Abstract
BACKGROUND:
Protein infusion in the small intestine results in intestinal brake activation: a negative feedback mechanism that may be mediated by the release of gastrointestinal peptides resulting in a reduction in food intake. It has been proposed that duodenum, jejunum and ileum may respond differently to infused proteins.
OBJECTIVE:
To investigate differences in ad libitum food intake, feelings of hunger and satiety and the systemic levels of cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), peptide YY (PYY), glucose and insulin after intraduodenal, intrajejunal and intraileal protein infusion.
METHODS:
Fourteen subjects (four male, mean age: 23±2.1 years, mean body mass index: 21.6±1.8 kg m−2) were intubated with a naso-ileal catheter in this double-blind, randomized, placebo-controlled crossover study. Test days (four in total, executed on consecutive days) started with the ingestion of a standardized breakfast, followed by the infusion of 15 g of protein in the duodenum, jejunum or ileum over a period of 60 min. Food intake was measured by offering an ad libitum meal and Visual Analogue Scale (VAS) scores were used to assess feelings of hunger and satiety. Blood samples were drawn at regular intervals for CCK, GLP-1, PYY, glucose and insulin analyses.
RESULTS:
Intraileal protein infusion decreased ad libitum food intake compared with both intraduodenal and placebo infusion (ileum: 628.5±63 kcal vs duodenum: 733.6±50 kcal, P<0.01 and placebo: 712.2±53 kcal, P<0.05). GLP-1 concentrations were increased after ileal infusion compared with jejunal and placebo infusion, whereas CCK concentrations were only increased after intraileal protein infusion compared with placebo. None of the treatments affected VAS scores for hunger and satiety nor plasma concentrations of PYY and glucose.
CONCLUSIONS:
Protein infusion into the ileum decreases food intake during the next meal compared with intraduodenal infusion, whereas it increases systemic levels of GLP-1 compared with protein infusion into the jejunum and placebo respectively.
Similar content being viewed by others
Introduction
Proteins are known to be more satiating compared with isocaloric amounts of either fat or carbohydrates.1, 2 An increase in the amount of dietary protein results in increased feelings of satiety with significant long-term weight loss. Therefore, high-protein diets are considered to be effective weight management tools.2, 3 The mechanisms underlying this satiating effect of proteins are complex and multifactorial, but it has been well established that the gastrointestinal (GI) tract plays an important role in generating the signals that mediate satiety.4, 5
When administered intraduodenally, pea protein induces a more pronounced decrease in food intake and increase in plasma cholecystokinin (CCK) levels as compared with oral ingestion of the same amount of pea protein.6 This difference has been explained by the activation of the so-called duodenal brake. Nutrients in the duodenum activate a negative feedback mechanism that not only modulates proximal gastrointestinal motility, secretion and mucosal peptide release but also feelings of satiety and food intake.7 Recently, several other human studies have also provided evidence for an inhibitory effect of intraduodenal administration of proteins on satiety and food intake.8, 9, 10 It has been suggested that infusion of nutrients into the ileum results in an even more pronounced effect on satiety and food intake when compared with infusion into the more proximal parts of the small intestine.11, 12
Evidence for a pronounced ileal brake effect on satiety and food intake is derived not only from human intubation studies but also from surgical procedures such as the Roux-en-Y gastric bypass and ileal transposition.13, 14 After Roux-en-Y gastric bypass the proximal intestine is bypassed thereby increasing exposure of the more distal small intestine to undigested nutrients.15 Direct administration of fat into the ileum via an intestinal catheter results in a more pronounced reduction in appetite, increase in fullness and delay in GI motility as compared with administration of the same amount of fat into the duodenum.11
Human data comparing the responsiveness of different parts of the small intestine to protein exposure are still lacking. Aim of the present study was therefore to investigate whether differences exist in ad libitum food intake, feelings of hunger and satiety and in the systemic levels of cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), peptide YY (PYY) when comparing intraduodenal vs intrajejunal vs intraileal protein administration. We hypothesized that intraileal protein infusion results in a more potent decrease in food intake and a stronger increase in both feelings of satiety and plasma GI peptide levels as compared with intraduodenal and intrajejunal protein infusion.
Materials and methods
This study was approved by the Medical Ethics Committee of the Maastricht University Medical Center+ (MUMC), Maastricht, The Netherlands, and performed in full accordance with the Declaration of Helsinki (latest amendment by the World Medical Association in 2013). All participants gave their written informed consent prior to participation. The study has been registered in the US National Library of Medicine (http://www.clinicaltrials.gov, NCT02500069).
Subjects
Healthy, lean volunteers were recruited by local advertisement. Subjects were screened using a standard health questionnaire and a physical examination. Exclusion criteria included: medical or surgical history that could have affected study outcome, medication use (except contraceptives), smoking and excessive alcohol consumption (>20 g of alcohol per day). All participants were weight stable for at least 2 months before participation, and were not following any energy restriction- or food supplementation diets. Number of subjects was determined by using the data of previous work.11 Power calculation, based on the primary outcome parameter energy intake, showed that fourteen study completers would allow us to detect a difference in energy intake of 37 kcal with an α of 0.05 and β of 0.8.
Study design
In this double-blind randomized placebo-controlled crossover study, subjects were studied on four randomly assigned consecutive test days. On these occasions, 15 g of casein was infused using a naso-ileal feeding catheter over a period of 60 min into one of the three different locations of the small intestine: (1) the duodenum, (2) the jejunum and (3) the ileum, or no protein was infused (placebo). During each intervention, protein infusion was accompanied by simultaneous infusion of tap water in the other two locations. For example, intraduodenal protein infusion was accompanied by simultaneous tap water infusion into the jejunum and ileum, respectively. The placebo treatment consisted of tap water infusion in all three locations.
Naso-ileal catheter
Subjects were intubated with a 290 cm long silicon 9-channel (8-lumen, 1 balloon inflation channel, outer diameter of 3.5 mm) custom-made catheter (Dentsleeve International, Mui Scientific, Mississauga, Canada). The catheter contained three sideholes per channel with 3-cm interspacing between consecutive sideholes, and had an inflatable balloon (maximum inflation capacity 10 ml) integrated into the distal tip. After local anesthesia of a nostril (xylocaine 10% spray; AstraZeneca, Zoetermeer, The Netherlands), the catheter was introduced into the stomach. Subsequently, the tip of the catheter was positioned in the duodenum under intermittent fluoroscopic control. Progression of the catheter into the ileum was performed as described previously.16 Correct positioning of the catheter was checked under fluoroscopy before starting each test day. The catheter includes radio-opaque markers at all sideholes, which enabled us to accurately determine the position of the catheter. The radio-opaque markers made it possible to select the infusion channel from this multi-lumen catheter that was located at the infusion location (duodenum, jejunum or ileum). Infusion into the duodenum took place 5 cm distal to the pylorus, while infusion into the jejunum took place 40–50 cm distal of the pylorus. Intraileal infusion was performed at least 120 cm distal to the pylorus.
Protein and placebo infusions
Casein (energy density: 3.4 kcal g−1, Dutch Protein Services, The Netherlands) was used as the protein source in this study. Fifteen grams of casein was dissolved in 180 ml of tap water and infused at a rate of 3 ml min−1 over a 60 min period. During placebo treatment, tap water was infused simultaneously at the same rate in all three locations.
Protocol
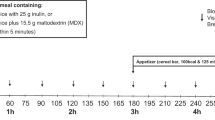
During four consecutive test days, subjects arrived at our laboratory at 0800 hours after an overnight fast. After checking correct positioning of the catheter as described above, test days started with the insertion of an intravenous catheter in a forearm vein for collection of blood samples. At 0830 hours basal blood samples and Visual Analogue Scale (VAS) scores for hunger and satiety were taken. Hereafter, subjects consumed a standard fixed sized liquid, dairy breakfast meal (155 kcal, Goedemorgen Drink Yoghurt, Friesland Campina, The Netherlands, energy composition: 2 g lipid, 23.5 g carbohydrates, 7.3 g protein). A liquid formula was chosen in order to make sure that the breakfast meal had emptied from the stomach (and had been absorbed) at the time the infusions started. One-hundred and fifty minutes after the ingestion of the breakfast meal (1100 hours), infusion of protein or placebo into the small intestine was started. Intestinal infusion took place over 60 min, at a rate of 3 ml min−1 and were blinded for both the subject and investigator. Thirty minutes after ending the infusion, volunteers received a standard ad libitum pasta lunch (Lasagna Bolognese, t=240 min, energy composition (per 100 g): 160 kcal, 9.4 g lipid, 11 g carbohydrates, 7.1 g protein). The ad libitum pasta lunch was served in excess (2000 g), in a room with only a chair and a table in order to make sure that the volunteer was fully focused on food consumption (mobile phones were collected by the investigator). Volunteers consumed the meal alone and had thirty minutes to eat. The meal was weighed before and after consumption and by subtracting these numbers, the amount eaten was calculated. After ingestion of the meal, the test day was finished (see Figure 1 for design of test day). Participants were allowed to return home with the naso-ileal catheter in situ.
Gastrointestinal peptides
Venous blood samples were drawn at regular intervals (baseline (0), 60, 120, 150, 165, 180, 195, 210, 240 min, respectively). For GLP-1 (7–36), PYY and CCK measurements blood was collected in ice chilled EDTA aprotinin coated tubes (Becton and Dickinson, New Jersey, USA) and 10 μl Dipeptidyl peptidase-4 inhibitor (DPP4-010, Merck Millipore, Massachusetts, USA) per 1 ml of whole blood was immediately added after blood collection to prevent proteolytic cleavage. Tubes were centrifuged at a rate of 3000 r.p.m., 4 °C for 15 min and plasma was transferred into aliquots and stored at −80 °C. Active GLP-1 (7–36) was determined using a glucagon-like peptide-1 (Active) ELISA kit with a range of 2–100 pmol l−1, an inter-assay coefficient variation (CV) of 11%, and an intra-assay CV of 6% (EGLP- 35 K, Merck Millipore, Massachusetts, USA). Total PYY (includes both peptide YY 1–36 and peptide YY 3–36) was measured using a human PYY (Total) ELISA kit with a range of 10–2000 pg ml−1, an inter-assay CV of 6% and an intra-assay CV of 3% (EZHPYYT66K, Merck Millipore). Plasma CCK (cholecystokinin 26–33) concentrations were measured with an optimized and validated commercial human RIA kit (EURIA CCK, RB302, Euro-Diagnostica, Malmö, Sweden).14 This improved assay system has been optimized to reach a high sensitivity of 0.05 pmol l−1 and to have no cross-reactivity to gastrin-17 or sulfated gastrin. The intra-assay CV was 8.9% at a concentration of 0.84 pmol l−1 and 4.9% at a concentration of 1.98 pmol l−1.
Glucose and insulin
Sodium fluoride- and SST II Plus gold tubes (Becton & Dickinson) were used for determination of glucose and insulin, respectively. SST II Plus gold tubes were stored at room temperature for 30 min before centrifugation at 3000 r.p.m., 20 °C for 15 min. Glucose measurements were performed on a Roche Cobas C701 analyzer (GLUC3, Roche, Mannheim, Germany) with a inter-assay variation of 0.02 mmol l−1 at glucose concentration 3.27 mmol l−1. Serum insulin was measured using the Immulite 1000 Insulin Kit (LKIN5) on the Immulite 1000 (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA) with a inter-assay variation of 4.09 pmol l−1 at insulin concentration 51.23 pmol l−1.
Visual Analogue Scales
Feelings of hunger, satiety, fullness, prospective food consumption, nausea, pain and bloating were measured using VAS (0–100 mm) anchored at the low end with the most negative or lowest intensity feelings (for example, not at all), and with opposing terms at the high end (for example, very high, extreme).17
Statistical analyses
Statistical analyses were performed using the SAS statistical software package (SAS version 9; SAS institute, Cary, NC, USA). Data were visually checked for normality and for constant variance of residuals by plots of residuals vs corresponding predicted values. If data were not normally distributed, log transformation was applied for further analysis of the data, as was the case for CCK, GLP-1 (7–36) and PYY. Regarding food intake, statistical analysis was performed on the amount of food eaten in kcal. VAS scores for hunger and satiety, CCK, GLP-1 (7–36) and PYY are displayed from the start of the test day (t=−15 min) until the last blood sample collected before the start of the ad libitum meal (t=240 min). The effects of each intervention on the VAS scores and levels of GI peptides were determined by analyzing the VAS scores and peptide levels from the start of the test day until ingestion of the ad libitum meal. Area under the curve (AUC) was calculated by the trapezoid rule, for the VAS scores and CCK and PYY. Incremental AUC (iAUC) was calculated for GLP-1 (due to baseline differences at t=150 min).
All variables were compared with a mixed analysis of variance model that included baseline as covariate and the fixed factors treatment (ileum, jejunum, duodenum and placebo). For the VAS and plasma parameters, time and the interaction between treatment and time were added to the model. Because of the crossover design, intervention effects within subjects were compared by including the random factor subject. If an intervention effect occurred, a post hoc Dunnett test was used to analyze differences in ad libitum meal intake. A post hoc Tukey–Kramer test was used to analyze differences in VAS scores and GI peptides (CCK, GLP-1 and PYY). Data are presented as the mean±s.e.m. (unless specified otherwise) and considered significant at P<0.05.
Results
Sixteen healthy volunteers were included. Two volunteers dropped out because of discomfort induced by the feeding catheter; 14 volunteers (four males; mean age: 23±2.1 years, mean body mass index: 21.6±1.8 kg m−2) completed the study.
Food intake
Intraileal protein infusion decreased ad libitum meal intake compared with placebo infusion and compared with duodenal protein infusion (main effect: P<0.05, post hoc analysis: ileum: 628.5±63 kcal vs placebo: 712.2±53 kcal; P<0.05 and vs duodenum: 733.6±50 kcal; P<0.01; Figure 2). Food intake did not differ between jejunal (668.3±59 kcal) and ileal protein infusion.
Food intake in kcal (mean+s.e.m.) of an ad libitum lunch ingested 30 min after ending the protein infusion into the ileum, jejunum, duodenum or placebo, respectively, over a 60 min period (n=14). A significant reduction in food intake was observed after ileal protein infusion compared with duodenal protein infusion (**P<0.01) and placebo infusion (*P<0.05) based on a mixed analysis of variance model (main effect: P<0.05) with a post hoc Dunnett correction.
VAS scores—appetite
Mean VAS scores for desire to eat, hunger, fullness and satiety are displayed from the start of the test day (t=−15 min) until the start of the ad libitum meal (t=240 min; Figure 3). Consumption of the liquid breakfast at time t=0 min decreased the desire to eat and hunger and increased fullness and satiety in all treatments. VAS scores for the desire to eat, hunger, fullness and satiety were not different after ileal protein infusion compared with duodenal or jejunal protein infusion or compared with placebo, respectively. AUC for the desire to eat, hunger, fullness and satiety did not differ between the four different treatments (data not shown).
VAS desire to eat, hunger, fullness and satiety (mean+s.e.m.) during and after protein infusion into the ileum, jejunum, duodenum and placebo, respectively. Intestinal infusion started 150 min after breakfast ingestion and lasted for 60 min. No differences in VAS scores were observed. These results were analyzed with a mixed analysis of variance model with a post hoc Tukey–Kramer correction.
VAS scores—symptoms
Mean VAS scores for nausea, pain and bloating are displayed from t=120 until the start of the ad libitum meal (t=240 min; Figure 4). VAS scores nausea, pain and bloating did not differ between the four interventions (ileum, jejunum, duodenum and placebo).
VAS nausea, pain and bloating (mean+s.e.m.) during and after protein infusion into the ileum, jejunum, duodenum and placebo, respectively. Intestinal infusion started 150 min after breakfast ingestion and lasted for 60 min. No differences in VAS scores were observed. These results were analyzed with a mixed analysis of variance model with a post hoc Tukey–Kramer correction.
GLP-1, CCK and PYY
The effects of ileal, jejunal and duodenal infusion on plasma concentrations of GLP-1, CCK and PYY and AUCs (150–240 min) are presented in Figure 5. Fasted plasma concentrations of CCK, GLP-1 and PYY did not differ between test days. CCK concentrations increased after ingestion of the liquid breakfast, whereas GLP-1 and PYY concentrations did not change. Ileal protein infusion increased GLP-1 concentrations compared with jejunal and placebo infusion, respectively (main effect: P<0.0001, post hoc analysis: P<0.0001, Figure 5a). Duodenal protein infusion increased GLP-1 concentrations compared with placebo infusion (P<0.001, Figure 5a). The iAUC (150–240 min) for GLP-1 was increased after ileal protein infusion compared with placebo infusion (Figure 5b). CCK concentrations were also increased after intraileal and intrajejunal protein infusion compared with placebo (P<0.05). Furthermore, an increase in CCK was observed after intrajejunal infusion compared with duodenal infusion (P<0.05, Figure 5c). Ileal and jejunal protein infusion increased the AUC (150–240 min) for CCK compared with placebo infusion (Figure 5d). None of the treatments affected plasma concentrations of PYY (Figure 5e and f).
Plasma concentrations of GLP-1, CCK and PYY (mean+s.e.m.) and GLP-1, CCK and PYY areas under the curve (AUC 150–240 min) during and after protein infusion in the ileum, jejunum, duodenum and placebo infusion, respectively. Intestinal infusion started 150 min after breakfast ingestion and continued for 60 min. (a) Significantly higher GLP-1 levels were observed after intraileal infusion of protein vs intrajejunal and placebo infusion (both ****P<0.0001). Intraduodenal protein infusion increased GLP-1 concentrations compared with placebo infusion (***P<0.001). (b) iAUC (150–240 min) for GLP-1 was significantly increased after ileal protein infusion compared with placebo infusion (*P<0.05). (c) Significantly higher CCK levels were observed after intraileal and intrajejunal infusion of protein vs placebo infusion; (***P<0.001). Intrajejunal infusion increased CCK levels compared with duodenal infusion; (*P<0.05). (d) AUC (150–240 min) of CCK during ileal and jejunal infusion was significantly increased compared with placebo infusion (*P<0.05 and ***P<0.001 respectively). (e, f) No differences in the release of PYY were observed. These results were analyzed with a mixed analysis of variance model with a post hoc Tukey–Kramer correction.
Glucose and insulin
Ingestion of the breakfast increased plasma insulin concentration, followed by a decrease in plasma glucose in all treatments (Figure 6). Insulin levels increased after intraileal, intrajejunal and intraduodenal protein infusion compared with placebo infusion (P<0.0001). No differences in glucose levels were observed.
Glucose and insulin concentrations (mean+s.e.m.) during and after protein infusion into the ileum, jejunum, duodenum and placebo infusion, respectively. Intestinal infusion was started 150 min after breakfast ingestion and lasted for 60 min. Intraileal, intrajejunal and intraduodenal infusion of protein increased insulin levels compared with placebo infusion (****P<0.0001). No differences in glucose concentrations were seen. These results were analyzed with a mixed analysis of variance model with a post hoc Tukey–kramer correction.
Discussion
To our knowledge this is the first study to demonstrate that administration of undigested proteins in the ileum induces a significantly more pronounced effect on food intake reduction compared with intraduodenal but not compared with intrajejunal administration of the same amount of proteins. Additionally, we showed that systemic GLP-1 levels were significantly higher after intraileal infusion compared with jejunal infusion of protein and placebo, respectively.
It has previously been shown that intraduodenal protein infusion results in a stronger reduction in food intake compared with oral protein ingestion.6 The present study shows that differences in food intake reduction not only exist between oral vs intestinal administration but also between the different small intestinal regions (duodenum, jejunum and ileum). We infused a small amount of casein (total dose of 51 kcal) at a rate of 0.9 kcal min−1 and found that intraileal administration results in a significant reduction in food intake compared to placebo, while no significant reduction was seen after intraduodenal or intrajejunal protein administration. Although other investigators have observed previously that an intraduodenal protein load of 3 kcal min−1 (total dose of 180 kcal) was needed in order to significantly affect energy intake, our findings provide clear evidence that a much lower threshold exists with respect to the ileum.8 It should be noted that a difference in threshold might also be related to the type of protein infused. The study by Ryan et al used whey as protein source while casein has been used in the present study. Casein and whey both contain all the essential amino acids but differ with respect to digestion and absorption characteristics.18 Some investigators indicate that whey is more satiating and results in a more pronounced release of GI peptides compared to casein.19, 20, 21, 22 It should be taken into account that these observations were based on studies with oral ingestion of casein and whey. We selected casein as protein source based on our recent report with significant effects of casein on food intake after intraileal infusion.16
It was anticipated that a reduction in energy intake would be accompanied by changes in hunger and satiety scores; however no significant differences between treatments were observed with regard to satiety scores. It is possible that the current study was not sufficiently powered to detect statistically significant differences in hunger and satiety scores. However, similar findings were also observed by Ryan et al. and Geraedts et al.6, 8 Interestingly, when intestinal infusion of nutrients is accompanied by simultaneous distension of the stomach, a more potent increase in feelings of satiety is observed compared with intestinal infusion alone, that is without stomach distension.23 These data highlight the important role of the stomach in the regulation of satiety and food intake.23, 24, 25 The present study was designed to study the effect of intestinal exposure to undigested protein, excluding the role of the stomach as regulator of satiety and food intake. Therefore, subjects consumed a light and liquid breakfast (150 kcal) that had been ingested 150 min before the start of the infusion. Consequently, the stomach of our volunteers was expected to have emptied the breakfast completely, leading to a non-distended stomach during the intestinal nutrient infusion experiment.
It has been suggested that exposing the distal small intestine to nutrients may result in the most potent brake effect on food intake. However, the mechanism of action of such an effect is not completely understood. Here we show an increase in systemic levels of the GI peptide GLP-1 after intraduodenal and intraileal protein infusion compared with placebo infusion.
Ryan et al. observed similar effects and showed that protein administration at a rate of 0.5 kcal min−1 transiently stimulated the release of GLP-1.8 In the present study protein was infused at a rate 0.9 kcal/min. Remarkably, a higher increase in GLP-1 levels was observed after intraileal protein infusion compared with infusion into the more proximal locations. A similar increase in GLP-1 has also been observed after Roux-en-Y gastric bypass and ileal transposition. Both procedures are known to increase the exposure of the distal small intestine to nutrients.26, 27, 28 Based on the changes seen after these procedures, it was hypothesized that a higher density of entero-endocrine L-cells, found in the mucosa of the distal small intestine could account for such a GLP-1 increase.29 These variations in density and absolute number of L-cells over the small intestine, may help to explain the observed differences in food intake and GI peptide release between the duodenum, jejunum and ileum in the current study.12
Ryan et al.8 found that an intraduodenal protein load of ⩾3 kcal min−1 was required in order to induce an increase in systemic CCK and PYY levels, while the calorie infusion rate in the present study was 0.9 kcal min−1. It is therefore not surprising that we did not observe differences in the release of PYY after protein infusion in any of the locations in the small intestine.
We showed that the protein-induced increase in insulin levels was not dependent on the location of protein infusion. Interestingly, these results are not in line with a recent human in vivo catheter study by Salinari et al. They reported that infusing a mixed meal (Nutrison, 524 kcal) in the mid-jejunum not only increased GLP-1 levels, but also enhanced insulin sensitivity and resulted in a less pronounced increase in insulin levels when compared with infusion in the duodenum and proximal jejunum.30 Several factors may account for the fact that insulin levels after intraileal protein administration were not different compared with the other locations in our study. First, we included lean, healthy and young adults, while the Salinari study was performed in obese subjects. The difference in glucose homeostasis between these groups might account for the discrepancy in insulin secretion. Second, the incretin effect on insulin secretion after glucose ingestion was shown to be dose dependent.31 As we only infused 15 g of protein (50 kcal) in the current study, it is possible that this amount was too small to establish effects on insulin levels.
Our study has some limitations that need to be addressed. First, a liquid breakfast was ingested 150 min before the start of the infusion. As such we intended to exclude intestinal exposure to breakfast-originated nutrients at 150 min and beyond and thereby assess the effects of intestinally infused protein only. Gastric emptying was not measured in this study and therefore we cannot fully exclude that the breakfast meal has interfered with the infused nutrients. However, low caloric and liquid meals as we employed are known to have emptied from the stomach in less than 90 min.32 Second, our subjects had a relatively low energy intake at the ad libitum lunch and since no palatability data nor 24-h food intake were collected we cannot rule out that intubating participants with a naso-ileal catheter for several consecutive days could have affected eating behavior and thus study outcome parameters. Third, we infused protein directly into the distal small intestine, hereby bypassing regions that are important for protein digestion. It is not clear whether distal protein infusion could lead to protein malabsorption and subsequently to increased colonic protein fermentation. If this would be the case, this may potentially lead to detrimental effects for the host’s physiology and health, possibly affecting outcome parameters in this study.33 Noteworthy, in the current study we did not observe adverse effects in any of the subjects after distal protein infusion. A recent study by Bojsen-Møller et al.34 also showed that protein digestion was not impaired after Roux-en-Y gastric bypass. This suggests that the distal small intestine is capable of effectively digesting and absorbing protein and its metabolites.
This study provides evidence for location-specific responsiveness in the small intestine to protein infusion with regard to intestinal brake activation. These results have implications for the design of future nutritional and/or surgical strategies for management of overweight and obesity. These strategies should aim to deliver nutrients to the more distal small intestine in order to exert the most potent brake effect.
This study shows the effects of a protein infusion into different locations of the small intestine. Ileal protein infusion results in the most pronounced and potent increase in GLP-1 and decrease in food intake.
In conclusion, protein infusion into the ileum decreases food intake during the next meal compared with duodenal infusion, whereas it increases systemic levels of GLP-1 compared with protein infusion into the jejunum.
Author contributions
MvA, FT, HH and AM designed the research; MvA conducted the research; MvA and DR analyzed data and performed the statistical analyses; DR performed the hormone analyses; MvA, DR, FT, HH and AM contributed to interpretation of the results; MvA, FT and AM wrote the manuscript. AM and FT had primary responsibility for the final content of the manuscript. All authors contributed to and approved the final manuscript.
References
Astrup A . The satiating power of protein—a key to obesity prevention? Am J Clin Nutr 2005; 82: 1–2.
Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M . Protein, weight management, and satiety. Am J Clin Nutr 2008; 87: 1558S–1561SS.
Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 2005; 82: 41–48.
Cummings DE, Overduin J . Gastrointestinal regulation of food intake. J Clin Invest 2007; 117: 13–23.
Mars M, Stafleu A, de Graaf C . Use of satiety peptides in assessing the satiating capacity of foods. Physiol Behav 2012; 105: 483–488.
Geraedts MC, Troost FJ, Munsters MJ, Stegen JH, de Ridder RJ, Conchillo JM et al. Intraduodenal administration of intact pea protein effectively reduces food intake in both lean and obese male subjects. PloS ONE 2011; 6: e24878.
Shin HS, Ingram JR, McGill AT, Poppitt SD . Lipids, CHOs, proteins: can all macronutrients put a 'brake' on eating? Physiol Behav 2013; 120: 114–123.
Ryan AT, Feinle-Bisset C, Kallas A, Wishart JM, Clifton PM, Horowitz M et al. Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 2012; 96: 474–482.
Ryan AT, Luscombe-Marsh ND, Saies AA, Little TJ, Standfield S, Horowitz M et al. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 2013; 98: 300–311.
Soenen S, Giezenaar C, Hutchison AT, Horowitz M, Chapman I, Luscombe-Marsh ND . Effects of intraduodenal protein on appetite, energy intake, and antropyloroduodenal motility in healthy older compared with young men in a randomized trial. Am J Clin Nutr 2014; 100: 1108–1115.
Maljaars J, Haddeman E, Peters H, Masclee AA . Comparison of ileal and duodenal brake mechanisms on satiety and gastrointestinal transport. Gastroenterology 2007; 132: A207–A208.
Maljaars PW, Peters HP, Mela DJ, Masclee AA . Ileal brake: a sensible food target for appetite control. A review. Physiol Behav 2008; 95: 271–281.
Atkinson RL, Whipple JH, Atkinson SH, Stewart CC . Role of the small bowel in regulating food intake in rats. Am J Physiol 1982; 242: R429–R433.
le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007; 246: 780–785.
DeMaria EJ . Bariatric surgery for morbid obesity. N Engl J Med 2007; 356: 2176–2183.
van Avesaat M, Troost FJ, Ripken D, Hendriks HF, Masclee AAM . Ileal brake activation: macronutrient-specific effects on eating behavior? Int J Obesity 2015; 39: 235–243.
Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A et al. Appetite control: methodological aspects of the evaluation of foods. Obes Rev 2010; 11: 251–270.
Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C, Astrup A . Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials. Adv Nutr 2013; 4: 418–438.
Calbet JA, Holst JJ . Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr 2004; 43: 127–139.
Lang V, Bellisle F, Oppert JM, Craplet C, Bornet FR, Slama G et al. Satiating effect of proteins in healthy subjects: a comparison of egg albumin, casein, gelatin, soy protein, pea protein, and wheat gluten. Am J Clin Nutr 1998; 67: 1197–1204.
Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP et al. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav 2009; 96: 675–682.
Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MP, Brummer RJ et al. A breakfast with alpha-lactalbumin, gelatin, or gelatin+TRP lowers energy intake at lunch compared with a breakfast with casein, soy, whey, or whey-GMP. Clin Nutr 2009; 28: 147–155.
Powley TL, Phillips RJ . Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol Behav 2004; 82: 69–74.
Feinle C, Christen M, Grundy D, Faas H, Meier O, Otto B et al. Effects of duodenal fat, protein or mixed-nutrient infusions on epigastric sensations during sustained gastric distension in healthy humans. Neurogastroenterol Motil 2002; 14: 205–213.
Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J . Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther 2011; 33: 880–894.
Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 2005; 90: 359–365.
le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006; 243: 108–114.
Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ . Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 2005; 288: E447–E453.
Lutz TA, Bueter M . Physiological mechanisms behind Roux-en-Y gastric bypass surgery. Dig Surg 2014; 31: 13–24.
Salinari S, Carr RD, Guidone C, Bertuzzi A, Cercone S, Riccioni ME et al. Nutrient infusion bypassing duodenum-jejunum improves insulin sensitivity in glucose-tolerant and diabetic obese subjects. Am J Physiol Endocrinol Metab 2013; 305: E59–E66.
Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986; 63: 492–498.
Sachdeva P, Kantor S, Knight LC, Maurer AH, Fisher RS, Parkman HP . Use of a high caloric liquid meal as an alternative to a solid meal for gastric emptying scintigraphy. Dig Dis Sci 2013; 58: 2001–2006.
Windey K, De Preter V, Verbeke K . Relevance of protein fermentation to gut health. Mol Nutr Food Res 2012; 56: 184–196.
Bojsen-Moller KN, Jacobsen SH, Dirksen C, Jorgensen NB, Reitelseder S, Jensen JB et al. Accelerated protein digestion and amino acid absorption after Roux-en-Y gastric bypass. Am J Clin Nutr 2015; 102: 600–607.
Acknowledgements
The research was funded by TI Food and Nutrition, a public-private partnership on pre-competitive research in food and nutrition. All funders had an input in the study design, whereas study conduct, data collection and analysis, as well as manuscript writing were the sole responsibility of the academic partners. Registered at clinicaltrials.gov: NCT02500069. Names for PubMed indexing: van Avesaat, Ripken, Hendriks, Masclee, Troost.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
van Avesaat, M., Ripken, D., Hendriks, H. et al. Small intestinal protein infusion in humans: evidence for a location-specific gradient in intestinal feedback on food intake and GI peptide release. Int J Obes 41, 217–224 (2017). https://doi.org/10.1038/ijo.2016.196
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.196
- Springer Nature Limited
This article is cited by
-
Small intestinal CaSR-dependent and CaSR-independent protein sensing regulates feeding and glucose tolerance in rats
Nature Metabolism (2024)
-
The metabolic impact of small intestinal nutrient sensing
Nature Communications (2021)