Abstract
As cadmium levels are increasing in the environment, the adverse effects of cadmium exposure specifically associated with chronic diseases are receiving increasing attention. Several population–based studies have been conducted on the association between cadmium and diabetes mellitus (DM) but have reported controversial results. Here, we aimed to evaluate the association between cadmium exposure and DM. In this meta-analysis, a random effects model was used because there was evidence of heterogeneity among studies. A dose-response relationship was assessed through a restricted cubic spline model with three knots. The results showed a positive association between cadmium levels in the body and DM (OR = 1.27; 95% CI, 1.07–1.52). The cadmium levels in the body were defined on the basis of combined urinary and blood cadmium. Subgroup analysis further indicated a positive association between urinary cadmium levels and DM (OR = 1.31; 95% CI, 1.02–1.69). The dose-response analysis results showed a positive association between levels of urinary cadmium above 2.43 μg/g creatinine and DM, and the risk of DM increased by 16% for each l μg/g creatinine increase in urinary cadmium levels. The results from our meta-analysis indicate that cadmium levels in the body are positively associated with DM, and urinary cadmium levels above 2.43 μg/g creatinine are associated with an increased risk of DM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic mellitus (DM) is a world-wide health concern with increasing morbidity and mortality rates. DM is a chronic metabolic disease caused by genetic and environmental factors, and its main pathophysiology is related to a lack of insulin and insulin resistance. Impaired insulin secretion not only is a hallmark of type 1 diabetes mellitus but also is involved in the development of type 2 diabetes mellitus (T2DM). Pancreatic β-cell dysfunction is the main reason for the decrease in insulin secretion. The precise regulation of β-cell function plays an important role in maintaining the balance of blood glucose (Mazur et al. 2013). Therefore, the cause of the pancreatic β-cell dysfunction warrants clarification. In addition to genetic factors, environmental and dietary factors play very important roles in the development of DM (Longnecker and Daniels 2001; Wajchenberg 2007). Exploring the environmental and dietary factors contributing to the reduced function of insulin-producing β cells is therefore crucial.
In industrialized countries, the concentration of cadmium in the environment has increased since the twentieth century (Jin et al. 2002). In addition to occupational exposure, smoking is the primary route of exposure to cadmium; one cigarette contains 1.70–2.12 μg cadmium (Afridi et al. 2015a, b). Diet is another important route through which cadmium enters the human body (Satarug et al. 2010). Common foods such as spinach, sunflower seeds, lettuce, shredded wheat, and peanuts contain cadmium at levels equal to or greater than the amount in beef liver, an organ abundant in cadmium (Edwards and Ackerman 2016). A safe intake limit of 7 μg cadmium/week/kg body weight has been proposed by the Food and Agriculture Organization/World Health Organization (WHO) Joint Expert Committee on Food Additives. However, the Agency for Toxic Substances and Disease Registry (ATSDR) has estimated that the minimum risk level of human exposure to cadmium through chronic oral ingestion is 0.1 μg/kg/day, a dose likely to avoid appreciable risk of adverse non-cancer health effects (Faroon et al. 2012). The WHO has recommended 5 μg/g creatinine in the urine as an occupational cadmium exposure biological threshold limit. However, several studies have provided evidence that cadmium affects diseases and health, such as cancer, cardiovascular diseases, and diabetes, at exposure levels (urinary cadmium levels of 0.5–2.0 μg/g creatinine) lower than the threshold limit (Akesson et al. 2014). These findings suggest that the current safe intake level does not provide sufficient health protection and consequently should be lowered. Therefore, the adverse effect of low-dose cadmium should receive more attention.
The low doses of cadmium enter the body through smoking and the diet affect the body’s glucose metabolism, and may even increase the morbidity from diabetes. In the past few years, research on this possibility has attracted increasing attention. The data from the Third National Health and Nutrition Examination Survey (NHNES III) have demonstrated a dose-response relationship between urinary cadmium levels and an increased risk of T2DM (Schwartz et al. 2003). The risk estimate for diabetes was 1.45 when comparisons were made between urinary cadmium levels of < 1 μg/g creatinine and ≥ 2 μg/g creatinine. A study conducted in Pakistan has shown that the average blood cadmium concentrations for both nonsmoking men and smokers with T2DM (4.3–7.1 g/L and 7.78–10.23 g/L) are significantly higher than those of their respective control groups (3.13–5.31 g/L and 4.02–6.68 g/L) (Afridi et al. 2008), thus indicating an association between cadmium and T2DM. Animal studies have shown that administration of cadmium compounds (0.84 mg/kg) or oral cadmium exposure in drinking water (32.5 ppm) to rats increases blood glucose levels (Bell et al. 1990; Trevino et al. 2015). However, research from China has shown an insignificant association between cadmium exposure and DM (Feng et al. 2015). Moreover, a study from the USA has shown that the association between cadmium levels and DM is still controversial (Menke et al. 2016). Therefore, we aimed to evaluate the association between cadmium exposure and risk of DM morbidity by conducting a meta-analysis to determine dose response.
Materials and methods
Search strategy and selection criteria
We performed a meta-analysis following a predetermined protocol in accordance with the MOOSE guidelines (Stroup et al. 2000). A systematic literature search was performed using the database of PubMed, MEDLINE and the Cochrane Library up to July 2018 for the cohort, cross-sectional, and case-control studies. And the following search items were used: “cadmium” in combination with “diabetes mellitus” with no restrictions.
The following inclusion criteria were used: (1) the cohort, cross-sectional, and case-control studies were included; (2) effect estimates with corresponding 95% confidence intervals (CIs) (or sufficient information to calculate them) had to be reported; (3) the study report cadmium level of body; (4) the outcome of interest was diabetes. Only studies published in English were considered.
Data extraction and quality assessment
Data were extracted for the first author’s name, year of publication, country or area of origin, number of participants, diagnosis criteria of DM, assay method of urinary/blood cadmium, the potential confounding variables in the adjustments, measurements of cadmium exposure, and effect estimates and their corresponding 95% CIs. Data extraction was performed independently by two investigators. Discrepancies were resolved by discussion.
The methodological quality of the studies included was assessed using an 11-item checklist which was recommended by Agency for Healthcare Research and Quality (AHRQ) (Rostom et al. 2004). An item would be scored “0” if it was answered “NO” or “UNCLEAR”; if it was answered “YES,” then the item scored “1.” Article quality was assessed as follows: low quality = 0–3; moderate quality = 4–7; high quality = 8–11 (Hu et al. 2015).
Statistical analysis
We combined odds ratio (OR) for the highest vs. lowest category of urinary cadmium level from each study. Data were analyzed using the Stata version 11.0 software (Stata Corporation, College Station, TX). The possible heterogeneity across included studies was assessed by the Cochrane Q test and I2 statistic (Higgins and Thompson 2002); P < 0.1 for the Q test or I2 > 50% was considered as significant heterogeneity. In the presence of significant heterogeneity, a random effects model was used to calculate the pooled effect size; otherwise, a fixed-effects model was applied (DerSimonian and Laird 1986). We further performed meta-regression analyses to explore the possible sources of heterogeneity. We also conducted a sensitivity analysis to explore the impact of a single trial on the overall risk estimate by omitting one study in each turn. Begg’s funnel plots and Egger’s regression test at the P < 0.10 were applied to assess the publication bias of this meta-analysis (Egger et al. 1997).
We performed a dose-response meta-analysis using the method developed by Greenland and Orsini et al. (Greenland and Longnecker 1992; Orsini et al. 2012). Only studies that reported the number of cases and control subjects and the OR and its variance estimate for at least 3 quantitative exposure categories were included. For each study, the mean or median level for each category of urine cadmium level was assigned to each corresponding OR. If the data was not available, we assigned the midpoint of the upper and lower boundaries in each category as the average concentration. If the lower or upper boundary of the category was open-ended, the width of the interval was assumed to be the same amplitude as the closest category. The restricted cubic spline was used to model the urinary cadmium level at fixed knots (including 10, 50, and 90% of the distribution). The P value for non-linearity was calculated using a null hypothesis test that the coefficient of the second spline was equal to zero.
Results
Search results
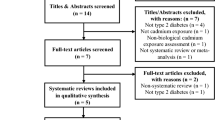
We initially retrieved 175 unique citations from the PubMed, MEDLINE, and the Cochrane Library database. Of these, majority were excluded after the first screening based on abstracts or titles, mainly because they were not observational studies or relevant to our analysis. After full-text review of 43 articles, 11 studies were excluded because the effect estimates were not reported or calculated, 4 studies were excluded because urinary cadmium level was not reported, 11 studies were excluded because the outcome of interest was not diabetes, and 2 studies were excluded because they were meta-analysis. We further excluded 2 studies in which the association was not evaluated. In total, 13 articles including 13 studies (Barregard et al. 2013; Borne et al. 2014; Feng et al. 2015; Haswell-Elkins et al. 2007; Li et al. 2017a, b; Liu et al. 2015; Menke et al. 2016; Moon 2013; Nie et al. 2016; Schwartz et al. 2003; Son et al. 2015; Swaddiwudhipong et al. 2010b; Tangvarasittichai et al. 2015) were selected for the final analysis. A flow diagram showing the selection process is presented in Fig. 1. All of the selected articles were assessed for methodological quality. The quality assessment showed the quality scores ranged from 7 to 10 (Table 1).
Study characteristics
Table 1 described the characteristics of the 13 selected studies (Barregard et al. 2013; Borne et al. 2014; Feng et al. 2015; Haswell-Elkins et al. 2007; Li et al. 2017a, b; Liu et al. 2015; Menke et al. 2016; Moon 2013; Nie et al. 2016; Schwartz et al. 2003; Son et al. 2015; Swaddiwudhipong et al. 2010b; Tangvarasittichai et al. 2015). These studies were published from 2003 to 2017, of which eight were conducted in Asia, two in North America, two in Europe, and one in Australia. There were 12 cross-sectional studies and one prospective cohort study. One study was conducted in occupational workers (Liu et al. 2015) and two in residents of abandoned metal mine(Swaddiwudhipong et al. 2010b; Tangvarasittichai et al. 2015), whereas others in residents of ordinary areas (Barregard et al. 2013; Feng et al. 2015; Haswell-Elkins et al. 2007; Li et al. 2017a, b; Menke et al. 2016; Moon 2013; Nie et al. 2016; Schwartz et al. 2003; Son et al. 2015). The number of participants ranged from 124 to 9447, with a sum of 45,062. Three studies (Feng et al. 2015; Menke et al. 2016; Son et al. 2015) only selected person with type 2 diabetes, and the other ten studies (Barregard et al. 2013; Borne et al. 2014; Haswell-Elkins et al. 2007; Li et al. 2017a, b; Liu et al. 2015; Moon 2013; Nie et al. 2016; Schwartz et al. 2003; Swaddiwudhipong et al. 2010b; Tangvarasittichai et al. 2015) did not distinguish between type 1 and type 2 diabetes mellitus. Eight studies examined cadmium in urine or blood only, while the other five studies detect the contents of various metals in urine or blood. Three assay techniques were used to measure urinary and blood cadmium level.
Main analysis
Of the 13 trials, 5 showed a significant association between the urinary or blood cadmium level and the risk of DM. Because there was evidence of heterogeneity (P < 0.001, I2 = 69.6%), we used a random effects model. The meta-analysis results showed a positive association between cadmium levels in the body and DM (OR = 1.27; 95% CI, 1.07–1.52) (Fig. 2).
Subgroup and sensitivity analysis
We stratified the studies by cadmium exposure measurements. The analysis, including nine studies, showed a positive association between urinary cadmium levels and DM (OR = 1.31; 95% CI, 1.02–1.69) (Fig. 3). Substantial heterogeneity was observed (P = 0.001, I2 = 69.0%). No significant association was found between blood cadmium levels and DM (OR = 1.29; 95% CI, 0.94–1.75), with four studies included in the analysis (Fig. 3).
When we stratified the analysis by geographical region, the overall results in Asia showed a positive association between cadmium levels in the body and DM (OR = 1.28; 95% CI, 1.03–1.60). In studies conducted in America and Europe, there was no significant association between body cadmium levels and DM (Table 2). In studies conducted in Asia, America, and Europe, no significant association was found between cadmium levels in the body and DM (Table 3).
We next conducted sensitivity analyses to examine the effect of a single trial on the overall risk estimate and explore potential sources of heterogeneity by omitting one trial at a time. Exclusion of any study did not significant alter the overall combined odds ratio, and the values ranged from 1.20 (95% CI, 1.02–1.44) and 1.35 (95% CI, 1.11–1.61). Exclusion of any of the studies had little effect on heterogeneity. However, after exclusion of three studies together (Haswell-Elkins et al. 2007; Li et al. 2017a, b; Tangvarasittichai et al. 2015), the heterogeneity was significantly decreased (P = 0.177, I2 = 26.5%).
Dose-response relationship
To investigate the precise relationship between urinary cadmium levels and the risk of DM, we performed a dose-response analysis. Six studies with quantitative exposure categories were included and used to evaluate the dose-response analysis of the associations between urinary cadmium levels and the risk of DM (Feng et al. 2015; Haswell-Elkins et al. 2007; Liu et al. 2015; Schwartz et al. 2003; Son et al. 2015; Swaddiwudhipong et al. 2010b). The characteristics of these studies are described in Table 4. Using a restricted cubic spline model, we observed significant linear association (Pnonlinear = 0.2453). The linear model indicated that for every l μg/g creatinine increase in urinary cadmium level, the risk of DM increased by 16% (1.16, 95% CI 1.04, 1.27) (Fig. 4). The analysis also indicated a positive association between urinary cadmium levels above 2.43 μg/g creatinine and DM (Fig. 4).
Association between urinary cadmium level and risk of DM, dose-response analysis (Odds ratios of DM according to the urinary cadmium concentrations (n = 23,881). Odds ratios (ORs) and 95% confidence intervals (95%CI) are derived from restricted cubic spline regression, with knots placed at the 10th, 50th, and 90th percentiles of the distribution of urine cadmium. The solid line and the long dash line represent the estimated odds ratio and their 95% CIs
Publication bias
We first conducted publication bias between cadmium level of body and risk of DM. The results of Begg’s (P = 0.047) and Egger’s (P = 0.021) tests indicated the evidence of publication bias. After excluding the study which had smallest sample size (Haswell-Elkins et al. 2007), no significant publication bias found in Begg’s (P = 0.371) and Egger’s (P = 0.124) tests. Funnel plots also showed no sign of publication bias.
We further conducted publication bias between urinary cadmium level and risk of DM. There was evidence of publication bias with Begg’s (P = 0.076) and Egger’s (P = 0.057) tests. By excluding the study which had smallest sample size (Haswell-Elkins et al. 2007), Begg’s (P = 0. 266) and Egger’s (P = 0. 130) tests showed no significant publication bias. Funnel plots were also examined.
Discussion
A total of two meta-analysis (Li et al. 2017b; Wu et al. 2017) on the risk of cadmium exposure and DM were published in 2017; the results prompted an increase in the attention paid to the adverse effects of cadmium exposure on DM. A meta-analysis (Wu et al. 2017) including seven studies (Barregard et al. 2013; Haswell-Elkins et al. 2007; Liu et al. 2015; Menke et al. 2016; Son et al. 2015; Swaddiwudhipong et al. 2010b; Tangvarasittichai et al. 2015) has revealed that urinary cadmium is not associated with increased DM risk. However, another meta-analysis (Li et al. 2017b), including seven studies (Feng et al. 2015; Liu et al. 2015; Menke et al. 2016; Schwartz et al. 2003; Son et al. 2015; Swaddiwudhipong et al. 2010a; Swaddiwudhipong et al. 2010b), has found a positive association between urinary cadmium concentrations and the risk of DM. Our meta-analysis including nine studies revealed a positive association between urinary cadmium level and DM (Barregard et al. 2013; Feng et al. 2015; Haswell-Elkins et al. 2007; Liu et al. 2015; Menke et al. 2016; Schwartz et al. 2003; Son et al. 2015; Swaddiwudhipong et al. 2010b; Tangvarasittichai et al. 2015). In a study by Li (Li et al. 2017b), a dose-response analysis including four studies (Feng et al. 2015; Liu et al. 2015; Schwartz et al. 2003; Son et al. 2015) has indicated that each l μg/g creatinine increase in urinary cadmium levels is associated with a 16% increased risk of DM. Our dose-response analysis involving six studies (Feng et al. 2015; Haswell-Elkins et al. 2007; Liu et al. 2015; Schwartz et al. 2003; Son et al. 2015; Swaddiwudhipong et al. 2010b) detected a positive association between levels of urinary cadmium above 2.43 μg/g creatinine and DM—levels far below the WHO standard of 5 μg/g creatinine. Another difference between this study and the previous studies was that we combined urinary and blood cadmium to assess the association between cadmium levels in the body and DM. The meta-analysis involving 13 studies indicated a positive association between cadmium levels in the body and DM.
Urinary cadmium is generally accepted to indicate renal injury from long-term exposure, especially at relatively low exposure levels, whereas blood cadmium levels are primarily indicative of recent exposure. Thus, urinary cadmium may be an appropriate indicator of long-term low-dose cadmium exposure in humans. Our dose-response analysis detected a positive association between levels of urinary cadmium above 2.43 μg/g creatinine and DM. In addition, studies have shown that urinary cadmium levels below the threshold limit may still cause health problems. Numerous studies have suggested that urinary cadmium levels of < 1 μg/g creatinine are associated with tubular and glomerular impairment (Akesson et al. 2006; Akesson et al. 2005). A study from Japan has shown that bone density is negatively correlated with urinary cadmium (mean, 2.9 μg/g creatinine) (Honda et al. 2003). The available information indicates that urinary cadmium levels as low as 0.5–2 μg/g creatinine are associated with bone effects (Akesson et al. 2014). These findings indicate that attention should be paid to the effects of low-dose cadmium exposure on DM.
The mechanism underlying the relationship between body cadmium levels and DM is not fully understood. Cadmium-induced pancreatic β-cell dysfunction may be a possible mechanism leading to DM. Wong’s study has reported the content of cadmium in an initial sample set of native human islets of Langerhans (Wong et al. 2017). Muayed’s study has also shown that cadmium accumulates in pancreatic β cells, and chronic low-level cadmium exposure impairs the function of insulin-producing β cells (El Muayed et al. 2012). The possible mechanisms through which cadmium induces pancreatic β-cell dysfunction may involve cadmium’s effects on energy metabolism, oxidative stress, calcium channel function, and cell-cell adhesion (Edwards and Ackerman 2016). We believe that the mechanisms involved in oxidative stress must be emphasized. A study of the effects of low doses of arsenic on pancreatic β-cell function may enable understanding of the mechanism through which cadmium affects pancreatic β-cell function through antioxidants (Fu et al. 2010). The results of that study indicate that low levels of arsenic provoke a cellular adaptive oxidative stress response that increases intracellular glutathione (GSH) and H2O2-scavenging activity, dampens ROS signaling involved in glucose-stimulated insulin secretion, and thus disturbs β-cell function. Cadmium is likely to play the same role as the arsenic. One study from the El Muayed lab has shown that low concentrations of CdCl2 do not alter the ROS levels in dispersed primary mouse islet cells but decrease the ratio of oxidized glutathione (GSSG) to glutathione (GSH), thus indicating an upregulation of cellular protective mechanisms (El Muayed et al. 2012). The cellular protective mechanisms induced by low concentrations of cadmium may play an important role in the process of pancreatic β-cell dysfunction. Therefore, more studies are needed to investigate the mechanism underlying the effects of prolonged exposure to low concentrations of cadmium on pancreatic β-cell function.
In addition, cadmium-induced insulin resistance may be another possible mechanism leading to DM. Studies in rodents exposed to cadmium have shown a decrease in the expression of GLUT4, a major transporter of glucose uptake in muscle cells, thus indicating that cadmium exposure increases insulin resistance (Han et al. 2003). Another study on rats exposed to cadmium through drinking water has shown that cadmium increases insulin resistance in the liver, adipose tissue, and cardiovascular system (Trevino et al. 2015). However, cadmium exposure has not been found to significantly alter insulin resistance in population-based studies (Menke et al. 2016; Moon 2013). Therefore, further studies are needed to clarify the relationship between cadmium and insulin resistance.
This meta-analysis was limited by considerable moderate heterogeneity across studies. Our sensitivity analyses suggested that three studies (Haswell-Elkins et al. 2007; Li et al. 2017a, b; Tangvarasittichai et al. 2015) were the main sources of heterogeneity. The difference between the three studies and the others was that their sample size was very small, with numbers of participants ranging from 124 to 551. In addition, the study that included 124 people (Haswell-Elkins et al. 2007) did not adjust for confounding factors.
There are additional limitations in this meta-analysis. First, the category of urinary cadmium levels was converted into a midpoint in the dose-response analysis. The calculation for a skewed distribution virtually decreased the precision. Second, four studies were not designed to measure cadmium exposure as the only outcome. Third, significant publication bias was found in this meta-analysis, owing to a study with a sample size of only 124 people (Haswell-Elkins et al. 2007). Therefore, the study with a small sample size was the main source of publication bias. Finally, it is important to emphasize that most of the studies included in this meta-analysis were cross-sectional studies, in which exposure and disease were measured simultaneously. Therefore, the results herein should be considered with caution, given the above limitations.
Conclusion
In summary, the results from our meta-analysis indicate a positive association between level of urinary cadmium above 2.43 μg/g creatinine and DM. In addition, further studies are needed to elucidate the mechanism of pancreatic beta cell dysfunction induced by cadmium.
References
Afridi HI, Kazi TG, Kazi N, Jamali MK, Arain MB, Jalbani N, Baig JA, Sarfraz RA (2008) Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res Clin Pract 80:280–288. https://doi.org/10.1016/j.diabres.2007.12.021
Afridi HI, Talpur FN, Kazi TG, Brabazon D (2015a) Assessment of toxic elements in the samples of different cigarettes and their effect on the essential elemental status in the biological samples of Irish hypertensive consumers. J Hum Hypertens 29:309–315. https://doi.org/10.1038/jhh.2014.87
Afridi HI, Talpur FN, Kazi TG, Brabazon D (2015b) Effect of trace and toxic elements of different brands of cigarettes on the essential elemental status of Irish referent and diabetic mellitus consumers. Biol Trace Elem Res 167:209–224. https://doi.org/10.1007/s12011-015-0308-5
Akesson A et al (2005) Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect 113:1627–1631
Akesson A et al (2006) Cadmium-induced effects on bone in a population-based study of women. Environ Health Perspect 114:830–834
Akesson A, Barregard L, Bergdahl IA, Nordberg GF, Nordberg M, Skerfving S (2014) Non-renal effects and the risk assessment of environmental cadmium exposure. Environ Health Perspect 122:431–438. https://doi.org/10.1289/ehp.1307110
Barregard L, Bergstrom G, Fagerberg B (2013) Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: a cross-sectional and prospective study in women. Environ Res 121:104–109. https://doi.org/10.1016/j.envres.2012.11.005
Bell RR, Early JL, Nonavinakere VK, Mallory Z (1990) Effect of cadmium on blood glucose level in the rat. Toxicol Lett 54:199–205
Borne Y et al (2014) Cadmium exposure and incidence of diabetes mellitus--results from the Malmo diet and Cancer study. PLoS One 9:e112277. https://doi.org/10.1371/journal.pone.0112277
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Edwards J, Ackerman C (2016) A review of diabetes mellitus and exposure to the environmental toxicant cadmium with an emphasis on likely mechanisms of action. Curr Diabetes Rev 12:252–258
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ Clin Res 315:629–634
El Muayed M et al (2012) Accumulation of cadmium in insulin-producing beta cells. Islets 4:405–416. https://doi.org/10.4161/isl.23101
Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, Rudisill C (2012) Toxicologicalprofile for cadmium. ATSDR. www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15. Accessed Sept 2012
Feng W, Cui X, Liu B, Liu C, Xiao Y, Lu W, Guo H, He M, Zhang X, Yuan J, Chen W, Wu T (2015) Association of urinary metal profiles with altered glucose levels and diabetes risk: a population-based study in China. PLoS One 10:e0123742. https://doi.org/10.1371/journal.pone.0123742
Fu J, Woods CG, Yehuda-Shnaidman E, Zhang Q, Wong V, Collins S, Sun G, Andersen ME, Pi J (2010) Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect 118:864–870. https://doi.org/10.1289/ehp.0901608
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
Han JC, Park SY, Hah BG, Choi GH, Kim YK, Kwon TH, Kim EK, Lachaal M, Jung CY, Lee W (2003) Cadmium induces impaired glucose tolerance in rat by down-regulating GLUT4 expression in adipocytes. Arch Biochem Biophys 413:213–220
Haswell-Elkins M, Imray P, Satarug S, Moore MR, O’Dea K (2007) Urinary excretion of cadmium among Torres Strait Islanders (Australia) at risk of elevated dietary exposure through traditional foods. J Expo Sci Environ Epidemiol 17:372–377. https://doi.org/10.1038/sj.jes.7500520
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Honda R, Tsuritani I, Noborisaka Y, Suzuki H, Ishizaki M, Yamada Y (2003) Urinary cadmium excretion is correlated with calcaneal bone mass in Japanese women living in an urban area. Environ Res 91:63–70
Hu J, Dong Y, Chen X, Liu Y, Ma D, Liu X, Zheng R, Mao X, Chen T, He W (2015) Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatry 61:78–89. https://doi.org/10.1016/j.comppsych.2015.05.001
Jin T et al (2002) Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad). Biometals 15:397–410
Li XT, Yu PF, Gao Y, Guo WH, Wang J, Liu X, Gu AH, Ji GX, Dong Q, Wang BS, Cao Y, Zhu BL, Xiao H (2017a) Association between plasma metal levels and diabetes risk: a case-control study in China. Biomed Environ Sci : BES 30:482–491. https://doi.org/10.3967/bes2017.064
Li Y, Zhang Y, Wang W, Wu Y (2017b) Association of urinary cadmium with risk of diabetes: a meta-analysis. Environ Sci Pollut Res Int 24:10083–10090. https://doi.org/10.1007/s11356-017-8610-8
Liu B, Feng W, Wang J, Li Y, Han X, Hu H, Guo H, Zhang X, He M (2015) Association of urinary metals levels with type 2 diabetes risk in coke oven workers. Environ Pollut (Barking, Essex : 1987) 210:1–8. https://doi.org/10.1016/j.envpol.2015.11.046
Longnecker MP, Daniels JL (2001) Environmental contaminants as etiologic factors for diabetes. Environ Health Perspect 109(Suppl 6):871–876
Mazur MA, Winkler M, Ganić E, Colberg JK, Johansson JK, Bennet H, Fex M, Nuber UA, Artner I (2013) Microphthalmia transcription factor regulates pancreatic beta-cell function. Diabetes 62:2834–2842. https://doi.org/10.2337/db12-1464
Menke A, Guallar E, Cowie CC (2016) Metals in urine and diabetes in U.S. adults. Diabetes 65:164–171. https://doi.org/10.2337/db15-0316
Moon SS (2013) Association of lead, mercury and cadmium with diabetes in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Diabet Med 30:e143–e148. https://doi.org/10.1111/dme.12103
Nie X, Wang N, Chen Y, Chen C, Han B, Zhu C, Chen Y, Xia F, Cang Z, Lu M, Meng Y, Jiang B, D. Jensen M, Lu Y (2016) Blood cadmium in Chinese adults and its relationships with diabetes and obesity. Environ Sci Pollut Res Int 23:18714–18723. https://doi.org/10.1007/s11356-016-7078-2
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175:66–73. https://doi.org/10.1093/aje/kwr265
Rostom A, Dubé C, Cranney A et al (2004) Celiac disease. Evid Rep Technol Assess (Summ) 104:1–6
Satarug S, Garrett SH, Sens MA, Sens DA (2010) Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 118:182–190. https://doi.org/10.1289/ehp.0901234
Schwartz GG, Il’yasova D, Ivanova A (2003) Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 26:468–470
Son HS, Kim SG, Suh BS, Park DU, Kim DS, Yu SD, Hong YS, Park JD, Lee BK, Moon JD, Sakong J (2015) Association of cadmium with diabetes in middle-aged residents of abandoned metal mines: the first health effect surveillance for residents in abandoned metal mines. Ann Occup Environ Med 27:20. https://doi.org/10.1186/s40557-015-0071-2
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Swaddiwudhipong W, Limpatanachote P, Nishijo M, Honda R, Mahasakpan P, Krintratun S (2010a) Cadmium-exposed population in Mae Sot district, Tak province: 3. Associations between urinary cadmium and renal dysfunction, hypertension, diabetes, and urinary stones. J Med Assoc Thail = Chotmaihet thangphaet 93:231–238
Swaddiwudhipong W, Mahasakpan P, Limpatanachote P, Krintratun S (2010b) Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: a population study. Environ Res 110:612–616. https://doi.org/10.1016/j.envres.2010.06.002
Tangvarasittichai S, Niyomtam S, Meemark S, Pingmuangkaew P, Nunthawarasilp P (2015) Elevated cadmium exposure associated with hypertension, diabetes and chronic kidney disease, in the population of cadmium-contaminated area. International Journal of Toxicological and Pharmacological Research 7(1):50–56
Trevino S, Waalkes MP, Flores Hernandez JA, Leon-Chavez BA, Aguilar-Alonso P, Brambila E (2015) Chronic cadmium exposure in rats produces pancreatic impairment and insulin resistance in multiple peripheral tissues. Arch Biochem Biophys 583:27–35. https://doi.org/10.1016/j.abb.2015.07.010
Wajchenberg BL (2007) Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 28:187–218. https://doi.org/10.1210/10.1210/er.2006-0038
Wong WP, Allen NB, Meyers MS, Link EO, Zhang X, MacRenaris KW, El Muayed M (2017) Exploring the association between demographics, SLC30A8 genotype, and human islet content of zinc, cadmium, copper, iron, manganese and nickel. Sci Rep 7:473. https://doi.org/10.1038/s41598-017-00394-3
Wu M, Song J, Zhu C, Wang Y, Yin X, Huang G, Zhao K, Zhu J, Duan Z, Su L (2017) Association between cadmium exposure and diabetes mellitus risk: a prisma-compliant systematic review and meta-analysis. Oncotarget 8:113129–113141. https://doi.org/10.18632/oncotarget.21991
Funding source
This work was financially supported by the National Natural Science Foundation of China [grant numbers: 81773414, 81673151].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, FF., Hu, ZY., Li, BY. et al. Evaluation of the association between urinary cadmium levels below threshold limits and the risk of diabetes mellitus: a dose-response meta-analysis. Environ Sci Pollut Res 26, 19272–19281 (2019). https://doi.org/10.1007/s11356-019-04943-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04943-3