Abstract
Background
The aim was to determine the effects of fat and protein preloads on pouch emptying (PE), caecal arrival time (CAT), glucose absorption, blood glucose (BSL), gut hormones, haemodynamics and gastrointestinal (GI) symptoms in subjects who had undergone Roux-en-Y gastric bypass (RYGB) >12 months previously.
Methods
Ten RYGB subjects were studied on three occasions, in randomised order, receiving 200-ml preloads of either water, fat (30 ml olive oil) or whey protein (55 g), 30 min before a mixed meal. PE, CAT, BSL, plasma 3-O-methyl-D-glucopyranose (3-OMG), insulin, glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide 1 (GLP-1) and glucagon, blood pressure (BP), heart rate (HR) and GI symptoms were assessed over 270 min.
Results
Although fat and protein preloads did not alter PE of either solids or liquids, the CAT of solids, but not liquids, was longer than that after the water preload (fat 68 ± 5 min and protein 71 ± 6 min vs. water 46 ± 5 min; P = 0.02). BSL elevated promptly after the meal on all days (P < 0.001), but after protein, the magnitude and integrated increases in the first 75 min were less than fat and water preloads (area under the curve (AUC(0–75 min)), 18.7 ± 18.2 vs. 107.2 ± 30.4 and 76.1 ± 19.3 mmol/L/min; P < 0.05). Compared to water preload, the protein and fat preloads were associated with greater increases in plasma insulin, GLP-1 and glucagon concentrations, a reduction in BP, and greater increases in HR, fullness, bloating and nausea. Plasma 3-OMG levels were lower after the protein than after the water and fat preloads (P < 0.001).
Conclusions
Given its effects to attenuate post-prandial glycaemia, reduce intestinal glucose absorption and potentiate the “incretin response”, without inducing more adverse post-prandial GI symptom, protein preload may prove clinically useful in RYGB patients and warrant further evaluation, particularly in those with type 2 diabetes (T2DM) and/or dumping syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the anatomical changes of Roux-en-Y gastric bypass (RYGB) [1], ingested nutrients can empty rapidly from the gastric pouch to reach the distal small intestine in greater quantities than normal leading to post-prandial hyperglycaemia and increased release of gut hormones, including cholecystokinin (CCK), peptide YY (PYY) and the so-called “incretin hormones” glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The presence of a large nutrient load in the intestine can also increase splanchnic blood flow and, in turn, lead to a reduction in systemic blood pressure (i.e. post-prandial hypotension) and adverse symptoms [2, 3]. This phenomenon is most prominent in the elderly and those with autonomic neuropathy [3, 4] and can be attenuated by slowing gastric emptying and the rate of nutrient delivery in to the small intestine.
In healthy lean and obese subjects who have intact stomach, both oral and intra-duodenal administration of nutrients have been shown to slow gastric emptying, suppress hunger and decrease subsequent energy intake [5], as well as modulate glycaemia [6–8]. These effects are triggered, at least in part, by the release of peptides including GLP-1, GIP, CCK and PYY [9, 10], which are known to stimulate pyloric contractions, inhibit both antral and proximal gastric motility and suppress appetite [6, 7, 11–13]. One of the consequences of such negative feedback by the presence of nutrients in the small intestine is the attenuation of post-prandial hyperglycaemia [10, 12]. Based on this concept, in patients with type 2 diabetes (T2DM), we have shown that consumption of a small amount of protein or fat 30 min before a meal is associated with slowing of gastric emptying, increased release of GIP and GLP-1 and a substantial reduction in the magnitude of post-prandial glycaemic excursion [8, 10]. Similarly, administration of lipid or protein into the duodenum in health slows both gastric emptying and small intestinal transit of a subsequent meal as well as the glycaemic response [10, 14]. Given that post-prandial hyperglycaemia and hypotension are relatively common after RYGB, manoeuvres that slow the rate of nutrients transit to the small intestine may attenuate post-prandial hyperglycaemia, hypotension and, possibly, gastrointestinal (GI) symptoms related to meal ingestion. The aim of the current study was to determine the effects of fat and protein preloads on pouch emptying (PE), post-prandial glycaemia, glucose absorption, gut hormones, blood pressure and gastrointestinal symptoms in RYGB patients without dumping syndrome.
Methods
Subjects
Ten subjects (5M/5F; age, 52.2 ± 2.5 years; body mass index (BMI), 31.1 ± 1.3 kg/m2) who had undergone RYGB over 12 months (mean duration = 2.6 ± 1.4 years ago, with a mean reduction in body mass index (BMI) of 14.2 ± 4.3 kg/m2) for morbid obesity were recruited. The RYGB was performed by a single bariatric surgeon who adopted the same surgical approach in all subjects [15]. Subjects were excluded if there was a history of significant respiratory, renal, or hepatic disease, diabetes mellitus, chronic alcohol abuse, smoking, the use of medication known to influence gastrointestinal (GI) motility or blood glucose, or a history of significant GI diseases, including lactose intolerance. The study protocol was approved by the local research ethics committee and each subject provided written informed consent.
Protocol
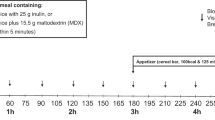
Each subject was studied in the sitting position on three occasions after an overnight fast, separated by at least 1 week. On each study day, a different “preload” was consumed in randomised order, 30 min before a mixed meal (total 1424 kJ) that consisted of a 50-g beef patty (584 kJ) labelled with 15 MBq 99mTc-sulfur colloid and a 150 ml glucose drink (50 g of glucose, 840 kJ), containing 3 MBq 67Ga-EDTA and 3 g 3-O-methyl-D-glucopyranose (3-OMG, Sigma-Aldrich Co, St Louis, USA) to assess carbohydrate absorption. The preloads were 200 ml in volume and consisted of (i) water (200 ml, 0 kJ), (ii) 30 ml olive oil + 170 ml water (fat preload; 1080 kJ) and (iii) 55 g whey protein (whey isolate) mixed with 50 ml skim milk (1.6 g protein, 2.4 g sugar, 67 kJ) and 50 ml water (protein preload; total 1080 kJ) (Fig. 1). The preloads were consumed within 5 min and the meal was ingested within 7 min. The tolerability of the mixed meal in RYGB subjects had been confirmed prior to the commencement of the study.
After insertion of an intravenous cannula, venous blood samples were taken before (t = −30 min) and after the ingestion of the preload drinks at regular intervals for measurements of blood glucose, 3-OMG, plasma insulin, GIP, GLP-1 and glucagon [16–18]. Sitting in front of the gamma camera, the mixed meal was ingested (beef patty first followed immediately by the glucose drink) and completed at t = 0. Mean blood pressure (MAP) and heart rate (HR) were also quantified with an automated oscillometric BP monitor (DINAMAP ProCare 100) at ~3-min intervals for the first 2 h and every 10 min thereafter [19]. Post-prandial hypotension was defined as a fall in systolic BP >20 mmHg that was sustained for >30 min [19, 20]. Perceptions of appetite, GI symptoms and symptoms of dumping were assessed using validated visual analogue scales (VASs) [16, 18] at regular intervals. The presence of dumping syndrome was assessed using Sigstad’s clinical diagnostic index, and if the score >7, the dumping syndrome was deemed to be clinically significant [21].
Measurements
Pouch Emptying and Caecal Arrival Time
The transit of labelled meal through the gastric pouch and the small intestine was quantified using scintigraphy [22, 23]. Data were corrected for subject movement, radionuclide decay and γ-ray attenuation. A region of interest that corresponded to the gastric pouch was identified.
Blood Glucose and Plasma 3-OMG, Insulin, GIP, GLP-1 and PYY
Blood glucose concentrations were determined immediately using a portable blood glucose meter (Medisense Companion 2 Meter, Medisense, Inc., Waltham, MA) [22, 23]. Plasma 3-OMG concentrations were measured using high-performance exchange chromatography [22]. As previously described [22, 23], total plasma GIP, GLP-1 and PYY were measured by radio-immunological assay (RIA), and plasma insulin was measured by ELISA.
Data Analysis
Data are presented as mean ± SD. Differences in changes of blood glucose, gut hormones, 3-OMG absorption, haemodynamics and GI symptoms after various preloads and the meal were evaluated by two-way repeated measures analysis of variance (ANOVA) with treatment and time as factors. In addition, differences in pouch emptying and changes in outcome measures (including blood glucose, insulin, glucagon, GIP and GLP-1) before and after the preloads (but prior to meal ingestion) were compared using Student’s paired t test. Analyses were performed using GraphPad Prism statistical software, version 6 (GraphPad Software Inc., La Jolla, CA, USA). Significance was accepted at a P value <0.05.
Results
The study was tolerated well without any untoward events. The mean glycated haemoglobin (HbA1c) in the cohort at the time of the study was 6.2 ± 0.2 %. No patient had dumping syndrome and mean Sigstad’s score was 3.8 ± 1.5.
Pouch Emptying and Caecal Arrival Time
The composition of the preload had no effect on pouch emptying of solids or liquids, which was extremely rapid in all groups (t50 of water vs. fat vs. protein for solid, 3.8 ± 0.9 vs. 4.1 ± 0.6 vs. 3.6 ± 0.5 min, and for liquid, 1.6 ± 0.7 vs. 1.1 ± 0.6 vs. 1.3 ± 0.5 min). Although caecal arrival time (CAT) of liquids was short and comparable amongst the preloads (water vs. fat vs. protein, 23 ± 4 vs. 26 ± 6 vs. 27 ± 5 min; P > 0.05), after the protein and fat preloads, CAT of solids was more prolonged than after the water preload (water vs. fat vs. protein, 46 ± 5 vs. 68 ± 5 vs. 71 ± 6 min; P < 0.05).
Blood Glucose, Glucose Absorption, Plasma Insulin, GIP, GLP-1 and Glucagon
There were no differences in baseline blood glucose, plasma insulin, GIP, GLP-1 or glucagon concentrations (Fig. 2a–e). When compared to fat and water preloads, the protein preload was associated with a small, but significant, increase in blood glucose (0.9 ± 0.3 mmol/L), and a marked increase in plasma insulin (P = 0.04). There was a prompt rise in blood glucose (P < 0.001) and plasma insulin (P < 0.001) after the meal on all days, but the magnitude and integrated increases in blood glucose in the first 75 min after the meal following the protein preload (area under the curve (AUC(0–75 min)), 18.7 ± 18.2 mmol/L/min) were less than those after fat (107.2 ± 30.4 mmol/L/min, P = 0.03) and water (76.1 ± 19.3 mmol/L/min; P = 0.048) preloads. In contrast to the comparable overall blood glucose between the groups, both peak (P < 0.001) and the AUC (P < 0.01) for plasma insulin were greater after the protein, than the water and fat, preloads, with no differences between water and fat.
Effects of 200 ml fat (30 ml olive oil; 1080 kJ) or whey protein (55 g; 1080 kJ) preloads ingested at t = −30 min, and a mixed meal ingested at t = 0 min on a blood glucose, b plasma insulin, c plasma GIP, d plasma GLP-1, e plasma glucagon and f glucose absorption, as assessed by plasma 3-OMG and AUC0–240 min after RYGB (n = 10). Data are mean ± SEM
Both protein and fat preloads increased plasma GIP (P < 0.01) and GLP-1 (P < 0.01). After the meal, there were rapid increases in plasma GIP (P < 0.01) and GLP-1 (P < 0.01) on all study days (P < 0.001). Whilst the magnitude of the post-prandial increase in plasma GIP was comparable between the nutrient preloads, the increase in plasma GLP-1 was greater after the fat, than the protein and water, preloads (P < 0.01). After the meal, plasma GLP-1 after the protein preload was higher than that after the water preload between 60 and 180 min (P < 0.01). Only the protein preload was associated with an increase in plasma glucagon (P < 0.01). In all groups, plasma glucagon increased after the meal, and the magnitude of the increase was least after the water preload (Fig. 2e). The post-prandial rise in plasma glucagon after the fat preload was higher than that after the water preload (P < 0.001), but less than after protein preload (P < 0.001).
Although neither the rate of increase nor peak plasma 3-OMG concentrations were different amongst the preloads, both integrated (AUC(0–240 min)) and plasma levels of 3-OMG after the protein were less than after the water and fat preloads (P < 0.001) (Fig. 2f). There was no difference in peak plasma or AUC(0–240 min) of 3-OMG between the fat and water preloads. There was a strong correlation between peak plasma 3-OMG and peak blood glucose levels after the protein (P = 0.02, r = 0.72), but not the fat or water, preloads.
Blood Pressure and Heart Rate
Although all preloads were associated with an increase in HR (P < 0.001; Fig. 3), only fat and protein preloads reduced mean arterial pressure (MAP) by ~10 mmHg (P < 0.001). The increase in HR was least after the water than that after the protein or fat preloads (P < 0.01). The changes in MAP and HR were most marked at 30 min after ingestion of protein and fat preloads. Meal ingestion did not reduce MAP but was associated with a small increase in HR in the first 30 min (P < 0.001). In all groups, both MAP and HR had returned to baseline by 45 min after meal ingestion.
Gastrointestinal Symptoms
Although there was suppression of sensations of hunger, desire to eat and the amount of food that could be eaten, as well as increases in fullness, bloating and nausea after the meal, the suppression of hunger was least after water preload with no effect on bloating and nausea and was highest after fat preload (Fig. 4).
Effects of 200 ml fat (30 ml olive oil; 1080 kJ) or whey protein (55 g; 1080 kJ) preloads ingested at t = −30 min on appetite sensations and GI symptoms after RYGB (n = 10): a hunger, b fullness, c bloating, d desire to eat, e nausea and f amount of food can be eaten, after a mixed meal ingested at t = 0 min. Data are mean ± SEM
Discussion
This is the first study to evaluate the effects of a small macronutrient preload of fat or protein given before a mixed meal after RYGB, with a particular focus on their potential to attenuate the post-prandial glycaemic excursion, stimulate incretin responses and reduce both haemodynamic disturbances and GI symptoms. The main observations were that, in unselected RYGB subjects without overt dumping syndrome, (i) nutrient preloads, especially protein, modified small intestinal transit (i.e. CAT), but not pouch emptying of solids, associated with a diminished post-prandial rise in blood glucose and plasma 3-OMG concentrations; (ii) the protein preload and, to a lesser extent, fat preload are associated with greater stimulation of plasma insulin, glucagon, GLP-1 and GIP than the water preload; and (iii) whilst both nutrient preloads were associated with a reduction in desire to eat and a comparable meal-induced hypotension (~10 mmHg) and tachycardia (~15 bpm), the protein preload did not lead to nausea and was associated with less bloating than the fat preload. Together, our findings suggest that protein, but not fat, preloads may be beneficial in RYGB subjects by modestly attenuating post-prandial hyperglycaemia, glucose absorption and reducing desire to eat, without inducing adverse GI symptoms. The effects of protein preload, therefore, warrant further evaluation in RYGB subjects, particularly those with either persistent T2DM and/or dumping syndrome.
Although the lack of difference in pouch emptying after the preloads is easily explained by the loss of small intestinal feedback on “gastric regulation” of nutrient delivery to the small intestine after RYGB [2, 6, 7], the prolonged intestinal transit of meal after nutrient preloads is intriguing and most likely related to the neuro-humoral effects of GLP-1, GIP and glucagon on small intestinal motility. The early heightened releases of these gut hormones after the nutrient preloads are probably responsible for the slower intestinal transit, as GLP-1, GIP and glucagon are known to induce small intestinal hypomotility [3, 4, 24] and slow transit [25]. Together with the heightened incretin responses, the slower intestinal transit may contribute to the observed lesser increase in post-prandial blood glucose and plasma 3-OMG after the protein preload.
It is also intriguing to note the different patterns of incretin hormones elicited by the protein and fat preloads, despite the comparable pouch emptying and caloric content of the preload. Ingestion of whey protein increased all regulatory (insulin, GIP and GLP-1) and counter-regulatory (glucagon) hormones, whereas the fat preload only increased GIP and GLP-1. When compared to water, post-prandial rises in plasma insulin, GIP, GLP-1 and glucagon were greater after the nutrient preloads, particularly after the protein preload, which may relate to direct stimulatory effects of whey protein in the small intestine [12, 14, 26, 27]. Furthermore, the discrepant increases in plasma GIP and GLP-1, as compared to the marked increases in plasma insulin and glucagon, after the protein preload may relate to the relatively low level of post-prandial hyperglycaemia (peak glucose ~10 mmol/L). The “stimulatory incretin” effects of GIP and GLP-1 on insulin release are glucose-dependent, with much greater “incretin” stimulation during hyperglycaemia [28]. In the current study, only subjects without T2DM were included, which is most likely to be responsible for the modest attenuation of post-prandial hyperglycaemia by the nutrient preloads. The results are likely to be different if RYGB patients with T2DM are included. Thus, the impact of these preloads in RYGB patients with T2DM would be more clinically relevant and warrants evaluation.
The use of protein preloads may have therapeutic potential given the association with reduced intestinal glucose absorption and increased early satiety. In contrast to fat, protein preloads appear to induce early post-prandial “fullness” without causing nausea, which predictably impacts adversely on quality of life [29]. In order to minimise the additional caloric content of the “nutrient preloads”, the use of snacks of high protein, rather than fat and carbohydrate, content between main meals may favour weight loss by inducing early satiety by releasing “appetite-related” gut hormones, such as GLP-1, and reducing glucose absorption.
Although no subject complained of overt dumping symptoms, the observed haemodynamic changes after exposure to a small nutrient preload in RYGB can provide the physiological basis for the hypotensive symptoms of dumping syndrome. In the current study, fat and protein had comparable effects, as would be predicted [30]. The mechanism(s) responsible for the hypotension or tachycardia remains unclear, but may be related to gut hormonal effects or direct stimulation to the sympathetic nerves [31–33].
There are a number of limitations of the current study, which should be recognised. While the number of subjects was relatively small, the studies were randomised, repeated over three occasions and technically demanding. More importantly, despite the small sample size, intra-subject differences between the preloads were observed and highly significant. As discussed, it would be of interest to assess RYGB patients with T2DM and dumping syndrome, in view of the potential clinical impact of nutrient preloads on glycaemia and post-prandial symptoms. We studied subjects who had RYGB surgery at least 1 year previously in view of the adaptive changes in GI function known to occur in the first 12 months [34, 35]. This is to miminise the impact of the variation induced by these adaptive changes. The attenuation of post-prandial rise of blood glucose after the protein preload may also be more pronounced if milk was avoided in the preload, as the carbohydrate content led to a small increase in blood glucose prior to the main meal. The protein content in our preload group (55 g) was high, as compared to the normal requirement per meal (~30 g), which represented a deliberate strategy as the study was “proof of principle”. Our findings and their applicability, therefore, should be viewed circumspectly. We believe that that lack of pre-RYGB data does not represent a major limitation given that the GI responses with “intact GI anatomy” (i.e. pre-RYGB) are substantially different from those after RYGB, as demonstrated by our group [23] and others [34, 35].The small intestinal transit time we observed was shorter than that reported in a study by Carswell et al. [36], which most likely reflects differences in the measurement technique and test meal. The sulphasalazine-sulphapyridine test, as used by Carswell et al. [36], has not been validated against the gold-standard scintigraphy; and compared to lactulose/hydrogen breath test, orocaecal transit assessed by sulphasalazine test has been reported to be more prolonged [37]. We have also adopted a mixed meal where the solid “beef patty” component was predictably “washed out” by the glucose drink and have shown that pouch emptying and, accordingly, small intestinal transit, are influenced by gravity [23]. In contrast, Carswell et al. [36] did not employ a test meal, and the amount of water used to swallow the 1 g of sulphasalazine tablet was not documented.
Conclusions
After RYGB, protein and, to a much lesser extent, a fat preload is associated with a more prolonged small intestinal transit, a smaller post-prandial glycaemic rise, slower intestinal glucose absorption, greater increases in plasma insulin glucagon and GIP, and diminished desire to eat, without inducing adverse post-prandial GI symptoms, as compared to fat and water preloads. Protein preloads may, therefore, have therapeutic value and warrant further evaluation in RYGB subjects particularly those with persistent T2DM and/or dumping syndrome.
References
Tice JA, Karliner L, Walsh J, et al. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121(10):885–93.
Lin HC, Doty JE, Reedy TJ, et al. Inhibition of gastric emptying by glucose depends on length of intestine exposed to nutrient. Am J Physiol. 1989;256(2 Pt 1):G404–11.
Sun Y, Song G, Yin J, et al. Effects and mechanisms of electroacupuncture on glucagon-induced small intestinal hypomotility in dogs. Neurogastroenterol Motil: Off J Eur Gastrointest Motil Soc. 2010;22(11):1217–23. e318.
Trahair LG, Horowitz M, Hausken T, et al. Effects of exogenous glucagon like peptide 1 on the blood pressure, heart rate, mesenteric blood flow and glycemiac responses to intraduodenal glucose in healthy older subjects. J Clin Endocrinol Metab. 2014;99(12):E2628–34.
Chapman IM, Goble EA, Wittert GA, et al. Effects of small-intestinal fat and carbohydrate infusions on appetite and food intake in obese and nonobese men. Am J Clin Nutr. 1999;69(1):6–12.
Lin HC, Doty JE, Reedy TJ, et al. Inhibition of gastric emptying by sodium oleate depends on length of intestine exposed to nutrient. Am J Physiol. 1990;259(6 Pt 1):G1031–6.
Lin HC, Elashoff JD, Gu YG, et al. Nutrient feedback inhibition of gastric emptying plays a larger role than osmotically dependent duodenal resistance. Am J Physiol. 1993;265(4 Pt 1):G672–6.
Ma J, Stevens JE, Cukier K, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32(9):1600–2.
Bowen J, Noakes M, Trenerry C, et al. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J Clin Endocrinol Metab. 2006;91(4):1477–83.
Gentilcore D, Chaikomin R, Jones KL, et al. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab. 2006;91(6):2062–7.
Asmar M. New physiological effects of the incretin hormones GLP-1 and GIP. Dan Med Bull. 2011;58(2):B4248.
Chapman MJ, Fraser RJL, Matthews G, et al. Glucose absorption and gastric emptying in critical illness. Crit Care. 2009;13:R140.
Read NW, Cammack J, Edwards C, et al. Is the transit time of a meal through the small intestine related to the rate at which it leaves the stomach? Gut. 1982;23(10):824–8.
Brennan IM, Luscombe-Marsh ND, Seimon RV, et al. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am J Physiol Gastrointest Liver Physiol. 2012;303(1):G129–40.
Nguyen NQ, Game P, Bessell J, et al. Outcomes of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. World J Gastroenterol: WJG. 2013;19(36):6035–43.
Pilichiewicz AN, Little TJ, Brennan IM, et al. Effects of load, and duration, of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul, Integr Comp Physiol. 2006;290(3):R668–77.
Little TJ, Doran S, Meyer JH, et al. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am J Physiol Endocrinol Metab. 2006;291(3):E647–55.
Pilichiewicz AN, Papadopoulos P, Brennan IM, et al. Load-dependent effects of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul, Integr Comp Physiol. 2007;293(6):R2170–8.
Gentilcore D, Doran S, Meyer JH, et al. Effects of intraduodenal glucose concentration on blood pressure and heart rate in healthy older subjects. Dig Dis Sci. 2006;51(4):652–6.
Nguyen NQ, Mangoni AA, Fraser RJ, et al. Prokinetic therapy with erythromycin has no significant impact on blood pressure and heart rate in critically ill patients. Br J Clin Pharmacol. 2007;63(4):498–500.
Mallory GN, Macgregor AM, Rand CS. The Influence of Dumping on Weight Loss After Gastric Restrictive Surgery for Morbid Obesity. Obes Surg. 1996;6(6):474–8.
Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption, and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22(9):2003–9.
Nguyen NQ, Debreceni TL, Burgstad CM, Wishart JM, Bellon M, Rayner CK, et al. Effects of Posture and Meal Volume on Gastric Emptying, Intestinal Transit, Oral Glucose Tolerance, Blood Pressure and Gastrointestinal Symptoms After Roux-en-Y Gastric Bypass. Obes Surg. 2014. doi:10.1007/s11695-014-1531-4.
Edholm T, Cejvan K, Abdel-Halim SM, et al. The incretin hormones GIP and GLP-1 in diabetic rats: effects on insulin secretion and small bowel motility. Neurogastroenterol Motil: Off J Eur Gastrointest Motil Soc. 2009;21(3):313–21.
Ogawa E, Hosokawa M, Harada N, et al. The effect of gastric inhibitory polypeptide on intestinal glucose absorption and intestinal motility in mice. Biochem Biophys Res Commun. 2011;404(1):115–20.
Gunnerud UJ, Heinzle C, Holst JJ, Ostman EM, Bjorck IM. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS One. 2012;7(9):e44731.
Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. The American journal of clinical nutrition. 2010;91(4):966–75.
Vollmer K, Gardiwal H, Menge BA, et al. Hyperglycemia acutely lowers the postprandial excursions of glucagon-like Peptide-1 and gastric inhibitory polypeptide in humans. J Clin Endocrinol Metab. 2009;94(4):1379–85.
Laurenius A, Olbers T, Naslund I, et al. Dumping syndrome following gastric bypass: validation of the dumping symptom rating scale. Obes Surg. 2013;23(6):740–55.
Gentilcore D, Hausken T, Meyer JH, et al. Effects of intraduodenal glucose, fat, and protein on blood pressure, heart rate, and splanchnic blood flow in healthy older subjects. Am J Clin Nutr. 2008;87(1):156–61.
Mehagnoul-Schipper DJ, Lenders JW, Willemsen JJ, et al. Sympathoadrenal activation and the dumping syndrome after gastric surgery. Clin Auton Res. 2000;10(5):301–8.
Saito A. Acute adaptational disturbances due to the imbalance of the autonomic nervous system. Tohoku J Exp Med. 1971;103(1):71–92.
Yamamoto H, Mori T, Tsuchihashi H, et al. A possible role of GLP-1 in the pathophysiology of early dumping syndrome. Dig Dis Sci. 2005;50(12):2263–7.
Umeda LM, Silva EA, Carneiro G, et al. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1 and glucagon secretion in type 2 diabetic patients. Obes Surg. 2011;21(7):896–901.
Jorgensen NB, Jacobsen SH, Dirsken C, et al. Acute and long-term effects of RYGB on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303(1):E122–31.
Carswell KA, Vincent RP, Belgaumakar AP, et al. The effect of bariatric surgery on intestinal absorption and transit time. Obes Surg. 2014;24(5):796–805.
Staniforth DH. Comparison of orocaecal transit times assessed by the lactulose/hydrogen and the sulphasalazine/sulphapyridine methods. Gut. 1989;30(7):978–82.
Acknowledgments
Research Support from the National Health and Medical Research Council (NHMRC). Recipient of a New Investigator Project Grant 2010-2013
Conflict of interest
Authors Nam Q Nguyen, Tamara L Debreceni, Carly M Burgstad, Melissa Neo, Max Bellon , Judith M Wishart, Scott Standfield, Dylan Bartholomeusz, Chris K Rayner, Gary Wittert, Michael Horowitz declare that they have no conflict of interest.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, N.Q., Debreceni, T.L., Burgstad, C.M. et al. Effects of Fat and Protein Preloads on Pouch Emptying, Intestinal Transit, Glycaemia, Gut Hormones, Glucose Absorption, Blood Pressure and Gastrointestinal Symptoms After Roux-en-Y Gastric Bypass. OBES SURG 26, 77–84 (2016). https://doi.org/10.1007/s11695-015-1722-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1722-7