Abstract
Background:
Genome-wide association studies in adults have identified genetic loci associated with adiposity measures. Little is known about the effects of these loci on growth and body fat distribution from early childhood onwards.
Methods:
In a population-based prospective cohort study among 4144 children, we examined the associations of weighted risk scores combining 29 known genetic markers of adult body mass index (BMI) and 14 known genetic markers of adult waist-hip ratio (WHR) with peak weight velocity, peak height velocity, age at adiposity peak and BMI at adiposity peak in early infancy and additionally with BMI, total fat mass, android/gynoid fat ratio and preperitoneal fat area at the median age of 6.0 years (95% range 5.7, 7.8).
Results:
A higher adult BMI genetic risk score was associated with a higher age at adiposity peak in infancy and a higher BMI, total fat mass, android/gynoid fat ratio and preperitoneal fat area in childhood (P=0.05, 1.5 × 10−24, 3.6 × 10−18, 4.0 × 10−11 and 1.3 × 10−5, respectively), with the strongest association for childhood BMI with a 0.04 higher s.d. score BMI (95% confidence interval 0.03, 0.05) per additional risk allele. A higher adult WHR genetic risk score was not associated with infant growth measures or childhood BMI and total fat mass, but was associated with childhood android/gynoid fat ratio and preperitoneal fat area (P<0.05).
Conclusion:
Genetic variants associated with BMI and WHR in adults influence growth patterns and general and abdominal fat development from early childhood onwards.
Similar content being viewed by others
Introduction
Childhood overweight and obesity is an important public health problem associated with adverse short- and long-term effects on blood pressure, lipid profiles, glucose metabolism and psychological well being.1, 2, 3, 4 Although environmental, lifestyle-related and behavioral factors contribute to childhood obesity, genetic susceptibility and gene–environment interactions also influence the risk of obesity. Heritability estimates range from 40 to 70%.5, 6, 7, 8 A recent genome-wide association study (GWAS) identified 32 genetic loci associated with adult body mass index (BMI),9 whereas another large GWAS identified 14 loci associated with adult waist-hip ratio (WHR) adjusted for BMI.10 To date, no GWAS on childhood BMI have been published, but two GWAS identified four genetic loci associated with severe childhood obesity.1, 11 These genetic loci had not been identified in adults before, suggesting that different genetic mechanisms influence growth and adiposity development in different stages of life.12 Rather than BMI, more detailed measures of body fat distribution may also reflect general and abdominal adiposity from childhood onwards. In infancy, the best anthropometric predictors for obesity in childhood and adulthood are infant growth patterns.13, 14 Studying detailed measures of early growth patterns and body fat distribution may therefore lead to further insight into the genetic causes of child adiposity.
Thus far, little is known about the effects of the 46 single-nucleotide polymorphisms (SNPs) previously identified for either adult BMI or adult WHR and the four SNPs previously identified for severe childhood obesity on detailed measures of early infant growth and adiposity in children. Therefore, we examined the association of these SNPs with infant growth patterns and childhood general and abdominal adiposity measures, both by studying SNPs individually and by combining them into genetic risk scores.
Materials and methods
Design and study population
This study was embedded in the Generation R Study, a population-based prospective cohort study of 9749 children and their parents from fetal life onwards in Rotterdam, The Netherlands.15 The study has been approved by Medical Ethical Committee of Erasmus MC, University Medical Center Rotterdam. All children were born between April 2002 and January 2006 and form a largely prenatally enrolled birth cohort that is currently being followed until young adulthood. Written consent was obtained from one of the parents for all participating children. Even with consent of the parents, when the child was not willing to participate actively, no outcome measurements were performed. During infancy, height and weight were repeatedly measured to obtain measures of infant growth. At the age of 6 years, all eligible children were invited to visit a dedicated research center for follow-up measurements. A GWA screen was available in 5733 children. The present analyses were limited to singleton live births for whom information on at least one of the outcomes under study was available (n=4151). A participant flowchart is given in the Supplementary Material, Supplementary Figure S1.
Genetic variants
DNA was isolated from cord blood samples. If DNA samples from cord blood were missing (in 6.3% of the participants), DNA was isolated from blood samples at follow-up measurements. GWA analysis was performed using the Illumina 610 Quad and 660 platforms.16 A stringent process of quality control was applied. Individuals with low sample call rates or sex mismatches were excluded. MACH software was used to impute genotypes to the cosmopolitan panel of HapMap II (release 22).17, 18 The quality of imputation ranged from 0.77 to 1.00 with an average of 0.97, indicating good imputation. Before imputation, SNPs were excluded in case of high levels of missing data (SNP call rate <98%), highly significant departures from Hardy–Weinberg equilibrium (P<1 × 10−6), or low minor allele frequencies (<1%).16 Of the 50 SNPs (32 adult BMI, 14 adult WHR, 4 severe childhood obesity), information on 46 was available in the GWAS data set. Information on rs887912, rs2890652, rs4836133 and rs4929949, all previously associated with adult BMI, was not available, but rs763712 was used as a perfect proxy for rs887912 (r2=1 and D’=1). For the other three SNPs, no perfect proxy was available in the GWAS data set, so these were excluded, giving a total of 47 SNPs in the analysis.
Infant growth
Measures of infant growth were derived from the weight and length/height data, obtained by well-trained staff. These measures took place at the ages of 1, 2, 3, 4, 6, 11, 14, 18, 24, 36 and 48 months, based on the national health care program in the Netherlands. Peak weight velocity and peak height velocity in infancy were derived using the Reed1 model for boys and girls separately, as described in detail previously.13, 14, 19, 20 The Reed1 model is a four-parameter model that is fitted by gender on all weight and height measurements in children aged 0–3 years, including birth weight and length. We assumed both a fixed and a random component for all four parameters. For each child, the first derivative of the fitted distance curve was taken to obtain the weight or height velocity curve. As having two measurements was inadequate to capture the shape of the growth curve, all analyses were restricted to children with a minimum of three measurements.
To obtain BMI and age at adiposity peak, a cubic mixed effects model was fitted on log(BMI) from 2 weeks to 1.5 years of age, adjusted for gender.14, 21 As children may lose up to 10% of body weight in their first 14 days of life, BMI growth was modeled from the age of 2 weeks. When fitting the model, age was centralized to 0.75 years. In addition to fixed effects, we included random effects for the constant and the slope of the model. We assumed autoregressive AR1 within-person correlation structure between the measurements. Then, BMI at adiposity peak and age at adiposity peak were derived for each child at the maximum point of the curve, which is the infant adiposity peak.
General and abdominal adiposity at school-age
Adiposity outcomes were measured in a dedicated research center by trained research staff, according to specific research protocols, as previously described.22 We calculated BMI (kg m−2) from height and weight, both measured without shoes and heavy clothing. Overweight and obesity were defined using age- and gender adjusted BMI criteria.23
Total fat mass and android/gynoid fat ratio were measured using Dual-energy X-ray absorptiometry (DXA) scanner (iDXA, GE-Lunar, 2008, Madison, WI, USA), and analyzed with the enCORE software v.12.6.21 DXA is able to accurately detect whole-body fat mass within less than 0.25% coefficient of variation. Children were placed on the DXA table in supine position without shoes, heavy clothing and metal objects with their hands flat and pronated. We calculated total fat mass (kg) as a percentage of total body weight (kg) measured by DXA. The android/gynoid fat ratio was calculated using android fat mass and gynoid fat mass measured by DXA. The android/gynoid fat ratio reflects the ratio of the central body fat distribution in the abdomen (android fat) and hip (gynoid fat) regions.24
Preperitoneal fat area, a measure of visceral abdominal fat, was measured by abdominal ultrasound examinations performed with the Philips/ATL HDI 5000, as described in detail previously.25 In brief, preperitoneal fat area thickness was measured perpendicular to the skin surface on the median upper abdomen with a linear (L12-5 MHz) transducer.26 We scanned longitudinally just below the xiphoid process to the navel along the linea alba. Preperitoneal fat area distance was measured as distance of the linea alba to the peritoneum on top of the liver. Preperitoneal fat area was measured as area of 2 cm length along the linea alba starting from the maximum preperitoneal distance in direction of the navel (PP-area). We measured this area three times and used the mean value of these measures. The intraobserver reproducibility and the intraclass correlation coefficients ranged from 0.93 to 0.97 from which we can conclude that our measurements for ultrasound were highly reproducible.22
Statistical analysis
First, we performed multiple linear regression analyses to examine the associations of the 47 SNPs (29 adult BMI, 14 adult WHR, 4 severe childhood obesity), with peak weight velocity, peak height velocity, BMI at adiposity peak and age at adiposity peak in infancy, and BMI, total fat mass, android/gynoid fat ratio and preperitoneal fat area in childhood, assuming additive genetic effects. As total fat mass, android/gynoid fat ratio and preperitoneal fat area were not normally disturbed, they were natural logarithm transformed for further analyses. To enable comparison of effect sizes of different outcome measures, we calculated s.d. scores (SDS) ((observed value-mean)/s.d.) for all measures by using the data of the study population. We did not construct age-adjusted SDS values, because of the small age range of the outcome measures. Only for BMI, we obtained age-adjusted SDS using Dutch reference growth curves (Growth Analyzer 3.0, Dutch Growth Research Foundation, Rotterdam, The Netherlands).
Second, we combined the 29 adult BMI SNPs and the 14 adult WHR SNPs into two separate genetic risk scores that summed the number of BMI- and WHR-increasing alleles, respectively, weighted by their previously reported effect sizes in adults. The risk scores were rescaled to a score ranging from zero to the maximum number of effect alleles and rounded to the nearest integer. Linear regression analyses were performed to examine the association of these risk scores with peak weight velocity, peak height velocity, BMI at adiposity peak and age at adiposity peak in infancy and BMI, total fat mass, android/gynoid fat ratio and preperitoneal fat area in childhood.
All analyses were performed in the full group and also in children with a European ethnicity only, as this was the largest ethnic subgroup. A child was classified as European if he/she was within four s.d. from the HapMap CEU panel mean value for all first four principal components, based on the genetic data.
All models were adjusted for sex, except for the sex-stratified models, and for the first four principal components (specific for the full group or for Europeans only). Models for all measures of general and abdominal adiposity were additionally adjusted for age. Models for total fat mass, android/gynoid fat ratio and preperitoneal fat area were additionally adjusted for height. We also tested for sex interaction. As a statistically significant sex interaction was found for some of the individual SNPs for measures of general and abdominal adiposity, but not for the risk scores, all models of individual SNPs for measures of generaral and abdominal adiposity were additionally run for boys and girls separately in the full group. To adjust for multiple testing in the analysis of the individual SNPs, Bonferroni correction was used (P<1.1 × 10−3 was considered statistically significant). All analyses were performed using the Statistical Package for the Social Sciences version 21.0 for Windows (SPSS IBM, Chicago, IL, USA).
Results
Characteristics of the study population
Table 1 shows the characteristics for all children and for the European children separately. In the full group, the prevalences of overweight and obesity were 12.9 and 4.1%, respectively. We observed lower prevalences in the European children.
Infant growth patterns
Of the 47 available SNPs, only rs2815752, previously associated with adult BMI, was associated with infant BMI at adiposity peak (P=1.1 × 10−4), with the direction of effect in line with the direction in adult GWAS. Results for all SNPs can be found in the Supplementary Material (Supplementary Tables S1 and S2 for all and European children only, respectively).
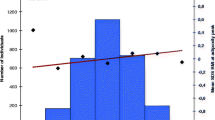
Combined in a weighted risk score, ranging from 13 to 38 with a mean of 26.0 (s.d. 3.6), the 29 available adult BMI SNPs were only borderline significantly associated with age at adiposity peak (P=0.05; Table 2). For each additional average risk allele, age at adiposity peak increased by 0.01 SDS (95% confidence interval (CI) 0.00, 0.02). The difference in mean age at adiposity peak between the two extreme risk groups (⩽16 and ⩾35 risk alleles) was 0.4 SDS (Figure 1d). There were no associations with any other measure of infant growth (Table 2; Figure 1).
(a–d) Effect of adult BMI genetic risk score on infant growth (N=3114)*. (a) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS peak weight velocity on the y axis on the right and a line representing the regression of the mean SDS peak weight velocity values for each category of the risk score. Along the y axis on the left a histogram is shown, representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2. (b) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS peak height velocity on the y axis on the right and a line representing the regression of the mean SDS peak height velocity values for each category of the risk score. Along the y axis on the left a histogram is shown, representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2. (c) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS BMI at adiposity peak on the y axis on the right and a line representing the regression of the mean SDS BMI at adiposity peak values for each category of the risk score. Along the y axis on the left a histogram is shown, representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2. (d) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS age at adiposity peak on the y axis on the right and a line representing the regression of the mean SDS age at adiposity peak values for each category of the risk score. Along the y axis on the left a histogram is shown, representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2. *Adjusted for gender and the first four principal components.
Combined in a weighted risk score, ranging from 6 to 23 with a mean of 14.0 (s.d. 2.4), the 14 adult WHR SNPs were not associated with any measure of infant growth (Table 2; Figure 2). Results in the European children were similar as those in the full group (Supplementary Material, Supplementary Table S3).
(a–d) Effect of adult WHR genetic risk score on infant growth (N=3114)*. (a) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS peak weight velocity on the y axis on the right and a line representing the regression of the mean SDS peak weight velocity values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2. (b) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS peak height velocity on the y axis on the right and a line representing the regression of the mean SDS peak height velocity values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2. (c) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS BMI at adiposity peak on the y axis on the right and a line representing the regression of the mean SDS BMI at adiposity peak values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2. (d) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS age at adiposity peak on the y axis on the right and a line representing the regression of the mean SDS age at adiposity peak values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2. *Adjusted for gender and the first four principal components.
General and abdominal adiposity at school-age
Of the 47 available SNPs, the adult BMI SNPs rs2867125, rs1558902, rs7138803 and rs713586 were associated with childhood BMI (all P<6.1 × 10−4). Rs2867125, rs1558902 and rs713586 were also associated with total fat mass (all P<7.4 × 10−5). The adult WHR SNP rs6861681 was associated with childhood android/gynoid fat ratio (P=4.5 × 10−5). The directions of effect were in line with those observed in previous GWAS. The 29 adult BMI SNPs together explained 2.4, 1.4, 1.2 and 0.5% of the variance in child BMI, total body fat, android/gynoid fat ratio and preperitoneal fat area, respectively. Similar results were observed among European children only. Results for all SNPs can be found in the Supplementary Material (Supplementary Tables S4 and S5 for all and European children only, respectively). There were no large sex differences in measures of general and abdominal adiposity (Supplementary Material, Supplementary Tables S6 and S7).
Combined in a weighted risk score, the 29 adult BMI SNPs were associated with childhood BMI (P=1.5 × 10−24; Table 2). For each additional average risk allele, BMI increased by 0.04 SDS (95% CI 0.03, 0.05). The difference in mean BMI between the two extreme risk groups (⩽16 and ⩾35 risk alleles) was 1.7 SDS BMI (Figure 3a). The BMI genetic risk score was also associated with total fat mass (P=3.6 × 10−18), android/gynoid fat ratio (P=4.0 × 10−11) and preperitoneal fat area (P=1.3 × 10−3; Table 2; Figures 3b and d). Similar results were observed among European children only (Supplementary Material, Supplementary Table S3). The risk score based on the 14 adult WHR SNPs was associated with child android/gynoid fat ratio (P=3.8 × 10−3). For each additional average risk allele, ln(android/gynoid fat ratio) increased by 0.02 SDS (95% CI 0.01, 0.03; Table 2). As shown in Figure 4c, the difference in mean ln(android/gynoid fat ratio) between the two extreme risk groups (⩽8 and ⩾21 risk alleles) was 0.4 SDS. The adult WHR genetic risk score was not associated with BMI and total fat mass but reached significance for preperitoneal fat area (P=0.01; Table 2; Figures 4a, b and d). Similar results were observed among European children only (Supplementary Material, Supplementary Table S3).
(a–d) Effect of adult BMI genetic risk score on childhood adiposity (N=4144)*. (a) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS BMI in kg m−2 on the y axis on the right and a line representing the regression line of the mean SDS BMI values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2. (b) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS-ln(total fat fat mass) on the y axis on the right and a line representing the regression of the mean SDS-ln(total fat mass) values for each category of the risk score. Along the y axis on the left a histogram is shown, representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2b**. (c) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS-ln(android/gynoid fat ratio) on the y axis on the right and a line representing the regression of the mean SDS-ln(android/gynoid fat ratio) values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2**. (d) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS-ln(preperitoneal fat area) on the y axis on the right and a line representing the regression of the mean SDS-ln(preperitoneal fat area) values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2**. *Adjusted for age, gender and the first four principal components. **Additionally adjusted for height.
(a–d) Effect of adult WHR genetic risk score on childhood adiposity (N=4144)*. (a) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS BMI in kg m−2 on the y axis on the right and a line representing the regression of the mean SDS BMI values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2. (b) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS-ln(total fat mass) on the y axis on the right and a line representing the regression of the mean SDS-ln(total fat mass) values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2**. (c) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS-ln(android/gynoid fat ratio) on the y axis on the right and a line representing the regression of the mean SDS-ln(android/gynoid fat ratio) values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2**. (d) Along the x axis, categories of the risk score (overall sum of risk alleles, weighted by previous reported effect sizes, rescaled and rounded to the nearest integer) are shown together with the mean SDS-ln(preperitoneal fat area) on the y axis on the right and a line representing the regression of the mean SDS-ln(preperitoneal fat area) values for each category of the risk score. Along the y axis on the left, a histogram is shown representing the number of individuals in each risk-score category. P-value is based on the continuous risk score, as presented in Table 2**. *Adjusted for age, gender and the first four principal components. **Additionally adjusted for height.
Discussion
In our study, a higher adult BMI genetic risk score was associated with a higher age at adiposity peak in infancy, and a higher BMI, total fat mass, android/gynoid fat ratio and preperitoneal fat area in childhood. A higher adult WHR genetic risk score was not associated with infant growth measures, but was associated with increased android/gynoid fat ratio and preperitoneal fat area in childhood. Results were similar for boys and girls and for European children separately.
Interpretation of main findings
Infant growth measures are known to be strongly associated with increased risk of overweight and obesity in childhood and adulthood.13, 14 Previous studies have shown that adult BMI SNPs were associated with measures of growth in early life, both individually and combined in a genetic risk score.27, 28 Two previous studies, one including over 9000 children and one including around 1000 participants included in childhood with almost 40 years follow-up, also studied the association of a genetic risk score based on the 32 known adult BMI SNPs with infant growth measures.27, 28 In both studies a higher genetic risk score was associated with an earlier age at adiposity rebound and a higher BMI at adiposity rebound.27, 28 In the larger study, the score was also associated with a higher BMI at adiposity peak.27 We have extended these studies by also testing genetic variants previously associated with adult WHR and extreme child obesity and by testing more detailed measures of adiposity.
In our study, a higher adult BMI genetic risk score tended to be associated with a higher age at adiposity peak in infancy, a finding that was previously reported for girls only.27 We did not observe an interaction with sex in our study for the growth outcomes. A higher adult BMI genetic risk score was not associated with any of the other infant growth measures in our study, whereas it was positively associated with BMI at adiposity peak in previous studies.27 This could be explained by the study population in which data on growth was available, which was larger in the previous study (n=9328) compared with the current study (n=3114), which may have affected the power of our study. Also we did not observe associations of the adult WHR genetic risk score with infant growth measures. To the best of our knowledge, our study is the first to examine the associations of this WHR risk score with measures of infant growth patterns. Further studies focused on the genetics of early infant growth in larger populations are required to further examine these associations.
Obese children are at a higher risk to remain obese throughout the life course and to develop cardiovascular and metabolic diseases in adulthood.2, 29, 30, 31 In line with previous studies in children and adolescents,9, 32 we observed that various adult BMI SNPs were associated with childhood fat measures. These associations were directionally consistent with results reported in previous GWAS among adults.9, 10 We did not observe associations of SNPs known to be associated with severe childhood obesity with measures of general and abdominal adiposity, suggesting that these SNPs have stronger effects on the extremes of the distribution. The adult BMI genetic risk score was significantly associated with childhood BMI in our study, which is in line with previous work.27, 28 In addition, we show for the first time that the adult BMI genetic risk score was also associated with total fat mass, android/gynoid fat ratio and preperitoneal fat area in childhood. The adult WHR genetic risk score was only associated with childhood android/gynoid fat ratio and preperitoneal fat area, suggesting that different loci may influence specific adiposity measures.
In our study, the adult BMI SNPs seem to better capture the variation in child adiposity measures than the adult WHR SNPs. The fact that the 29 SNPs previously associated with adult BMI combined accounted for 2.4% of the explained variance of child BMI, whereas in adults 32 SNPs (including these 29) only explained 1.5%, suggests that the effect of these loci may differ over the life course.
Underlying mechanisms
The risk scores are based on a combination of SNPs, all located in or near genes with different functions. Many of the genes close to significantly associated SNPs in our study, including TMEM18, FTO and NEGR1, the nearest genes to rs2867125, rs1558902 and rs2815752, respectively, previously associated with adult BMI,9 are highly expressed in the brain. The association of these SNPs with child adiposity may be the result of a neuronal effect on the energy metabolism,33, 34, 35 with multiple potential influences on body weight regulation, including on appetite and energy expenditure.36 As both the adult BMI and WHR genetic risk scores were associated with general and abdominal adiposity in childhood, it is suggested that the underlying mechanisms for adult obesity start to influence body fat development from early childhood onwards. However, there is still limited understanding of the biological function of the identified genes and gene–environment and gene–gene interactions may have a role. Therefore, further research, including functional studies, is required to establish the mechanisms of these genes related to obesity and to determine whether the genes described are indeed the causally related genes. In addition, to date, a large meta-analysis of GWAS of child BMI is lacking. Such a study could shed more light on the relative roles of known SNPs, as well as identify new adiposity loci specific to this age group.
Methodological considerations
This study was embedded in a population-based prospective cohort study including a large number of children in which measures of infant growth patterns and childhood general and abdominal adiposity were prospectively measured. A major strength of the current study is the large number of available detailed phenotypes. To the best of our knowledge, this study is the first that examined the associations of two risk scores and of 47 individual loci with such detailed measures of infant growth and child adiposity. However, owing to the relatively limited sample size, power for some of the associations was limited, especially for the individual SNPs and in the subgroup of European children. The SNPs were originally identified in GWAS among a much larger number of subjects. Therefore, our main conclusions are based on the genetic risk scores rather than the individual SNPs. For the genetic risk scores, for both BMI and WHR, we had 80% power to detect a difference of 0.04 SDS.
In population-based cohort studies such as ours, loss to follow-up is considered a more serious threat to the internal validity than nonparticipation at baseline.37 Of all children with genetic information, we had information on measures of childhood general and abdominal adiposity available in 72.3%. Children with no information available for these measures had a significantly higher BMI at adiposity peak, peak weight velocity and peak height velocity (all P<0.04) and a significantly lower age at adiposity peak (P=0.04) as compared with those with information available for these measures. Data on growth were only collected in a subgroup of the study population.15 Of all children with genetic information, we had information on measures of growth available in 54.3%. Those children with no information on measures of growth available had a significantly higher BMI, total fat mass and android/gynoid fat ratio (all P<0.002) and a significantly lower preperitoneal fat area (P=0.001) as compared to those with information on measures of growth available. This might have affected the distribution of the outcome measures in our study population, but we consider it unlikely to have strongly affected effect estimates.
We performed detailed measurements of childhood abdominal adiposity. Both DXA and abdominal ultrasound are valid methods for epidemiological studies. DXA is able to accurately measure total fat mass with high precision.22 Abdominal ultrasound has been validated against computed tomography in a previous study, showing that ultrasound measurements can be used to approximate visceral fat in children, although preperitoneal fat measurements do not perfectly capture visceral fat. This may have caused some measurement error, which was likely random and may therefore have diluted some of the observed associations.25 Finally, not all SNPs were available in the GWAS data set. Of the total of 50 SNPs, only four SNPs were not available of which one was replaced by a perfect proxy, so we have captured the vast majority of known SNPs in our study.
Conclusion
Our findings suggest that genetic variants related to BMI and WHR identified in adults influence growth and adiposity from early childhood onwards.
References
Bradfield JP, Taal HR, Timpson NJ, Scherag A, Lecoeur C, Warrington NM et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet 2012; 44: 526–531.
Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L et al. Health consequences of obesity. Arch Dis Child 2003; 88: 748–752.
Schonbeck Y, Talma H, van Dommelen P, Bakker B, Buitendijk SE, Hirasing RA et al. Increase in prevalence of overweight in Dutch children and adolescents: a comparison of nationwide growth studies in 1980, 1997 and 2009. PLoS One 2011; 6: e27608.
WHO. Obesity and overweight. Fact sheet N°311, 2013. Available at http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on September 2014).
Maes HH, Neale MC, Eaves LJ . Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 1997; 27: 325–351.
Wardle J, Carnell S, Haworth CM, Plomin R . Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr 2008; 87: 398–404.
Jansen PW, Roza SJ, Jaddoe VW, Mackenbach JD, Raat H, Hofman A et al. Children's eating behavior, feeding practices of parents and weight problems in early childhood: results from the population-based Generation R Study. Int J Behav Nutr Phys Act 2012; 9: 130.
Birch LL, Davison KK . Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr Clin North Am 2001; 48: 893–907.
Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010; 42: 937–948.
Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, Steinthorsdottir V et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 2010; 42: 949–960.
Scherag A, Dina C, Hinney A, Vatin V, Scherag S, Vogel CI et al. Two new Loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and german study groups. PLoS Genet 2010; 6: e1000916.
Graff M, Ngwa JS, Workalemahu T, Homuth G, Schipf S, Teumer A et al. Genome-wide analysis of BMI in adolescents and young adults reveals additional insight into the effects of genetic loci over the life course. Hum Mol Genet 2013; 22: 3597–3607.
Tzoulaki I, Sovio U, Pillas D, Hartikainen AL, Pouta A, Laitinen J et al. Relation of immediate postnatal growth with obesity and related metabolic risk factors in adulthood: the northern Finland birth cohort 1966 study. Am J Epidemiol 2010; 171: 989–998.
Mook-Kanamori DO, Durmus B, Sovio U, Hofman A, Raat H, Steegers EA et al. Fetal and infant growth and the risk of obesity during early childhood: the Generation R Study. Eur J Endocrinol 2011; 165: 623–630.
Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol 2012; 27: 739–756.
Jaddoe VW, van Duijn CM, van der Heijden AJ, Mackenbach JP, Moll HA, Steegers EA et al. The Generation R Study: design and cohort update 2010. Eur J Epidemiol 2010; 25: 823–841.
Li Y, Willer C, Sanna S, Abecasis G . Genotype imputation. Annu Rev Genomics Hum Genet 2009; 10: 387–406.
Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR . MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol 2010; 34: 816–834.
Sovio U, Bennett AJ, Millwood IY, Molitor J, O'Reilly PF, Timpson NJ et al. Genetic determinants of height growth assessed longitudinally from infancy to adulthood in the northern Finland birth cohort 1966. PLoS Genet 2009; 5: e1000409.
Berkey CS, Reed RB . A model for describing normal and abnormal growth in early childhood. Hum Biol 1987; 59: 973–987.
Sovio U, Mook-Kanamori DO, Warrington NM, Lawrence R, Briollais L, Palmer CN et al. Association between common variation at the FTO locus and changes in body mass index from infancy to late childhood: the complex nature of genetic association through growth and development. PLoS Genet 2011; 7: e1001307.
Gishti O, Gaillard R, Manniesing R, Abrahamse-Berkeveld M, van der Beek EM, Heppe DH et al. Fetal and infant growth patterns associated with total and abdominal fat distribution in school-age children. J Clin Endocrinol Metab 2014; 99: 2557–2566.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH . Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240–1243.
Helba M, Binkovitz LA . Pediatric body composition analysis with dual-energy X-ray absorptiometry. Pediatr Radiol 2009; 39: 647–656.
Mook-Kanamori DO, Holzhauer S, Hollestein LM, Durmus B, Manniesing R, Koek M et al. Abdominal fat in children measured by ultrasound and computed tomography. Ultrasound Med Biol 2009; 35: 1938–1946.
Suzuki R, Watanabe S, Hirai Y, Akiyama K, Nishide T, Matsushima Y et al. Abdominal wall fat index, estimated by ultrasonography, for assessment of the ratio of visceral fat to subcutaneous fat in the abdomen. Am J Med 1993; 95: 309–314.
Warrington NM, Howe LD, Wu YY, Timpson NJ, Tilling K, Pennell CE et al. Association of a body mass index genetic risk score with growth throughout childhood and adolescence. PLoS One 2013; 8: e79547.
Belsky DW, Moffitt TE, Houts R, Bennett GG, Biddle AK, Blumenthal JA et al. Polygenic risk, rapid childhood growth, and the development of obesity: evidence from a 4-decade longitudinal study. Arch Pediatr Adolesc Med 2012; 166: 515–521.
Daniels SR . The consequences of childhood overweight and obesity. Future Child 2006; 16: 47–67.
Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C . Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005; 331: 929.
Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011; 365: 1876–1885.
den Hoed M, Ekelund U, Brage S, Grontved A, Zhao JH, Sharp SJ et al. Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes 2010; 59: 2980–2988.
Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 2009; 41: 25–34.
Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007; 318: 1469–1472.
Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 2009; 41: 18–24.
Berthoud HR, Morrison C . The brain, appetite, and obesity. Annu Rev Psychol 2008; 59: 55–92.
Nohr EA, Frydenberg M, Henriksen TB, Olsen J . Does low participation in cohort studies induce bias? Epidemiology 2006; 17: 413–418.
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives and pharmacies in Rotterdam The generation and management of GWAS genotype data for the Generation R Study were done at the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, The Netherlands. The generation and management of GWAS genotype data for the Generation R Study were done at the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, The Netherlands. We thank Karol Estrada, Dr Tobias A Knoch, Anis Abuseiris, Luc V de Zeeuw and Rob de Graaf for their help in creating GRIMP, BigGRID, MediGRID and Services@MediGRID/D-Grid, (funded by the German Bundesministerium fuer Forschung und Technology; grants 01 AK 803A-H, 01 IG 07015 G) for access to their grid computing resources. We thank Mila Jhamai, Manoushka Ganesh, Pascal Arp, Marijn Verkerk, Lizbeth Herrera and Marjolein Peters for their help in creating, managing and QC of the GWAS database. Also, we thank Karol Estrada for their support in creation and analysis of imputed data. The general design of Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (ZonMw), the Netherlands Organisation for Scientific Research (NWO), the Ministry of Health, Welfare and Sport and the Ministry of Youth and Families. This research also received funding from the European Union’s Seventh Framework Programme (FP7/2007–2013), project EarlyNutrition under grant agreement no. 289346. VWJ received an additional grant from the Netherlands Organization for Health Research and Development (VIDI 016.136.361).
Author Contributions
SV, CM, JFF and VWVJ designed and conducted the research and wrote the paper. SV analyzed the data. CM, RG, CMR, AH, VWVJ and JFF provided comments and consultation regarding the analyses, interpretation of the results and manuscript. SV, JFF and VWVJ had primary responsibility for final content. All authors gave final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Vogelezang, S., Monnereau, C., Gaillard, R. et al. Adult adiposity susceptibility loci, early growth and general and abdominal fatness in childhood: the Generation R Study. Int J Obes 39, 1001–1009 (2015). https://doi.org/10.1038/ijo.2015.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.12
- Springer Nature Limited
This article is cited by
-
Shared genetic underpinnings of childhood obesity and adult cardiometabolic diseases
Human Genomics (2019)
-
Genetic contribution to waist-to-hip ratio in Mexican children and adolescents based on 12 loci validated in European adults
International Journal of Obesity (2019)
-
Perirenal fat is related to carotid intima-media thickness in children
International Journal of Obesity (2018)
-
Associations of adult genetic risk scores for adiposity with childhood abdominal, liver and pericardial fat assessed by magnetic resonance imaging
International Journal of Obesity (2018)
-
Associations of genetic risk scores based on adult adiposity pathways with childhood growth and adiposity measures
BMC Genetics (2016)