Abstract
A simple and rapid cloud point extraction methodology has been developed for the separation and determination of palladium, after complexation with benzil mono-(2-pyridyl) hydrazone in acidic medium, using Triton X-114 as nonionic surfactant. Under the optimum experimental conditions, the calibration curve was linear in the range of 0.05–25 µg L−1. The enrichment factor was 104 for a 50 mL sample volume. The limits of detection, based on three times the standard deviation of the blank signal by seven replicate measurements was 0.12 µg L−1. The relative standard deviation (RSD) for seven replicate determination at 5 µg L−1 of palladium was 1.85 %. Under the presence of foreign ions and EDTA as a strong complexing agent, no significant interference was observed. The accuracy of the results was verified by analyzing spiked water samples. The proposed method has been applied for the separation and determination of palladium from synthetic highly active radioactive waste with satisfactory results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Highly active liquid waste (HLW) generated during the irradiation of reactor fuel is a valuable resource of platinum groups metals (PGMs), namely palladium (Pd), ruthenium (Ru) and rhodium (Rh) [1]. Most of these metals are stable during reactor operation and are being targeted now as valuables. The projected estimates [2] indicate that about 2,500–3,000 tonnes of fission PGMs will be produced by the year 2030 as a result of nuclear reactor operation worldwide and only 7,000 tonnes of natural reserves will be left in the same period.
The applications of PGMs in various chemical, pharmaceutical, petroleum and electronic industries and research institutes are well documented and, currently, there is a heavy demand of these metals for various applications [2, 3]. Since the natural abundance of PGMs in the earth crust is very low and those present in mines are also likely to be consumed in another few decades, there is an increased interest towards recovering PGMs from nuclear wastes. In addition, separation of PGMs from waste may indirectly exclude some concerns associated with the management of high-level radioactive waste. These metals increase the melting point of waste glass formed and tend to separate as distinct phase during vitrification leading to a non-homogeneous glass matrix [4]. This problem is more serious and undesirable when the waste originates from reprocessing of fast reactor fuels, in which the amount of fission platinoids are much higher due to the employment of 239Pu as fissile element and higher burn-up [2]. Therefore, removal of PGMs from HLW is desirable prior to immobilization.
To recover the PGMs from HLW, several extraction methods have been reported in the literature [5–17], with particular interest in the separation of Pd.
Cloud point extraction (CPE) is probably one of the most versatile and simple methods for the preconcentration and extraction of metal ions. It has attracted a considerable attention because it complies with the “Green Chemistry” principles [18], as the amount of organic solvent is much less than that of traditional liquid extraction. Moreover, it is simple, cheap, highly efficient, rapid and of lower toxicity than those used organic solvents. The first application of CPE for metal determination was reported by Watanabe et al. [19]. Since then, it has been used for separation and preconcentration of many metal ions in different matrices [20]. The separation mechanism is based on the clouding phenomena of surfactant. Upon heating a micellar solution of a non-ionic surfactant, the surfactant will change from water-soluble to oil-soluble. Above a certain temperature, called the cloud point, it will completely water insoluble and, hence, the surfactant molecules will separate out from the aqueous phase. As a result, the clear solution becomes turbid and phase separation occurs. At the cloud point, the homogenous surfactant-rich phase contains much of the surfactant while the other phase, called the water (or aqueous) phase, contains mostly water and surfactant monomers at a concentration near its critical micelle concentration. Some hydrophobic compounds or organometallic complexes initially present in the solution and bound to the micelles can be favorably extracted and concentrated in a small volume of surfactant-rich phase [21]. No data is available in the literature for the separation of Pd(II) from HLW using CPE.
Several research groups [22–28] have proposed CPE systems for the analysis of Pd(II) (Table 1). Most of the systems are effective at weak acidic to basic pH values. However, at these conditions, the selectivity of the method is hindered by the interference from some metal ions [29] as well as the precipitation of many transition metals as hydroxides from the aqueous solution [30], making the method not applicable for the determination of Pd(II) in acidic media.
In this article, we describe a CPE system for the separation and preconcentration of Pd(II). In the developed system benzil mono-(2-pyridyl) hydrazone (BMPH), scheme 1, was used as the chelating agent and Triton X-114 as a non ionic surfactant. The experimental parameters affecting the CPE efficiency were investigated in detail. The analytical figures of merit and interfering ions tolerance are presented. Finally, the method was successfully applied for the separation of Pd(II) from synthetic HLW.
Experimental
Reagents and solutions
All aqueous solutions were prepared with ultrapure water that had been obtained by Milli-Q water purification system (Millipore, Billerica, MA, USA). Reagents used were of analytical grade from Sigma–Aldrich (St. Louis, MO, USA), Fluka (Buches, Switzerland) or Merck (Darmstadt, Germany). The laboratory glassware was kept overnight in 10 % v/v HNO3 solution. Before the use, the glassware was washed with deionized water and dried in a dust free environment. Stock solution of Pd(II) was prepared by dissolving 0.1664 g of PdCl2 in 100 mL 0.1 mol L−1 HCl solution and was standardized gravimetrically by dimethylglyoxime method [31]. Working standard solutions were obtained by appropriate dilution of the stock standard solutions. BMPH was prepared according to the method of Chiswell [32] by heating under reflux equal stoichiometric amounts of benzil and 2-hydrazinopyridine (0.1 mol L−1) in ethanol (50 mL) in the presence of 1 mL glacial acetic acid for 30 min. One mmol L−1 solution of BMPH was prepared by dissolving appropriate amounts in 100 mL ethanol. Buffer solutions were prepared by 1 mol L−1 hydrochloric acid—1 mol L−1 sodium acetate (pH 0.5–3.5); 0.2 mol L−1 acetic acid—0.2 mol L−1 sodium acetate (pH 4.0–7.0); 2 mol L−1 ammonium chloride—2 mol L−1 ammonium hydroxide (pH 7.5–12.0). Triton X-114 was used without further purification.
Apparatus
A Perkin Elmer atomic absorption spectrophotometer (Model AAnalyst 800) with a longitudinal Zeeman background correction furnished with a transversely heated graphite atomizer (THGA) was used for determination of Pd(II). Sample solutions were injected into the atomizer using AS-800 auto sampler. The sample injection volume was 20 μL. The system is equipped with win Lab 32 software. The instrumental parameters and temperature program for the graphite atomizer were summarized in Table 2. The pH of the solution was adjusted using a Hanna instrument model 8519 digital pH meter(Hanna Instruments, Germany). A thermostated water-bath with a temperature control within ±1 °C (Hänigsen, Germany) Model Kottermann 3006, maintained at the desired temperature, was employed for the cloud-point temperature experiments. A CH90-2 centrifuge (Hinotek Technology Co. Ltd., China) was used to accelerate the phase separation process.
Synthetic HLW
Synthetic HLW was prepared in a manner similar to that reported in literature [33], with the addition of other metal ions such as Th, Hf, Ho and V. The concentration of various metal ions in simulated highly active nuclear waste (nitrate salts preferred) is given in Table 3. Metal powder, metal oxide, sulfate and chloride salts were used in the absence of nitrate salts. Each of them was dissolved separately in hot conc. nitric acid before their addition to one another. Acidity of the simulated HLW was ascertained by alkalimetry in the presence of neutral saturated K2C2O4 solution. The overall acidity of simulated HLW was adjusted by evaporating the solution to near dryness, and then adding 3 M HNO3. Before applying the recommended CPE procedure and to reduce the ionic strength, 5 mL of the HLW was taken and the pH was adjusted to 2 by 0.5 mol L−1 NaOH and the solution was brought to a final volume of 50 mL with deionized water. One mL of this diluted solution were analyzed by the prescribed procedure.
Recommended procedure of CPE
An aliquot of 50 ml of a solution containing Pd(II), buffered with pH 2, Triton X-114 (0.05 % w/v) and 10−5 mol L−1 of BMPH were kept for 10 min in a thermostatic bath at 40 °C. The surfactant-rich phase typically settles through the aqueous phase. The phase separation is accelerated by centrifuging at 4,000 rpm for 10 min. The phases were cooled down in an ice bath in order to increase the viscosity of the surfactant rich phase. The bulk aqueous phase was decanted by inverting the tube and dried in water bath. The surfactant rich phase in the tube was made up to 0.5 ml by adding mixture of methanol/conc. HNO3 (5:1). Twenty µL of the samples were introduced into GFAAS for the determination of Pd(II).
Results and discussion
Optimization of CPE procedure
In order to obtain maximum extraction efficiency by CPE method, several parameters need to be taken into account before analysis of the real samples. The most important are: (1) pH of the sample solution (2) ligand concentration, (3) surfactant concentration, (4) temperature and duration of reaching equilibrium, and (5) ionic strength. All optimization experiments were carried out on standard solution of Pd(II).
Effect of pH
The separation of metal ions by CPE method involves prior formation of a complex with sufficient hydrophobicity to be extracted into the small volume of surfactant rich phase. The pH plays an important role on metal–chelates formation and subsequent extraction. The effect of the pH on metal ions extraction was assessed by varying the pH from 0.5 to 12 using the suitable buffer solution. As can be seen in Fig. 1, with the increase in the pH range, the extraction recovery of Pd(II) increased gradually, and the maximum extraction efficiency was obtained at pH ranging from 2.0 to 4.0. Thus, pH 2.0 was used as the optimum pH for extraction of Pd(II) for the subsequent experiments.
Effect of BMPH concentration
BMPH forms many complexes with different metal ions and has many applications in trace element separation and preconcentration [29, 34, 35]. In general, the concentration of the chelating reagent has a remarkable effect on the extraction efficiency. In order to select the optimal concentration of BMPH, the effect of BMPH concentration in the range of 1–60 µmol L−1 was investigated, while the other experimental parameters remaining constant. The results obtained (Fig. 2) show that the percentage recoveries of Pd(II) were enhanced by increasing the concentration of BMPH up to 10−5 mol L−1, and reaches the plateau afterwards. Thus, BMPH concentration of 10−5 mol L−1 was employed throughout the work.
Effect of Triton X-114
Triton X-114 was chosen because of its commercial availability in a high purified homogeneous form, low toxicological properties and cost. Also, the high density of the surfactant rich phase facilitates phase separation by centrifugation. Additionally, the cloud point (23–26 °C) of Triton X-114 permits its use in the preconcentration of a large number of molecules and chelates. For these reasons, most CPE systems developed for Pd(II) are designed around this non-ionic surfactant (Table 1). The variation on the extraction recovery, as a function of the Triton X-114 concentration is expressed in Fig. 3, when 10 ml solution containing Pd(II) and all the reagents in the presence of 0.01-0.1 % (w/v) Triton X-114 was extracted. Extractions close to 100 % were observed for a surfactant concentration higher than 0.05 w/v. At lower concentrations, the extraction efficiency of the complex was low, due to the inadequacy of the assembly to entrap the complex. The lowest concentration of Triton X-114 possible was chosen in this experiment to minimize the volume of the surfactant rich phase, which should positively impact the preconcentration factor. Hence, a concentration of 0.05 % (w/v) was chosen for further studies.
Effect of equilibrium temperature and time
Equilibrium temperature and time seem to play important roles to achieve easy phase separation. Especially, temperature plays additional role in enhancing preconcentration efficiency and enrichment factors. When the temperature increases, dehydration occurs and results in decreasing the volume of the surfactant-rich phase, increasing the phase–volume ratio. Another important point equilibrium time is necessary to achieve easy separation, preconcentration, and complete extraction. At equilibrium time, metals react with chelating agents and they transport inside the surfactant-rich phase kinetically. It is therefore essential to maintain the equilibrium time and temperature lowest quantitative extraction. The dependence of extraction efficiency upon equilibration temperature was studied in the time range of 20–80 °C, and was found in the temperature range of 40–60 °C the extraction is completed (Fig. 4). Above this, temperatures lead to the decomposition of complexes and the reduction of extraction efficiency. Therefore, an equilibrium temperature of 40 °C was further used in all experiments. The effect of equilibrium time on the extraction efficiency was studied in the range of 5–30 min (Fig. 5), and it was found that equilibrium time of 10 min was adequate for the extraction of Pd(II).
Effect of centrifugation time and rate
Generally, centrifugation time and rate never affect micelle formation but accelerate the separation process. The effect of the centrifugation time on the extraction efficiency was studied at 5–25 min at 4,000 rpm. A centrifugation time of 10 min was selected. Since the extraction of Pd(II) is almost quantitative, no appreciable improvements were observed for longer time.
Effect of ionic strength
The cloud point of micellar solutions can be controlled by addition of salts, alcohols, non-ionic surfactants and some organic compounds (salting-out effects). To date, most of the studies conducted have shown that ionic strength has no appreciable effect on the extraction efficiency [36]. Therefore, to investigate the influence of ionic strength on extraction efficiency, various experiments were performed by adding different amounts of NaNO3 (0–1 mol L−1) and the rest of the experimental conditions were kept constant. Based on the obtained results, the addition of NaNO3 within the interval of 0–0.5 mol L−1 had no significant effect on the CPE efficiency. The analytical signal decreased considerably by increasing NaNO3 concentrations (>0.5 mol L−1). This effect might be explained by the additional surface charge when the NaNO3 concentration is very high, thus changing the molecular architecture of the surfactant and consequently the micelle formation process [25]. These results indicate that samples of high ionic strength such as HLW must be diluted before applying the procedure to decrease the ionic strength.
Effect of methanol
In the phase separation step, the surfactant-rich phase with high viscosity was settled. The addition of a diluent, such as methanol/conc. HNO3 mixture (5:1) reduces the surfactant phase viscosity. There is an optimum volume (0.5 mL) with respect to the metal ion recovery. Larger volumes of acidified methanol, dilution are clearly predominated resulting in a gradual absorbance reduction. 0.5 mL of the methanol/conc. HNO3 mixture is therefore used throughout the remaining experiments.
Interferences
In order to investigate the selectivity of the method, 10 mL of the sample solution containing 5 µg L−1 of Pd(II) was extracted under the specified experimental conditions in the presence of high concentration of various cations and anions, usually present in real samples. An ion was considered to be interfering when it caused a variation greater than ±5 % in the recovery percentage of the samples. The results are tabulated in Table 4. As can be seen, the recoveries of the Pd(II) in these cases were almost quantitative, meaning that the method described is applicable to the analysis of Pd (II) in different samples. Also, in the present study, the addition of EDTA as a strong chelating agent did not affect the separation process with any ratio in the range of the ionic strength (1 mol L−1). So, the method is also applicable in the presence of complexing agents without digestion step to remove the organic matrix.
Analytical performance
The analytical characteristics of the optimized method, including linear range, limit of detection (LOD), repeatability and enrichment factor are summarized in Table 5. The results indicate that the graph of absorbance versus metal ion concentration was linear in the range of 0.05–25 µg L−1. The correlation coefficients (R 2) values was 0.9993. According to the IUPAC recommendations, LOD was calculated as the concentration of the analyte yielding a signal equivalent to three times the standard deviation of the reagent blank signal (n = 7). Under the optimum conditions, the LOD value for Pd(II) was 0.12 µg L−1. Furthermore, for preconcentration of 50 ml of the working standard solution based on the slope ratio of calibration curve with and without preconcentration, an enrichment factor of 104 was obtained. The relative standard deviations (RSD) of our method, obtained for seven determinations of Pd(II) was 1.85 %.
Analysis of spiked water samples
The proposed CPE methodology was applied to the determination of Pd(II) in spiked water samples to estimate the accuracy of the procedure. Water samples (i.e., tap water, sea water, river water and mineral water) were filtered using a 0.45 µm pore size membrane filter to remove suspended particulate matter and aliquots of water (10 mL) were subjected to the recommended procedure. Each type of water was spiked with variable amounts of Pd(II) to assess matrix effects. The results are shown in Table 6. Good agreement was obtained between the added and found analyte contents using the recommended procedure.
Comparison with other methods
The analytical characteristics of the proposed method were compared with methods in the literature (Table 1). The proposed methodology shows significant improvement regarding the limit of detection and the enrichment factor, compared to the methods already published. The working in more acidic conditions (pH 2) is another advantage of our procedure, to avoid the interferences caused by the precipitation of transition metal hydroxides in the extraction process [29].
Palladium separation from synthetic HLW
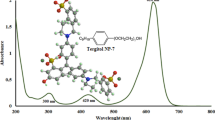
The study was applied for Pd(II) recovery from a synthetic HLW with composition shown in Table 3. This is the composition of HLW that is based on the fission product inventory of spent fuel from a pressurized heavy water reactor with burn-up of 6,500 MWd/ton of UO2 after three years of cooling [32]. The quantity of waste produced is assumed to be 800 L/ton of fuel [37]. Results presented in Table 7 show that this method gives very good precision (1.68 %SD) and high, more than 98 %, accuracy. This indicated that the separation procedure developed is free of interferences from the HLW constituents. The above results suggest the interesting feasibility of using CPE methodology for the recovery of Pd(II) from HLW.
Conclusion
The proposed approach is a sensitive method for Pd(II) preconcentration. The method is simple, easy to use and economic. Another interesting feature of the method is speed. It leads to excellent extraction efficiencies. The method works in strongly acidic conditions (pH 2), avoiding the interferences caused by the precipitation of transition metal hydroxides in the Pd(II) extraction process. Cloud point extraction also has an advantage over traditional solvent extraction because toxic solvents are replaced by minimal quantities of surfactant, leading to a high preconcentration factor. As can be shown in Table 1, the characteristic data of the present method are better or comparable with those reported in the literature. This technique provides good precision, simplicity, ease of operation, low detection limits and good recovery within a short time compared to the other techniques.
References
Jenson GA, Platt AM, Mellinger GB, Bjorklund WJ (1984) Recovery of Noble metals from fission products. Nucl Technol 65:305–324
Ache HJ, Baestle LH, Bust RP, Nechaev AF, Popik VP, Ying Y (1989) Feasibility of separation and utilization of ruthenium, rhodium, and palladium from high level waste, vol 308. IAEA Technical Report Series, Vienna
Kolarik Z, Renard EV (2005) Potential applications of fission platinoids in industry. Platin Met Rev 49:79–90
Sundaram SK, Perez JM Jr (2000) Noble metals and spinel settlings in high level waste glass melters. PNNL-13347. Pacific Northwest National Laboratory, Richland
Kolarik Z, Renard EV (2003) Recovery of valuable fission platinoids from spent fuel: Part I. General considerations and basic chemistry. Platin Met Rev 47:74–87
Kolarik Z, Renard EV (2003) Recovery of valuable fission platinoids from spent fuel: Part II separation processs. Platin Met Rev 47:123–131
Ruhela R, Singh KK, Tomar BS, Sharma JN, Kumar M, Hubli RC, Suri AK (2012) Amberlite XAD-16 functionalized with 2-acetyl pyridine group for the solid phase extraction and recovery of palladium from high level waste solution. Sep Pur Technol 99:36–43
Jayakumar M, Venkatesan KA, Srinivasan TG, VasudevaRao PR (2009) Studies on the feasibility of electrochemical recovery of palladium from high-level liquid waste. Electrochim Acta 54:1083–1088
Giridhar P, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2006) Extraction of fission palladium by aliquat 336 and electrochemical studies on direct recovery from ionic liquid phase. Hydrometallurgy 81:30–39
Dakshinamoorthy A, Dhami PS, Naik PW, Dudwadkar NL, Munshi SK, Dey PK, Venugopal V (2008) Separation of palladium from high level liquid waste of PUREX origin by solvent extraction and precipitation methods using oximes. Desalination 232:26–36
Zhang A, Jiang J, Chai Z (2013) Preparation of a macroporous silica-based multidentate soft-ligand material and its application in the adsorption of palladium and the others. Sep Sci Technol 48:1500–1509
Zhang A, Wang X, Chai Z (2010) Synthesis of a macroporous silica-based derivative of pyridine material and its application in separation of palladium. AIChE J 56:3074–3083
Zhang A, Xue W, Chai Z (2012) Preparation of a macroporous silica–pyridine multidentate material and its adsorption behavior for some typical elements. AIChE J 58:3517–3525
Zhang A, Kuraoka E, Hoshi H, Kumagai M (2004) Synthesis of two novel macroporous silica-based impregnated polymeric composites and their application in highly active liquid waste partitioning by extraction chromatography. J Chromatogr A 1061:175–182
Zhang A, Kuraoka E, Kumagai M (2006) Removal of Pd(II), Zr(IV), Sr(II), Fe(III), and Mo(VI) from simulated high level liquid waste by extraction chromatography utilizing the macroporous silica-based polymeric materials. Sep Purif Technol 50:35–44
Zhang A, Kuraoka E, Kumagai M (2007) Preparation of a novel macroporous silica-based 2,6-bis(5,6-diisobutyl-1,2,4-triazine-3-yl)pyridine impregnated polymeric composite and its application in the adsorption for trivalent rare earths. J Radioanal Nucl Chem 274:455–464
Zhang A, Zhu Y, Liu Y, Chai Z (2011) Preparation of a macroporous silica-based pyridine impregnated material and its adsorption for palladium. Ind Eng Chem Res 50:6898–6905
Anastas P, Eghbali N (2010) Green Chemistry: Principles and Practice. Chem Soc Rev 39:301–312
Watanabe H, Tanaka H (1978) A non-ionic surfactant as a new solvent for liquid–liquid extraction of zinc(II) with 1-(2-pyridylazo)-2-naphthol. Talanta 25:585–589
Ojeda CB, Rojas FS (2012) Separation and preconcentration by cloud point extraction procedures for determination of ions: recent trends and applications. Microchim Acta 177:1–21
Pytlakowska K, Kozik V, Dabioch M (2013) Complex-forming organic ligands in cloud-point extraction of metal ions: a review. Talanta 110:202–228
Bakircioglu D (2012) Cloud point extraction for the preconcentration of palladium and lead in environmental samples and determination by flow injection flame atomic absorption spectrometry. Environ Sci Pollut Res 19:2428–2437
Tong S, Jia Q, Song N, Zhou W, Duan T, Bao C (2011) Determination of gold(III) and palladium(II) in mine samples by cloud point extraction preconcentration coupled with flame atomic absorption spectrometry. Microchim Acta 172:95–102
Tavallali H, Yazdandoust S, Yazdandoust M (2010) Cloud point extraction for the preconcentration of silver and palladium in real samples and determination by atomic absorption spectrometry. Clean-Soil, Air, Water 38:242–247
Ghaedi M, Shokrollahi A, Niknam K, Niknam E, Najibi A, Soylak M (2009) Cloud point extraction and flame atomic absorption spectrometric determination of cadmium(II), lead(II), palladium(II) and silver(I) in environmental samples. J Hazard Mater 168:1022–1027
Tavakoli L, Yamini Y, Ebrahimzadeh H, Nezhadali A, Shariati S, Nourmohammadian F (2008) Development of cloud point extraction for simultaneous extraction and determination of gold and palladium using ICP-OES. J Hazard Mater 152:737–743
Shokoufi N, Shemirani F, Shokoufi M (2009) Laser induced-thermal lens spectrometry after cloud point extraction for the determination of trace amounts of palladium. Spectrochim Acta A 74:761–766
Lian Y, Zhen W, Tai Z, Yang Y, Song J, Li Z (2012) Cloud point extraction and flame atomic absorption spectrometry analysis of palladium, platinum, and gold ions from industrial polluted soil. Rare Met 31:512–516
Hassanien MM (2009) FAAS determination of palladium after its selective recovery by silica modified with hydrazone derivative. Microchim Acta 167:81–89
Labrecque C, Potvin S, Whitty-Le´ veille´ L, Lariviere D (2013) Cloud point extraction of uranium using H2DEH[MDP] in acidic conditions. Talanta 107:284–291
Jeffery GH, Bassett J, Mendham J, Denney RC (1989) Vogel’s Textbook of Quantitative Chemical Analysis, 5th edn. Longman Scientific and Technical, New York, p 463
Chiswell V, Lions F, Tomlinson ML (1964) Tridentate Chelate Compounds. IV. Metal complexes from α-diketone mono-α-pyridyl hydrazone Type Ligands. Inorg Chem 3:492–499
Chitnis RR, Wattal PK, Ramanujam A, Dhami PS, Gopalakrishnan V, Mathur JN, Murali MS (1998) Separation and recovery of uranium, neptunium, and plutonium from high level waste using tributyl phosphate: countercurrent studies with simulated waste solution. Sep Sci Technol 33:1877–1887
Pflaum RT, Tucker ES (1971) Spectrophotometric determination of cobalt with benzil mono (2-pyridyl)hydrazone. Anal Chem 43:458–466
Berger SA (1983) The solvent extraction of Cu(II), Ni(II), and Co(II) with benzil-mono-(2-pyridylhydrazone). Microchim Acta 81:333–340
Safavi A, Abdollahi H, Hormozi Nezhad MR (2004) Cloud point extraction, preconcentration and simultaneous spectrophotometric determination of nickel and cobalt in water samples. Spectrochim Acta, Part A 60:2897–2901
Dakshinamoorthy A, Dhami PS, Naik PW, Dudwadkar NL, Munshi SK, Deya PK, Venugopal V (2008) Separation of palladium from high level liquid waste of PUREX origin by solvent extraction and precipitation methods using oximes. Desalination 232:26–36
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassanien, M.M., Mortada, W.I. & Kenawy, I.M. Selective separation of palladium from synthetic highly active liquid waste by cloud point extraction using benzil mono-(2-pyridyl)hydrazone and Triton X-114. J Radioanal Nucl Chem 303, 261–269 (2015). https://doi.org/10.1007/s10967-014-3430-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3430-5