Abstract
Background
Both Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) decrease the latency of food delivery to the proximal small intestine. This is implicated in exaggerated post-prandial release of glucagon-like peptide 1 (GLP-1), which provokes early satiety and reductions in food intake. Altered stomach anatomy also creates a deficit in enzymatic pre-processing. The impact of this state effect as a modulator of gut hormone responses remains underexplored.
Methods
A double-blind cross-over trial study was conducted in 13 healthy subjects assigned to receive in the fasted state and in random order at 1 week apart, a direct jejunal infusion of either intact casein or a casein hydrolysate. Downstream effects on GLP-1 release, ratings of hunger and fullness and food and water intake on each study day were recorded when an ad libitum meal was provided 30 min after the infusion.
Results
Circulating GLP-1 was increased 25 min after infusions and peaked to a similar degree at 15 min post-meal initiation. The hormone surge had no impact on ratings of hunger and fullness ahead of the ad libitum meal. The kinetic and magnitude of satiation following each infusion was not significantly different. Food and water intake were likewise not differentially impacted by the two infusion types.
Conclusions
Protein macronutrient state upon arrival in the small intestine does not in isolation impact upon GLP-1 responses and subsequent onset of satiety. This potentially points to rate of delivery being the dominant factor in exaggerated post-prandial GLP-1 responses in patients post-RYGB and VSG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Analysis of randomised controlled trials of bariatric surgery demonstrates comparable short-term efficacy on the primary outcome of weight loss between Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) [1]. Some but not all studies suggest that both procedures are associated with enhanced weight loss and improved weight loss maintenance relative to laparoscopic adjustable gastric banding (LAGB) [2, 3].
While all bariatric procedures include an element of reduced gastric capacity, loss of pyloric sphincter function and responsiveness to feedback signals from the small intestine may be of particular importance in RYGB and VSG as these changes result in early nutrient transfer to the small intestine. Early and exaggerated circulating post-prandial levels of satiety gut hormone signals such as glucagon-like peptide 1 (GLP-1) occur following both RYGB and VSG but not following LAGB [4–6]. These exaggerated hormonal responses may have their basis in enhanced stimulation of secretion from intestinal L-cells occurring secondary to accelerated nutrient transfer. Thus, what the small intestine sees, how quickly it sees it and how it responds following RYGB and VSG may play a key mechanistic role in the long-term weight loss obtained with both procedures [7, 8].
Body weight is regulated via cooperation between short-term afferent signals from the gut and long-term energy storage signals from the adipose tissue [9]. The proportional impact of short-term signals is titrated in favour of food intake or meal termination in the fasted and fed state, respectively. Orexigenic gut hormones (e.g. ghrelin) and anorexigenic gut hormones (e.g. GLP-1) converge and interact in the brain with the longer-term key energy storage signal leptin which is secreted from adipose tissue in proportion to fat mass [9]. Together, these hormones control food intake and energy expenditure. Modulation of the activation of central hunger, satiety and food reward pathways are key to the former, and GLP-1 has been implicated in all these phenomena, particularly in relation to the induction of satiety [10]. GLP-1 reduces food intake through direct and indirect actions at multiple sites including via activation of pro-opiomelanocortin (POMC) neurons of the arcuate nucleus of the hypothalamus with subsequent inhibition of orexigenic agouti-related peptide (AGRP)/neuropeptide Y (NPY) neurone activity [11]. GLP-1 is also implicated in enhancement of satiety via the nucleus tractus solitarius of the hind-brain which may in part also be linked to altered activation of the mesolimbic centres resulting in dampened food reward [12]. Further supportive evidence for a role for GLP-1 in controlling food intake comes from controlled physiological studies in man. A blunted post-prandial GLP-1 response is observed in morbidly obese patients, blockade of GLP-1 release in recipients of bariatric surgery acutely decreases satiety and increased food intake and satiety and food intake are increased and decreased respectively following administration of the stable GLP-1 analogue liraglutide [4, 13, 14]. The early post-RYGB increase in GLP-1 release in response to mixed macronutrient and high fat liquid meals has been shown to occur independently of the ensuing post-operative hypocaloric state with total release optimally augmented by mixed macronutrient stimulus [15]
While the effects of exaggerated gut hormone responses are now being elucidated, a critical question remains as to whether it is the rate and/or the state of the nutrient that is being transferred to the small intestine post-RYGB and VSG that exerts the more powerful stimulatory influence on their release. Rate has been shown to be of importance [16] but the effect of state remains underexplored. The question of state comes to the fore given that reduced functional and reservoir capacity of the stomach following RYGB and VSG necessarily imposes a limit on the degree to which macronutrient can be pre-processed. In the case of protein macronutrient, reduced gastric pepsin-mediated pre-processing with attendant reductions in the generation of absorbable short peptides in the proximal small intestine could lead to delivery of undigested nutrient to the downstream small intestine, the physiochemical make-up of which may provide different degrees of stimulation to GLP-1 secreting enteroendocrine L-cells.
This question of rate versus state is not only of interest to the purist investigator of mechanisms of bariatric surgery. It is also of importance in the applied setting in relation to the rational development of satiety inducing functional food supplements designed to harbour bariatric mimetic properties.
We therefore set out to address the question of whether the state of nutrient delivery to the small intestine acts as an important determinant of GLP-1 responses and a potential modulator of food intake post-RYGB and VSG. We hypothesised that ahead of intake of an ad libitum mixed meal-and versus delivery of matched protein hydrolysate with proven GLP-1 secretagogue activity in vitro, direct jejunal delivery of undigested protein macronutrient (casein) would provoke an exaggerated GLP-1 response and a relative acceleration in time to onset of reductions in hunger and induction of satiety. We predicted that these phenomena would translate into a reduction in ad libitum food intake in participants on the day of intact casein administration. Ingestion of casein protein as a component of test meal studies has been shown to exert a more marked satiating effect than other protein test compounds in volunteers with intact gastric processing increasing GLP-1, reducing food intake and causing a relative delay in gastric emptying [17].
Methods
Study Design
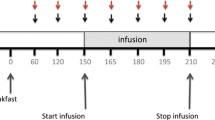
The hypothesis articulated above was interrogated in a randomised, double-blinded cross-over trial in 13 healthy volunteers (Fig. 1). Participants visited on two separate study days separated by at least 1-week wash-out period and received in double-blinded randomised order either hydrolysed or intact casein. The protein bolus was delivered to the intestinal lumen by means of a naso-jejunal tube (NJ) advanced directly into the jejunum thus allowing the bolus to ‘bypass’ pepsin-mediated hydrolysis in the stomach and proximal digestive events in the small intestine. Participants were afforded ad libitum access to a mixed meal at 30 min after delivery of the protein bolus as described in further detail below in ‘Study Protocol’ section and in Fig. 1. The primary end-point was delta change in gut hormone responses at 25 min post-infusion and secondary end-points were related to recognised knock on correlates of GLP-1 bioactivity namely feelings of hunger and fullness (visual analogue scales, VAS) and food intake (kilocalories consumed during an ad libitum meal).
Study design. The study was conducted as a randomised, double-blinded cross-over trial wherein healthy volunteers (n = 13) were administered 15 g of intact or hydrolysed casein over a 2-min period via a naso-jejunal tube advanced directly into the jejunum, thus ‘bypassing’ exposure to endogenous gastric proteolytic enzymes. Participants attended on two separate study days in order to receive both infusions and allow for paired analysis of the relative impact of both infusions on the end-points. Participants and investigators were blinded to order of infusions until study close. Following infusions, patients’ libitum mixed meal of pyttipanna ‘Swedish hash’ consisting of fried beef, potato, onion and egg (1.5 kcal/g) was given. The end-points examined were plasma glucagon-like peptide 1 levels (pg/ml) and visual analogues scale (VAS) ratings of hunger and fullness all beginning from 60 min prior to meal initiation and total quantity of food consumed (g)
Recruitment
Ethical approval for the study was granted by the Regional Ethics Review Board in Gothenburg (Dnr: 753–13) (Table 1). All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was received from all participants in the study. Thirteen healthy volunteers of both sexes between the ages of 18 and 35 were recruited. Exclusion criteria were as follows: pregnancy, sensitivity to milk proteins or local anesthetics, vegetarianism, narcotic addiction or other conditions that may have decreased study compliance without necessarily generating biological confounders.
Protein Source
In the present study, we investigated the effect of exogenously hydrolysed and intact preparations of the milk protein casein. Under normal circumstances, dietary casein is pre-processed by gastric pepsin. Through collaboration with the Irish consortium of Food for Health Ireland, it was possible to obtain a well-defined casein hydrolysate and corresponding unprocessed casein. The casein hydrolysate was selected as it had been shown in in vitro bioassay to be a potent stimulator of GLP-1 release from cultured enteroendocrine L-cells (unpublished data).
Both the casein hydrolysate and the casein control solution are produced under Irish (&EU) food grade manufacturing regulations. Briefly, to produce the hydrolysate, sodium caseinate (≈90 % protein) was hydrolysed with a food grade enzyme preparation >3 h. Enzyme inactivation was achieved by heating the sample to 85 °C for 30 s. The sample was then concentrated and spray-dried to yield a sample containing 90 % protein (including dipeptide and tripeptide fractions), 5 % moisture and ash. The control solution contained the corresponding amount of casein that had been pasteurised only.
Study Protocol
Each participant was studied on two occasions at 1 week apart (Fig. 1). Abstinence from alcohol and avoidance of strenuous exercise for the 24 h before and after each study day was required. Food intake for the 24 h prior to each study day was similar between participants as per recommendations. Compliance was confirmed before the commencement of each study. All participants consumed an identical meal (fish or pasta meal delivered from the laboratory) between 1900 and 2000 h on the night before the study. Fasting was observed from 2000 h the night before each study visit.
On study days, participants arrived at the facility at 0830 h. A venous cannula was inserted into one forearm for the collection of blood. A NJ tube was advanced to the proximal jejunum, participants relaxed for 30 min before the start of the study protocol. Correct positioning was ensured by fluoroscopy. All time cues were removed from the study room, so that the participants were unaware of the time. Throughout the study period, the participants were encouraged to relax.
The participants received a 2-min infusion through the NJ tube of either casein hydrolysate or intact casein in solutions with a total protein content of 15 g, followed by a flush with 20 ml of 37 °C tap water after which the tubing was removed and 30 min later access to ad libitum meal was initiated. The standard meal was ‘Swedish Hash’ pytt-i-panna consisting of fried beef, potato and onion with a calorific value of 1.5 kcal per gram [18]. Food was made available for consumption ad libitum with quantities sufficient to exceed the upper range of normal appetites.
Basal blood samples were taken before and at 15 min after NJ tube placement and 5 min prior to meal initiation. Blood was collected thereafter at 30-min intervals out to 120 min using 6-ml EDTA tubes containing 5000 kallikrein inhibitor units (0.12 ml) of aprotonin. Plasma was separated immediately by centrifugation and then stored at −70 °C until analysis (assessment of plasma GLP-1 levels).
Subjective Measurement of Hunger and Fullness
Hunger and fullness ratings were recorded by participants throughout the study on standard 10 cm visual analogue scale anchored from 0 to 10 with the text expressing the absence (0) and maximal presence (10) of hunger and fullness. We have previously validated the use of VAS scales in cross-over feeding studies in bariatric patient populations (4).
Food and Water Intake
The amounts of food and water intake were recorded in grams and millilitres.
Measurement of Plasma GLP-1
Plasma total GLP-1 levels were measured by enzyme-linked immunosorbent assay (Multi-Species GLP-1 Total ELISA, Merck Millipore, Germany) validated to detect in the picomolar range with intra-assay and inter-assay variability of <5 and <12 %, respectively.
Statistical Analyses
Data are expressed as mean ± SEM. Longitudinal time and treatment effects and interactions were assessed by two-way ANOVA. Paired t tests were used to examine intra-individual study differences in 25-min plasma GLP-1 levels, 15-min post-meal initiation ratings of hunger and fullness and total food and water intake. Pearson correlation and Spearman rank correlation was used to assess absolute levels of food and water intake and rank order by participants across study days. Statistical significance was set at p = 0.05.
Results
Participants
Seven female and six male participants completed the cross-over study (Table 1). The mean age of participants was 28.2 ± 1.5 years, mean height was 1.72 ± 0.03 m and mean weight was 70.4 ± 3.7 kg. Mean body mass index was 23.4 ± 0.57 kg/m2.
Equivalent Stimulus Potency Between Intact Casein and Casein Hydrolysate in Relation to GLP-1 Release
Circulating GLP-1 levels were measured and compared from 60 min prior to until 120 min post-initiation of the ad libitum meal on both study days (Fig. 2a). At baseline, 60 min prior to meal initiation, GLP-1 levels were 26.7 ± 2.7 and 25.7 ± 2.2 pg/ml on the intact casein and casein hydrolysate treatment days, respectively. Insertion of the NJ tube did not alter the GLP-1 concentrations (p = 0.81).
Time-dependent changes in plasma GLP-1. Healthy volunteers (n = 13) were administered in a double-blinded cross-over fashion 15 g of intact or hydrolysed casein over a 2-min period via a naso-jejunal tube advanced directly into the proximal jejunum then offered an ad libitum mixed meal of pyttipanna ‘Swedish hash’ consisting of fried beef, potato, onion and egg (1.5 kcal/g). a Plasma glucagon-like peptide 1 levels (pg/ml, ±SEM) were monitored at the time of intubation (−60), 15 min later (−45), 5 min prior to initiation of the ad libitum meal (25 min post-infusion) and then at 15, 30, 60, 90 and 120 min after meal initiation. p < 0.01 for time-dependent alterations in GLP-1 levels. Treatment and time-treatment interaction were not significant (p = 064 and p = 0.89, respectively). b Plasma GLP-1 (pg/ml, ±SEM) at 25 min post-infusion on both study days (p = 0.29). NGT naso-gastric tube, INF infusion and ALM ad libitum meal
A significant effect of time on GLP-1 levels was noted (p < 0.01) but no treatment effect (p = 0.82) or time-treatment interaction was observed (p = 0.61). Twenty-five minutes after delivery of intact and hydrolysed casein samples and 5 min prior to meal initiation, circulating GLP-1 levels rose to 39.9 ± 4.4 and 36.4 ± 3.8 pg/ml, respectively (p = 0.29 treatment comparison, Fig. 2b), representing respective increases over baseline of 51 and 37 % following only a 60 kcal stimulus. GLP-1 did not show any further increases despite ad libitum meal (504 ± 40 kcal and 459 ± 49 kcal) consumption on intact casein nor the casein hydrolysate administration days, respectively. The highest GLP-1 concentrations recorded occurred at 15 min post-meal initiation at 42.2 ± 4.7 and 40 ± 3.3 pg/ml GLP-1 on the intact casein and casein hydrolysate administration days, respectively.
Intact Casein and Casein Hydrolysate Reduce Hunger and Increase Fullness to an Equivalent Degree
VAS scored levels of hunger and fullness were also measured and compared from 60 min before until 120 min after initiation of the ad libitum meal on both study days (Fig. 3). At baseline on the intact casein administration day, at 60 min prior to meal initiation ratings of hunger and fullness (from a maximum of 10) were 5.5 ± 20.7 and 2.6 ± 0.5, respectively, as compared to 5.0 ± 20.6 and 2.0 ± 0.5, respectively, at baseline on the day of hydrolysate administration. There was no appreciable alteration in ratings prior to initiation of food intake but peak alterations from baseline in both parameters were observed 15 min after meal initiation. Hunger ratings decreased by 88 % to 0.7 ± 0.2 and fullness rating increasing by 300 % to 7.8 ± 0.4 on the day of intact casein administration. Fifteen minutes after meal initiation on the day of hydrolysate administration, hunger ratings decreased by 81 % to 1.0 ± 0.5 and fullness rating increasing by 354 % to 7.2 ± 0.5. Direct paired comparison of hunger and fullness ratings at 15 min demonstrated that responses were not significantly different between treatment days (p = 0.40 and p = 0.12, respectively). There were, however, statistically significant time-dependent changes in both parameters from baseline across the duration of both study visits (p < 0.01) but no evidence of a significant treatment effect (p = 0.43) or time-treatment interaction (p = 0.97).
Time-dependent changes in measures of hunger and fullness. Healthy volunteers (n = 13) were administered in a double-blinded cross-over fashion 15 g of intact or hydrolysed casein over a 2-min period via a naso-jejunal tube advanced directly into the proximal jejunum, then offered an ad libitum mixed meal of pyttipanna ‘Swedish hash’ consisting of fried beef, potato, onion and egg (1.5 kcal/g). VAS assessed hunger (a) and fullness (b) were monitored at the time of intubation (−60), 15 min later (−45), 5 min prior to initiation of the ad libitum meal (25 min post-infusion) and then at 15, 30, 60, 90 and 120 min after meal initiation. p < 0.01 for time-dependent alterations in both hunger and fullness levels. Treatment and time-treatment interaction were not significant for hunger (p = 0.82 and p = 0.65) or fullness (p = 0.97) ratings. NJT naso-jejunal tube, INF infusion and ALM ad libitum meal
Intact Casein and Casein Hydrolysate Result in Ad Libitum Food Intake to an Equivalent Degree
Food and water intake were compared across study days (Fig. 4). On the day of intact casein administration, 336 ± 26.8 g (504 ± 40 kcal) of food was consumed versus 306 ± 32.9 g (459 ± 49 kcal) consumed following the casein hydrolysate administration (p = 0.21). A positive intra-individual correlation between study day food intake in absolute (Pearson’s r 2 = 0.51, p = 0.05) and ranking terms (Spearman’s r = 0.67, p = 0.01) was observed. Water intake on the day of intact casein administration was 335 ± 34 ml versus 331 ± 37 ml following casein hydrolysate administration (p = 0.40). A significant positive intra-individual correlation between study day water intake in absolute terms (Pearson’s r 2 = 0.52, p = 0.05) and a trend in ranking terms (Spearman’s r = 0.67, p = 0.07) was observed.
Ad libitum food and water intake following duodenal infusion of intact and hydrolysed casein. Healthy volunteers (n = 13) were administered in a double-blinded cross-over fashion 15 g of intact or hydrolysed casein over a 2-min period via a naso-jejunal tube advanced directly into the proximal jejunum then offered an ad libitum mixed meal of pyttipanna ‘Swedish hash’ consisting of fried beef, potato, onion and egg (1.5 kcal/g). Food (g) and water intake (ml) was recorded at point of voluntary meal termination at both visits. There was no statistical difference between study days in food intake (p = 0.21, panel a) or for water intake (p = 0.40, panel c). Rank order of participants for both food and water intake was retained across study days and moderate direct correlations existed for individual participants between absolute quantities of food (R 2 0.51, p = 0.05, panel b) and water intake (R 2 0.52, p = 0.05, panel d) consumed across study days
Discussion
The present study set out to address in a controlled feeding study whether the state of digestion of protein macronutrient influenced the extent and duration of GLP-1 responses in the small intestine in association with a coherent impact on subjective ratings of hunger and fullness and absolute food and water intake. We hypothesised that direct jejunal delivery of undigested protein would provoke an exaggerated GLP-1 response and a relative acceleration in the time to onset of and intensity of reductions in hunger, induction of satiety and consequent decrements in quantity of ad libitum food intake. The research question and hypothesis related to a potential mechanism via which RYGB, and to a degree VSG, might enhance satiety not only due to early transit of nutrient (rate) but also as a consequence of reduced gastric pre-processing of nutrient and subsequent proximal small intestinal digestion (state). Both phenomena being linked to the induction of an exaggerated gut hormone response (in which GLP-1 plays a fundamental role) physiologically designed to act as an ‘alarm’ to detect supposed overload or dysfunction and thus reduce gastric emptying and signal meal termination as an adaptive response.
The range of dietary-derived factors that can influence L-cell secretory response is diverse but studies directly examining state of nutrient are lacking [19]. Casein administration has been shown before to cause enhancement of GLP-1 release [17]. Our results show that jejunal exposure to intact or hydrolysed casein is sufficient to provoke an increase in circulating GLP-1 prior to the onset of feeding equivalent both in terms of kinetic and magnitude at peak. This indicates that protein macronutrient may stimulate L-cells secretory activity without the need for gastric pepsin hydrolysis but not to an enhanced degree relative to stimulation with pre-processed protein. Coherent with the similar kinetic and magnitude of GLP-1 release in response to intact and hydrolysed nutrient, ratings of hunger and fullness did not show divergence between study days. This indicates that state does not by some GLP-1-independent manner influence satiety.
Interestingly, prior to the onset of feeding but 25 min after jejunal stimulation, GLP-1 levels were similar to those obtained at the peak post-prandial time-point (15 min) yet show no impact on subjective ratings of hunger and fullness at this time-point. Therefore, GLP-1 release, or at least the stimulus as provided, does not appear to exert an impact on the verbal report of the desire for food in 12 h fasted subjects. This could be due to a dominant pre-prandial effect of orexigenic hormones such as ghrelin. Whether pre-prandial GLP-1 elevation via direct nutrient stimulation of the jejunum precipitates a decrease in subsequent ad libitum food intake relative to saline vehicle by decreasing the latency to titration in anorexigenic signals was not specifically addressed in this study, but this may be credibly proposed to be the case. It does raise the interesting question, however, as to whether any luminal exposure to protein solution, even if it was of endogenous rather than dietary macronutrient origin (e.g. cephalic phase exocrine pancreatic secretions), may be able to provoke a pre-meal GLP-1 response without impacting on conscious feelings of satiety until food intake is initiated, whereupon it combines with other stimuli to control quantity of food ingested. Dysfunction of such an axis would be interesting to explore in obesity or exploit as a means of optimising satiety. Nonetheless, the lack of an additional GLP-1 response after the substantial ad libitum meal consumed above the GLP-1 response achieved with only 60 kcal of hydrolysed casein protein would suggest that the NJ tube delivered macronutrients had a very substantial effect on the endocrine L-cell.
Our findings do not exclude a role for nutrient state to influence onset of satiety in the context of mixed meal. Considering the role of other macronutrients and state, for example release of free-fatty acids from triglycerides by the action of gastric lipase, serves to induce the release of a potent satiety-inducing enteroendocrine hormone from I cells in the form of cholecystokinin (CCK) [20]. In the absence of this effect due to impaired gastric pre-processing in RYGB and VSG, one could propose that satiety induction would be impaired. However, the same phenomenon also impairs the intestinal phase of exocrine pancreatic secretion which could result in alterations in food intake secondary to state-dependent early meal termination occurring as a consequence of conditioned avoidance secondary to negative visceral consequences of impaired fat digestion and absorption. Such a mechanism may be active in the mechanism of action in obesity of the pancreatic lipase inhibitor orlistat [21] and to a degree in bariatric surgery [22].
The conclusions of the present study are necessarily limited in their scope to the context of a single macronutrient (gastric amylase/lipase also likely important in a mixed meal setting) and examined only the cardinal satiety hormone GLP-1 without also examining other satiety hormones such as peptide-YY and CCK. However, if there had been a differential impact on other satiety hormones, we would have expected to see a change in VAS score or food intake in the presence equivalent changes in GLP-1 but this was not observed. This strongly indicates that there was not a differential effect of the infusions on other gut-derived satiety signals.
The inclusion of a saline control group was not a prerequisite for us to address the question in hand but the failure to do so does impact to a degree how confidently we can make inferences on the effect of pre-meal direct stimulation of GLP-1 release and food intake. This said, the controlled study of one variable is also the strength of the current study, and our results demonstrate that in isolation, changes in protein state do not differentially activate L-cell responses in situ in the human gut. Identification of the interplay between rate and state and mixed pre-prandial exposure to macronutrient exposure could form the basis for more complex follow-up studies.
References
Vidal P, Ramon JM, Goday A, et al. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy as a definitive surgical procedure for morbid obesity. Mid-term results. Obes Surg. 2013;23(3):292–9.
Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8, CD003641.
Trastulli S, Desiderio J, Guarino S, et al. Laparoscopic sleeve gastrectomy compared with other bariatric surgical procedures: a systematic review of randomized trials. Surg Obes Relat Dis. 2013;9(5):816–29.
le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5.
Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013. PubMed Epub 2013/07/10. Eng.
Papamargaritis D, le Roux CW, Sioka E, et al. Changes in gut hormone profile and glucose homeostasis after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2013;9(2):192–201.
Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306(4):E424–32.
Dirksen C, Jorgensen NB, Bojsen-Moller KN, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55(7):1890–901.
Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15(6):367–78.
Sobrino Crespo C, Perianes Cachero A, Puebla Jimenez L, et al. Peptides and food intake. Front Endocrinol. 2014;5:58.
Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138(10):4445–55.
Richard JE, Anderberg RH, Goteson A, et al. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One. 2015;10(3), e0119034.
Faerch K, Torekov SS, Vistisen D, et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes. 2015;64(7):2513–25.
van Can J, Sloth B, Jensen CB, et al. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2014;38(6):784–93.
Evans S, Pamuklar Z, Rosko J, et al. Gastric bypass surgery restores meal stimulation of the anorexigenic gut hormones glucagon-like peptide-1 and peptide YY independently of caloric restriction. Surg Endosc. 2012;26(4):1086–94.
Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity. 2014;22(9):2003–9.
Marsset-Baglieri A, Fromentin G, Airinei G, et al. Milk protein fractions moderately extend the duration of satiety compared with carbohydrates independently of their digestive kinetics in overweight subjects. Br J Nutr. 2014;112(4):557–64.
Laurenius A, Larsson I, Bueter M, et al. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes. 2012;36(3):348–55.
Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587(Pt 1):27–32.
Beglinger C, Degen L. Fat in the intestine as a regulator of appetite—role of CCK. Physiol Behav. 2004;83(4):617–21.
Ackroff K, Sclafani A. Effects of the lipase inhibitor orlistat on intake and preference for dietary fat in rats. Am J Physiol. 1996;271(1 Pt 2):R48–54.
le Roux CW, Bueter M, Theis N, et al. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1057–66.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interest.
Funding
This study was funded by Enterprise Ireland (TC20130001), Science Foundation Ireland (12/YI/B2480) and an ALF grant to LF at Sahlegrenska University Hospital, Gothenburg.
Human Rights Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
le Roux, C.W., Engström, M., Björnfot, N. et al. Equivalent Increases in Circulating GLP-1 Following Jejunal Delivery of Intact and Hydrolysed Casein: Relevance to Satiety Induction Following Bariatric Surgery. OBES SURG 26, 1851–1858 (2016). https://doi.org/10.1007/s11695-015-2005-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-2005-z