Abstract
Data directly associating cadmium (Cd) with metabolic syndrome (MetS) are sparse and inconsistent. We aimed to quantitatively assess the association of Cd exposure with risk of MetS and its individual components. Literature searching was performed in PubMed, EMBASE, and MEDLINE-OVID through September, 2021. Weighted odds ratios (ORs) for MetS and its components were pooled by comparing the highest to the lowest category of Cd exposure using random-effects models. Eleven (10 from Asia and 1 from the US) cross-sectional studies (33,887 participants and 7176 cases) were identified. Overall, Cd exposure was not associated with risk of MetS [OR: 1.08, 95% confidence interval (CI): 0.92, 1.28]. However, the association became significant when pooling Asian studies (OR: 1.18, 95% CI: 1.02, 1.35), and it was more pronounced with Cd measured in blood (OR: 1.24, 95% CI: 1.05, 1.45). Additionally, Cd exposure was significantly associated with reduced HDL-cholesterol (OR: 1.27, 95% CI: 1.05, 1.54) and elevated triglyceride (OR: 1.17, 95% CI: 1.05, 1.30), but not other components. This meta-analysis indicates that Cd exposure is associated with risk of MetS among Asian populations, which is mainly explained by Cd’s association with dyslipidemia. Further studies are needed to better understand the mechanism of action.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS), defined by abdominal obesity, elevated fasting glucose level, dyslipidemia, and increased blood pressure, is a constellation of metabolic abnormalities that are related to risk of cardiovascular disease and type 2 diabetes mellitus [1,2,3]. MetS is one of the leading public health challenges worldwide and its prevalence increased dramatically in the past decades [4, 5]. More than one third of Americans are suffering from MetS [6]. Although unhealthy diet and sedentary lifestyle may play critical roles in the pathogenesis of MetS [7, 8], increasing evidence suggests that environmental factors, e.g., some heavy metal exposure, may promote risk of MetS [9, 10].
Cadmium (Cd) is a toxic metal that distributes ubiquitously in soil. It contaminates foods and drinking water, which are the major sources of exposure in the general populations in addition to smoking. Once absorbed, Cd accumulates in human body for decades with only 0.01-0.02% excreted through urine and feces each day [11, 12]. Cd tends to accumulate in liver and kidney [13], causing chronic inflammation in liver by activating Kupffer cells [14] and oxidative damage that leads to renal tubular dysfunction [15]. Thus, Cd may have greater toxicity in metabolic system [16].
Studies directly relating Cd exposure to MetS are limited and the findings are inconsistent [17, 18], although the associations of Cd with some individual components of MetS have been observed [19,20,21]. A recent meta-analysis synthesized data from 10 cross-sectional studies and reported that Cd exposure was not associated with MetS risk [22]. Of note, the associations of Cd exposure with individual components were not investigated, and major errors might occur in the previous meta-analysis due to the following reasons: 1) included studies using data that are substantially overlapped. In particular, two included studies (Noor et al. [18] and Bulka et al. [23]) used data from the National Health and Nutrition Examination Survey (NHANES) 2011–2014; 2) combined results with Cd exposure measured in different biospecimens, e.g., blood and urine; and 3) a couple of eligible studies were not included. Therefore, we conducted this study for more comprehensive analyses by including two more studies with consideration on the types of biomarker for Cd exposure and possible ethnic disparities. Also, we further examined the associations between Cd exposure and individual components of MetS.
Methods and materials
Search strategy
This study was conducted in line with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. Electronic databases (PubMed, EMBASE, and MEDLINE-OVID) were searched comprehensively through September, 2021 using the combination of the terms: “Metabolic Syndrome X” or “metabolic syndrome” or “syndrome X” or “insulin resistance syndrome”, and “cadmium” or “metal” or “heavy metal”, with no language restriction. All identified records were downloaded to a reference list in Endnote X9 and the duplicate publications were excluded using the function “Find Duplicates”. Google Scholar and the reference lists of published articles were manually searched for additionally relevant publications. Literature screening was independently conducted by LL and YL and checked by CC.
Inclusion criteria
Studies were included in this study if they met the following criteria: (a) investigating the association between Cd exposure and MetS risk; (b) the associations of interest were reported as hazard ratio, relative risk or odds ratio (OR) with corresponding 95% confidence interval (CI), or these risk estimates could be calculated using the information in the articles; and (c) Cd exposure was measured in biomarkers, rather than estimated from the environment. Studies were excluded if they: (a) were reviews, abstracts, letter-to-editors, posters, or case-reports; (b) were laboratory studies; or (c) reported risk estimates were not able to be combined with that from other primary studies. If study populations were substantially overlapped, we included the study with large sample size and more comprehensive information. Finally, 11 cross-sectional studies were identified in this meta-analysis.
Quality assessment
Three authors (LL, YL, and CC) independently evaluated the identified studies by using the modified Newcastle–Ottawa Quality Assessment Scale (NOS) for cross-sectional studies [24]. Each study was scored based on 7 questions from the following items: sample representativeness, sample size, non-respondents, exposure ascertainment, adjusted covariates, outcome assessment, and statistical methods. The maximum score was 10 points.
Data extraction
Two authors (LL and YL) independently extracted the following information using a pre-determined data collection form: the first author, publication year, region, sex, age, number of all participants and MetS cases, diagnosis criteria of MetS, measurement of Cd, risk estimates of MetS and its components in the highest Cd exposure group as compared to those in the lowest exposure group, and the adjusted covariates. According to the modified National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) definition (2005 revision) [25], the individual MetS components included abdominal obesity defined as waist circumference (WC) ≥102 centimeter (cm) for men and ≥88 cm for women, reduced high-density lipoprotein cholesterol (HDL-c) defined as HDL-c < 40 milligram/deciliter (mg/dl) for men and <50 mg/dl for women, elevated triglyceride (TG) defined as TG ≥ 150 mg/dl, elevated blood pressure (BP) defined as systolic BP ≥ 130 millimeters of mercury (mmHg) or diastolic BP ≥ 85 mmHg, and elevated glucose levels defined as fasting blood glucose ≥ 100 mg/dl.
Statistical analysis
All analyses were performed using STATA software version 16.0 (STATA Corporation LP, College Station, TX, USA). A two-sided P value ≤ 0.05 was considered to be statistically significant. We pooled ORs of MetS comparing the highest to the lowest category of Cd exposure using DerSimonian and Laird random-effects models [26, 27]. Similarly, in 7 studies with available data [10, 17, 18, 28,29,30,31], we pooled ORs for each MetS component using DerSimonian and Laird random-effects models. The weighted ORs were presented for the overall associations of Cd exposure with MetS risk and its individual components. In addition, the analyses were stratified by the types of Cd biomarker (i.e., blood or urine) and study populations. Heterogeneities among studies were evaluated by calculating the I2 statistic along with Cochran’s Q test [32]. I2 values <50%, 50–75%, and >75% were considered to represent low, moderate, and high heterogeneity, respectively. Publication bias were assessed by using Egger’s regression asymmetry test [33].
Results
Literature searching
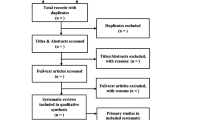
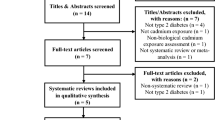
A total of 132 potentially relevant articles were identified from the aforementioned databases after removing 148 duplicated records in Endnote X9. Among these studies, 92 irrelevant studies were further excluded by reviewing the titles and abstracts. Of the remaining 40 studies, 32 studies were removed after full-text screening for the following reasons: (a) it did not report risk estimates of MetS (n = 10); (b) it was a review or an abstract (n = 10); (c) Cd was not an exposure of interest (n = 6); (d) it was not conducted in human (n = 1); (e) its reported results could not be combined with that from other primary studies (n = 3); or (f) its study population substantially overlapped with other primary studies (n = 2). Three additional studies were identified from Google scholar or the reference lists of previous publications. Finally, 11 studies [10, 17, 18, 28,29,30,31, 34,35,36,37] were included in this meta-analysis. The flow of literature searching was shown in Fig. 1.
Characteristics of published studies
The 11 included studies were all cross-sectional studies published from 2013 to 2020. The detailed characteristics of the studies were displayed in Table 1. These studies consisted of 33,887 participants and 7,176 cases of MetS from USA (n = 1) [18], Korea (n = 6) [10, 17, 28, 30, 34, 35], China (n = 3) [31, 36, 37], and Thailand (n = 1) [29]. All the included studies were published in English, except for one in Korean [30] and one in Chinese [36]. Four studies recruited only male [10, 17, 31] or female [29] participants, and the rest recruited both sexes. Cd was measured in urine in two studies [35, 37], in blood in eight studies [10, 17, 28,29,30,31, 34, 36], and in both biomarkers in one study [18]. The reported ORs of MetS were based on quantiles of Cd exposure, including quintiles [18], quartiles [28, 31, 34,35,36], tertiles [10, 17, 29, 37] and dichotomous levels [30]. MetS was ascertained according to Chinese Diabetes Society (CDS) criteria in two Chinese studies [31, 36], and according to NCEP ATP III criteria [35] and its modified version [10, 17, 18, 28,29,30, 34, 37] in the rest. One study reported crude ORs [36] and the rest reported adjusted ORs [10, 17, 18, 28,29,30,31, 34, 35, 37].
Quality assessment
The quality of each included study was evaluated by using NOS tool (Supplemental Table 1). All studies achieved high quality with scores ranging from 7 to 9 points. They all reported sufficient information on sample size, exposure ascertainment, outcome assessment, and statistical methods. However, not all studies provided data on sample representativeness, non-respondent rate, or adjusted covariates.
Results of meta-analysis
Overall, Cd exposure was not associated with MetS risk (weighted OR: 1.08, 95% CI: 0.92, 1.28; Fig. 2) when pooling data from all 11 primary studies. Moderate heterogeneity (I2 = 63.5%, p = 0.002) was observed. However, Cd exposure was significantly associated with risk of MetS when pooling data from the 10 studies conducted in Asian populations (weighted OR: 1.18, 95% CI: 1.02, 1.35; Fig. 3a), and the association was more pronounced with Cd exposure measured in blood (weighted OR: 1.24, 95% CI: 1.05, 1.45; Fig. 3b). Low heterogeneity was observed in the 10 Asian studies (I2 = 43.4%, p = 0.069).
a Weighted ORs (95% CIs) of MetS comparing the highest to the lowest Cd exposure category in the 10 studies conducted in Asian populations. OR for individual study are shown as solid diamond (♦), and the weighted ORs are shown as the open diamond (♢). Horizontal lines represent 95% CIs for the study-specific ORs. b Weighted ORs (95% CIs) of MetS comparing the highest to the lowest blood Cd exposure category in the 8 studies conducted in Asian populations with blood Cd measurements. OR for individual study are shown as solid diamond (♦), and the weighted ORs are shown as the open diamond (♢). Horizontal lines represent 95% CIs for the study-specific ORs.
In the analyses with MetS components (Fig. 4), Cd exposure was associated with risk of reduced HDL-c levels (weighted OR: 1.27, 95% CI: 1.05, 1.54) and elevated TG levels (weighted OR: 1.17, 95% CI: 1.05, 1.30), and the association was borderline significant with elevated BP (weighted OR: 1.22, 95% CI: 0.99, 1.52). Cd exposure was not associated with the other two individual components (WC and glucose levels). Heterogeneity was considered to be high for WC (I2 = 80.1%, p < 0.001), moderate for HDL-c (I2 = 69.7%, p = 0.003), BP (I2 = 69.8%, p = 0.001), and glucose levels (I2 = 74.6%, p < 0.001), and low for TG levels (I2 = 0%, p = 0.489). When we pooled studies with Cd exposure measured in blood, the associations with WC and BP were strengthened and became either borderline significant or significant (data now shown).
Egger’s regression asymmetry test suggested that publication bias was unlikely in our analyses (in all 11 primary studies: p = 0.41; in 9 studies with Cd measured in blood: p = 0.42; in 10 Asian studies: p = 0.14; in 8 Asian studies with Cd measured in blood: p = 0.12; Supplemental Figs. 1–4).
Discussion
In this meta-analysis with updated literature, we found that Cd exposure was significantly associated with risk of MetS among Asian populations and the association was more pronounced in studies with Cd exposure measured in blood. Also, the observed association might be mainly explained by Cd’s association with dyslipidemia measured by elevated TG and reduced HDL-c levels. A recent published meta-analysis [38] reported that Cd exposure was not associated with risk of MetS. However, that study did not consider ethnic disparities and types of Cd biomarker in the analyses. Also, it did not examine Cd exposure in relation to individual component of MetS. In addition, a couple of eligible studies were not included. With extensive literature review and comprehensive analyses, our study provides important additional information to the literature.
The potential toxicity of Cd exposure on MetS and its components is also supported by some other cross-sectional studies that do not meet the inclusion criteria of the present meta-analysis. For example, in a study of Korea National Health and Nutrition Examination Survey (KNHANES) 2010 [39], blood Cd concentration was associated with increased fasting glucose levels. In an updated analysis using KNHANES 2016 data, blood Cd concentration was significant higher in participants with MetS compared to other participants [38]. In a Lebanese cross-sectional study [40], serum Cd level was significantly associated with a higher risk of dyslipidemia, but not with diabetes or obesity. In a case-control study from the Iran Hoveyzeh cohort, participants with higher urinary Cd concentrations had higher odds of MetS and low HDL-c levels. However, two other cross-sectional studies reported no association of hair or urinary Cd levels with MetS and its components [23, 41].
Laboratory studies have provided some evidence that may explain the potential toxicity of Cd on dyslipidemia [42], although the underlying mechanisms have not been fully elucidated. Studies suggest that Cd exposure inhibits or disturbs numerous enzymes involved in the lipid metabolism [43, 44]. In a rat model [43], Cd administration increased hepatic TG synthesis by inducing the overexpression of glycerol-3-phosphate acyltransferase mitochondrial isoform, an enzyme participating in TG synthesis. Cd administration also facilitated the synthesis of liver fatty acids by increasing the activities of related enzymes, e.g., fatty acid synthase and isocitrate dehydrogenase. In addition, Cd exposure damaged the cellular antioxidant defense systems by depleting glutathione and reducing antioxidant enzyme activities [45, 46]. Thus, Cd may indirectly generate reactive oxygen species and induce oxidative stress [47], which may contribute to defects in the synthesis and secretion of lipoprotein in liver, leading to decreased HDL-c and increased TG [48]. Moreover, Cd exposure may induce inflammatory responses in many organs (e.g., liver, kidney, and cardiac tissue) by activating multiple intracellular signaling pathways in immune cells and up-regulating inflammation markers and mediators (e.g., NF-κB, IL-6, TNF-ɑ, and C-reactive protein) [49]. Inflammation induces dyslipidemia [50] probably through regulating the levels of apolipoproteins and the activities of relevant enzymes [51]. Since elevated TG/HDL-c ratio and inflammation predicts the development of hypertension [52] and diabetes [53, 54], the toxicity of Cd exposure on other individual components of MetS such as BP and glucose levels is possible, but may require a higher exposure level and prolonged exposure duration.
Notably, Cd exposure may induce metabolic disorders via damaging endothelium and affecting energy balance. Cd may infiltrate into the endothelial cells of vessel walls, leading to the endothelial injury through the proinflammatory intracellular signaling pathway [55, 56]. Endothelial dysfunction contributes to the development of metabolic disorders, including obesity, insulin resistance, dyslipidemia, hypertension, atherosclerosis, and diabetes mellitus [57, 58]. Additionally, Cd is able to interfere with metabolic and energy balance, probably through affecting the metabolism of carbohydrates and lipids [59, 60]. It has been demonstrated that Cd may affect glucose homeostasis by mediating some corresponding gene expression in islet both in vivo and in vitro [61]. In rat model, Cd exposure induced decreased glycogen synthesis and increased de novo lipogenesis in liver, which was associated with low expression of GSK 3β-pS9 and strong expression of SREBP-1c [62].
Some inherent limitations in the primary studies and the existing dataset need to be highlighted. First, most of the primary studies measured Cd in blood, which may not reflect the long-term Cd exposure [63]. Our analysis suggests that the associations of interest may vary depending on the type of Cd biomarkers. More studies with biomarkers reflecting long-term exposure, e.g., nail Cd concentrations, are needed [64]. Second, the heterogeneity among studies should be considered. The definition of MetS is not consistent across all primary studies, which may partially explain the observed high heterogeneity among these studies. Also, a study used self-reported data on individual components of MetS [35]. Notably, all included studies except one were conducted in Asia. The definitions of MetS in Asian studies were modified from the NCEP ATP III definition and slightly different across countries. For example, the cutoff points for WC in most of the included Asian studies were tighter than that in the American study (e.g. WC ≥ 90 cm for men and ≥85 cm for women in Korean studies vs WC ≥ 102 cm for men and ≥ 88 cm for women in the American study). The heterogeneity may also be explained by the geographical variation of Cd exposure. For example, in the American study [18], the highest quintile of urinary Cd concentration was greater than 0.60 μg/L, similar to the median level (0.65 μg/L) of urinary Cd in a Chinese study [37]. Notably, although no significant association between cd exposure and MetS was observed in the American study, a significant positive association between urinary Cd and MetS odds was found in the current smokers, who had a relatively higher urinary Cd concentration than the general population. Third, all primary studies are cross-sectional design, thus causal inference cannot be established based on the existing literature. In addition, since the analysis is based on observational studies, the potential inherent bias or residual confounding (e.g. measurements error) from the primary studies cannot be ruled out. Fourth, the possibility of publication bias, e.g., publications in other languages, cannot be completely ignored, though there is insufficient evidence of publication bias involved in our analyses. Finally, the limited eligible studies may not provide sufficient statistical power to our analyses. Clearly, more studies especially well-designed prospective cohort studies are warranted.
Conclusions
In summary, our meta-analysis indicated that Cd exposure, especially measured in blood, was associated with risk of MetS among Asian populations. This association may be largely explained by Cd’s association with dyslipidemia measured by elevated TG and reduced HDL-c. Further studies are needed to confirm our findings and better understand how Cd being involved in fat metabolism. Particular attention should be paid to the potential ethnic disparities, geographic variations, and biomarker difference.
Data availability
All the STATA codes for performing the analyses and generating the figures will be made available upon request pending from the corresponding author.
References
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28.
Alberti KG, Zimmet P, Shaw J. The metabolic syndrome-a new worldwide definition. Lancet. 2005;366:1059–62.
O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12.
Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9.
Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-12. JAMA. 2015;313:1973–4.
Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-6. JAMA. 2020;323:2526–8.
Di Marzo V, Silvestri C. Lifestyle and metabolic syndrome: contribution of the endocannabinoidome. Nutrients. 2019;11:1956 https://doi.org/10.3390/nu11081956.
Gallardo-Alfaro L, Bibiloni MDM, Mascaró CM, Montemayor S, Ruiz-Canela M, Salas-Salvadó J, et al. Leisure-time physical Activity, sedentary behaviour and diet quality are associated with metabolic syndrome severity: the PREDIMED-Plus study. Nutrients. 2020;12:1013 https://doi.org/10.3390/nu12041013.
Planchart A, Green A, Hoyo C, Mattingly CJ. Heavy metal exposure and metabolic syndrome: evidence from human and model system studies. Curr Environ Health Rep. 2018;5:110–24.
Lee BK, Kim Y. Blood cadmium, mercury, and lead and metabolic syndrome in South Korea: 2005-10 Korean National Health and Nutrition Examination Survey. Am J Ind Med. 2013;56:682–92.
Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, et al. Toxicological profile for cadmium. 2012. https://stacks.cdc.gov/view/cdc/12391.
Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19:529–35.
Kjellström T. Exposure and accumulation of cadmium in populations from Japan, the United States, and Sweden. Environ Health Perspect. 1979;28:169–97.
Yamano T, DeCicco LA, Rikans LE. Attenuation of cadmium-induced liver injury in senescent male fischer 344 rats: role of Kupffer cells and inflammatory cytokines. Toxicol Appl Pharm. 2000;162:68–75.
Thévenod F. Nephrotoxicity and the proximal tubule. Insights from cadmium. Nephron Physiol. 2003;93:p87–93.
Go YM, Sutliff RL, Chandler JD, Khalidur R, Kang BY, Anania FA, et al. Low-Dose cadmium causes metabolic and genetic dysregulation associated with fatty liver disease in mice. Toxicol Sci. 2015;147:524–34.
Lee BK, Kim Y. Association of blood cadmium level with metabolic syndrome after adjustment for confounding by serum ferritin and other factors: 2008-12 Korean National Health and Nutrition Examination Survey. Biol Trace Elem Res. 2016;171:6–16.
Noor N, Zong G, Seely EW, Weisskopf M, James-Todd T. Urinary cadmium concentrations and metabolic syndrome in U.S. adults: the National Health and Nutrition Examination Survey 2001-14. Environ Int. 2018;121:349–56.
Gallagher CM, Meliker JR. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:1676–84.
Tangvarasittichai S, Niyomtam S, Pingmuangkaew P, Nunthawarasilp P. Dyslipidemia in the elevated cadmium exposure population. Int J Toxicolcol Pharmacol. 2015;7:92–98.
Xiao L, Li W, Zhu C, Yang S, Zhou M, Wang B, et al. Cadmium exposure, fasting blood glucose changes, and type 2 diabetes mellitus: A longitudinal prospective study in China. Environ Res. 2021;192:110259.
Xu P, Liu A, Li F, Tinkov AA, Liu L, Zhou JC. Associations between metabolic syndrome and four heavy metals: a systematic review and meta-analysis. Environ Pollut. 2021;273:116480.
Bulka CM, Persky VW, Daviglus ML, Durazo-Arvizu RA, Argos M. Multiple metal exposures and metabolic syndrome: a cross-sectional analysis of the National Health and Nutrition Examination Survey 2011-4. Environ Res. 2019;168:397–405.
Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:1–17.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–45.
Moon S-S. Additive effect of heavy metals on metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-10. Endocrine 2014;46:263–71.
Sirivarasai J, Chunhabundit R, Chansirikarnjana S, Chottivitayatarakorn S, Prasanatikom W, Khansakorn N, et al. Environmental Lead and Cadmium Exposure and Metabolic Syndrome in Women. Thai J Toxicol. 2016;31:20–35.
Lee J-M, Seok K-J, Ryu J-Y, Jung W-S, Park J-B, Shin K-H, et al. Association between heavy metal exposure and prevalence of metabolic syndrome in adults of South Korea. Korean J Fam Pract. 2017;7:172–8.
Guo X, Yang Q, Zhang W, Chen Y, Ren J, Gao A. Associations of blood levels of trace elements and heavy metals with metabolic syndrome in Chinese male adults with microRNA as mediators involved. Environ Pollut. 2019;248:66–73.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Rhee SY, Hwang Y-C, Woo J-T, Sinn DH, Chin SO, Chon S, et al. Blood lead is significantly associated with metabolic syndrome in Korean adults: an analysis based on the Korea National Health and Nutrition Examination Survey (KNHANES), 2008. Cardiovasc Diabetol. 2013;12:9.
Shim YH, Ock JW, Kim YJ, Kim Y, Kim SY, Kang D. Association between heavy metals, bisphenol A, volatile organic compounds and phthalates and metabolic syndrome. Int J Environ Res Public Health. 2019;16:671.
Jin Y-N, Fu H-L, Liao S, Fan X-M, Zhao Y, Xu H-X, et al. Relationship between 23 serum elements and metabolic syndrome among rural residents. Chinese J Public Health. 2013. 1834–8.
Xu Y, Wei Y, Long T, Wang R, Li Z, Yu C, et al. Association between urinary metals levels and metabolic phenotypes in overweight and obese individuals. Chemosphere. 2020;254:126763.
Park Y, Oh CU. Association of lead, mercury, and cadmium with metabolic syndrome of young adults in South Korea: The Korea National Health and Nutrition Examination Survey (KNHANES) 2016. Public Health Nurs. 2021;38:232–8.
Yoo I-Y. The association of blood heavy metal concentration and components of metabolic syndrome in Korean male adults. Indian J Sci Technol. 2015;8:467–74.
Ayoub N, Mantash H, Dhaini HR, Mourad A, Hneino M, Daher Z. Serum cadmium levels and risk of metabolic syndrome: a cross-sectional study. Biol Trace Elem Res. 2021;199:3625–33.
Park SB, Choi SW, Nam AY. Hair tissue mineral analysis and metabolic syndrome. Biol Trace Elem Res. 2009;130:218–28.
Samarghandian S, Azimi-Nezhad M, Shabestari MM, Azad FJ, Farkhondeh T, Bafandeh F. Effect of chronic exposure to cadmium on serum lipid, lipoprotein and oxidative stress indices in male rats. Interdiscip Toxicol. 2015;8:151–4.
Larregle EV, Varas SM, Oliveros LB, Martinez LD, Antón R, Marchevsky E, et al. Lipid metabolism in liver of rat exposed to cadmium. Food Chem Toxicol. 2008;46:1786–92.
Rogalska J, Brzóska MM, Roszczenko A, Moniuszko-Jakoniuk J. Enhanced zinc consumption prevents cadmium-induced alterations in lipid metabolism in male rats. Chem Biol Interact. 2009;177:142–52.
Lopez E, Arce C, Oset-Gasque M, Canadas S, Gonzalez M. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic Biol Med. 2006;40:940–51.
Almenara CC, Broseghini-Filho GB, Vescovi MV, Angeli JK, Faria TDO, Stefanon I, et al. Chronic cadmium treatment promotes oxidative stress and endothelial damage in isolated rat aorta. PLoS ONE. 2013;8:e68418.
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, et al. Cadmium stress: an oxidative challenge. Biometals. 2010;23:927–40.
Murugavel P, Pari L. Diallyl tetrasulfide protects cadmium-induced alterations in lipids and plasma lipoproteins in rats. Nutr Res. 2007;27:356–61.
Olszowski T, Baranowska-Bosiacka I, Gutowska I, Chlubek D. Pro-inflammatory properties of cadmium. Acta Biochim Pol. 2012;59:475–82.
Feingold KR, Grunfeld C The effect of inflammation and infection on lipids and lipoproteins. In: Endotext; 2019. https://www.ncbi.nlm.nih.gov/sites/books/NBK326741/. Accessed 1 Sep 2021.
Feingold KR, Grunfeld C. Effect of inflammation on HDL structure and function. Curr Opin Lipidol. 2016;27:521–30.
Tohidi M, Hatami M, Hadaegh F, Azizi F. Triglycerides and triglycerides to high-density lipoprotein cholesterol ratio are strong predictors of incident hypertension in Middle Eastern women. J Hum Hypertens. 2012;26:525–32.
Cabrera de León A, Domínguez Coello S, Almeida González D, Brito Diaz B, del Castillo Rodríguez J, González Hernández A, et al. Impaired fasting glucose, ancestry and waist‐to‐height ratio: main predictors of incident diagnosed diabetes in the Canary Islands. Diabet Med. 2012;29:399–403.
Salazar MR, Carbajal HA, Espeche WG, Leiva Sisnieguez CE, March CE, Balbín E, et al. Comparison of the abilities of the plasma triglyceride/high-density lipoprotein cholesterol ratio and the metabolic syndrome to identify insulin resistance. Diab Vasc Dis Res. 2013;10:346–52.
Fittipaldi S, Bimonte VM, Soricelli A, Aversa A, Lenzi A, Greco EA, et al. Cadmium exposure alters steroid receptors and proinflammatory cytokine levels in endothelial cells in vitro: a potential mechanism of endocrine disruptor atherogenic effect. J Endocrinol Investig. 2019;42:727–39.
Lin HC, Hao WM, Chu PH. Cadmium and cardiovascular disease: an overview of pathophysiology, epidemiology, therapy, and predictive value. Rev Port Cardiol (Engl Ed). 2021;40:611–7.
Pi X, Xie L, Patterson C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ Res. 2018;123:477–94.
Alexander RW. Hypertension and the pathogenesis of atherosclerosis: oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension 1995;25:155–61.
Bimonte VM, Besharat ZM, Antonioni A, Cella V, Lenzi A, Ferretti E, et al. The endocrine disruptor cadmium: a new player in the pathophysiology of metabolic diseases. J Endocrinol Investig. 2021;44:1363–77.
Haverinen E, Fernandez MF, Mustieles V, Tolonen H. Metabolic syndrome and endocrine disrupting chemicals: an overview of exposure and health effects. Int J Environ Res Public Health. 2021;18:13047.
Wong WPS, Wang JC, Schipma MJ, Zhang X, Edwards JR, El Muayed M. Cadmium-mediated pancreatic islet transcriptome changes in mice and cultured mouse islets. Toxicol Appl Pharmacol. 2021;433:115756.
Sarmiento-Ortega VE, Moroni-González D, Díaz A, Eduardo B, Samuel T Oral subacute exposure to cadmium LOAEL dose induces insulin resistance and impairment of the hormonal and metabolic liver-adipose axis in Wistar rats. Biol Trace Elem Res. 2021; https://doi.org/10.1007/s12011-021-03027-z.
Gallagher CM, Meliker JR. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:1676–84.
Rahimzadeh MR, Rahimzadeh MR, Kazemi S, Moghadamnia A. Cadmium toxicity and treatment: an update. Casp J Intern Med. 2017;8:135–45.
Author contributions
LL was responsible for conducting the research, screening potentially eligible studies, extracting and analyzing data, quality assessment, interpreting results, updating reference lists, creating tables and figures, and writing original draft preparation. YL was responsible for conducting the research, screening potentially eligible studies, extracting and analyzing data, quality assessment, interpreting results, writing review, and revising the manuscript. CC was responsible for quality assessment, interpreting results, writing review, and revising the manuscript. YZ was responsible for creating tables and figures, writing review, and revising the manuscript. WG was responsible for writing review and revising the manuscript. SZ was responsible for writing review and revising the manuscript. KK was responsible for conceptualization, supervision, writing review, and revising the manuscript.
Funding
This study was partially supported by the grants from the National Institutes of Health (R01DK116603 and RF1AG056111).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This meta-analysis does not require ethical approval.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, L., Li, Y., Chen, C. et al. Associations of cadmium exposure with risk of metabolic syndrome and its individual components: a meta-analysis. J Expo Sci Environ Epidemiol 33, 846–854 (2023). https://doi.org/10.1038/s41370-022-00444-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-022-00444-7
- Springer Nature America, Inc.
Keywords
This article is cited by
-
Environmental exposure to lead and cadmium are associated with triglyceride glucose index
Scientific Reports (2024)
-
Elevated serum lead and cadmium levels associated with increased risk of dyslipidemia in children aged 6 to 9 years in Shenzhen, China
Environmental Science and Pollution Research (2023)
-
Association of blood cadmium and metabolic syndrome: a cross-sectional analysis of National Health and Nutrition Examination Survey 2017–2020
Environmental Science and Pollution Research (2022)