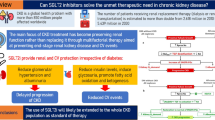
Abstract
Chronic kidney disease (CKD) is a major public health issue and an independent risk factor for cardiovascular and all-cause mortality. Diabetic kidney disease develops in 30–50% of diabetic patients and it is the leading cause of end-stage renal disease in the Western world. Strict blood pressure control and renin-angiotensin system (RAS) blocker use are the cornerstones of CKD treatment; however, their application in everyday clinical practice is not always ideal and in many patients CKD progression still occurs. Accumulated evidence in the past few years clearly suggests that sodium-glucose co-transporter-2 (SGLT-2) inhibitors present potent nephroprotective properties. In clinical trials in patients with type 2 diabetes mellitus, these agents were shown to reduce albuminuria and proteinuria by 30–50% and the incidence of composite hard renal outcomes by 40–50%. Furthermore, their mechanism of action appears rather solid, as they interfere with the major mechanism of proteinuric CKD progression, i.e., glomerular hypertension and hyperfiltration. The present review summarizes the current evidence from human trials on the effects of SGLT-2 inhibitors on nephroprotection and discusses their position in everyday clinical practice.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) represents a major public health issue with a prevalence between 10 and 13% in Western Societies [1]. The presence of CKD is a long-established independent risk factor for cardiovascular disease (CVD) and all- cause mortality [2,3,4], therefore, delaying CKD progression was previously suggested to be of importance for cardioprotection [5, 6]. In addition, elevated levels of urine albumin excretion (UAE) previously characterized as “microalbuminuria” and currently as “moderately increased albuminuria” for levels between 30 and 299 mg/day or equivalent or macroalbuminuria or “severely increased albuminuria for UAE levels ≥300 mg/day or equivalent are associated with increased risk in both incident CVD and progression toward ESRD [7, 8].
Diabetes mellitus (DM) is recognized as one of the most common chronic diseases in nearly all countries, causing premature death and disability [9]. The prevalence of diabetes has increased significantly during the past decades, mainly as a consequence of the continuous rise in the incidence of type 2 diabetes mellitus (T2DM); it is predicted that the global number of adult patients with T2DM will rise to 642 million by year 2040 [10, 11]. Chronic hyperglycemia in diabetic patients causes several macrovascular and microvascular complications. Diabetic kidney disease (DKD) is one of diabetes’ microvascular complications, and develops in ~30–50% of patients with T2DM [12]. DKD has been identified as the leading cause of end-stage renal disease (ESRD) during the last decades in United States, Europe, and Australia–New Zealand, whereas recent analyses suggest that it has also climbed to be the primary cause of ESRD in some South-Asian and South-American countries [12,13,14].
Therapeutic strategies reducing albumin or protein excretion in the urine have been long associated with renoprotective outcomes, since they were shown to slow the rate of eGFR decline [8, 15,16,17]. Renin-angiotensin system (RAS) blockers, including angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are the cornerstone of treatment for proteinuric CKD, including DKD for many years [8, 18]. According to the Kidney-Disease-Improving-Global-Outcomes (KDIGO) 2012 Clinical Practice Guidelines for the Evaluation and Management of Chronic Kidney Disease, diabetic, and nondiabetic patients with moderately or severely increased albuminuria should receive an ACEI or ARB [8]. When combined with strict glycemic and blood pressure (BP) control, optimal RAS blockade may reduce the rate of eGFR decline in proteinuric CKD from 10–12 to 2–3 ml/min/1.73 m2 per year [16]. However, in healthy individuals the decline is around 1–1.5 ml/min/1.73 m2 per year, while several factors may interfere with maximum use of RAS blockers in everyday clinical practice, including the risk of acute kidney injury and hyperkalemia [19, 20]. Hence, there is need for novel treatments that will further delay the onset and progression of CKD.
Sodium-glucose co-transporter-2 (SGLT-2) inhibitors, is a relatively novel class of oral antidiabetic agents, that reduce plasma glucose levels by inhibiting glucose reabsorption [21]. Data from clinical trials in T2DM suggest that SGLT-2 inhibitors have potent renoprotective effects, including reductions of 30–50% in albuminuria or proteinuria and clear beneficial effects on hard renal outcomes such as doubling of serum creatinine (SCr), eGFR decline higher than 40%, ESRD, initiation of renal replacement treatment, and death due to renal disease [22,23,24]. This nephroprotective effect seems to arise from reversal of the major mechanism of proteinuric DKD, i.e., glomerular hypertension and hyperfiltration through modulation of the tone of the afferent arteriole [24]. This review will present the evidence regarding the renoprotective properties of SGLT-2 inhibitors, evolving from clinical studies on the effects of these agents on albuminuria/proteinuria and hard renal outcomes in patients with T2DM and discuss their current position in relation to established renoprotective therapies.
Current status of pharmacologic management in proteinuric kidney disease
Effects of ACE inhibitors and ARBs on albuminuria/proteinuria and hard renal outcomes
The effect of ACE inhibitors and ARBs on hard renal outcomes and surrogates has been thoroughly investigated. Major clinical trials in the field of proteinuric diabetic CKD have shown that this class of antihypertensive treatment can delay the onset and the progression of kidney injury more effectively compared with placebo or other therapy. The Collaborative Study Group was the first relevant study [25]; it randomized 409 individuals with type 1 DM (T1DM) and overt nephropathy to receive captopril or placebo. Captopril was associated with 43% reduction in the risk of the primary end-point of doubling of SCr, 50% reduction in the combined end-point of death, need for dialysis, and transplantation, and 30% reduction in UAE compared with placebo. Small differences in BP favoring the captopril group were noted, but did not affect outcomes [25]. The Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) study included 1513 participants with T2DM and overt nephropathy who were randomly assigned to receive treatment either with losartan or placebo [26]. Losartan was associated with 16% reduction in doubling of SCr, ESRD or death, 35% reduction in ACR and 15% decrease in the rate of creatinine clearance reduction. In Irbesartan Diabetic Nephropathy Treatment (IDNT) study 1715 hypertensive patients with T2DM and overt nephropathy randomly allocated to receive irbesartan, amlodipine, or placebo [27]. Irbesartan resulted in a 20% reduction compared with placebo and 23% reduction compared with amlodipine in doubling of SCr, ESRD or death; proteinuria decreased by 33% with irbesartan vs. 6% with amlodipine, and 10% with placebo [27].
Similar results about were observed in studies including nondiabetic patients with proteinuric nephropathy. In the Ramipril Efficacy In Nephropathy (REIN) trial, ramipril was associated with a lower rate of eGFR decrease per month and a greater reduction in proteinuria when compared with placebo, independently of the antihypertensive effect [28]. Finally, in the African-American Study on Kidney Disease (AASK) study, which assigned 1094 African-American individuals to receive ramipril or metoprolol or amlodipine, the ACE inhibitor was associated with a 22% reduction compared with metoprolol and 38% compared with amlodipine in the risk of the composite endpoint (reduction of GFR ≥ 50%, ESRD or death), along with relevant differences in protein excretion [29].
Studies on the renoprotective effect of double RAS blockade or the use of mineralocorticoid receptor antagonists (MRAs)
The limited number of therapeutic choices for the treatment of proteinuric CKD led several investigators to study the potential renoprotective effects of dual RAS blockade. The Aliskiren-Trial-in-Type-2-Diabetes-Using-Cardiorenal-Endpoints (ALTITUDE) study compared the effects of a combination of aliskiren and ACEI or ARB vs. ACE or ARB alone on cardiovascular and renal outcomes in 8561 patients with T2DM [30]. The study was prematurely terminated at 69% of events due to renal complications, hyperkalemia, and hypotension in the aliskiren group, without allowing any significant differences in hard renal ourcomes despite a greater reduction in UACR levels [30]. Similar results were observed in the VA-NEPHRON-D trial which randomized 1448 patients with T2DM, macroalbuminuria and CKD stage 2 & 3 to lisinopril or placebo on top of losartan treatment. The study was prematurely ended at a median follow-up period of 2.2 years due to safety reasons; RAAS blockade increased the risks of hyperkalemia and AKI, while the incidence of the primary renal outcome did not differ significantly between groups [31]. Following the above findings all relevant guidelines recommended against the combined use ACEi and ARBs in the treatment of hypertension or proteinuric CKD [32,33,34].
In addition to the above several clinical trials suggest that MRAs, including the older spironolactone and eplerenone and the newer finerenone can reduce albuminuria by 25–30% and proteinuria up to 50% on top of ACE inhibitors or ARBs [35, 36]. The fear of associated hyperkalemia, particularly in patients with DM that are prone to this condition [37], is perhaps the main reason for the limited use of MRAs in CKD. However, results from a sub-analysis of the CRIB-II study including patients with non-diabetic CKD and eGFR of 30–89 ml/min/1.73 m2 suggest that the use of spironolactone 25 mg on top of concurrent ACE inhibitor or ARB treatment was well tolerated, as <1% of the patients developed potassium levels >6 mmol/L [38]. Furthermore, current evidence suggest that finerenone may be associated with particularly low rates of hyperkalemia [39], while the availability of safe potassium binding medications, such as patiromer and zirconium sicilate have opened more possibilities for MRA use in individuals prone to hyperkalemia, such as those with CKD and DM [20]. Two ongoing clinical trials evaluating the effect of finerenone on hard renal outcomes are awaited to elucidate this field [40, 41].
SGLT-2 receptors and SGLT-2 inhibitors
The SGLT1 and SGLT2 receptors belong to the SLC5 active glucose transporters family and were initially known due to their association with congenital glucose-galactose malabsorption and familial renal glycosuria, respectively [42]. The SGLT1 and SGLT2 consist of 664 and 672 amino acids, accordingly, and they have a similar protein structure [43]. This structure is important for their stepped molecular mode of transport; binding extracellular sodium causes protein transformation, which allows to trap extracellular glucose and after flipping releases sodium and glucose intracellularly and flips over to its original form [44]. SGLT1 and SGLT2 are located in the transporting epithelial cells that regulate glucose transfer from the external environment to the body [45]. SGLT-2 receptor is primarily located in the S1 segment of the proximal convoluted tubule (PCT) and is responsible for 90% of renal glucose reabsorption at the kidneys [44, 46]. SGLT1 is located in the S2/S3 segment of the PCT and reabsorbs about 10% of the filtered glucose, as it has a lower affinity for glucose and does not transport galactose [47].
SGLT-2 inhibitors are considered as a relatively new antidiabetic drug category available for the treatment of T2DM [48]. Inhibition of SGLT-2 has been described as a mechanism for decreasing hyperglycemia several years ago. The first tested compound, a naturally occurring glucoside extracted from the root bark of apple trees, namely phlorizin, was never approved because of its poor absorption when administered orally and severe gastrointestinal side-effects [49]. Research data during the past decade on the kidney’s role in glucose homeostasis and hyperglycemia control resulted in the development of other SGLT-2 inhibitors [50], such as dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, luseogliflozin, tofogliflozin, and ertugliflozin, marketed currently in various countries worldwide for patients with T2DM. Following the recent publication of the DEPICT-1 trial [51], the European Medicines Agency approved the use of dapagliflozin also for patients with T1DM in March 2019 [52].
The mechanistic background of SGLT-2 inhibition derives through their effect on SGLT-2, which are responsible for active co-transportation of glucose and sodium in a 1:1 ratio, through the apical cell membrane of the PCT cells [46]. The co- transporters reabsorb almost all the glucose that is freely filtered daily at the glomeruli, up to a 375 mg/min [53]. In the setting of hyperglycemia (glucose levels >200–250 mg/gl) the SGLTs family proteins approach saturation and result in renal excretion of the excessive filtered glucose [53]. Rate of glucose reabsorption is increased by 20% in DM patients compared with healthy individuals, due to the expression of up to three times more SGLT-2 co-transporters, as an “adaptive” effort of the kidney to preserve glucose accumulation [54]. This pathophysiologic change leads to the minimization of glucosuria, which increases hyperglycemia. The inhibition of these receptors in the kidneys results in glycosuria, plasma glucose levels reduction, and body weight decrease [55].
Blockade of the SGLT-2 leads also to an inhibition of sodium reabsorption in the PCT and consequently to higher urine sodium excretion and increased diuresis [56]. This diuretic effect is mild, but increased tubular lumen glucose concentration also produces an osmotic effect and enhances water and sodium excretion, an effect similar to that of osmotic diuretics [57]. A growing body of evidence suggest that SGLT-2 inhibition can lead to significant decreases in salt-sensitive BP and normalize abnormal BP dipping profile [58]. Moreover, decrease in actual plasma volume [59], body weight and total visceral fat [60], as well as serum uric acid levels possibly through the glycosuria-induced alteration of uric acid transportation in renal tubules [61], reduction of the sympathetic nervous system overdrive [62], as well as improvement in arterial stiffness [63], endothelial dysfunction and vascular resistance decrease [64] have been proposed as additional mechanisms further contributing to the overall BP lowering effect of SGLT-2 inhibitors.
Effects of SGLT-2 inhibitors on albuminuria and proteinuria
As discussed above, albuminuria and proteinuria are long-established risk factors for CKD progression [3, 7, 15]. A previous meta-analysis of clinical trials, by Heerspink et al. showed that a pharmaceutically-induced reduction in albuminuria of 30% is translated to a long-term reduction in the risk of ESRD of 23.7% [65]. The effects of SGLT-2 inhibitors on albuminuria and proteinuria were examined as secondary efficacy outcomes in several clinical trials with these agents in patients with T2DM. In a recent meta-analysis of data from 15 clinical trials (17,540 participants with T2DM) was shown that SGLT-2 inhibitors were associated with significant reductions in UACR and UAE levels, by ~25% and 40% respectively, when compared with placebo or other antidiabetic treatment including insulin regimens (Fig. 1) [23]. The aforementioned results were mainly driven by data for dapagliflozin and empagliflozin while no significant changes in albuminuria were observed in patients receiving canagliflozin, ipragliflozin, or lusogliflozin. A discussion of key SGLT-2 inhibitor studies examining their effects on albuminuria or proteinuria follows directly below; these studies are summarized in Table 1.
SGLT-2 inhibitors vs. other antidiabetic agents. Reprinted with Permission from Piperidou et al., J Hypertens 2019 [23].
Studies with empagliflozin
In a study by Ridderstrale et al. evaluating the efficacy and safety of empagliflozin vs. glimepiride as add-on therapy to metformin in 1549 patients with T2DM, after a 104-weeks of treatment empagliflozin produced UACR reductions of −9 and −483.5 mg/g in patients with baseline micro- and macroalbuminuria compared with −56.5 and −380.1 mg/g reductions in the glimepiride group [66]. In a subsequent pooled analysis including T2DM patients with albuminuria from five clinical trials treatment with empagliflozin significantly reduced UACR levels in both patients with micro- and macroalbuminuria (mean difference in change from baseline: −32%, p < 0.001; and −41%, p < 0.001; accordingly) compared with placebo [67]. In another study, 7028 patients with T2DM and CVD were randomized to empagliflozin 10 mg, 25 mg, or placebo (ratio 1:1:1) in addition to standard of care and results showed that empagliflozin treatment was associated with significant decrease in UACR after a 164-weeks in patients with micro- (−49%, 95% CI: −60 to −36%, p < 0.0001), macro- (−42%, 95% CI −49 to −34%, p < 0.0001) or normoalbuminuria (−15%, 95% CI −22 to −1%, p = 0.0004) compared with baseline [68].
Studies with canagliflozin
In a study of 269 patients with T2DM and CKD [estimated glomerular filtration rate (eGFR) 30–50 ml/min/1.73 m2) randomized to canagliflozin (100 or 300 mg) or placebo and UACR decreased by −0.9 μg/mg (95% CI −8.1–2.2 μg/mg) and −6.9 μg/mg (95% CI −59.3–0.2 μg/mg) in the canagliflozin groups respectively, while it increased in the placebo group by 2.2 mg/dl (95% CI −1.0–14.7 μg/mg) [69]. A secondary analysis of the CANTATA-SU study, in which 1450 individuals with T2DM were randomly assigned to canagliflozin 100 or 300 mg, or glimepiride 6–8 mg on-top of metformin, showed that canagliflozin decreased UACR levels (−5.7%, 95% CI -2.3–13.1%, p = 0.16 and 11.2% 95% CI 3.6–18.3% p = 0.01 for the 100 or 300 mg accordingly) compared with glimepiride, while differences were even higher in patients with micro- or macroalbuminuria (31.7%, 95% CI 8.6–48.9%; p = 0.01 and 49.3%, 95% CI 31.9–62.2%; p = 0.001 for the 100 or 300 mg accordingly) relative to glimepiride [70]. The data assessment of 10,142 patients who were included in the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program (described in detail below) showed that mean UACR was 18% lower (95% CI 16–20%) in the canagliflozin compared with the control group, while mean between-group differences for patients with normo-, micro- macroalbuminuria were −9% (95% CI −12 to −7%), −34% (95% CI −38 to −29%) and −36% (95% CI −43 to −28%) accordingly [71].
Studies with dapagliflozin
In a study by Nauck et al., including 814 T2DM patients, 52-week treatment with dapagliflozin decreased UACR by 19.0 mg/g compared with a reduction of 0.8 mg/g achieved with glipizide [72]. Another study randomized 252 patients with inadequately controlled T2DM and moderate renal impairment (eGFR 30–59 ml/min/1.7 m2) to dapagliflozin 5 or 10 mg or placebo and showed higher change in UACR from baseline only in the 10 mg and not in the 5 mg group (78.0 ± 112.5 vs. −11.69 ± 48.6 vs. 69.7 ± 80.1 mg/g, accordingly) compared with baseline [73]. A subsequent pooled analysis of 12 studies and 4545 T2DM patients with eGFR 60–90 mL/min/1.73 m2 showed that dapagliflozin (three doses 2.5 mg, 5 mg, 10 mg) was not associated with higher reductions in UACR levels from baseline compared with placebo (−10.3 ± 10.34, p = 0.0924 vs. 9.2 ± 7.15, p = 0.7538 vs. −10.8 ± 7.95, p = 0.098 vs. 3.9 ± 8.54, accordingly) [74]. A post-hoc analysis of two studies including patients with T2DM and hypertension receiving treatment with ACEIs or ARBs compared the effect of dapagliflozin vs. placebo on albuminuria and found that dapagliflozin reduced UACR by an additional −33.2% (95% CI −45.4 to −18.2%) compared with placebo, after 12-weeks [75]. A sub-analysis of the Multicenter-Trial-to-Evaluate-the-Effect-of-Dapagliflozin-on-the-Incidence-of-Cardiovascular-Events (DECLARE- TIMI 58) study, in which 17,160 patients with T2DM were randomized to receive either dapagliflozin 10 mg or placebo showed that only 2% of the participants in the treatment arm vs. 3.3% in the placebo group developed microalbuminuria (HR 0.59, 95% CI 0.39–0.87, p < 0.0082), and 5.2% of the patients treated with dapagliflozin developed macroalbuminuria compared with 12.8% in the control group [hazard ratio (HR) 0.38, 95% CI 0.25–0.58, p < 0.0001] [76]
Studies with ipragliflozin, luseogliflozin, ertugliflozin, and tofogliflozin
So far, most of the evidence regarding the effects of ipragliflozin in renal function and albuminuria derive from studies in the Japanese populations. A total of 245 T2DM patients were randomized to ipragliflozin or placebo in the EMIT study, and results suggested a similar decrease in UACR between the two groups (−24.83 ± 197.300 vs. −6.48 ± 37.384 mg/g) [77]. In the SPOTLIGHT study, patients with inadequately controlled T2DM were randomized to ipragliflozin or placebo as an add-on to pioglitazone and differences between groups in UACR were again not significant (−6.26 ± 46.724 vs. −7.24 ± 35.430 mg/g) [78]. The LANTERN study randomized 164 patients with poor T2DM control (HBA1c: 6.9–8.9%) and impaired renal function (eGFR 30–60 ml/min/1.73 m2) to ipragliflozin or placebo and showed reduction of UACR in the ipragliflozin and increase in the placebo group (−23.72 ± 229.100 vs. 4.28 ± 62.635 mg/g) [79]. A study investigating the effects of luseogliflozin on albuminuria showed no difference in UACR change between the active group (42.71, 95% CI −35.1–120.6) and placebo (67.81, 95% CI −12.7–148.3 mg/g) [80]. A more recent study compared the effects of ertugliflozin (5 mg and 15 mg) with placebo and/or glimepiride on albuminuria and showed similar changes between in UACR between the active and the control groups, but in patients with albuminuria at baseline, the ertugliflozin groups had greater reductions in UACR (−29.5%, 95% CI: −44.8 to −9.8; p < 0.01 and −37.6% −51.8 to −19.2; p < 0.001 for relevant to placebo changes from baseline in the 5 mg and the 15 mg ertugliflozin groups accordingly) [81]. To the best of our knowledge, no study so far has ever evaluated the association of tofogliflozin with renal outcomes.
Effects of SGLT-2 inhibitors on hard renal outcomes
As of this writing, four major studies have announced results on the effects of SGLT-2 inhibitors on hard renal outcomes. All of them included patients with T2DM. The first of these studies was the EMPA-REG OUTCOME study, which randomized 7028 patients with T2DM to 10 or 25 mg of empagliflozin vs. placebo and were followed-up from a mean 3.1 years [82]. A total of 5665 of included participants were receiving treatment with an ACEI or an ARB. According to the analysis of the pre- specified renal outcomes of the EMPA-REG OUTCOME trial, individuals treated with empagliflozin showed reduced incidence in the combined end-point of progression to macroalbuminuria, initiation of renal- replacement therapy or death from renal disease (HR 0.61; 95% CI 0.53–0.70; P < 0.001) compared with patients receiving placebo. In addition, patients of the intervention treatment arm had lower rates of a post-hoc defined renal composite outcome [initiation of renal- replacement therapy, doubling of SCr, or death from renal disease (HR 0.54; 95% CI 0.40–0.75; P < 0.001) [Table 2]. Of note, significant between-group differences of approximately the same magnitude were observed in the individual analyses of all the above components. Interestingly, the number of hyperkalemia or acute kidney failure episodes in patients treated with empagliflozin were lower or similar to those with placebo, regardless the kidney function status at baseline [82].
A probable criticism was that the basic characteristics of the total population included in the EMPA-REG OUTCOME trial did not resemble that of classical studies with renal endpoints (i.e., proteinuric DKD), mainly due to the fact that the main aim of the trial was the evaluation of the cardiovascular effects. Following that, differences in the described components were mainly driven by doubling of SCr since the other kidney-associated outcomes were less frequent. According to the baseline data, there were 5201 individuals with an eGFR > 60 ml/min/1.73 m2 (64% had no albuminuria, 27% had microalbuminuria, and 8.5% had macroalbuminuria). Following, there were 1819 patients with an eGFR < 60 ml/min/1.73 m2. With regards to the albuminuria status of these patients: 47% had no albuminuria, 34% had microalbuminuria, and 19% macroalbuminuria [82]. Thus, one can argue, that with more than 3000 patients with either eGFR < 60 ml/min/1.73 m2 or micro- and macroalbuminuria the study had adequate power to assess significant differences in renal endpoints.
The CANVAS program consisted of two double-blind, randomized trials (CANVAS and CANVAS-R) and aimed to evaluate the long-term renal effects of canagliflozin in the context of a pre-specified explanatory analysis [71]. In CANVAS study 4330 individuals with T2DM were randomly assigned to receive 300 mg canagliflozin, 100 mg canagliflozin, or placebo at a 1:1:1 ratio [83]. In CANVAS-R study 5812 participants with T2DM were randomly assigned (1:1) to either canagliflozin 100 mg or matching placebo; up-titration to 300 mg of canagliflozin was optional based on the clinician’s recommendations and 20.1% of the treated patients had an eGFR < 60 mL/min/1.73 m2 at baseline measurements [84]. A total of 4593 of included participants were receiving treatment with RAAS blockers. Canagliflozin showed a significant decrease in the pre-specified primary renal endpoint of 40% reduction in eGFR levels, need for renal-replacement therapy, or death from renal causes compared with placebo (HR:0.60; 95% CI 0.47–0.77). The effect of canagliflozin was consistent across all four eGFR categories (baseline eGFR ≥90, 60–<90, 45–<60, and <45 mL/min/1.73 m2) (p heterogeneity = 0.28 and >0.50, respectively) irrespectively of the presence of CKD or not [85]. A recent explanatory analysis of the CANVAS study aiming to assess the effects of canagliflozin on a wide range of renal components, showed a comparable reduction in the outcome of doubling of SCr, ESRD, and renal death in the intervention arm vs. placebo (HR:0.53; 95% CI 0.33–0.84) [86]. In contrast to EMPAREG-OUTCOME, where all components were decreased with empagliflozin, in this analysis canagliflozin significantly reduced the doubling of SCr (HR:0.50; 95% CI 0.30–0.84) but not the incidence of ESRD (HR:0.77; 95% CI 0.30–1.97).
The DECLARE- TIMI 58 was a randomized, double-blind, multi-national phase III trial which studied as secondary outcome the effects of dapagliflozin on hard renal outcome [87]. The primary renal composite outcome of ≥40% decrease in eGFR to <60 mL/min/1.73 m2, ESRD, or renal death was observed in 1.5% patients in the dapagliflozin group compared with 2.8% patients in the placebo group (HR 0·53 [95% CI 0·43–0·66]; p < 0·0001). The separate analyses of the components of the renal composite showed significantly lower rates of the incidence of ≥40% decrease in eGFR to <60 mL/min/1.73 m2 in the intervention arm vs. the placebo arm (HR 0.54 [95% CI 0·43–0·67]; p < 0.0001). ESRD occurred in 0.1% of patients treated with dapagliflozin vs. 0.2% of patients treated with placebo (HR 0.31 [0.13–0.79], p = 0.013) whereas death from renal cause was observed in 0.1% of patients in both treatment categories (HR 0.60 [0.22–1.65], p = 0.32) [87].
Τhe recent CREDENCE study, the first SGLT-2 inhibitor study with a combined renal end-point as a primary outcome, randomized 4401 patients with T2DM, CKD, and albuminuria (ratio of albumin [mg] to creatinine [g], >300–5000) who were already treated with RAS-blockers in canagliflozin and placebo [86]. In total, 4395 study participants were on ACEI or ARB treatment. This study was prematurely terminated after a planned interim analysis on the recommendation of the data and safety monitoring committee due to clear benefit of canagliflozin vs. placebo. The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (HR 0.66; 95% CI 0.53–0.81; P < 0.001), and the relative risk of ERSD was lower by 32% (HR 0.68, 95% CI 0.54–0.86 P = 0.002) [86].
Two hard renal outcome studies with SGLT-2 inhibitors are recruiting patients with CKD independently of diabetic status. The Study-to-Evaluate-the-Effect-of-Dapagliflozin-on-Renal-Outcomes-and-Cardiovascular-Mortality-in-Patients-With-Chronic-Kidney-Disease (Dapa-CKD) is an event-driven, randomized, double-blind study, evaluating the effect of dapagliflozin vs. placebo in addition to standard of care (maximum tolerated labeled dose with ACEI or ARB), to prevent the progression of CKD or cardiovascular/renal death in patients with eGFR ≥ 25 and ≤75 mL/min/1.73 m2, and UACR ≥ 200 and ≤5000 mg/g. The primary outcome is a composite of ≥50% sustained decline in eGFR or reaching ESRD or CV death or renal death [88]. The study started was planned to enroll 4000 participants and to complete at November 2020 but was prematurely terminated due to benefit; its results are awaited in a few months [89]. Another ongoing study, the Study-of-Heart-and-Kidney-Protection-With-Empagliflozin (EMPA-KIDNEY) aims to investigate the effect of empagliflozin on kidney disease progression or cardiovascular death vs. placebo on top of standard treatment in patients with CKD (eGFR ≥ 20 to <45 mL/min/1.73 m² or eGFR ≥ 45 to <90 mL/min/1.73 m² with UACR ≥ 200 mg/g or protein:creatinine ratio ≥300 mg/g) [90]. The composite primary outcome consists of time to first occurrence of (i) kidney disease progression (defined as ESRD, a sustained decline in eGFR to <10 mL/min/1.73 m², renal death, or a sustained decline of ≥40% in eGFR from randomization) or (ii) cardiovascular death. The study plans to enroll 5000 participants from November 2018 and to be completed in June 2022.
Mechanisms for the nephroprotective actions of SGLT-2 inhibitors
Patients with T2DM typically present salt-sensitive hypertension, due to the inherent disability of the kidneys to perform natriuresis, leading in mild volume overload and suppression of renin activity described as “hyporeninemic hypoaldosteronism” [91]. The salt-sensitivity of T2DM patients is mediated by a number of mechanisms, most important of which seems to be insulin resistance and associated hyperinsulinemia resulting in increased sodium reabsorption by actions both in the proximal tubule (through stimulation of Na + -H + exchange and Na+-K+-ATPase) [91] and the distal tubule (through stimulation of the epithelial sodium channel (ENaC)) [92]. Treatment with SGLT-2 inhibitors is associated with mild but also clinically meaningful natriuretic and diuretic effects, which may produce an 4–5/2–3 mmHg BP reduction in these patients [21]. As mentioned above, SGLT-2 inhibition is also associated with decreases body weight and total visceral fat and serum uric acid levels [60, 61].
In addition to the indirect beneficial effects that BP, weight, and uric acid reduction may exert on the kidney, several mechanisms of renoprotective actions of SGLT-2 inhibitors have been hypothesized. Among them, some experiments suggest that SGLT-2 inhibitors have anti-inflammatory and antifibrotic effects and may reverse renal hypoxia [22, 93,94,95,96,97]. However, the most solid mechanism refers to a direct reduction of intraglomerular pressure [22, 93]. It is long known, that DM promotes proteinuric renal injury through afferent vasodilation via several pathways; this leads to consequent increase in intraglomerular pressure and albuminuria [15, 98]. Recent data suggest that one of these pathways is increased proximal tubular sodium and glucose reabsorption through SGLT-2, which follows the increase in the transporter concentration and activity, as discussed above. An increased proximal tubular reabsorption would result in decreased sodium chloride delivery to the macula densa, suppression of the tubuloglomerular feedback and afferent arteriolar dilation (Fig. 2) [99, 100]. This was exemplified by Cherney et al. in a study including, among others, 27 patients with T1DM and hyperfiltration during hyperinsulinemic euglycemic clamp; in these individuals empagliflozin treatment for 8 weeks resulted in reduction of baseline inulin-measured GFR by 33 ml/min/1.73 m2, with concomitant reduction in renal blood flow measured with paraaminohippurate [101]. These observations suggest that SGLT-2 inhibitors, through increased sodium delivery to the macula densa and restoration of the tubuloglomerular feedback are able to reduce afferent arteriole vasodilation, the main pathophysiological culprit of proteinuric diabetic nephropathy. This action clearly explains the characteristic slight eGFR drop within few weeks of SGLT-2 inhibition, which is fully reversible after discontinuation of the drug [22] and typically the associated reduction in intraglomerular pressure, albuminuria, and renal injury progression. Such an effect through reversal of afferent arteriole dilatation would be irrelevant from the efferent arteriole vasodilation induced by RAS blockade, offering a complementary major pathway for renoprotection [21, 24]. However, a recent randomized trial examining the effects of dapagliflozin on renal microcirculation in 44 patients with T2DM, did not confirm the above findings [102]. In that study dapagliflozin also reduced GFR but did not increase renal vascular resistance, suggesting that the reduction in intraglomerular pressure with SGLT-2 inhibitors is not due to vasoconstriction of the afferent but to vasodilation of the efferent arteriole. The main explanation offered by the authors is that in middle-aged and elderly T2DM patients the afferent arteriole is already constricted and the whole regulation of glomerular microcirculation by the macula densa mechanism may be different compared with young T1DM patients [102]. Although this could be possible, several limitations may hamper the conclusions of the later study [103], and thus, the working hypothesis of the SGLT-2 inhibitor renoprotective action rather remains that of the modulation of afferent arteriole tone. In any case, the mechanistic details of the SGLT-2 inhibitor action need to be further investigated by larger future studies. One important issue for future research is whether SGLT-2 inhibitors offer significant nephroprotection in patients without glomerular hyperfiltration and albuminuria and through which mechanisms this may occur.
In physiological conditions the vast majority of the filtered glucose is reabsorbed through SGLT-2 receptors, along with filtered sodium and the normal vascular tone of the afferent arteriole is preserved through normal tubuloglomerular feedback. In diabetes increased reabsorption of sodium and glucose through the SGLT-2 receptors leads to decreased amounts of sodium and chloride delivered to the macula densa, which in turn leads to afferent arteriole vasodilation. Via SGLT-2 inhibition the increased sodium reabsorption is reversed, and consequently increased amounts of sodium are delivered to the macula densa which restore the normal tubuloglomerular feedback and cause the reversal of the arteriole vasodilation.
Conclusions
Within the past 20–25 years, i.e., since the publication of seminal ACE inhibitor and ARB trials, nephrology did not meet any breakthrough discoveries leading to major changes in everyday clinical practice. With the prevalence of CKD continuously growing all over the world and the well-documented associations of CKD with cardiovascular and all-cause mortality the need for novel therapies to retard CKD progression grew more and more urgent [1, 2, 4]. The appearance of strong data from human trials showing SGLT-2 inhibitors to potently decrease intermediate and hard renal outcomes on top of RAS blockade in patients with T2DM completely changed the landscape in the treatment of DKD, with major international bodies recommending the use of these agents in patients with T2DM and CKD, within their licensed indications [24, 104, 105]. Ongoing renal outcome trials in patients with CKD of various etiologies, together with mechanistic studies on the ways of nephroprotection are expected to shed more light and expand the indications of SGLT-2 inhibitors for benefit of our patients.
Summary table
-
Prevalence of CKD is continuously growing and the close association between CKD and increased cardiovascular and all-cause mortality makes the need for novel therapies to retard CKD progression urgent.
-
Over the past decades, breakthrough discoveries to decrease this association are scarce.
-
Longitudinal data suggest that SGLT-2 inhibitors present significant cardio- and reno-protective effects, on top of RAS blockade, in patients with T2DM.
-
Ongoing renal outcome trials in patients with CKD together with mechanistic studies on the ways of nephroprotection are expected to expand the indications of the treatment with SGLT-2 inhibitors in additional populations.
References
Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis. 2003;42:677–84.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305.
Sarafidis PA, Bakris GL. Microalbuminuria and chronic kidney disease as risk factors for cardiovascular disease. Nephrol Dial Transplant. 2006;21:2366–74.
Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012;380:807–14.
Couser WG, Riella MC, Abraham G, Beerkens P, Feehally J, Garcia Garcia G, et al. World Kidney Day 2011: protect your kidneys, save your heart. Rev Investig Clin. 2011;63:8–11.
Ruiz-Hurtado G, Sarafidis P, Fernández-Alfonso MS, Waeber B, Ruilope LM. Global cardiovascular protection in chronic kidney disease. Nat Rev Cardiol. 2016;13:603–8.
Khosla N, Sarafidis PA, Bakris GL. Microalbuminuria. Clin Lab Med. 2006;26:635–53.
KDIGO. 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
WHO. Global report on diabetes; 2016. https://apps.who.int/iris/handle/10665/204871 Accessed 4 May 2020.
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011;378:31–40.
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73:A7–a8.
Kramer A, Pippias M, Noordzij M, Stel VS, Andrusev AM, Aparicio-Madre MI, et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: a summary. Clin Kidney J. 2019;12:702–20.
Stel VS, Awadhpersad R, Pippias M, Ferrer-Alamar M, Finne P, Fraser SD, et al. International comparison of trends in patients commencing renal replacement therapy by primary renal disease. Nephrol (Carlton). 2019;24:1064–76.
Sarafidis PA, Khosla N, Bakris GL. Antihypertensive therapy in the presence of proteinuria. Am J Kidney Dis. 2007;49:12–26.
Sarafidis PA, Ruilope LM. Cardiorenal disease development under chronic renin-angiotensin-aldosterone system suppression. J Renin Angiotensin Aldosterone Syst. 2012;13:217–9.
Sarafidis PA, Ruilope LM. Aggressive blood pressure reduction and renin-angiotensin system blockade in chronic kidney disease: time for re-evaluation? Kidney Int. 2014;85:536–46.
Lasaridis AN, Sarafidis PA. Diabetic nephropathy and antihypertensive treatment: what are the lessons from clinical trials? Am J Hypertens. 2003;16:689–97.
Sarafidis PA, Blacklock R, Wood E, Rumjon A, Simmonds S, Fletcher-Rogers J, et al. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin J Am Soc Nephrol. 2012;7:1234–41.
Sarafidis PA, Georgianos PI, Bakris GL. Advances in treatment of hyperkalemia in chronic kidney disease. Expert Opin Pharmacother. 2015;16:2205–15.
Loutradis C, Papadopoulou E, Angeloudi E, Karagiannis A, Sarafidis P. The beneficial actions of SGLT-2 inhibitors beyond management of hyperglycemia. Curr Med Chem. 2019. epub ahead of print.
Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26–39.
Piperidou A, Sarafidis P, Boutou A, Thomopoulos C, Loutradis C, Alexandrou ME, et al. The effect of SGLT-2 inhibitors on albuminuria and proteinuria in diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019;37:1334–43.
Sarafidis P, Ferro CJ, Morales E, Ortiz A, Malyszko J, Hojs R, et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transpl. 2019;34:208–30.
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–62.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9.
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60.
The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet . 1997;349:1857–63.
Wright JT Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421–31.
Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–13.
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–903.
KDIGO. Clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337–414.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 2018;71:1269–324.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the management of Arterial Hypertension. J Hypertens. 2018;36:2284–309.
Sarafidis PA, Memmos E, Alexandrou ME, Papagianni A. Mineralocorticoid receptor antagonists for nephroprotection: current evidence and future perspectives. Curr Pharm Des. 2018;24:5528–36.
Alexandrou ME, Papagianni A, Tsapas A, Loutradis C, Boutou A, Piperidou A, et al. Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019;37:2307–24.
Loutradis C, Tolika P, Skodra A, Avdelidou A, Sarafidis PA. Prevalence of hyperkalemia in diabetic and non-diabetic patients with chronic kidney disease: a nested Case-Control Study. Am J Nephrol. 2015;42:351–60.
Edwards NC, Steeds RP, Chue CD, Stewart PM, Ferro CJ, Townend JN. The safety and tolerability of spironolactone in patients with mild to moderate chronic kidney disease. Br J Clin Pharmacol. 2012;73:447–54.
Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of Finerenone on Albuminuria in patients with diabetic nephropathy: a randomized clinical trial. Jama 2015;314:884–94.
Ruilope LM, Agarwal R, Anker SD, Bakris GL, Filippatos G, Nowack C, et al. Design and baseline characteristics of the Finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am J Nephrol. 2019;50:345–56.
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Nowack C, et al. Design and baseline characteristics of the Finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. Am J Nephrol. 2019;50:333–44.
Wright EM. Glucose transport families SLC5 and SLC50. Mol Asp Med. 2013;34:183–96.
Wright EM, Loo DD, Hirayama BA, Turk E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiol (Bethesda). 2004;19:370–6.
Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab. 2017;26:27–38.
Lee YJ, Lee YJ, Han HJ. Regulatory mechanisms of Na(+)/glucose cotransporters in renal proximal tubule cells. Kidney Int Suppl. 2007:S27–S35.
DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5–14.
Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261:32–43.
American Diabetes Association Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S73–S85.
Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335–80.
Abdul-Ghani MA, Norton L, DeFronzo RA. Efficacy and safety of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Curr Diab Rep. 2012;12:230–8.
Dandona P, Mathieu C, Phillip M, Hansen L, Tschöpe D, Thorén F, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care 2018;41:2552–9.
Forxiga®. Summary of product characteristics. 2012. https://www.ema.europa.eu/en/documents/product-information/forxiga-epar-product-information_en.pdf Accessed 4 May 2020.
Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflug Arch. 2004;447:510–8.
Vlotides G, Mertens PR. Sodium-glucose cotransport inhibitors: mechanisms, metabolic effects and implications for the treatment of diabetic patients with chronic kidney disease. Nephrol Dial Transplant. 2015;30:1272–6.
Chao EC, Henry RR. SGLT2 inhibition–a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–9.
Filippatos TD, Tsimihodimos V, Elisaf MS. Mechanisms of blood pressure reduction with sodium-glucose co-transporter 2 (SGLT2) inhibitors. Expert Opin Pharmacother. 2016;17:1581–3.
Sarafidis PA, Georgianos PI, Lasaridis AN. Diuretics in clinical practice. Part I: mechanisms of action, pharmacological effects and clinical indications of diuretic compounds. Expert Opin Drug Saf. 2010;9:243–57.
Tamura K, Wakui H, Azushima K, Uneda K, Umemura S. Circadian blood pressure rhythm as a possible key target of SGLT2 inhibitors used for the treatment of Type 2 diabetes. Hypertens Res. 2016;39:396–8.
Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–62.
Cefalu WT, Stenlof K, Leiter LA, Wilding JP, Blonde L, Polidori D, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia 2015;58:1183–7.
Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, Nakanishi T, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35:391–404.
Jordan J, Tank J, Heusser K, Heise T, Wanner C, Heer M, et al. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J Am Soc Hypertens. 2017;11:604–12.
Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–93.
Muskiet MH, Tonneijck L, Smits MM, Kramer MH, Heerspink HJ, van Raalte DH. Pleiotropic effects of type 2 diabetes management strategies on renal risk factors. Lancet Diabetes Endocrinol. 2015;3:367–81.
Heerspink HJ, Kropelin TF, Hoekman J, de Zeeuw D. Drug-induced reduction in albuminuria is associated with subsequent renoprotection: a meta-analysis. J Am Soc Nephrol. 2015;26:2055–64.
Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700.
Cherney D, Lund SS, Perkins BA, Groop PH, Cooper ME, Kaspers S, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 2016;59:1860–70.
Cherney DZI, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, von Eynatten M, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–21.
Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–27.
Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28:368–75.
Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704.
Nauck MA, Del Prato S, Meier JJ, Duran-Garcia S, Rohwedder K, Elze M, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 2011;34:2015–22.
Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–71.
Kohan DE, Fioretto P, Johnsson K, Parikh S, Ptaszynska A, Ying L. The effect of dapagliflozin on renal function in patients with type 2 diabetes. J Nephrol. 2016;29:391–400.
Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18:590–7.
Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–17.
Kashiwagi A, Akiyama N, Shiga T, Kazuta K, Utsuno A, Yoshida S, et al. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetol Int. 2015;6:125–38.
Kashiwagi A, Shiga T, Akiyama N, Kazuta K, Utsuno A, Yoshida S, et al. Efficacy and safety of ipragliflozin as an add-on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study (the SPOTLIGHT study). Diabetol Int. 2015;6:104–16.
Kashiwagi A, Takahashi H, Ishikawa H, Yoshida S, Kazuta K, Utsuno A, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–60.
Haneda M, Seino Y, Inagaki N, Kaku K, Sasaki T, Fukatsu A, et al. Influence of renal function on the 52-week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in japanese patients with type 2 diabetes mellitus. Clin Ther. 2016;38:66–88.e20.
Cherney DZI, Heerspink HJL, Frederich R, Maldonado M, Liu J, Pong A, et al. Effects of ertugliflozin on renal function over 104 weeks of treatment: a post hoc analysis of two randomised controlled trials. Diabetologia 2020;63:1128–40.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)–a randomized placebo-controlled trial. Am Heart J. 2013;166:217–223.e211.
Neal B, Perkovic V, Matthews DR, Mahaffey KW, Fulcher G, Meininger G, et al. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19:387–93.
Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation 2018;138:1537–50.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
A study to evaluate the effect of dapagliflozin on renal outcomes and cardiovascular mortality in patients with chronic kidney disease (Dapa-CKD). ClinicalTrials.gov Identifier: NCT03036150. https://clinicaltrials.gov/ct2/show/NCT03036150 Accessed 5/5/2020.
Farxiga Phase III DAPA-CKD trial will be stopped early after overwhelming efficacy in patients with chronic kidney disease. 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/farxiga-phase-iii-dapa-ckd-trial-will-be-stopped-early-after-overwhelming-efficacy-in-patients-with-chronic-kidney-disease.html Accessed 5 May 2020.
EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin). ClinicalTrials.gov Identifier: NCT03594110. https://clinicaltrials.gov/ct2/show/NCT03594110 Accessed 5 May 2020.
Sarafidis PA, Bakris GL. The antinatriuretic effect of insulin: an unappreciated mechanism for hypertension associated with insulin resistance? Am J Nephrol. 2007;27:44–54.
Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Haring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12:721–37.
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose Cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016;134:752–72.
Terami N, Ogawa D, Tachibana H, Hatanaka T, Wada J, Nakatsuka A, et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 2014;9:e100777.
O’Neill J, Fasching A, Pihl L, Patinha D, Franzen S, Palm F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Ren Physiol. 2015;309:F227–234.
Chang YK, Choi H, Jeong JY, Na KR, Lee KW, Lim BJ, et al. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS ONE 2016;11:e0158810.
Górriz JL, Navarro-González JF, Ortiz A, Vergara A, Nuñez J, Jacobs-Cachá C, et al. Sodium-glucose cotransporter 2 inhibition: towards an indication to treat diabetic kidney disease. Nephrol Dial Transplant. 2020;35:i13–i23.
Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–7.
Norton L, Shannon CE, Fourcaudot M, Hu C, Wang N, Ren W, et al. Sodium-glucose co-transporter (SGLT) and glucose transporter (GLUT) expression in the kidney of type 2 diabetic subjects. Diabetes Obes Metab. 2017;19:1322–6.
Solini A, Rossi C, Mazzanti CM, Proietti A, Koepsell H, Ferrannini E. Sodium-glucose co-transporter (SGLT)2 and SGLT1 renal expression in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:1289–94.
Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–97.
van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020;97:202–12.
Soler MJ, Porrini E, Fernandez-Fernandez B, Ortiz A. SGLT2i and postglomerular vasodilation. Kidney Int. 2020;97:805–6.
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of Hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–701.
Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 update to: management of Hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487–93.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AP have not any financial or other relationships, which might lead to a conflict of interest regarding this paper. CL received scholarship from the Hellenic Society for Medical Education. PS is an advisor/speaker to Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Elpen Pharmaceuticals, Genesis Pharma, Menarini, Innovis Pharma, Winmedica and has received research support for an Investigator-Initiated Study from Astra Zeneca.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piperidou, A., Loutradis, C. & Sarafidis, P. SGLT-2 inhibitors and nephroprotection: current evidence and future perspectives. J Hum Hypertens 35, 12–25 (2021). https://doi.org/10.1038/s41371-020-00393-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-00393-4
- Springer Nature Limited
This article is cited by
-
Budget impact analyses for treatment of heart failure. A systematic review
Heart Failure Reviews (2024)
-
Effects of renal denervation on kidney function in patients with chronic kidney disease: a systematic review and meta-analysis
Journal of Human Hypertension (2023)
-
Role of hypertension in progression of pediatric CKD
Pediatric Nephrology (2023)
-
The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management
Cardiovascular Diabetology (2022)
-
Herzinsuffizienz
Die Kardiologie (2022)