymphoid structures in breast cancer prognosis: a systematic review and meta-analysis. Cancer Cell Int. 21, 536 (2021).
Cipponi, A. et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 72, 3997–4007 (2012).
Rodriguez, A. B. & Engelhard, V. H. Insights into tumor-associated tertiary lymphoid structures: novel targets for antitumor immunity and cancer immunotherapy. Cancer Immunol. Res. 8, 1338–1345 (2020).
Vanhersecke, L. et al. Standardized pathology screening of mature tertiary lymphoid structures in cancers. Lab Investig. 103, 100063 (2023).
Zhao, H. et al. ImmunoPET imaging of human CD8(+) T cells with novel (68)Ga-labeled nanobody companion diagnostic agents. J. Nanobiotechnol. 19, 42 (2021).
Sautès-Fridman, C. et al. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Front. Immunol. 7, 407 (2016).
Sun, R., Gao, D. S., Shoush, J. & Lu, B. The IL-1 family in tumorigenesis and antitumor immunity. Semin. Cancer Biol. 86, 280–295 (2022).
Zhou, X. et al. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral. Oncol. 53, 27–35 (2016).
Gan, X. et al. Spatial multimodal analysis revealed tertiary lymphoid structures as a risk stratification indicator in combined hepatocellular-cholangiocarcinoma. Cancer Lett. 581, 216513 (2024).
Wu, Z. et al. CD20(+)CD22(+)ADAM28(+) B cells in tertiary lymphoid structures promote immunotherapy response. Front. Immunol. 13, 865596 (2022).
Acknowledgements
This study is supported by Natural Science Foundation of Shandong Province, China (Grant no. ZR2021MH353) and Health Science and Technology Team Building Project of Shandong Province. We would also like to thank Home for Researchers (www.home-for-researchers.com) and Figdraw (https://www.figdraw.com/) for their support, as we used Figdraw that belongs to Home for Researchers to draw figures in this review.
Author information
Authors and Affiliations
Contributions
Zhao Lianyu and Wang Xuan conceived the general idea and framework of the project; Zhang Zhe, Chen Zhanwei, and Wang Xiaohui collected relevant literature and materials; Zhao Lianyu, Jin Song, and Wang Shengyao completed the writing. Wu Haiwei, Huang Shengyun, and Zhang Dongsheng reviewed and corrected the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, L., Jin, S., Wang, S. et al. Tertiary lymphoid structures in diseases: immune mechanisms and therapeutic advances. Sig Transduct Target Ther 9, 225 (2024). https://doi.org/10.1038/s41392-024-01947-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-024-01947-5
- Springer Nature Limited