Abstract
For the first time, air-assisted cloud point extraction (AACPE) was presented to preconcentrate metal ions. The procedure was conjugated with inductively coupled plasma-optical emission spectroscopy for determination of samarium. In this procedure, samarium ions were complexed with aluminon and extracted into Triton X-114 in the presence of potassium iodide. The mixture was repeatedly sucked and dispersed with a syringe (three times) to create cloud solution. Experimental factors that affect the extraction competence of the AACPE procedure, such as pH, amount of aluminon and Triton X-114, salt addition, number of suction/injection cycles, and centrifugation rate and time, have been investigated and optimized. A linear calibration curve from 0.2 to 200.0 μg L−1 with enrichment factor and detection limit of 102 and 0.06 μg L−1, respectively, was established under the optimum experimental conditions. The approach was used to determine samarium in wastewater and rock samples, with recoveries ranging from 98% to 99%.
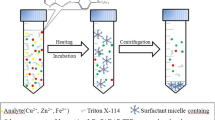
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Samarium (Sm) exists naturally in many minerals such as monazite, bastnasite, and samarskite along with other rare earth elements. Samarium iodide (SmI2) and samarium oxide (Sm2O3) are used as catalysts. Samarium oxide is also used for the production of optical and infrared adsorbing glass. Samarium cobalt alloy (SmCo5) is used in making powerful magnets [1].
The most accurate techniques for determining Sm are neutron activation analysis (NAA) [2], inductively coupled plasma-mass spectrometry (ICP-MS) [3], and inductively coupled plasma-optical emission spectrometry (ICP-OES) [4]. Generally, the direct measurement is limited due to background interference from sample matrix. To overcome this problem, several extraction procedures are used including solid phase extraction (SPE) [5], liquid–liquid extraction [6] and precipitation [7].
In the past few decades, interest in environmentally friendly separation procedures has increased. Among them microextraction procedures have performed well to improve separation, achieve high efficiency, and reduce environmental hazard effects caused by the use of toxic organic solvents [8, 9]. Examples of eco-friendly extraction procedures are cloud point extraction (CPE) [10,11,12,13], dispersive liquid–liquid microextraction (DLLME) [14] and solidification of floated organic drop [15].
The characteristics of CPE include simplicity, high efficiency, safety, and versatility [16]. It is based on the analyte being extracted from the aqueous sample and introduced into a phase with a high surfactant content [17]. Because the use of organic extractants has been replaced by surfactants in CPE, it becomes eco-friendly technique [18]. The procedure was used for extracting of many f-block elements [19,20,21,22]. The procedure was applied to extract Sm3+ using various complexing agents including Alizarin Red S [19], diglycolamide [21], 8-hydroxyquinoline [23], and 1-(2-thenoyl)-3,3,3-trifluoracet [24].
Recently, a new approach known as air-assisted CPE (AACPE) has been developed for preconcentration of heterocyclic aromatic amines before analysis by high performance liquid chromatography [25]. The procedure uses air agitation, as a dispersion tool, in conjunction with conventional CPE to improve extraction efficiency and expedite the rapid extraction process. The strategy combines the benefits of both DLLME and the CPE [26].
Aluminon, ammonium salt of aurintricarboxylic acid, is a triphenyl methane derivative that have important applications. It is used as a colorimetric reagent for aluminum, acid–base indicator [27]. Medically, the compound and its derivatives can be used as antiviral [28] and inhibitor for apoptosis [29]. Despite the fact that it forms stable complexes with a wide range of metal ions, it has received little attention as a complexing agent in separation processes [30].
In this study, the AACPE was applied, for the first time, to preconcentrate metal ions prior to determination by ICP-OES. Samarium was selectively extracted into Triton X-114 at pH 2.5 after complexation with aluminon. The dispersion process is enhanced by air agitation using a syringe. Various experimental parameters that influenced extraction efficiency were studied. The approach was employed for quantification of Sm in wastewater and rock samples.
Experimental
Apparatus
A Genway 7300 spectrophotometer (Cole-Parmer Ltd., Staffordshire, UK) was used to record UV–visible spectra. ICP-OES analysis was performed with an Agilent 5100 ICP-OES (Agilent Technologies, Melbourne, Australia). Table 1 shows the operating conditions of ICP-OES for Sm3+ determination. A digital pH meter was used to take the readings (Hanna Instruments Inc, RI, USA). To speed up the phase separation, a commercial centrifuge (Hinotek Technology Co., Ningbo, China) was used.
Chemicals
Ultrapure chemicals were used in the study and purchased from Merck (Darmstadt, Germany) or Sigma-Aldrich (St. Louis, MO, USA). The stock standard solution of Sm3+ (1000 mg L−1) was prepared by dissolving 0.2956 g of Sm(NO3)3·6H2O (99.9%) in 5.0 mL of HNO3 (1.0 mol L−1) and the volume was made up to 100 mL with double distilled water. The stock solution of aluminon (10–2 mol L−1) was prepared by dissolving 0.4734 g in 100 mL of double distilled water. The pH was controlled using the following solutions: HCl/KCl (pH 1.0–2.0), acetate buffer (pH 3.0–6.0), and hexamine buffer (pH 7.0–8.0). Hard Rock Mine Waste standard reference material (SRM 2780a) from the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA) was processed to evaluate the accuracy.
General procedure for AACPE
To aqueous solution of standard or sample, 2.0 mL buffer solution (pH 2.5), 0.5 mL of 10–2 mol L−1 aluminon, 1.0 mL of 5.0% (v/v) Triton X-114 and 1.0 mL of 1 mol L−1 KCl were added in the same sequence and the volume was adjusted to 50 mL by double distilled water. The contents were then rapidly sucked into a 50-mL syringe and injected into the tube (3 times) using a needle. To increase the viscosity of the surfactant-rich phase, the tubes were first placed in ice bath for 10 min before being centrifuged at 3000 rpm for 5 min. The upper aqueous phase was decanted, and the residual surfactant-rich phase was removed by micro-syringe and its volume was made up to 0.5 mL by 1.0 mol L−1 of HNO3 prior to aspiration into ICP-OES. The extraction recovery (R), which may be computed using the following formula, was used to measure the extraction efficiency.
where Cs is the concentration of Sm3+ in the surfactant-rich phase of volume Vs, Ci is its initial concentration and Vi is the initial volume.
Environmental samples
Rock samples were taken from Abu-Tartour phosphate mine (New Valley, Egypt) and collected in polyethylene bags. Rock samples were crushed and powdered to less than 120 mesh using agate mortar. In Teflon vessels, accurately weighed samples or SRM (0.2–0.3 g) were mixed with a mixture of HF, HNO3 and double distilled water (2.0 mL each). The microwave digestion was proceeded based on our previously optimized program: 145 °C (5 min), 165 °C (5 min) and 170 °C (20 min) [31]. Following cooling, 20 mL of boric acid (5% w/v) was added to neutralize the excess HF and the volume was completed to 50 mL with double distilled water. Industrial wastewater samples, from Sinmar Chemicals Factory (Port-Said, Egypt), were collected in acid-washed polyethylene vessels. The water samples were filtered through 0.45 μm cellulose nitrate membrane (Millipore, Bedford, MA, USA), acidified to pH 2 with HNO3.
Results and discussion
Stoichiometry of the complex
The UV–Vis spectrum of samarium-aluminon complex shows maximum absorbance at 557 nm (Fig. 1a). The stoichiometry of the complex was evaluated using continuous variation and mole-ratio methods. As indicated in Fig. 1b, maximum absorbance was achieved at the ratio between CAluminon and CAluminon + CSm equal 0.67 suggesting a 1:2 metal ligand complex. This finding was confirmed by an inflection at CAluminon/CSm of 2.0 in molar ratio plot (Fig. 1c).
Optimization of AACPE procedure
Effect of pH
The effect of sample pH on AACPE of Sm3+ was investigated within the range of 1.0–8.0. The results in Fig. 2a showed that Sm3+ was separated at quantitative value (˃95%) in the pH range 2.5–5.0. The extraction was slightly decreased at higher pH because of the possible hydrolysis of the metal ions. As a result, the optimum pH was selected as 2.5 during the experiments.
The effect of aluminon concentration
The influence of aluminon concentration on Sm3+ extraction by the suggested procedure was studied from 0.01 to 0.5 mmol L−1. The results shown in Fig. 2b illustrated that the maximum extraction was attained when the concentration of aluminon was 0.1 mmol L−1 or more. Therefore, 0.1 mmol L−1 aluminon was defined as the optimum concentration for the AACPE of Sm3+ during this work.
The effect of Triton X-114 concentration
Triton X-114 is a nonionic surfactant that is widely employed in CPE due to its benefits, which include commercial availability in pure form, low cost and toxicity, high density, which makes phase separation by centrifugation easier, and a relatively low cloud point temperature [32]. Figure 2c displayed the impact of Triton X-114 concentration on extraction efficiency of the presented procedure. As shown, increasing the amount of TritonX-114 to from 0.01 to 0.1% (v/v) increased the extraction of Sm3+ and the further increase did not improve the extraction. As a result, for subsequent experiments, 0.1% (v/v) of Triton X-114 was used.
Salting-out effect
Strong electrolytes facilitate dehydration of Triton X-114’s poly(oxyethylene) chains, which aids phase separation by raising the density of the aqueous phase and reducing the surfactant's cloud point temperature [33, 34]. The effect of salt concentration on AACPE of Sm3+ was evaluated using KCl as a salting-out agent. The obtained data denoted that the extraction was enhanced at room temperature by addition of KCl and accomplished a plateau at concentration of 0.1 mol L−1.
Effect of number of suction/injection cycles
In the present study, the mixture of analyte, complexing agent, Triton X-114 and KCl was rapidly withdrawn into a 50 mL syringe and then injected into the tube. It is observed that increasing the number of suction/injection cycles enhances the turbidity of the solution resulting from the dispersion of micelles in the aqueous phase. As a result, the effect of number of suction/injection cycles on the extraction of Sm3+ was investigated in the range of 1–5 cycles to achieve the optimum status. The results in Fig. 3 showed that increasing the number of suction/injection cycles increased the extraction efficiency until reached a plateau at the third cycle. Therefore, 3 suction/injection cycles were chosen for further experiments. This step was completed in less than 15 s, indicating that the extraction procedure is very rapid.
Centrifugation conditions
To attain quantitative extraction in a short period, the influence of centrifugation time was studied in the range from 3 to 10 min at 2000 to 3000 rpm. Table 2 indicated that perfect extraction was obtained by centrifugation at 3000 rpm for 5 or 10 min. Therefore, centrifugation for 5 min at 3000 rpm was adopted as optimum centrifugation conditions in the study.
Selectivity
The impact of common concomitant ions on the extraction of Sm3+ (100 µg L−1) was investigated. The tolerable limits of interfering ions are summarized in Table 3. The tolerated level of each ion is the concentration that resulted in a recovery alteration of ± 5%. It has been shown that, under optimal conditions, the existence of other cations and anions at a certain ratio has no impact on AACPE of Sm3+. Therefore, the procedure is suitable for preconcentration of Sm3+ and its determination in real samples under the chosen conditions.
Analytical figures of merits
Linearity, accuracy, limit of detection (LOD), limit of quantification (LOQ), and enrichment factor were used to evaluate the analytical performance of the optimized procedure. The calibration curve exhibited linearity across the concentration range of 0.2–200.0 µg L−1 for a sample volume of 50.0 mL. For 10 replicates measurements of 1.0 and 5.0 µg L−1 of Sm3+, the relative standard deviations (RSD) were 1.8 and 2.4%, respectively. The LOD and LOQ were 0.06 and 0.20 µg L−1, defined as the concentration of Sm3+ equivalent to three times and ten times the standard deviation of the blank divided by slope of the calibration graph, respectively. The enrichment factor was 102.0 when estimated as the ratio of the slopes of the calibration graphs with and without CPE. Table 4 compares the analytical characteristics of the presented study to those of other preconcentration procedures coupled with ICP-OES analysis. When compared to most existing methods, the suggested AACPE procedure performs better in terms of LOD and linearity. An additional advantage of the current procedure is rapidness. The heating step that is required for traditional CPE is useless in AACPE. It neither need stirring nor toxic solvents that are usually required for SPE.
Analytical application
The proposed procedure for determination of Sm3+ was applied to a certified reference material (SRM 2780a) of Hard Rock Mine Waste to test its accuracy. The t value was calculated based on the following relation:
where X and Xo are the average measured and certified values for Sm3+, respectively, s is the standard deviation and n is the number of measurements. The results show the agreement between the measured value (4.6 ± 0.2 µg g−1) and certified one (4.7 µg g−1). Moreover, the t value (1.0) is smaller than the critical t value at 95 percent confidence (3.182) for degree of freedom of 3, indicating the accuracy of the procedure for the determination of Sm3+. The proposed AACPE procedure was used for trace analysis of Sm3+ by ICP-OES in wastewater and rock samples. Table 5 presented the analytical findings as well as the spiking sample analysis. The recovery (R) from spiked sample was determined using the following formula:
As can be shown, the proposed approach quantitatively recovered the added Sm3+ from wastewater and rock samples (R = 98.0–99.0%). Furthermore, the RSD was less than 3.0%, showing high precision. These findings support the method's suitability for determining Sm3+ in wastewater and rock samples.
Conclusion
A simple and innovative extraction technique, AACPE, was presented and combined, for the first time, with ICP-OES for preconcentration of Sm3+. The procedure is quick, precise, efficient, and sensitive. When compared to other preconcentration procedures such as SPE and traditional CPE, the extraction time is minimal. The procedure exhibits good analytical features for Sm3+ including low LOD, wide dynamic analytical range and acceptable preconcentration factor. Finally, the proposed approach was employed to determine Sm3+ in wastewater and rock samples at trace levels. We expect that the procedure will be used to extract other metal ions in different samples.
Data availability
All data generated during this study are included in the article.
References
J. Emsley, Nature’s building blocks: an AZ guide to the elements (Oxford University Press, Oxford, 2011)
I. Ribeiro, F. Genezini, M. Saiki, G. Zahn, M. Secco, J. Radioanal. Nucl. Chem. 305, 299–303 (2015)
S. Hirata, T. Kajiya, M. Aihara, K. Honda, O. Shikino, Talanta 58, 1185–1194 (2002)
M.C. Zuma, P.N. Nomngongo, N. Mketo, Minerals 11, 1103 (2021)
E. Zolfonoun, S.R. Yousefi, J. Braz. Chem. Soc. 27, 2348–2353 (2016)
B. Masry, M. Aly, N. Khalifa, A. Zikry, M. Gasser, J. Daoud, J. Arab, Nucl. Sci. Appl. 48, 1–16 (2015)
H. Onoda, Y. Kurioka, J. Environ. Chem. Eng. 4, 4536–4539 (2016)
W.I. Mortada, Microchem. J. 157, 105055 (2020)
Y.-X. Gu, T.-C. Yan, Z.-X. Yue, F.-M. Liu, J. Cao, L.-H. Ye, Microchem. J. 176, 107241 (2022)
M.M. Elnagar, M.A. Hashem, M.M. Hassanien, M.A. Ismail, Sep. Purif. Technol. 265, 118534 (2021)
W. Mortada, I. Kenawy, G. El-Gamal, S. Moalla, J. Radioanal. Nucl. Chem. 313, 69–77 (2017)
R. Ridha, E. Azooz, S.S. Tarish, Anal. Bioanal. Chem. Res. 9, 251–258 (2022)
E. Adnan Azooz, F. Abd Wannas, R. Kadhim Ridha, S. Kadhim Jawad, E.A.J. Al-Mulla, Anal. Bioanal. Chem. Res. 9, 133–140 (2022)
M. Laosuwan, S. Mukdasai, S. Srijaranai, Molecules 25, 552 (2020)
G.G. El-Gamal, W.I. Mortada, M.M. Hassanien, A.A. Ibrahim, Y.G. Abou El-Reash, J. Anal. At. Spectrom. 36, 1306–1312 (2021)
M.M. Hassanien, W.I. Mortada, I.M. Kenawy, J. Radioanal. Nucl. Chem. 303, 261–269 (2015)
S. Mandal, S. Lahiri, Microchem. J. 175, 107150 (2022)
W.I. Mortada, M.M. Hassanien, A.A. El-Asmy, Anal. Methods 5, 530–535 (2013)
M.M. Hassanien, I.M. Kenawy, M.E. Khalifa, M.M. Elnagar, Microchem. J. 127, 125–132 (2016)
M.E. Khalifa, W.I. Mortada, M.M. El-defrawy, A.A. Awad, Microchem. J. 151, 104214 (2019)
C. Labrecque, D. Larivière, Anal. Methods 6, 9291–9298 (2014)
A. Saha, S.B. Deb, A. Sarkar, M.K. Saxena, B. Tomar, RSC Adv. 6, 20109–20119 (2016)
Y. Li, B. Hu, J. Hazard. Mater. 174, 534–540 (2010)
F.D.S. Depoi, F.R.S. Bentlin, M.F. Ferrão, D. Pozebon, Anal. Methods Lond. 4(9), 2809–2014 (2012)
J. Vichapong, R. Burakham, S. Srijaranai, Food Anal. Methods 10, 1645–1652 (2017)
E.A. Azooz, H.S.A. Al-Wani, M.S. Gburi, E.H.B. Al-Muhanna, Open Chem. 20, 525–540 (2022)
R.A. Clark, G.L. Krueger, J. Histochem. Cytochem. 33, 729–732 (1985)
Y. Chen, A. Bopda-Waffo, A. Basu, R. Krishnan, E. Silberstein, D.R. Taylor, T.T. Talele, P. Arora, N. Kaushik-Basu, Antivir. Chem. Chemother. 20, 19–36 (2009)
H. Cho, D.Y. Lee, S. Shrestha, Y.S. Shim, K.C. Kim, M.-K. Kim, K.-H. Lee, J. Won, J.-S. Kang, Mol. Cells 18, 46 (2004)
F. Saad, M. Elghalban, J.H. Al-Fahemi, N. Yarkandy, N.M. El-Metwaly, K. Abou-Melha, G. Al-Hazmi, K. Saleh, J. Therm. Anal. Calorim. 128, 1565–1578 (2017)
P.B. Kurt-Karakus, Environ. Int. 50, 47–55 (2012)
S. Wang, S. Meng, Y. Guo, J. Spectrosc. 2013, 735702 (2013)
J.B. Ghasemi, B. Hashemi, M. Shamsipur, J. Iran. Chem. Soc. 9, 257–262 (2012)
A.R. Zarei, Anal. Biochem. 369, 161–167 (2007)
S.R. Yousefi, E. Zolfonoun, M.R. Pourjavid, S.J. Ahmadi, Anal. Methods 6, 3694–3699 (2014)
L. Farzin, M. Amiri, J. Nanoanal. 5, 241–248 (2018)
D. Li, X. Chang, Z. Hu, Q. Wang, R. Li, X. Chai, Talanta 83, 1742–1747 (2011)
M.H. Mallah, F. Shemirani, M.G. Maragheh, Environ. Sci. Technol. 43, 1947–1951 (2009)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the data in this paper have not been published elsewhere or considered for publication in any other journal. All the authors have approved this submission. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mortada, W.I., Awad, A.A., El-Defrawy, M.M. et al. Air-assisted cloud point extraction coupled with inductively coupled plasma optical emission spectroscopy for determination of samarium in environmental samples. ANAL. SCI. 38, 1489–1495 (2022). https://doi.org/10.1007/s44211-022-00181-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-022-00181-9