Abstract
Antimony (Sb) is listed as a priority pollutant in many countries worldwide. Sb accumulates in the environment, and displays harmful effects. The toxical and biological properties of Sb vary depending on Sb chemical form and oxidation state. Here, Sb speciation analysis allows to distinguish the different Sb forms and oxidation states. Although Sb environmental science is increasing, Sb is still a poorly studied element in land and water ecosystems. Analytical methods for Sb include extraction, selective hydride generation, coprecipitation, chromatography, electrochemistry, kinetics and spectrometry. Cloud point extraction is the most widely used extraction method for reliable separation and preconcentration of one of the most toxic species of inorganic antimony, Sb(III). This extraction technique is based on phase separation when the temperature of the micellar solution of a nonionic surfactant is increased above a certain threshold value defined as the cloud point. There is little knowledge on cloud point extraction coupled to electrothermal atomic absorption spectrometry, hydride generation atomic absorption spectrometry, inductively coupled plasma optical emission spectrometry, inductively coupled plasma mass spectrometry and spectrophotometry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Antimony
- Biological samples
- Cloud point extraction
- Environmental samples
- Extraction techniques
- Preconcentration
- Separation
- Speciation analysis
- Spectrometric methods
- Surfactant
3.1 Introduction
Antimony (Sb) is a toxic metalloid with a wide range of industrial applications and annual world production of 18 × 104 tons (Jackson 2020). Consequently, considerable amounts of Sb are emitted to the environment every year and it is often present at elevated concentrations in each environmental compartment (Deng et al. 2020). There is a growing concern surrounding the possible adverse effects of Sb on natural ecosystems and human health, which leads to drafting of new analytical procedures for a reliable quantification of the element. The focus not only lies on quantification of the total concentration of Sb in different matrices, yet equally on reliable quantification of its individual species. Considering the speciation analysis of complex matrix samples, a combination of a suitable separation procedure with a reliable quantification method is required in order to deliver accurate results. A large variety of sample preparation procedures have been developed for separation and preconcentration of different Sb species, among which the extraction procedures occupy an irreplaceable position in environmental and biological analysis (Gál et al. 2006). Primary techniques were based on liquid-liquid and solid-liquid extraction with different arrangements. Later on, procedures derived from cloud point extraction have been added to the repertoire of methods employed in a speciation analysis of Sb. For quantification of Sb, spectrometric methods, such as hydride generation atomic absorption spectrometry (HG-AAS), electrothermal atomic absorption spectrometry (ETAAS), inductively coupled plasma optical emission spectrometry (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS) and hydride generation atomic fluorescence spectrometry (HG-AFS) are amongst the most frequently used ones.
Since the year 2000, nearly 550 publications on speciation of Sb have been registered (Fig. 3.1). According to Scopus database information, on average 18 studies were published annually in the period of 2000–2009 and this number has doubled from 2010 to 2019. In comparison with arsenic, which it resembles both chemically and physically, antimony still remains the less studied element. A search on the Scopus database reveals that there are seven times more publications on speciation of arsenic than on speciation of antimony identified over the same period of interest. One reason might be that although belonging to the same periodic group, Sb appears to be much less toxic than its sister element compared on a molar basis (Maher 2009).
Whereas separation techniques based on liquid-liquid extraction and solid-phase extraction are well documented in the current literature (even for Sb speciation), the objective of this article is to provide a more detailed view on the recent cloud point extraction procedures combined with spectrometric methods that have been used in speciation analysis of Sb in environmental and biological samples.
3.2 Reviews on Antimony
The published literature contains different studies and comprehensive reviews on antimony-related topics. Some of these papers attempt to provide a clearer picture of natural biogeochemical cycles of Sb. Antimony is a global contaminant whose presence and continually increasing concentrations in each environmental compartment are of considerable interest. Nonetheless, many questions about the environmental chemistry of Sb still remain unanswered (Babula et al. 2008). It is important to note that the natural biogeochemical cycles of Sb need to be understood before an assessment of the impact of global contamination is made (Maher 2009). This is most definitely not possible without reliable quantification of total Sb and its species in all studied matrices. The literature reviewed in the course of this work can be further divided into the following sections: (1) antimony in the environment, (2) human exposure to antimony, (3) conventional and innovative techniques for antimony removal (4) different approaches to speciation of antimony and (5) antimony in bottled water. Papers dealing with particular fields are referred to in the appropriate sections below.
3.2.1 Antimony in the Environment
The key to understanding the behaviour of Sb in the environment lies in knowing its chemical form, concentration levels and speciation in various environmental compartments, its transformations, mobility, toxicity, possible interactions with other substances and many other aspects. Reviews on the presence of Sb in the environment are summarized in this section. Filella et al. (2002a, b, 2007) have focused their overviews mostly on Sb in natural waters. The authors discuss Sb occurrence (Filella et al. 2002a), its relevant solution chemistry (Filella et al. 2002b) and its relevant interactions with microbiota (Filella et al. 2007) in an aqueous environment. Their last paper on this topic (Filella et al. 2009) broadens the scope of the research on Sb behaviour in the different environmental compartments, its speciation, toxicity and cycling between compartments.
Smichowski (2008) aimed at providing a focused review on Sb present in volcanic ashes, fly ashes, road dust, airborne particulate matter, atmospheric aerosols and related matrices, and the methods for its reliable quantification in these types of samples. There is a lack of review papers on speciation analysis of Sb in atmospheric aerosols, most likely due to scarce information on this particular topic in the available literature. A possible reason might relate to the very low concentration of this element in atmospheric aerosols, which makes analytical determination of Sb in such matrices quite problematic.
The paper published by Oorts and Smolders (2009) reports a summary of the ecotoxicity data available for various Sb substances. Proceeding from the obtained data, they discuss the derivation of ecological threshold concentrations for Sb in freshwater and soils. However, ecotoxicity data are only accessible for a limited number of Sb substances. Furthermore, the information on the influence of abiotic factors on the bioavailability and toxic effects of Sb on either water or soil biota is also lacking.
Review by Tschan et al. (2009b) highlights that Sb is readily taken up by terrestrial plants, although little is known about the mechanism by which this occurs. The results of their original work (Tschan et al. 2009a) show that plant uptake of Sb increases linearly with Sb in solution or soluble Sb in soil over a wide range of concentrations until it is limited by toxicity. Antimony may thus be accumulated by plants on heavily contaminated soils at concentrations that may adversely affect human health. Later on, Feng et al. (2013) summarized studies in which possible mechanisms of plant uptake of Sb(V) and Sb(III) were described.
Natasha et al. (2019) bring a comprehensive look at a biogeochemical role of Sb in soil-plant system and the impact of Sb on human health related to consumption of crops and vegetables contaminated with this element. They aim to provide a conceivable link between exposure conditions, Sb chemical speciation, phytoavailability in soil, uptake and accumulation by plants, phytotoxicity, detoxification inside the plant and associated health risks.
Herath et al. (2017) present a critical overview of natural geochemical processes which trigger the mobilization of Sb from its host mineral constituents and related rocks to the surrounding areas. They underline the necessity of identification of regions and aquifer sediments most vulnerable to Sb-contamination in order to ensure the installation of safe drinking water wells. Recent advances in understanding the biogeochemical processes and ecological effects of Sb were summarized by He et al. (2019).
Article published by Vojteková et al. (2014) offers a quick overview on Sb levels in various environmental media, Sb chemistry and toxicity and interactions with organic and inorganic components. A short overview of chemical methods and procedures for speciation of Sb in different types of water samples, soils, sediments and fly ashes is also included. Recently, Bagherifam et al. (2019) published an interesting paper in which they evaluated and compared the central steps for derivation of soils, water and sediments regulatory guidelines with a focus on Sb. The authors point out that there are substantial variations in Sb toxicity guidelines (threshold values) in environmental systems that can be explained by the differences in geographical, ethnological, regulatory framework, scientific bases, site specific properties, selected software models and ecotoxicological criteria used for developing these guidelines. A number of factors suggest that there is a low to negligible likelihood for the potential global harmonization of all guideline values in the future.
3.2.2 Human Exposure to Antimony
One may be exposed to Sb by breathing air, drinking tap or bottled water, or eating food that contains Sb. Another possible route of exposure is skin contact with soil, water and other substances that contain Sb. Filella et al. (2009, 2012, 2013a, b) published several extensive reviews on human exposure to Sb. Their research largely focused on the Sb sources and its intake by humans (Belzile et al. 2011), Sb contents in some human tissues often used in biomonitoring (Filella et al. 2012), Sb contents in some human excreted biofluids (Filella et al. 2013a) and Sb concentration in human blood (Filella et al. 2013b). In their work, the authors present and critically discuss the literature information on Sb concentrations in water, air, food, hair, nails, teeth, urine, milk, saliva and human blood. Arain and Neitzel (2019) report on the risks associated with both formal and informal electronic waste (e-waste) recycling. Humans can be exposed to dangerous chemicals and (semi)metals, such as Sb, that are present in e-waste via multiple routes. Typically, exposure occurs by one of three exposure routes – ingestion, inhalation and dermal absorption (Nithya et al. 2020). The published data presented in reviewed studies showed that Sb concentrations in various types of biomarkers such as blood, hair and urine were consistently higher for occupationally exposed groups than for the reference and control groups.
3.2.3 Conventional and Innovative Techniques for Antimony Removal
Antimony is present in the environment as a result of natural processes and human activities both of which can lead to its increased concentration in the air, water, soils, sediments and other natural matrices (Escudero et al. 2019). Depending on the degree and nature of contamination, the effective removal techniques should be considered. Mubarak et al. (2015) provide a comprehensive overview of methods and procedures proposed for the removal of Sb from various contaminated matrices. Remediation techniques such as phytoremediation, bioremediation, coagulation-flocculation-sedimentation, adsorption, electrodialysis, ion exchange, chemical fixation, membrane separation and many others are included. Technologies stated above are also mentioned in a review published by Li et al. (2018). The authors show the weak points of particular removal procedures and conclude that new technologies are urgently needed to be developed and implemented to preserve sustainability of the environment. A closer look on As and Sb in wastewater and the options for their removal from this medium was taken by Ungureanu et al. (2015). The authors put a special emphasis on the adsorption and on the latest findings in the broad topic of alternative low-cost adsorbents. Deng et al. (2017) reviewed the application of iron-based materials for the removal of Sb from contaminated water. They discuss the interaction of Sb with the sorbents in relation to adsorption performance, influencing factors, mechanism, modelling of adsorption (isotherm, kinetic and thermodynamic models), advantages, drawbacks and the recent achievements in the field.
3.2.4 Different Approaches to Measure Speciation of Antimony
Reliable quantification of Sb species is an essential part of the studies aimed at elucidating Sb behaviour in various biogeochemical cycles. While speciation analysis of trace Sb in complex matrices is mostly based on combination of an efficient separation technique with a reliable quantification method, speciation analysis of inorganic antimony (iSb) in water samples relies on hydride generation process. In this case, optimization of the hydride generation process for selective quantification of Sb(III) is usually carried out and total iSb is quantified after an effective reduction of Sb(V) to Sb(III) (Ferreira et al. 2014). Strategies developed for speciation analysis of iSb using spectroanalytical techniques and hydride generation process were reviewed by Ferreira et al. (2014). Later on, Ferreira et al. (2019) published a summary of non-chromatographic and chromatographic procedures for speciation analysis of Sb in environmental matrices, where hydride generation atomic fluorescence spectrometry (HG-AFS) was used for Sb quantification.
The coupling of chromatographic techniques such as gas chromatography and high performance liquid chromatography with highly sensitive and selective quantification methods such as mass spectrometry, atomic fluorescence spectrometry, atomic absorption spectrometry or optical emission spectrometry has been widely exploited and accepted for the speciation analysis of trace Sb in complex matrices. Non-chromatographic techniques, mostly extractions, in combination with quantification methods listed above are also extensively used in speciation analysis of trace Sb in complicated matrices. The analytical methods for separation and/or quantification of Sb can be grouped in four main categories: chemical methods, chromatographic methods, electrochemical methods and kinetic methods (Smichowski et al. 1998).
Methodologies for the determination of total Sb in terrestrial environmental samples along with procedures for the speciation of Sb in aqueous solutions can be found in a review published by Nash et al. (2000). Krachler and Emons (2001a) focused on hyphenated instrumental techniques employed in speciation of both volatile and non-volatile Sb compounds. Related issues such as biomethylation, biological significance, occupational and environmental exposure were also discussed. Evaluation of selective hydride generation procedures, extraction techniques and chromatographic techniques in combination with atomic spectrometric methods for speciation of Sb in natural waters can be found in an article published by Hagarová and Kubová (2008). In the review on the speciation of As, Sb and Se published by Wu and Sun (2016), the performance of both chromatographic and non-chromatographic techniques was compared. Methods for quantification of total Sb together with procedures suitable for its speciation, including electrochemical techniques, were reviewed by Chomisteková et al. (2016). Yu et al. (2019) aimed to update the recent research progress in the speciation analysis of trace As, Hg, Se and Sb in environmental and biological samples with an emphasis on the recent applications of high performance liquid chromatography hyphenated to atomic spectrometry or mass spectrometry.
3.2.5 Antimony in Bottled Water
Although some consider the bottled water industry a marketing trick of the century, there is no doubt that it has become a big business. According to The Guardian, the past two decades made clear that water in bottles has become the fastest growing drinks market in the world. Among the factors which might affect water quality, the type of material used for a bottle is very likely to alter the chemistry of water it holds. Regarding Sb contamination, elevated concentrations of Sb in water arise from the use of antimony trioxide (Sb2O3) as a catalyst in the manufacture of polyethylene terephthalate, the prevailing type of material used for water bottles (Shotyk and Krachler 2007). There are various catalysts that can be used in the synthesis, but Sb2O3 is probably the most suitable and widespread one. It has a high catalytic activity, does not colour the product and it is cost effective (Mihucz and Záray 2016). Bach et al. (2012) agree that Sb is the most relevant element being leached out of polyethylene terephthalate bottles, but according to the published data obtained from the studies reviewed the authors concluded that Sb concentration never exceeded specific migration limit established by European Union. The overview published by Diduch et al. (2011) shows the available information on levels of inorganic constituents (including Sb) and organic contaminants in bottled water samples in the context of sample preparation procedures and analytical techniques.
3.3 Extraction Procedures Used in Speciation Analysis of Antimony
According to IUPAC, “speciation analysis” is defined as analytical activities of identifying and measuring the quantities of one or more individual chemical species in a sample (Templeton et al. 2000). In the speciation analysis of (ultra)trace elements in complex matrices, a combination of a suitable separation technique with a reliable quantification method is usually necessary. Chromatographic techniques (such as gas chromatography or high performance liquid chromatography) are often coupled with highly sensitive and selective spectrometric methods. In this case, a major challenge is the remarkably high cost of instrumentation, which makes these methods almost inaccessible to a number of analytical laboratories. In an attempt to use cheaper alternatives that deliver reliable results, many analytical chemists focus on the development of new non-chromatographic separation techniques or modification of existing ones. Undoubtedly, extraction techniques remain the hot topic of research, particularly when sample containing only a few simple chemical forms is to be analysed (Pyrzynska 2020).
One of the latest trends in modification of traditional extraction procedures is towards miniaturization of analytical systems. Miniaturization and automation of the methodologies are the basic strategies for greening the analytical methods. In such arrangements, a strong reduction in energy and reagents consumption, waste generation and time taken is expected (Armenta et al. 2015). Traditional liquid-liquid extraction in its miniaturized form, so-called liquid-phase microextraction (LPME), was introduced by Rezaee et al. (2006). Depending on the separation mode, there are three main variants of LPME: single drop microextraction (SDME), dispersive liquid-liquid microextraction (DLLME) and membrane mediated liquid phase microextraction (such as hollow fiber liquid phase microextraction (HF-LPME) and solvent bar microextraction (SBME)) (Ibrahim et al. 2017; Nemček and Hagarová 2020). These techniques have many other modifications (Werner et al. 2018) and have been successfully applied to separation and preconcentration of various analytes, including (ultra)trace Sb and its species. The following provide some examples. A new sensitive SDME method for Sb(III) determination using N-benzoyl-N-phenylhydroxylamine and 1-butyl-3-methylimidazolium hexafluorophosphate has been presented by Huang et al. (2018). A simple and fast ultrasound-assisted ionic liquid DLLME (UA-IL-DLLME) method for preconcentration of trace Sb and Sn in beverage samples has been developed by Biata et al. (2017). Two modes of LPME, HF-LPME and DLLME have been investigated by Marguí et al. (2013) to preconcentrate Sb prior to its determination by total reflection X-ray fluorescence (TXRF) analysis.
The techniques based on liquid-phase microextraction are very diversified. Their modification often involves preconcentration based on the use of tailor-designed or carefully selected extractants, e.g. ionic liquids or low-melting-point organic solvents. However, some concerns were raised with regard to the usage of ionic liquids. This intensifies the search for new solvents such as deep eutectic solvents, which are commonly accepted in green separation processes and are now widely acknowledged as a new class of ionic liquids analogues because they share many characteristics and properties with ionic liquids (Smith et al. 2014). Regardless of the solvent used, the common advantages of all LPME techniques are lower volumes of solvents and less laborious operations in comparison to traditional liquid-liquid extraction, as well as lower waste generation.
Solid-phase extraction is a popular separation and preconcentration technique both in organic and inorganic analysis. The analytes are usually extracted on a solid sorbent by selective sorption. This technique allows sorbent to be packed inside the cartridges, syringe barrels, microcolumns or extraction disks. Owing to limitations of column arrangements, dispersive solid-phase extraction (DSPE) gains on popularity. There are various kinds of sorbents that can be used in DSPE methodology. In recent years, variety of nanomaterials have been evaluated as potential sorbents for the separation/preconcentration and speciation of various trace elements in a number of samples. The high surface area of nano-sized materials, their high chemical activity, high adsorption capacity, fast adsorption dynamics and good mechanical and chemical stability are all properties that are relevant for the development of effective DSPE procedures suitable for separation and preconcentration of various analytes (Hu et al. 2015). Considerable attention has been particularly directed to metallic nanoparticles, metal oxide nanoparticles, silica-based nanomaterials and carbon-based nanomaterials (Pyrzynska 2020). In regard to Sb speciation, Lopéz-García et al. (2017) described an interesting procedure that required magnetic particles covered with silver nanoparticles functionalized with the sodium salt of 2-mercaptoethanesulphonate. After separation of the solid by means of a magnetic field, the solid phase was directly introduced into ETAAS; or alternatively, the material was slurried and then injected into the atomizer. Speciation of Sb(III) and Sb(V) was achieved by means of two extractions carried out at different acidity.
Solid-phase microextraction (SPME), introduced by Arthur and Pawliszyn (1990), is a non-exhaustive technique based on the partition equilibrium of the analytes between the sample matrix and the extraction phase. In its most known configuration, the SPME device consists of an extraction phase coated onto a fused-silica rod; the extraction phase can be exposed directly to the sample media (direct immersion) or to its headspace (Souza-Silva et al. 2015). The application of headspace solid-phase microextraction (HS-SPME) for a direct analysis of volatile Sb compounds such as stibine (SbH3), monomethylantimony, dimethylantimony and trimethylantimony was reported by Smith et al. (2002). The authors used SPME with polydimethylsiloxane fibres in combination with gas chromatography mass spectrometry (GC-MS). Another example of SPME involves the use of novel polystyrene oleic acid imidazole polymer in a micropipette tip of a syringe system and was described by Panhwar et al. (2018). Their simple, rapid and sensitive SPME in combination with ETAAS was used for speciation of iSb in mineral water, tap water and spring water samples and quantification of total Sb in acid digested soil and food samples.
In regard to SPME, various sorbents and configurations were described and used in different applications for a wide variety of sample matrices and analytes. This technique is considered a solvent-free because it is carried out without a solvent for isolation of the analytes. Another advantage is that it requires little or no solvent for the desorption step. Furthermore, most solid sorbents in SPME can be used repeatedly, thus greatly reducing waste generation and the cost of analysis. The possibility of an on-line set-up is an additional advantage that allows SPME to be performed at higher accuracy and precision, and lower labour consumption as a result of automation. Amongst obvious pros is also the miniaturized size and portability of SPME devices which greatly facilitates implementation of on-site sampling and optionally on-site analysis when coupled to portable analytical instruments. There is also a substantial reduction of errors associated with sample transportation and possible alterations during storage (Pérez-Fernández et al. 2017).
Cloud point extraction, first introduced by Watanabe (1974), is an excellent alternative to liquid-liquid extraction, where toxic organic solvents are replaced with nonionic surfactants. The phase separation is generally achieved by heating an aqueous solution containing nonionic surfactants, chelating reagents and target analytes over a critical temperature or using various additives to trigger the cloud point. Since the first work published, this extraction technique has gained popularity that is reflected in the thousands of papers and dozens of reviews published on this topic. Recent reviews on cloud point extraction are summarized by Gavazov et al. (2019).
Growing interest in Sb and its speciation in various matrices is illustrated by recent growth in the number of published studies concerning different analytical methods for the separation and quantification of Sb species. The extraction techniques are targeted in a fairly large group of studies as well. For this reason, it is impossible to mention all the papers on Sb speciation based on extraction procedures. Since procedures based on liquid-liquid extraction and solid-phase extraction (in their different miniaturized forms and modes) were discussed in the review recently published by Yu et al. (2019), the main objective of the present paper is to bring a comprehensive overview on recent cloud point extraction procedures used for separation and preconcentration of Sb in environmental and biological samples. Their relation to spectrometric methods is discussed further with several examples in the next section.
At this point, it could be useful to mention some Sb species which were identified and quantified in environmental and biological samples. In an aquatic environment, Sb predominantly exists in its inorganic forms as Sb(III) and Sb(V). In regard to methylantimony species, they have been found to be formed in both fresh and sea water. These organic species usually account for less than 10% of the total dissolved Sb (Krachler and Emons 2001a). Methylated Sb compounds were identified and quantified in biological samples (Krachler and Emons 2001b; Quiroz et al. 2011) as well as in soil and sediment samples (Krachler and Emons 2001b). The main Sb species identified in environmental and biological matrices can be seen in Table 3.1.
3.4 Speciation Procedures Based on Cloud Point Extraction and Spectrometric Quantification
Cloud point extraction is based on the following phenomenon: an aqueous solution of neutrally charged surfactant (nonionic or zwitterionic) at a concentration higher than critical micellar concentration becomes turbid and separates into two isotropic phases if an external condition is changed (e.g. temperature, pressure, pH or ionic strength). The surfactant solution becomes turbid because it attains the cloud point (i.e. incomplete solubilization) (Bezerra et al. 2005). At this point, the original surfactant solution separates into a surfactant phase of a small volume, which is rich in surfactant and contains an analyte trapped by micellar structures (so-called surfactant-rich phase) and a bulk diluted aqueous phase (so-called surfactant-poor phase or equilibrium solution).
The methodology for the separation and preconcentration of metallic ions can be briefly summarized as follows. In the first step, a chelating agent is usually added to a system to obtain the hydrophobic chelate which remains in the hydrophobic core of the micelles in surfactant-rich phase. This is followed by the addition of a surfactant and heating in a water bath to induce cloud point formation. In this moment, two isotropic phases are formed and their separation is accelerated by centrifugation. Afterwards, the system is usually cooled in an ice-bath in order to increase the viscosity of the surfactant-rich phase. The final step of cloud point extraction is decantation of an aqueous phase. The highly viscous surfactant-rich phase is obtained and conventionally diluted by methanol or ethanol solution of mineral acid (mainly HNO3 or HCl). Such diluted sample is now ready for measurement. In order to increase the reliability of the procedure, some optimization of the experimental conditions needs to be done, including optimizing pH (which plays a crucial role in metal-chelate formation and subsequent extraction), concentration of a surfactant, concentration of a chelating agent, incubation temperature and time, and centrifugation time. In some cases, the addition of salts, alcohols, some other surfactants and some organic compounds may also be helpful (salting-out effect). Selection of a suitable diluting agent for decreasing viscosity of the surfactant-rich phase is another important step. In speciation studies, the experimental conditions must be optimized with respect to the selective separation and preconcentration of selected species. The particular steps of the speciation analysis of inorganic antimony (iSb) using cloud point extractions will be discussed further in this section.
Nearly all procedures described here are associated with the use of nonionic surfactant Triton X-114 (octyl phenoxy polyethoxy ethanol) as an extracting agent. In general, the majority (about 80%) of studies in which cloud point extraction procedures are employed for separation, preconcentration and speciation of inorganic analytes utilize this nonionic surfactant. It is due to its excellent physicochemical characteristics, such as low cloud point temperature (23–25 °C; which is particularly important for the extraction of thermally unstable metallic chelates) and high density of the surfactant-rich phase (1.052 g/ml; which facilitates phase separation by centrifugation). Triton X-114 is available in high purity at a relatively low price and shows low toxicity which favors it over other surfactants.
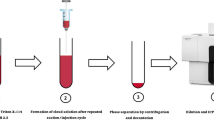
The majority of speciation studies dedicated to iSb using cloud point extraction requires optimization of the experimental conditions for selective separation and preconcentration of Sb(III). The total iSb is quantified by the same protocol after reduction of Sb(V) to Sb(III) using L-cysteine or iodide as reducing agents. Diagram showing the cloud point extraction adopted for selective separation and preconcentration of Sb(III) can be seen in Fig. 3.2. For quantification of Sb, spectrometric methods (such as flame atomic absorption spectrometry (FAAS), electrothermal atomic absorption spectrometry (ETAAS), hydride generation atomic absorption spectrometry (HG-AAS), inductively coupled plasma optical emission spectrometry (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS) or spectrophotometry) are usually used (Vojteková et al. 2014; Astolfi et al. 2006; Lebedev et al. 2003). Coupling of cloud point extraction with quantification methods listed above will be discussed in the following paragraphs.
3.4.1 Combination of Cloud Point Extraction and Flame Atomic Absorption Spectrometry
Since highly viscous surfactant-rich phase cannot be directly injected into the flame, a dilution of the surfactant-rich phase with a non-viscous solvent is required. The most commonly used eluents are dilute methanol or ethanol solutions of mineral acids. The effect of organic solvents on the flame in flame atomic absorption spectrometry (FAAS) has been a subject of several studies. It has been observed that low-surface-tension organic solvents may affect the nebulization process. There are reports indicating an appreciable enhancement in FAAS sensitivity, but at the same time, there are studies that found little or no sensitivity improvements in FAAS signal. Nevertheless, the application of low-surface-tension organic solvents is, in most cases, recommended (Stalikas 2002). Among these, methanolic or ethanolic solutions are proven to be efficient for lowering the viscosity of surfactant-rich phase.
One of the first researchers to apply cloud point extraction in the speciation analysis of inorganic antimony (iSb) in artificial seawater and wastewater was Fan (2005) and he did so in an attempt to extract and separate Sb(III) from an aqueous solution. The methodology was based on the formation of hydrophobic Sb(III)-N-benzoyl-N-phenylhydroxylamine complex trapped in a micellar phase of a nonionic surfactant. This complex was then extracted into the surfactant-rich phase at a temperature above the cloud point temperature, while Sb(V) remained in an aqueous phase. The concentration of Sb(III) in the surfactant-rich phase was then determined by FAAS. Total Sb was determined by the same procedure after reduction of Sb(V) to Sb(III) by L-cysteine; the amount of Sb(V) was calculated from the difference between values for total Sb and Sb(III).
Altunay and his co-workers published a series of papers where Victoria Pure Blue BO (Altunay and Gürkan 2015a), Pyronin B (Altunay and Gürkan 2015b), azomethine-H (Altunay and Gürkan 2016b), 2-(2-thiazolylazo)-p-cresol and 4-(2-thiazolylazo)resorcinol (Gürkan and Eses 2016) and morin (Altunay et al. 2016) were selected as complexing agents in cloud point extractions. The proposed procedures were tried out for separation and preconcentration of iSb species in various beverages, milk samples, fruit-flavored milk products and biological samples (such as blood serum and blood plasma). The authors highlight the benefits of these procedures which include minimum organic solvent consumption, experimental convenience and safety (for cloud point extraction), and fast and cheap analysis (for FAAS quantification). The combination of cloud point extraction and FAAS quantification was successfully utilized in their research work, showing good accuracy and precision, low quantification limits and high preconcentration factors in a wide linear range.
3.4.2 Combination of Cloud Point Extraction and Electrothermal Atomic Absorption Spectrometry
Since organic solvents and surfactants are both compatible with electrothermal atomic absorption spectrometry (ETAAS), no particular difficulties are anticipated in implementing ETAAS with cloud point extraction for element analysis. The contact angle of water with the surface of the graphite tube used in ETAAS is 85.7° while that of organic solvents is between 0 and 10°. Thus, the water-graphite system is expected to show shear behavior, whereas an organic solvent-graphite system has better interface compatibility. Both organic solvents and surfactants can efficiently decrease the contact angle between an aqueous solution and graphite material. These agents reduce the surface tension of liquid sample droplets allowing them to spread out evenly over the graphite surface before measurement. In order to use ETAAS for quantification of the target analyte following the cloud point extraction, the selection of an appropriate chemical modifier and an optimization of the furnace conditions need to be carried out.
To design a reliable cloud point extraction for selective quantification of Sb(III) in different types of natural waters, the experimental parameters were systematically investigated by Hagarová et al. (2008). In this study, ammonium pyrrolidine dithiocarbamate (APDC) was used as a complexing agent for Sb(III) separation and the ETAAS measurements were carried out in the presence of Pd(NO3)2 (used as a chemical modifier). In their later work, authors used the optimized procedure for systematic investigation of interferences caused by various coexisting ions and excess Sb(V) (Hagarová et al. 2012). While coexisting ions studied were found to have negligible effects on the extraction recovery rates, excess Sb(V) occurred to have a significant impact on recovery rates when Sb(V):Sb(III) ratio increased over 40:1; increasing the ratio led to increasing recovery rates, reaching as high as 160%. It is possible that certain amount of Sb(V) was reduced to Sb(III) which was then extracted.
The same complexing agent (APDC) was used in a procedure optimized by Jiang et al. (2010). Total Sb was determined after reduction of Sb(V) to Sb(III) using L-cysteine. The procedure was successfully applied for speciation of inorganic antimony (iSb) in leachates obtained from different food packaging materials. Andrade et al. (2017) optimized the cloud point extraction for the selective determination of Sb(III) in Brazilian mineral waters stored in polyethylene terephthalate bottles. Again, the preconcentration step was carried out by selective extraction of Sb(III) after its complexation with APDC, followed by separation of a surfactant-rich phase and subsequent quantification by ETAAS. The factors affecting separation and preconcentration were analyzed using univariate and multivariate approach. Optimization of an extraction procedure for selective separation and preconcentration of Sb(III) in water and blood serum samples using multivariate design was presented also by Souza and Tarley (2008). The cloud point extraction was based on the formation of Sb(III) complexes with O,O-diethyl dithiophosphate (DDTP) in the presence of Triton X-114. Total Sb was determined after reduction of Sb(V) to Sb(III) using L-cysteine. The latter two papers point out that using multivariate optimization reduces the number of experiments which results in lower reagent consumption, lower waste generation, and saving experimental time and cost.
3.4.3 Combination of Cloud Point Extraction and Hydride Generation Atomic Absorption Spectrometry
Hydride generation is probably the most popular technique for generation of volatile Sb compounds. In combination with atomic absorption spectrometry, HG-AAS offers fast measurement times and low detection limits for Sb in a wide variety of samples, including environmental and biological ones (Dědina 2007). It is based on the reaction of Sb compounds with tetrahydroborate in acid media to produce stibine (SbH3). It is well-known that Sb(III) is reduced fast and easily in strongly acidic to near neutral media, while Sb(V) is only reduced at a low pH at much slower rates (Ferreira et al. 2014). This fact is of utmost importance in speciation studies of inorganic antimony (iSb) which require thorough control of experimental conditions for hydride generation. By setting the conditions (e.g. optimization of tetrahydroborate concentration, presence of acid) under which HG-AAS measurement is taken, it is possible to distinguish Sb(III) from total iSb. However, this sample pretreatment technique (which involves the use of conventional quartz tube atomizers) does not allow for sample preconcentration. An optimized cloud point extraction has emerged as a useful method not only for separation but also for the preconcentration of analytes. Complications associated with the procedure of cloud point extraction and hydride generation systems may arise from the presence of surfactant; it can promote foam formation during the hydride transport, thus making the release of hydride from solution more difficult (Bezerra et al. 2005).
In 2016, Altunay and Gürkan (2016a) proposed a simple and cost efficient method for simultaneous separation and preconcentration of iSb and Se in water, beverage and food samples by means of ultrasound-assisted cloud point extraction followed by their determination by HG-AAS analysis. The method involved the use of PONPE 7.5 as an extractant and neutral red as a complexing agent for Sb(III) and Se(IV). The authors investigated the main factors affecting extraction efficiency and the behavior of analytes during the hydride formation.
3.4.4 Combination of Cloud Point Extraction and Inductively Coupled Plasma Optical Emission Spectrometry
Introducing organic solvents into plasma-based techniques may give rise to a number of operating difficulties. Their adverse effects on various parameters including excitation conditions, plasma stability, nebulizer flows and torch dimensions have been well recognized and documented. As far as the presence of surfactants in the plasma was concerned, the reported effects on sample transport and analytical sensitivity were little or modest (Stalikas 2002). In case of plasma-based methods developed for an element quantification after cloud point extraction, the surfactant-rich phase is usually diluted directly by mineral acids.
Electrothermal vaporization is one of the sample introduction techniques employed in ICP-OES and ICP-MS that was reported to improve sample consumption, transport efficiency and absolute detection limit. An on-line cloud point extraction in combination with electrothermal vaporization inductively coupled plasma optical emission spectrometry (ETV-ICP-OES) for quantification of inorganic antimony (iSb) species in environmental and biological samples has been proposed by Li et al. (2006). Sb(III) forms a hydrophobic complex with ammonium pyrrolidine dithiocarbamate (APDC) and subsequently enters the surfactant-rich phase; surfactant-rich phase is then retained in a microcolumn packed with absorbent cotton. The surfactant-rich phase is then eluted with acetonitrile and determined by ETV-ICP-OES. In this particular case, APDC was employed not only as a chelating agent in cloud point extraction but also as a chemical modifier in ETV-ICP-OES. The method was applied to the speciation of iSb in water and urine samples. Utilizing the flow analysis has been shown to avoid the time-consuming process of batch procedures and to minimize waste. However, induction of the cloud point and retention of the surfactant-rich phase remain two of the most critical aspects of cloud point extraction coupled to flow injection analysis. Nevertheless, the search for solutions to technical difficulties arising from these two issues may lead to the invention and development of more time-efficient procedures.
The application of cloud point extraction and hydride generation for quantification of Sb and Se using inductively coupled plasma optical emission spectrometry (ICP-OES) was discussed by dos Santos Depoi and Pozebon (2012). The complexing agent used to separate both analytes was O,O-diethyl dithiophosphate (DDTP). Experimental conditions for selective separation and preconcentration of Sb(III) and Se(IV), reduction of Sb(V) and Se(VI), hydrides generation and interferences were investigated in detail to examine the possibilities of reliable and selective quantitative measurement of Sb(III) and Se(IV) in natural waters. Special attention was given to the performance of hydride generation and pneumatic nebulization, the two were compared with regard to their efficiency in introducing the surfactant-rich phase into the plasma. Samples tested for Sb(III) were taken from estuarine water, seawater near the Rio Grande do Sul's coast and mineral water stored in polyethylene terephthalate bottles. For the analysis of water samples and fruit juices, Dundar et al. (2018) used ICP-OES coupled to an ultrasonic nebulizer. Separation of Sb(III) from Sb(V) was achieved by complexation of Sb(III) with dithizone. The effects of coexisting ions as well as all experimental parameters were studied and optimized. Recently, Biata et al. (2019) presented an ultrasound-assisted cloud point extraction combined with ICP-OES as a tool for separation, preconcentration and quantification of Sb, Sn and Tl in food and water samples. The optimization of experimental conditions was carried out by means of fractional factorial design and response surface methodology. The complexing agent for the analytes was 1-2(-pyridylazo)-2-napthol and dissolution of the surfactant-rich phase was achieved by 10% HNO3.
3.4.5 Combination of Cloud Point Extraction and Inductively Coupled Plasma Mass Spectrometry
From all the detection methods discussed above, inductively coupled plasma mass spectrometry (ICP-MS) is proven to be one of the most efficient and robust methods for element quantification. This technique is characterized by high sensitivity, large dynamic range, high capability of multi-element analysis and capability to perform isotopic measurements.
Coupling of flow injection analysis with ICP-ultrasonic nebulization represents a major breakthrough in the field of extraction technologies in terms of improvements in sample introduction into the plasma (without plasma fluctuation), transport and nebulization of the surfactant and organic phase (Silva et al. 2000). Other specific actions such as cooling of the nebulization spray chamber or use of a special plasma torch which can operate at a low argon flow rate can provide convenient remedies to possible issues arising from the presence of organic vapors in the plasma (Stalikas 2002). The combination of cloud point extraction and electrothermal vaporization inductively coupled plasma mass spectrometry (ETV-ICP-MS) is a powerful tool for simultaneous speciation of inorganic antimony (iSb) and Se in water samples (Li et al. 2008). Sb(III) and Se(IV) form complexes with diethyldithiocarbamate (DDTC) at pH 6.0 which can be quantitatively extracted into the surfactant-rich phase, whereas Se(VI) and Sb(V) remain in an aqueous solution. The proposed method is characterized with mild separation conditions (pH 6 is close to pH of natural water), simplicity, selectivity (good anti-disturbance ability), safety (no organic solvents) and low reagent cost.
3.4.6 Combination of Cloud Point Extraction and Spectrophotometry
In comparison with other spectrometric methods, spectrophotometry is still amongst most popular and attractive ones because of its simplicity, low-cost and compactness. Its main limitation is that it is not suitable for the measurement of (ultra)trace elements in complex matrices. If a reliable separation and preconcentration procedure is used, spectrophotometry can be successfully applied in (ultra)trace analysis.
Samadi-Maybodi and Rezaei (2012) reported on a new simple and versatile method for the cloud point extraction of ultra-trace amounts of Sb followed by spectrophotometric quantification of this element in seawater, human blood serum and Glucantime (anti-leishmania drug). The method was based on the color reaction of Sb(III) with iodide in acidic medium, in the presence of ascorbic acid as a reducing agent. An important detail about the experimental procedure is that researchers included a long thin neck container for extraction. Thanks to the special design of the vessel, no centrifugation and no cooling afterwards were required for separation of the extraction phase. As a result of different densities of the surfactant-rich phase and an aqueous phase, surfactant-rich phase separated as a top layer in a thin neck of the container. The surfactant-rich phase was then collected by a syringe-pipette and transferred to a quartz cuvette of the spectrophotometer. After adding methanol to the surfactant-rich phase in order to reduce viscosity, the absorbance of the solution was measured at the wavelength of 330 nm.
Cloud point extraction as a preconcentration step for Sb(III) detection prior to its spectrophotometric quantification was successfully tested also by El-Sharjawy and Amin (2016). The cloud point extraction was based on the reaction of Sb(III) with 3-dichloro-6-(3-carboxy-2-hydroxy-1-naphthylazo)quinoxaline in the presence of cetyltrimethylammonium bromide and KI at pH 4.5. The surfactant-rich phase was diluted with acetonitrile and the measurements were performed at 653 nm. The total amount of Sb in blood plasma, urine, water and biological samples was measured after Sb(V) to Sb(III) conversion using the same protocol.
A rapid, sensitive and more environmentally friendly analytical procedure utilizing cloud point extraction in a lab-in-syringe flow system aiming at the spectrophotometric quantification of Sb was developed by Frizzarin et al. (2016). The key feature of the method was the formation of an ion-pair between [SbI4]− and H+ which was subsequently extracted with Triton X-114. The multivariate optimization of some extraction parameters was carried out to identify interactions between the variables. The amount of total Sb in freshwater samples and antileishmanial drugs was determined after reduction of Sb(V) to Sb(III). The absorbance measurements were made at 345 nm. A lab-in-syringe flow system can be considered a revolutionary tool designed to make the procedure faster and easier; it allows for mechanization in all the steps involved in the analytical procedure, efficient mixing of multiple reagents solutions inside the syringe, and direct measurement in the surfactant-rich phase without the requirement for a filter or an eluent solution. It also reduces the risks of contamination. These characteristics make the proposed procedure more attractive compared to the existing batch or flow-based approaches.
3.5 Comparison of Analytical Characteristics of Procedures for Antimony Determination
The comparison of some experimental parameters of several reviewed extraction procedures can be seen in Table 3.2. For precision, relative standard deviations reported are in the range of 1.6–7.3% regardless of the detection method used and provide sufficient evidence of the reproducibility of the results. Traditional cloud point extractions generally involve a complex number of steps that must be undertaken to complete the task. After separation in the centrifuge, an aqueous phase has to be separated by decantation which is often considered the most problematic step. This step requires considerable attention so that reproducible results can be obtained. The reported relative standard deviations so far indicate that implementation of the optimized procedures would allow for precise and highly reproducible results.
The limit of detection is another important analytical parameter evaluated. It has been found to be highly dependent on the preconcentration factor (the higher the preconcentration factor the lower the limit of detection). For Sb(III) determined by cloud point extraction in combination with flame atomic absorption spectrometry, the obtained limits of detection were in the range of 0.03–5.15μg/l (Fan 2005; Altunay and Gürkan 2015a, b, 2016b; Gürkan and Eser 2016; Altunay et al. 2016). The lowest limit of detection reported for Sb(III) determined by such combination of techniques is comparable to limits of detection provided by combination of cloud point extraction and electrothermal atomic absorption spectrometry (range: 0.02–0.08μg/l) (Hagarová et al. 2008, 2012; Souza and Tarley 2008; Jiang et al. 2010; Andrade et al. 2017), cloud point extraction and inductively coupled plasma optical emission spectrometry or inductively coupled plasma mass spectrometry (range: 0.01–0.09μg/l) (Li et al. 2006, 2008; Depoi and Pozebon, 2012; Biata et al. 2019; Dundar et al. 2018). If the cost per analysis plays a critical role in deciding which detection method will be preferred for quantifying the analyte of interest, flame atomic absorption spectrometry seems to be the best choice.
A rapid, simple and low-cost detection method such as spectrophotometry has also been explored for its potential in speciation analysis of Sb after the selective separation and preconcentration of Sb(III) using optimized extraction procedures (Samadi-Maybodi and Rezaei 2012; El-Sharjawy and Amin 2016). From all methods listed in Table 3.2, the lowest limit of detection was obtained using cloud point extraction coupled to hydride generation atomic absorption spectrometry (Altunay and Gürkan 2016a). By means of hydride generation atomic absorption spectrometry, the hydride generation technique, the analyte can be separated from the liquid sample in the form of volatile hydride. The hydride generation efficiency can approach 100% under optimized experimental conditions and the limit of detection of the method is 20 times lower than the values reported for liquid sample nebulization when considering the efficiency of conventional nebulizers to be around 5% (Zurynková et al. 2018). The high preconcentration factor that can be obtained for Sb(III) using cloud point extraction leads to considerable improvement in a limit of detection, in particular when cloud point extraction is coupled to hydride generation atomic absorption spectrometry.
The remarkable outcomes were reported by Li et al. (2006) who applied the on-line flow-based cloud point extraction combined with electrothermal vaporization inductively coupled plasma optical emission spectrometry for Sb speciation in water and urine samples. In order to achieve the highest preconcentration factor attainable, 25, 50, 75, 100 and 150 ml volumes of sample were passed through the microcolumn at an optimum flow rate and different volumes of solutions were used for elution of the analyte. The preconcentration factor was 872 for analyte ions when the final sample volume was about 100 ml and the volume of acetonitrile was 100μl, which makes it probably the highest preconcentration factor ever obtained for inorganic Sb.
3.6 Conclusion
The majority of speciation studies on inorganic Sb that employ cloud point extraction methodology focus on the optimization of experimental conditions for selective separation and preconcentration of the analyte of interest. Of particular interest is the selective formation of a chelate-Sb(III) hydrophobic complex (at an optimal pH) which is subsequently retained in the hydrophobic cores of the micelles, while Sb(V) remains in an aqueous phase; thus, in the surfactant rich phase, only Sb(III) can be concentrated and separated. Total Sb is quantified by the same procedure after reduction of Sb(V) to Sb(III), and the amount of Sb(V) is calculated from the difference between the values for total Sb and Sb(III). In the case of relatively simple matrices such as natural waters, an error in the calculated concentrations of Sb(III) and Sb(V) seems to be negligible due to the fact that organic Sb species are identified very rarely in such types of samples.
Generally, only little attention is given to the stabilization of Sb species after water collection. Water samples are often analyzed directly with little or no pretreatment, samples for metal(loid) analysis are usually acidified with a drop of concentrated HNO3. One of the few scientists who indicated that care and preservation of freshwater samples prior to analysis is a very important aspect of the sampling process were Frizarrin et al (2016). After the addition of ethylenediaminetetraacetic acid (EDTA) to stabilize the analyte, they stored the samples at 4 °C till further use. However, the majority of researchers agree that speciation analysis should be conducted as soon as possible after water collection for these species, with immediate analysis being preferred, in order to minimize the risk of oxidation of Sb(III) to Sb(V) before measurement.
Food and biological tissues must be decomposed prior to analysis. The most common way to solubilize biological sample is digestion with a concentrated acid. The choice of acid is critical and so is reaction order, preferred acids being HNO3 – HClO4 mixture (Altunay and Gürkan 2015a), HNO3 – HCl mixture (Altunay and Gürkan 2015b; Altunay et al. 2016), HNO3 – H2SO4 – HClO4 mixture (Altunay and Gürkan 2015a) and an external heat source. The mixture of HNO3 and H2O2 (Altunay and Gürkan 2016a, 2016b; Gürkan and Eser 2016; Altunay et al. 2016; Depoi and Pozebon 2012; Biata et al. 2019) is commonly used in procedures carried out in closed vessels at elevated pressure, heated by microwave energy. After this step, Sb is present in its +5 oxidation state only and if optimized cloud point extractions were proposed for Sb(III), reduction of Sb(V) to Sb(III) must be done. For this purpose, a mixture of KI and ascorbic acid (Depoi and Pozebon 2012; El-Sharjawy and Amin 2016; Altunay and Gürkan 2016b; Gürkan and Eser 2016) or L-cysteine (Li et al. 2006, 2008; Souza and Tarley 2008; Altunay and Gürkan 2016a) are often used. By analysis of such decomposed samples only total Sb can be quantitatively determined and therefore the term “speciation analysis” should be avoided in this instance.
In traditional cloud point extractions, clouding is usually initiated by heating the mixture consisting of sample, complexing agent and surfactant in a water bath. The other type of energy which can be used to accelerate the reactions and clouding phenomena is ultrasound. The heat generated by the high-frequency sound waves enhances intensity and rate of interactions between the surfactant and an aqueous phase. The rise in popularity of ultrasound in chemistry can be attributed to its ease of use, low cost and green nature. Ultrasonic process plays an important role in an ultrasound-assisted cloud point extraction successfully applied in speciation analysis of Sb (Gürkan and Eser 2016; Altunay and Gürkan 2016b; Altunay et al. 2016; Biata et al. 2019). The faster the cloud point is induced, the more rapid extraction procedures can be envisioned for the future.
If duration of the procedure is an essential criterium for selecting the most appropriate pretreatment technique, then flow-based cloud point extraction seems to be a good choice (Li et al. 2006). A comprehensive review dedicated to cloud point extraction in flow-based systems was published by Melchert and Rocha (2016). This technique combines the advantages of flow analysis with that of cloud point extraction. The time-consuming steps such as centrifugation and cooling are omitted here. The use of cloud point extraction in a lab-in-syringe system can be considered a higher level of flow arrangement. The outstanding advantages of the method, such as mechanization of cloud point extraction, liquid-liquid extraction without organic solvents, mixing of reagent solutions with sample solution inside the syringe, reduction of the contamination risk and direct measurement of Sb in the surfactant-rich phase, make it rapid and environmentally friendly procedure. The pioneering exploitation of the lab-in-syringe system for cloud point extraction of Sb in water samples and antileishmanial drugs was discussed by Frizzarin et al. (2016).
Optimization of the cloud point extraction for reliable separation of the target analyte can take some time. A number of researchers use the univariate optimization strategy to find the best experimental conditions. This methodology is supposed to have an easier interpretation but requires more experiments which increase the consumption of reagents and time for analyses. Multivariate optimization is faster, more economical and effective, and allows for more than one variable to be optimized simultaneously. Several multivariate approaches such as fractional factorial design, Doehlert design and Box-Behnken design were exploited to identify interactions between the variables (Souza and Tarley 2008; Frizzarin et al. 2016; Biata et al. 2019).
There is no doubt that cloud point extraction represents a green analytical approach that limits or eliminates the use of toxic organic solvents. The optimization leads to development of new effective extraction procedures with high recovery rates and high preconcentration factors. As pointed out in the literature, cloud point extraction combined with spectrometric methods brings improvements in the limits of detection as well as in other analytical characteristics. The optimized cloud point extractions have a potential to be routinely used in many laboratories analysing environmental, biological, pharmaceutical and food samples for (ultra)trace organic and inorganic species. The potential of cloud point extraction for selective separation and preconcentration of Sb(III) has been documented by studies reviewed in this paper. Since the traditional cloud point extractions can be time- and labour-consuming and thus energy demanding, researchers are now investigating how to modify experimental conditions to ensure compliance with green chemistry principles. The processing time can be shortened by reducing the number of steps needed for a procedure (lab-in-syringe cloud point extraction); the consumption of chemicals can be reduced by miniaturization and/or using flow-based arrangement (flow-based cloud point extraction); an energy needed to accelerate the reaction and clouding phenomena can be produced in less environmentally harmful ways (ultrasound assisted cloud point extraction).
Abbreviations
- APDC:
-
ammonium pyrrolidine dithiocarbamate
- DDTC:
-
diethyldithiocarbamate
- DDTP:
-
O,O-diethyl dithiophosphate
- DLLME:
-
dispersive liquid-liquid microextraction
- DSPE:
-
dispersive solid-phase extraction
- ETAAS:
-
electrothermal atomic absorption spectrometry
- ETV-ICP-MS :
-
electrothermal vaporization inductively coupled plasma mass spectrometry
- ETV-ICP-OES:
-
electrothermal vaporization inductively coupled plasma optical emission spectrometry
- FAAS:
-
flame atomic absorption spectrometry
- GC-MS:
-
gas chromatography mass spectrometry
- HF-LPME:
-
hollow fiber liquid phase microextraction
- HG-AAS:
-
hydride generation atomic absorption spectrometry
- HG-AFS:
-
hydride generation atomic fluorescence spectrometry
- HS-SPME:
-
headspace solid-phase microextraction
- ICP-MS:
-
inductively coupled plasma mass spectrometry
- ICP-OES:
-
inductively coupled plasma optical emission spectrometry
- LPME:
-
liquid-phase microextraction
- SBME:
-
solvent bar microextraction
- SDME:
-
single drop microextraction
- SPME:
-
solid-phase microextraction
- TXRF :
-
total reflection X-ray fluorescence
- UA-IL-DLLME:
-
ultrasound-assisted ionic liquid dispersive liquid-liquid microextraction
References
Altunay N, Gürkan R (2015a) A new cloud point extraction procedure for determination of inorganic antimony species in beverages and biological samples by flame atomic absorption spectrometry. Food Chem 175:507–515. https://doi.org/10.1016/j.foodchem.2014.12.012
Altunay N, Gürkan R (2015b) A simple, inexpensive and convenient procedure for determination of inorganic Sb species in milk and beverage samples in PET containers by flame atomic absorption spectrometry. Anal Methods 7:9850–9860. https://doi.org/10.1039/C5AY01669J
Altunay N, Gürkan R (2016a) Separation/preconcentration of ultra-trace levels of inorganic Sb and Se from different sample matrices by charge transfer sensitized ion-pairing using ultrasonic-assisted cloud point extraction prior to their speciation and determination by hydride generation AAS. Talanta 159:344–355. https://doi.org/10.1016/j.talanta.2016.06.054
Altunay N, Gürkan R (2016b) Simultaneous determination of antimony and boron in beverage and dairy products by flame atomic absorption spectrometry after separation and pre-concentration by cloud point extraction. Food Addit Contam A 33:271–281. https://doi.org/10.1080/19440049.2015.1131335
Altunay N, Gürkan R, Yildirim E (2016) A new ultrasound assisted-cloud point extraction method for the determination of trace levels of tin and antimony in food and beverages by flame atomic absorption spectrometry. Food Anal Methods 9:2960–2971. https://doi.org/10.1007/s12161-016-0487-5
Andrade JK, de Andrade CK, Felsner ML, Quinia SP, dos Anjos VE (2017) Pre-concentration and speciation of inorganic antimony in bottled water and natural water by cloud point extraction with electrothermal atomic absorption spectrometry. Microchem J 133:222–230. https://doi.org/10.1016/j.microc.2017.03.043
Arain AL, Neitzel RL (2019) A review of biomarkers used for assessing human exposure to metals from e-waste. Int J Environ Res Public Health 16:1802. https://doi.org/10.3390/ijerph16101802
Armenta S, Garrigues S, de la Guardia M (2015) The role of green extraction techniques in Green Analytical Chemistry. TrAC Trends Anal Chem 71:2–8. https://doi.org/10.1016/j.trac.2014.12.011
Arthur CLP, Pawliszyn J (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62:2145–2148. https://doi.org/10.1021/ac00218a019
Astolfi ML, Canepari S, Catrambone M, Perrino C, Pietrodangelo A (2006) Improved characterisation of inorganic components in airborne particulate matter. Environ Chem Lett 3:186–191. https://doi.org/10.1007/s10311-005-0029-7
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2008) Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett 6:189–213. https://doi.org/10.1007/s10311-008-0159-9
Bach C, Dauchy X, Chagnon MC, Etienne S (2012) Chemical compounds and toxicological assessments of drinking water stored in polyethylene terephthalate (PET) bottles: a source of controversy reviewed. Water Res 46:571–583. https://doi.org/10.1016/j.watres.2011.11.062
Bagherifam S, Brown TC, Fellows CM, Naidu R (2019) Derivation methods of soils, water and sediments toxicity guidelines: a brief review with a focus on antimony. J Geochem Explor 205:106348. https://doi.org/10.1016/j.gexplo.2019.106348
Belzile N, Chen YW, Filella M (2011) Human exposure to antimony. I. Sources and intake. Crit Rev Environ Sci Technol 41:1309–1137. https://doi.org/10.1080/10643381003608227
Bezerra AM, Arruda MAZ, Ferreira SLC (2005) Cloud point extraction as a procedure of separation and pre-concentration for metal determination using spectroanalytical techniques: a review. Appl Spectrosc Rev 40:269–299. https://doi.org/10.1080/05704920500230880
Biata NR, Nyaba L, Ramontja J, Mketo N, Nomngongo PN (2017) Determination of antimony and tin in beverages using inductively coupled plasma-optical emission spectrometry after ultrasound-assisted ionic liquid dispersive liquid-liquid phase microextraction. Food Chem 237:904–911. https://doi.org/10.1016/j.foodchem.2017.06.058
Biata NR, Mashile GP, Ramontja J, Mketo N, Nomngongo PN (2019) Application of ultrasound-assisted cloud point extraction for preconcentration of antimony, tin and thallium in food and water samples prior to ICP-OES determination. J Food Compos Anal 76:14–21. https://doi.org/10.1016/j.jfca.2018.11.004
Chomisteková Z, Culková E, Bellová R, Melicherčíková D, Durdiak J, Beinrohr E, Rievaj M, Tomčík P (2016) Methods and procedures for the determination of antimony as an environmentally important analyte. Chem List 110:671–677
Dědina J (2007) Atomization of volatile compounds for atomic absorption and atomic fluorescence spectrometry: on the way towards the ideal atomizer. Spectrochim Acta, Part B 62:846–872. https://doi.org/10.1016/j.sab.2007.05.002
Deng RJ, Jin CS, Ren BZ, Hou BL, Hursthouse AS (2017) The potential for the treatment of antimony–containing wastewater by iron-based adsorbents. Water 9:794. https://doi.org/10.3390/w9100794
Deng RJ, Tang ZE, Hou BL, Ren BZ, Wang ZH, Zhu CQ, Kelly S, Hursthouse A (2020) Microbial diversity in soils from antimony mining sites: geochemical control promotes enrichment. Environ Chem Lett 18:911–922. https://doi.org/10.1007/s10311-020-00975-1
Depoi SF, Pozebon D (2012) The use of cloud point extraction and hydride generation for improving the Sb and Se limits of detection in ICP OES. J Braz Chem Soc 23:2211–2221. https://doi.org/10.1590/S0103-50532012001200010
Diduch M, Polkowska Z, Namiesnik J (2011) Chemical quality of bottled waters: a review. J Food Sci 76:R178–R196. https://doi.org/10.1111/j.1750-3841.2011.02386.x
Dundar MS, Kaptan F, Caner C, Altundag H (2018) Speciation of antimony using dithizone ligand via cloud point extraction and determination by USN-ICP-OES. At Spectrosc 39:100–105. https://doi.org/10.46770/AS.2018.03.002
El-Sharjawy AAM, Amin AS (2016) Use of cloud point preconcentration for spectrophotometric determination of trace amounts of antimony in biological and environmental samples. Anal Biochem 492:1–7. https://doi.org/10.1016/j.ab.2015.08.008
Escudero LB, Quintas PY, Wuilloud RG, Dotto GL (2019) Recent advances on elemental biosorption. Environ Chem Lett 17:409–427. https://doi.org/10.1007/s10311-018-0816-6
Fan ZF (2005) Speciation analysis of antimony(III) and antimony(V) by flame atomic absorption spectrometry after separation/preconcentration with cloud point extraction. Microchim Acta 152:29–33. https://doi.org/10.1007/s00604-005-0426-4
Feng R, Wei C, Tu S, Ding Y, Wang R, Guo J (2013) The uptake and detoxification of antimony by plants: a review. Environ Exp Bot 96:28–34. https://doi.org/10.1016/j.envexpbot.2013.08.006
Ferreira SLC, dos Santos WNL, dos Santos IF, Junior MMS, Silva LOB, Barbosa UA, de Santana FA, Queiroz AFDS (2014) Strategies of sample preparation for speciation analysis of inorganic antimony using hydride generation atomic spectrometry. Microchem J 114:22–31. https://doi.org/10.1016/j.microc.2013.11.019
Ferreira SLC, dos Anjos JP, Assis Felix CS, da Silva Junior MM, Palacio E, Cerda V (2019) Speciation analysis of antimony in environmental samples employing atomic fluorescence spectrometry – review. TrAC Trends Anal Chem 110:335–343. https://doi.org/10.1016/j.trac.2018.11.017
Filella M, Belzile N, Chen YW (2002a) Antimony in the environment: a review focused on natural waters. I. Occurrence. Earth Sci Rev 57:125–176. https://doi.org/10.1016/S0012-8252(01)00070-8
Filella M, Belzile N, Chen YW (2002b) Antimony in the environment: a review focused on natural waters. II. Relevant solution chemistry. Earth Sci Rev 59:265–285. https://doi.org/10.1016/S0012-8252(02)00089-2
Filella M, Belzile N, Lett MC (2007) Antimony in the environment: a review focused on natural waters. III. Microbiota relevant interactions. Earth Sci Rev 80:195–217. https://doi.org/10.1016/j.earscirev.2006.09.003
Filella M, Williams PA, Belzile N (2009) Antimony in the environment: knows and unknows. Environ Chem 6:95–105. https://doi.org/10.1071/EN09007
Filella M, Belzile N, Chen YW (2012) Human exposure to antimony. II. Contents in some human tissues often used in biomonitoring (hair, nails, teeth). Crit Rev Environ Sci Technol 42:1058–1115. https://doi.org/10.1080/10643389.2011.556540
Filella M, Belzile N, Chen YW (2013a) Human exposure to antimony. III. Contents in some human excreted biofluids (urine, milk, saliva). Crit Rev Environ Sci Technol 43:162–214. https://doi.org/10.1080/10643389.2011.604257
Filella M, Belzile N, Chen YW (2013b) Human exposure to antimony. IV. Contents in human blood. Crit Rev Environ Sci Technol 43:2071–2105. https://doi.org/10.1080/10643389.2013.790741
Frizzarin RM, Portugal LA, Estela JM, Rocha FRP, Cerdà V (2016) On-line lab-in-syringe cloud point extraction for the spectrophotometric determination of antimony. Talanta 148:694–699. https://doi.org/10.1016/j.talanta.2015.04.076
Gál J, Hursthouse AS, Cuthbert SJ (2006) Chemical availability of arsenic and antimony in industrial soils. Environ Chem Lett 3:149–153. https://doi.org/10.1007/s10311-005-0022-1
Gavazov K, Hagarová I, Halko R, Andruch V (2019) Recent advances in the application of nanoparticles in cloud point extraction. J Mol Liq 281:93–99. https://doi.org/10.1016/j.molliq.2019.02.071
Gürkan R, Eser M (2016) Application of ultrasonic–assisted cloud point extraction/flame atomic absorption spectrometry (UA-CPE/FAAS) for preconcentration and determination of low levels of antimony in some beverage samples. J Iran Chem Soc 13:1579–1591. https://doi.org/10.1007/s13738-016-0874-2
Hagarová I, Kubová J (2008) Speciation of antimony in waters using separation coupled with atomic spectrometry. Chem List 102:782–790
Hagarová I, Kubová J, Matúš P, Bujdoš M (2008) Speciation of inorganic antimony in natural waters by electrothermal atomic absorption spectrometry after selective separation and preconcentration of antimony(III) with cloud point extraction. Acta Chim Slov 55:528–534
Hagarová I, Bujdoš M, Matúš P, Čanecká L (2012) The use of two extraction procedures in combination with electrothermal AAS for speciation of inorganic antimony in natural waters. Chem List 106:136–142
He M, Wang N, Long X, Zhang C, Ma C, Zhong Q, Wang A, Wang Y, Pervaiz A, Shan J (2019) Antimony speciation in the environment: recent advances in understanding the biogeochemical processes and ecological effects. J Environ Sci (China) 75:14–39. https://doi.org/10.1016/j.jes.2018.05.023
Herath I, Vithanage M, Bundschuh J (2017) Antimony as a global dilemma: geochemistry, mobility, fate and transport. Environ Pollut 223:545–559. https://doi.org/10.1016/j.envpol.2017.01.057
Hu B, He M, Chen B (2015) Nanometer-sized materials for solid-phase extraction of trace elements. Anal Bioanal Chem 407:2685–2710. https://doi.org/10.1007/s00216-014-8429-9
Huang XS, Guan MX, Lu ZLZ, Hang YP (2018) Determination of trace antimony(III) in water samples with single drop microextraction using BPHA-[C4mim][PF6] system followed by graphite furnace atomic absorption spectrometry. Int J Anal Chem 2018:8045324. https://doi.org/10.1155/2018/8045324
Ibrahim ASA, Al-Farawati R, Hawas U, Shaban Y (2017) Recent microextraction techniques for determination and chemical speciation of selenium. Open Chem 15:103–122. https://doi.org/10.1515/chem-2017-0013
Jackson T (2020) The periodic table: a visual guide to the elements. White Lion Publishing, London. ISBN 9781781319307
Jiang XM, Wen SP, Xiang GQ (2010) Cloud point extraction combined with electrothermal atomic absorption spectrometry for the speciation of antimony(III) and antimony(V) in food packaging materials. J Hazard Mater 175:146–150. https://doi.org/10.1016/j.jhazmat.2009.09.141
Krachler M, Emons H (2001a) Speciation of antimony for the 21st century: promises and pitfalls. TrAC Trends Anal Chem 20:79–90. https://doi.org/10.1016/S0165-9936(00)00065-0
Krachler M, Emons H (2001b) Urinary antimony speciation by HPLC-ICP-MS. J Anal At Spectrom 16:20–25. https://doi.org/10.1039/B007903K
Lebedev A, Sinikova N, Nikolaeva S, Poliakova O, Khrushcheva M, Pozdnyakov S (2003) Metals and organic pollutants in snow surrounding an iron factory. Environ Chem Lett 1:107–112. https://doi.org/10.1007/s10311-002-0004-5
Li YJ, Hu B, Jiang ZC (2006) On-line cloud point extraction combined with electrothermal vaporization inductively coupled plasma atomic emission spectrometry for the speciation of inorganic antimony in environmental and biological samples. Anal Chim Acta 576:207–214. https://doi.org/10.1016/j.aca.2006.06.018
Li YJ, Hu B, He M, Xiang GQ (2008) Simultaneous speciation of inorganic selenium and antimony in water samples by electrothermal vaporization inductively coupled plasma mass spectrometry following selective cloud point extraction. Water Res 42:1195–1203. https://doi.org/10.1016/j.watres.2007.09.002
Li J, Zheng B, He Y, Zhou Y, Chen X, Ruan S, Yang Y, Dai C, Tang L (2018) Antimony contamination, consequences and removal techniques: a review. Ecotoxicol Environ Saf 156:125–134. https://doi.org/10.1016/j.ecoenv.2018.03.024
Lopéz-García I, Rengevicova S, Muñoz-Sandoval MJ, Hernández-Córdoba M (2017) Speciation of very low amounts of antimony in waters using magnetic core-modified silver nanoparticles and electrothermal atomic absorption spectrometry. Talanta 162:309–315. https://doi.org/10.1016/j.talanta.2016.10.044
Maher WA (2009) Antimony in the environment – the new global puzzle. Environ Chem 6:93–94. https://doi.org/10.1071/EN09036
Marguí E, Sagué M, Queralt I, Hidalgo M (2013) Liquid phase microextraction strategies combined with total reflection X–ray spectrometry for the determination of low amounts of inorganic antimony species in waters. Anal Chim Acta 786:8–15. https://doi.org/10.1016/j.aca.2013.05.006
Melchert WR, Rocha FRP (2016) Cloud point extraction in flow-based systems. Rev Anal Chem 35:41–52. https://doi.org/10.1515/revac-2015-0022
Mihucz VG, Záray G (2016) Occurrence of antimony and phthalate esters in polyethylene terephthalate bottled drinking water. Appl Spectrosc Rev 51:183–209. https://doi.org/10.1080/05704928.2015.1105243
Mubarak H, Chai LY, Mirza N, Yang ZH, Pervez A, Tariq M, Shaheen S, Mahmood Q (2015) Antimony (Sb) – pollution and removal techniques – critical assessment of technologies. Toxicol Environ Chem 97:1296–1318. https://doi.org/10.1080/02772248.2015.1095549
Nash MJ, Maskall JE, Hill SJ (2000) Methodologies for determination of antimony in terrestrial environmental samples. J Environ Monit 2:97–109. https://doi.org/10.1039/A907875D
Natasha SM, Khalid S, Dumat C, Pierart A, Niazi NK (2019) Biogeochemistry of antimony in soil–plant system: ecotoxicology and human health. Appl Geochem 106:45–59. https://doi.org/10.1016/j.apgeochem.2019.04.006
Nemček L, Hagarová I (2020) The recent strategies employed in chemical analysis of contaminated waters, sediments and soils as a part of the remediation process: extraction. In: Prasad R (ed) Environmental pollution and remediation. Springer, Singapore
Nithya R, Sivasankari C, Thirunavukkarasu A (2020) Electronic waste generation, regulation and metal recovery: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01111-9
Oorts K, Smolders E (2009) Ecological threshold concentrations for antimony in water and soil. Environ Chem 6:116–121. https://doi.org/10.1071/EN08109
Panhwar AH, Tuzen M, Hazer B, Kazi TG (2018) Solid phase microextraction method using a novel polystyrene oleic acid imidazole polymer in micropipette tip of syringe system for speciation and determination of antimony in environmental and food samples. Talanta 184:115–121. https://doi.org/10.1016/j.talanta.2018.03.004
Pérez-Fernández V, Rocca LM, Tomai P, Fanali S, Gentili A (2017) Recent advancements and future trends in environmental analysis: sample preparation, liquid chromatography and mass spectrometry. Anal Chim Acta 983:9–41. https://doi.org/10.1016/j.aca.2017.06.029
Pyrzynska K (2020) Nanomaterials in speciation analysis of metals and metalloids. Talanta 212:120784. https://doi.org/10.1016/j.talanta.2020.120784
Quiroz W, Arias H, Bravo M, Pinto M, Lobos MG, Cortés M (2011) Development of analytical method for determination of Sb(V), Sb(III) and TMSb(V) in occupationally exposed human urine samples by HPLC-HG-AFS. Microchem J 97:78–84. https://doi.org/10.1016/j.microc.2010.06.015
Rezaee M, Hosseini Y, Aghaee MMR, Ahmadi E, Sana BF (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9. https://doi.org/10.1016/j.chroma.2006.03.007
Samadi-Maybodi A, Rezaei V (2012) A cloud point extraction for spectrophotometric determination of ultra–trace antimony without chelating agent in environmental and biological samples. Microchim Acta 178:399–404. https://doi.org/10.1007/s00604-012-0852-z
Shotyk W, Krachler M (2007) Contamination of bottled waters with antimony leaching from polyethylene terephthalate (PET) increases upon storage. Environ Sci Technol 41:1560–1563. https://doi.org/10.1021/es061511+
Silva MAM, Frescura VLA, Curtius AJ (2000) Determination of trace elements in water samples by ultrasonic nebulization inductively coupled plasma mass spectrometry after cloud point extraction. Spectrochim Acta Part B 55:803–813. https://doi.org/10.1016/S0584-8547(00)00147-6
Smichowski P (2008) Antimony in the environment as a global pollutant: a review on analytical methodologies for its determination in atmospheric aerosols. Talanta 75:2–14. https://doi.org/10.1016/j.talanta.2007.11.005
Smichowski P, Madrid Y, Cámara C (1998) Analytical methods for antimony speciation in waters at trace and ultratrace levels. A review. Fresenius J Anal Chem 360:623–629. https://doi.org/10.1007/s002160050770
Smith LM, Maher WA, Craig PJ, Jenkins RO (2002) Speciation of volatile antimony compounds in culture headspace gases of Cryptococcus humicolus using solid phase microextraction and gas chromatography-mass spectrometry. Appl Organomet Chem 16:287–293. https://doi.org/10.1002/aoc.303
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082. https://doi.org/10.1021/cr300162p
Souza OJM, Tarley CRT (2008) Preconcentration and speciation of Sb(III) and Sb(V) in water samples and blood serum after cloud point extraction using chemometric tools for optimization. Anal Lett 41:2465–2486. https://doi.org/10.1080/00032710802352522
Souza-Silva EA, Jiang R, Rodriguez-Lafuente A, Gionfriddok E, Pawliszyn J (2015) A critical review of the state of the art of solid-phase microextraction of complex matrices 1. Environmental analysis. TrAC Trends Anal Chem 71:224–235. https://doi.org/10.1016/j.trac.2015.04.016
Stalikas CD (2002) Micelle-mediated extraction as a tool for separation and preconcentration in metal analysis. TrAC Trends Anal Chem 21:343–355. https://doi.org/10.1016/S0165-9936(02)00502-2
Templeton DM, Ariese F, Cornelis R, Danielsson LG, Muntau H, Van Leeuwen HP, Łobiński R (2000) Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl Chem 72:1453–1470. https://doi.org/10.1351/pac200072081453
Tschan M, Robinson BH, Nodari M, Schulin R (2009a) Antimony uptake by different plant species from nutrient solution, agar and soil. Environ Chem 6:144–152. https://doi.org/10.1071/EN08103
Tschan M, Robinson BH, Schulin R (2009b) Antimony in the soil – plant system – a review. Environ Chem 6:106–115. https://doi.org/10.1071/EN08111
Ungureanu G, Santos S, Boaventura R, Botelho C (2015) Arsenic and antimony in water and wastewater: overview of removal techniques with special reference to latest advances in adsorption. J Environ Manag 151:326–342. https://doi.org/10.1016/j.jenvman.2014.12.051
Vojteková V, Poperníková Z, Abusenaina AMM (2014) Antimony in various environment components. Chem List 108:135–140. https://doi.org/10.1007/s10311-010-0281-3
Watanabe H (1974) Spectrophotometric determination of cobalt with 1-(2-pyridylazo)-2-naphthol and surfactants. Talanta 21:295–302. https://doi.org/10.1016/0039-9140(74)80007-X
Werner J, Grzeskowiak T, Zgola-Grzeskowiak A, Stanisz E (2018) Recent trends in microextraction techniques used in determination of arsenic species. TrAC Trends Anal Chem 105:121–136. https://doi.org/10.1016/j.trac.2018.05.006
Wu D, Sun S (2016) Speciation analysis of As, Sb and Se. Trends Environ Anal Chem 11:9–22. https://doi.org/10.1016/j.teac.2016.05.001
Yu X, Liu C, Guo Y, Deng T (2019) Speciation analysis of trace arsenic, mercury, selenium and antimony in environmental and biological samples based on hyphenated techniques. Molecules 24:926. https://doi.org/10.3390/molecules24050926
Zurynková P, Dědina J, Kratzer J (2018) Trace determination of antimony by hydride generation atomic absorption spectrometry with analyte preconcentration/atomization in a dielectric barrier discharge atomizer. Anal Chim Acta 1010:11–19. https://doi.org/10.1016/j.aca.2018.01.033
Acknowledgements
The work was supported by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences under the contract VEGA 1/0153/17.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hagarová, I., Nemček, L. (2021). Recent Advances in Speciation Analysis of Trace Antimony in Environmental and Biological Samples Based on Cloud Point Extraction and Spectrometric Methods. In: Lichtfouse, E. (eds) Sustainable Agriculture Reviews 52. Sustainable Agriculture Reviews, vol 52. Springer, Cham. https://doi.org/10.1007/978-3-030-73245-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-73245-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73244-8
Online ISBN: 978-3-030-73245-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)