Abstract
Oil-in-water nanoemulsion (NE) formulations of mustard oil known for prominent antimicrobial activities have been prepared, characterized and tested against anthracnose pathogens Colletotrichum musae and Colletotrichum capsici. Physico-chemical properties viz., appearance, stability, pH, centrifugation, storage-stability, thermodynamic stress, persistent foam, droplets size and zeta potential were tested for the NE formulations. The NE formulations were prepared using low energy emulsification process with the optimized composition (w/w) of mustard oil (5%), Tween 20 and sodium dodecyl benzene sulfonate emulsifiers blend (20–30%), co-surfactants (4%), and de-ionized water. The required hydrophile-lipophile balance value for emulsifier blends was determined as 11.39–11.74. Only 3 (viz., NF2, NF3 and NF6) out of 15 NE formulations with transparent faint yellow color (pH 6.3) passed all the physico-chemical parameters tested. Among these, NF2 (foam height < 1 mL) was selected for further study. The average diameter (23 nm), polydispersity index (0.38) and zeta potential (− 12 mV) of mustard oil droplets in NF2 indicated the formation of a stable and homogeneous NE formulation. Application of NF2 exhibited low level of sensitivity to C. capsici and C. musae with maximum growth inhibition (6.2–7.3%) at 1.0% dose. Out of 18 phytochemicals detected in mustard oil by gas chromatography-mass spectrometry, 11 have been reported with antifungal properties including trans-13-octadecenoic acid with the highest relative abundance. The observed antifungal activity may be attributed to the presence of at least some or all the identified phtytochemicals in mustard oil. Further bio-assay on sensitive plant pathogenic fungi is underway to explore the application of the developed NE formulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Application of nanotechnology as efficient and economic delivery system has become popular in various fields viz., agricultural production, animal feed production, crop protection, food processing, food additives and pharmaceuticals (Peters et al. 2016). The nanotechnology has brought radical change in current mode of pesticides application minimizing the dosage of synthetic chemical or biochemical inputs used in plant protection (Fortunati et al. 2016). Nanoformulation of pesticides particularly the nanoemulsion (NE) is preferred to overcome the demerits of conventional emulsifiable concentrate (EC) formulation. The colloidal dispersion state of the extremely small droplets (≤ 100 nm diameter) produced in NE make the system transparent or translucent and thermodynamically stable (Sole et al. 2012; Gupta et al. 2016) by resisting the physical destabilization caused by gravitational separation, flocculation and coalescence (Ostertag et al. 2012).
Among many protection and preservation methods, anti-microbials of plant origin and its delivery in nanoform assumes significance in inhibiting the pathogenic microbes on food items either in raw or in processed form. Out of eight species of mustard (Brassica spp.; Family: Brassicaceae), the oil extracted from three species (Brassica nigra L., Brassica juncea L. and Brassica alba L.) exhibited prominent antimicrobial activities against many fungal and bacterial pathogens responsible for diseases in animals and plants (Aguilar-gonzalez et al. 2015; Reyes-Jurado et al. 2019). But their application in plant protection was limited due to their low solubility in water (Bhargava et al. 2015). The primary anti-microbial component of mustard oil, a non-phenolic volatile compound, allyl isothiocyanate, AIC (54.8–68.8%) was reported to inhibit a variety of pathogenic microorganisms under the normal delivery system (Kim et al. 2015).
Colletotrichum spp. (Family: Glomerellaceae) have been considered notorious among the ten most economically important fungal plant pathogens in the world causing anthracnose disease in more than 121 genera from 45 families (Farr et al. 2016) leading to heavy losses in affected crops like bananas, cassavas, legumes, and cereals worldwide (Dean et al. 2012; Rizwana 2018). Some species of this genus also infect crop at pre-harvest stage, remain as latent and express symptom on crops particularly fruits after the harvest or during storage or transit for the market. Colletotrichum musae and Colletotrichum capsici are two such pathogens of banana and chilli respectively causing serious postharvest anthracnose disease and reducing their commercial shelf-life to a great extent (Diao et al. 2017; Vilaplana et al. 2018). Postharvest treatment of banana and chilli by dipping in synthetic chemicals may lead to residual toxicity (Palou 2018). Attempts have also been made using botanical solutions for the control of anthracnose disease (Bazie et al. 2014; Rashid et al. 2015). But, till date, no biochemical fungicides of plant origin have been registered across the globe for use in pre and postharvest control of anthracnose in banana and chilli (Kumar and Kudachikar 2017).
The present experimentation was, therefore, intended to develop a stable ready to use NE formulation of mustard oil from Brassica nigra; and to evaluate its in-vitro antifungal properties against two important postharvest anthracnose pathogens viz., C. musae and C. capsici; and also to understand the active chemical constituents, other than AIC, present in mustard oil responsible for the antimicrobial effect.
Materials and methods
Oil of black mustard (Brassica nigra) was collected from the oil processing unit of Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, Nadia, West Bengal, India. Reagent like sodium dodecyl benzene sulfonate (SDBS) was purchased from India Glycol, India; Tween-20 from Sigma Aldrich, India and glycerin from Merck, India. Reagents were used without further purification. De-ionised water (Elix EE-22, Milli-Q, Merck) was used for all the formulations. Anhydrous calcium chloride (0.304 g) and magnesium chloride hexa-hydrate (0.139 g) were dissolved in distilled water and made up to 1 L to prepare standard hard water of 342 ppm (CIPAC MT 181995).
GC–MS analysis of mustard oil
Gas chromatography-mass spectrometry (GC–MS) is a technique for the quantitative and qualitative analysis of secondary metabolites of plant extract (Purkait et al. 2019). The crude mustard oil was diluted with HPLC grade hexane in a ratio of 1:200 and centrifuged at 8000 rpm for 5 min. Supernatant was filtered through 0.2µ Nylon 66 membrane filter paper with the help of syringe (SGE Analytical Science Pvt. Ltd, India). The filtrate obtained was transferred into a vial for GC–MS analysis.
Thermo Fisher Scientific GC (Trace 1300) instrument, equipped with the mass selective detector with triple quadruple analyzer (TSQ Duo 8000) and triplus RSH autosampler controlled by Xcalibur V3.1 software was used for the identification of phyto-chemical constituents. The electron ionization energy of 70 eV, ion-source temperature of 250 °C and the interface temperature of 290 °C were maintained. A splitless injection with 285 °C injector temperature was employed. A fused silica column made up of 5% phenyl methyl polysiloxane (TG-5MS, 30 m × 0.25 mm i.d. and 0.25 µm film thickness, Thermo Scientific, USA) was used. The oven temperature was programmed as follows: initially 70 °C hold for 1 min then increased up to 150 °C @ 25 °C/min, then increased up to 200 °C @ 5 °C/min and again increased up to 300 °C @ 12 °C/min holding for 6 min. Total run time was 28.65 min. Identification of components of mustard oil was done by tuning the obtained mass spectral data with NIST & Wiley library search.
Preparation of nanoemulsion (NE) formulation
NE preparation can be classified as low and high energy emulsification. Emulsification was performed using a modification of the low energy emulsification method proposed by Zhang et al. (2019) by mixing an oily phase (blends of emulsifier and oil) and an aqueous phase (blends of glycerin and propylene glycol in de-ionised water). Oily phase was formed by blending of two emulsifiers (SDBS and Tween 20) with mustard oil. Blends of Tween 20 and SDBS were in a range of 1:1–1:6 ratios. Mustard oil (5% w/w) was added drop wise into different blends of emulsifier with slow mixing by magnetic stirrer at 50–60 °C for final preparation of oily phase. Aqueous phase was formed by adding two co-surfactants, propylene glycol (2% w/w) and glycerol (2% w/w) in de-ionised water. Propylene glycol acts as an anti-freezing agent and glycerol acts for better stability and dispersion of the organic phase into continuous phase.
The oil-in-water NE formulations were prepared using mustard oil (5% w/w), blends of emulsifier (Tween 20 and SDBS, 20–30% w/w), blends of co-surfactant (4% w/w) and de-ionised water (61–71% w/w) to make 100%. A total of fifteen mustard oil NEs (NF1–NF15) were prepared by mixing oil and blend of emulsifiers in three different ratios (1:4, 1:5 and 1:6) as shown in Table 1.
The two emulsifiers were blended in five different ratios (1:1–1:4 and 1:6) and mixed with oils @ 20, 25 and 30%, w/w. Transparent faint yellow stable NE was formed by adding oily phase into aqueous phase under mixing in a magnetic stirrer at 500–600 rpm at room temperature (32 °C). All the NE formulations were tested for stability and subjected to further physico-chemical characterization.
Determination of hydrophilic–lipophilic balance (HLB)
The HLB value of emulsifier appears as a critical step for the development of emulsion formulations. The blends of emulsifiers with a wide range of HLB values can provide a satisfactory HLB value for the development of stable emulsions of mustard oil. This approach relies on the fact that stable formulations with low mean droplet size can be obtained when the HLB value of oil coincides with HLB values of blended emulsifiers (Fernandes et al. 2013). A set of emulsions were prepared by blending a couple of emulsifiers in a wide range of HLB value. Thus, the required HLB of emulsifiers was determined by calculating the HLB value of two emulsifiers (A and B) blends as shown in equation (i) which plays a significant role to develop the stable formulation (Pawignya et al. 2016).
where, WA and WB is the mass fraction of the emulsifier A and B, respectively.
Physico-chemical characteristics of nanoemulsion (NE)
Studies on physico-chemical properties like pH, persistency in foam, emulsion stability and centrifugation of developed nanoemulsions were evaluated following the protocol laid down in Collaborative International Pesticides Analytical Council (CIPAC) and Indian Standard (IS) specifications (BIS 1997). All the experiments were repeated thrice.
Measurement of pH
The pH value of NEs was measured using a pH meter (Systronics, model 335, Gujarat, India) and immersing the electrode into 1% aqueous solution of the formulated product at 25 ± 1 °C. Before the readings were recorded, pH meter was calibrated using buffer solutions viz., pH 7, 4 and 9.2 (Merck, India).
Determination of foam persistency
Persistency of foam is a measure of the amount of foam likely to be present in a spray tank after dilution of the product with water. Formulated product (2 mL) was taken in graduated cylinder (100 mL) containing hard water and made up the volume to 100 mL mark. The cylinder was stoppered, inverted 30 times at 180° and kept undisturbed at 32 °C for 1 min. The volume of foam formed at the top was measured (CIPAC MT 47.21995).
Determination of emulsion stability
Emulsion stability is a measure of uniformity of the dispersion throughout the solution in a spray tank after dilution of the product with water. After foam test, the emulsion was kept undisturbed at 32 °C for one hour to check the formation of any creamed layer at the top and deposition of any sediment at the bottom (CIPAC MT 36.32003).
Observation on phase separation after centrifugation
Each formulation was centrifuged at 5000 rpm for 30 min at room temperature (32 °C) and observed for phase separation, if any.
Determination of storage stability
The formulations which were stable on centrifugation and passed the emulsion stability tests were further tested for storage stability as per the described protocol of CIPAC MT 46.3 (2000). The formulations were stored at three different temperatures (4, 25 and 54 °C) for 14 days, which is equivalent to 2 years shelf-life at ambient temperature (27 ± 2 °C) (CIPAC MT 46.3 2000). After 14 days the formulations were checked for the formation of phase separation or any change in appearance (Table 1). The test was performed in triplicate at three different temperatures for all the formulations developed.
Testing of thermodynamic stability
The formulated NE was subjected to the following different thermodynamic stress tests.
Heating–cooling cycle
This was carried out by keeping the NE for 48 h at 4 °C followed by 48 h at 40 °C temperature. The cycle was repeated thrice.
Freezing–thawing stress
This was performed by keeping the formulated NE alternatively at 25 °C for 48 h and at – 18 °C for 48 h. The cycle was repeated thrice.
Pseudo-ternary phase diagram
The combination of mustard oil (MO), emulsifiers and co-surfactant and water which produced stable NE formulation is presented using the pseudo-ternary phase diagram (Azeem et al. 2009). Every corner of the diagram corresponds to 100% of oil, emulsifier blends and water. The formulations were prepared using a fixed percent of MO (5% w/w), variable percentage of emulsifiers and co-surfactant (24–34% w/w) and the rest part was filled with de-ionized water. In the ternary phase diagram, co-surfactant was assumed in the water part. The phase diagram was drawn using MS Excel.
Droplet size distribution, polydispersity index (PDI) and Zeta-potential
Mean droplet size and size distribution (polydispersity index) of developed formulation were determined by dynamic light scattering (DLS) at 90° using a Zetasizer (NanoS90, Malvern Instruments, UK). Measurements were performed in quintuplicate and average droplet size was expressed as the mean diameter.
The zeta potential was determined by Electrophoretic Light Scattering (ELS), using a Zetasizer (Nano-ZS, Malvern Instruments, UK). This equipment measures the velocity and direction of charged particles when an electrical field is applied (electrophoretic mobility). The formulation was diluted with ultra-pure Milli-Q water (1:25) prior analysis to minimize the multiple scattering effects caused due to the viscosity of formulated product (Riquelme et al. 2019). All experiments were performed in triplicates and the average values were accepted.
In-vitro bioassay against Colletotrichum musae and Colletotrichum capsici
Isolation and maintenance of pure culture
The banana anthracnose pathogen C. musae was isolated from ripe infected banana (cv. Martaman) following single spore isolation technique and was identified based on the colony morphology, morphometric characteristic of acervuli, seta, conidia and conidiophores according to the method described by Prittesh et al. (2016). C. capsici infected chilli fruits (cv. Beldanga) were cut from the margin of lesions into small pieces (5 mm diameter). The pieces were surface sterilized in an aqueous solution of 0.1% mercuric chloride (w/v), washed five–six times, dipped in streptocyclin and transferred on potato dextrose agar (PDA) culture plate. Mycelial bids from culture plates were transferred later onto PDA slants and allowed 8–10 days for sporulation. The pure culture of C. capsici was prepared by single spore isolation technique following the same procedures as described above and was identified based on the colony morphology, by measuring mycelia, conidia, conidiophores, acervuli as per standard description given by Booth and Sutton (1984).
The pure culture of fungi was maintained on PDA medium at 4 °C. Pathogen inocula were prepared by using the conidia of 10 day old cultures growing on PDA in petri plates. Conidia were dislodged from the surface of the media by flooding the plates with sterile distilled water and gentle scraping with a sterile slide. The suspensions were filtered through thin layer of absorbent cotton wool to remove the mycelial fragments and adjusted the spore number in suspension to 106 conidia/mL with a hemocytometer. Such spore suspension was used for inoculation of banana finger and chili in in-vitro testing of pathogenicity.
In-vitro bioassay of nanoemulsions (NE)
The in-vitro bioassay of the NE was conducted to evaluate the antifungal activity following poison food technique based on the inhibition in mycelial radial growth of C. musae and C. capsici on PDA (Jang and Kulk 2018). The selected formulation (NF2) with three doses (0.1, 0.5 and 1.0%) was added to conical flasks containing previously sterilized and cooled PDA medium. Unamended medium (without formulation) was used as control. After thorough mixing, 15 mL of medium at a temperature of 40–45 °C was poured into sterilized petri plates (9 cm diameter). Mycelial discs (7 mm diameter) from five-day-old C. musae and C. capsici cultures growing on PDA plates were lifted aseptically and placed separately at the centre of PDA plates. All the treatments and control plates were replicated thrice. The plates were then incubated at 28 ± 1 °C. The test pathogens were allowed to grow till full growth of the mycelia was attained in control plates. Final data on mycelial growth of C. musae and C. capsici were recorded from both the treated and control plates at 6 and 8 days, respectively after inoculation when the full mycelial growth (9 cm) of each control plates was attained. Percentage inhibition of mycelial growth was calculated using the following formula (Dutta et al. 2019);
where, dc is the average radial growth (cm) of test fungus in control; dt is average radial growth (cm) of the test fungus in treated plates.
Results
Properties of nanoemulsion (NE) formulation
The formulations were prepared using low energy methods without application of external mechanical force but using the intrinsic physical properties of the system. Based on the ratios of emulsifier blends, percent content of emulsifiers and ratio of oils and emulsifier, fifteen different formulations (NF1–NF15) were prepared (Table 1). The HLB values obtained for Tween 20 and SDBS were 16.72 and 10.50 respectively. The value of HLB blend was found constant (11.39) for a fixed ratio of emulsifiers (1:6). But the values of HLB blend increased from 11.39 to 13.61 with the change in the ratio of Tween 20 and SDBS from 1:6 to 1:1 (Table 1).
The pH of the formulations was found in the slightly acidic range (6.23–6.68). However, the formulations become almost neutral at application concentration (0.1–1%). Six formulations (NF1, NF7, NF10, NF11, NF13 and NF14) were separated into the oil phase and failed the stability test at room temperature. Nine formulations (NF2 to NF6, NF8, NF9, NF12 and NF15) passed the stability test (after 1 h) at room temperature of which four formulations were transparent-faint yellow (NF2, NF3, NF5 and NF6) and rest five were translucent. Out of the four transparent formulations, three formulations (NF2, NF3 and NF6) passed all the tests considered including storage and thermodynamic stability test (Table 1). There was no flocculation observed and no creamy layer was separated after 14 days of storage.
With the change in the ratio of oil and emulsifier from 1:4 to 1:6, the properties like, appearance and stability of formulation at room temperature, stability of emulsion, pH, centrifugation, cold test, storage and thermodynamic stability were changed. There were four types of appearance of the formulation viz., transparent faint yellow, translucent, opaque, and phase separation. The ratio of oil and emulsifier at 1:4 in formulation failed to qualify all the physico-chemical tests conducted. With the increase in emulsifier content to 1:5 and 1:6 the quality of the formulations improved and passed all the tests as observed in NF2, NF3 and NF6 (Table 1). Among the improved formulations, NF2 was the best one as it produced less foam height (< 1 mL) compared to NF3 and NF6 (Table 1).
Droplet size and zeta potential of nanoemulsion (NE)
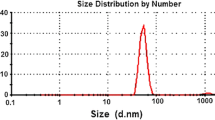
A complete picture of droplet size population and distribution in NF2 was obtained by analysis of data generated by DLS. The mustard oil NE appeared transparent and had an average particle size of 23 nm with polydispersity index (PDI) of 0.38 as measured by DLS (Fig. 1). From the figure, it was reflected that thirty percentage of droplet having sizes ~ 3 nm. The surface of NE was negatively charged with an average Zeta Potential of − 12 mV as measured by DLS Zetasizer.
Phase diagram of nanoemulsions (NE)
Behavior of the equilibrium phase of three component systems in the NE i.e. water/emulsifier/mustard oil is presented using the pseudo-ternary phase diagram (Fig. 2). The region of the phase diagram with small circles represents the compositions of transparent monophasic NE formulation consisting of mustard oil (5% w/w), emulsifier blends and co-surfactant (24–34% w/w) and water (61–71% w/w).
GC–MS analysis of mustard oil
Analytical chromatogram of mustard oil obtained by GC–MS analysis revealed the presence of several phytochemicals (Fig. 3). Five major [2-Decenal, (Z); 2,4-Decadienal, (E,E); 2-Undecanal; trans-13-Octadecenoic acid; Oleic acid, 3-(octadecyloxy) propyl ester] and thirteen minor peaks of bioactive constituents [Sitosterol; Campesterol; 6-Methyloctadecane; 17-Octadecynoic acid; 8-Heptadecene; 9-Hexadecenoic acid; Octadecane, 6-methyl; trans-13-Octadecenoic acid, methyl ester; cis-Vaccenic acid; Olein, 2 mono; cis-13-Octadecenoic acid; Glycidyl oleate; Ethyl iso-allocholate] were identified by comparing data with the NIST & Wiley library as listed in Table 2.
Antifungal activity of mustard oil nanoemulsion (NE)
The effect of mustard oil nanoformulation at three concentrations (0.1, 0.5 and 1.0%) was evaluated on radial growth and percent inhibition of two post harvest plant pathogens viz., C. musae and C. capsici as compared to control. Mean radial growth of the said two fungi decreased gradually with the increment in concentrations (0.1–1.0%) of nanoformulation (Table 3). The mean radial growth of tested fungi at higher concentration (1%) differed significantly (p < 0.05) over the other treatments (0.1 and 0.5%) as well as over the control set. The growth inhibition of C. capsici and C. musae was found to be 0.6–6.2% and 0.3–7.3%, respectively after six and eight days of inoculation (Table 3). Mustard oil NE exhibited maximum growth inhibition of 7.3% in C. musae and 6.2% in C. capsici at the highest concentration (1%).
Discussion
NE droplets with diameter in the order of 100 nm and transparent or translucent appearance are generally prepared using high and low energy methods. In the present investigation we used low energy method for the preparation of NE of mustard oil. Out of fifteen formulations (NF1 to NF15) prepared, two formulations (NF3 and NF6) using oil and emulsifier blend in the ratio of 1:6 and one formulation (NF2) using 1:5 ratio passed all the physico-chemical parameters considered. Out of the three short listed formulations, NF2 was selected as the best as it produced low foam height (< 1 mL) compared to NF3 and NF6. The formation of less foam which might be due to its low emulsifier content is most desirable attribute in terms of persistency and stability during the intended shelf life (Chang et al. 2012) and low phyto-toxicity (Galvez et al. 2018; Lechuga et al. 2016). Emulsifiers used in the low energy emulsification system lowered the interfacial tension between aqueous and oil phase and executed the formation of oil droplets in nano-scale (Hu et al. 2016). The HLB value (11.39) of the finally selected formulation (NF2) falls within the acceptable range of 8–18 for the stable oil-in-water NE (Mohamed et al. 2017).
DLS study confirmed the formation of nano droplets of mustard oil in NF2 formulation with average droplet size of 23 nm was better than the previously reported droplet size of 430 nm (Ghosh et al. 2012). The extremely small size of the nano-droplets will resist gravity separation, flocculation, coalescence and creaming due to Brownian motion making the formulation kinetically stable for at least 1 year at room temperature (Roy and Guha 2018). The PDI value (range: 0.08–0.70; average: 0.38) of the droplet ensures homogeneous distribution of droplets size and also confirms transparent or translucent nature of NE. A PDI value greater than 0.7 indicates a very broad and heterogeneous distribution of droplet size (Danaei et al. 2018).
The magnitude of zeta potential can predict the stability of emulsion. Emulsion with higher magnitude of zeta potential exhibits increased stability due to large electrostatic repulsion between particles. The zeta potential of ± 10 is considered critical because of its instability and the values higher than the critical range makes the formulation stable (Luesakul et al. 2019). Therefore, the mustard oil NE (NF2) was stable due to its measured zeta potential of − 12 mV. The negative charge of mustard oil NE might have been originated from the free fatty acids and other polar constituents present in the oil phase (Table 2) which may adsorb on to the surface of the emulsion (Bhargava et al. 2015). To the best of our knowledge, this is the first report showing mustard oil NE with a reduced droplet diameter by low energy emulsification method.
Among the 18 phytochemicals identified in mustard oil using GC–MS followed by comparison with NIST and Willey library, 11 compounds have been reported to possess antifungal properties (Table 2; Fig. 4). However, allyl isothiocyanate (AIC) could not be detected in the crude oil as it was not listed in the NIST & Wiley library. Among the antifungal compounds, trans-13-octadecenoic acid (X) with the highest relative abundance (peak area: 37.26%, Fig. 3) has also been reported earlier for antifungal activity (Mohy El-Din et al. 2018; Parera-Valadez et al. 2019). Occurrence of other antifungal compounds viz., Campesterol (XVII) and Sitosterol (XVIII) have also been reported in mustard oil by GC–MS analysis (Khan et al. 2016). The fatty acids alkyl ester (XII) has been reported for prominent antimicrobial activity against different plant pathogens viz., Penicillium digitatum, Botrytis cinerea and powdery mildew fungi (Savage et al. 1991; Jumina et al. 2019; Pinto 2017) and also exhibited a potent synergy with fungicides (Coleman 2002). Moreover, the fatty acids with prominent insecticidal activities have also been reported (Aider et al. 2016). Therefore, the presence of at least some or all the above mentioned phytochemicals may be instrumental for the observed antifungal activity of the mustard oil nanoformulation.
Chemical structure of antifungal compounds identified in mustard oil using GC–MS (Pubchem 2019)
The antimicrobial activities of mustard oil have mostly been reported against animal pathogenic bacteria and animal pathogenic fungi (Peng et al. 2014; Reyes-Jurado et al. 2019). A few plant pathogenic fungi viz., Aspergillus niger, Penicillium expansum, Rhizopus stolonifer, Botryotinia fuckeliana, etc. (Clemente et al. 2019) and a plant pathogenic bacteria (viz., Pectobacterium carotovorum) showed sensitivity to antimicrobial compounds present in mustard oil (Clemente et al. 2016). The observed mycelial growth inhibition of two plant pathogenic fungi C. musae and C. capsici by mustard oil NE @ 1.0% was low (6.2–7.3%) but significant (< 0.05) as compared to control and the lower doses (0.1–0.5%). Different species of fungi or even the isolates of the same fungal species may differ in their sensitivity against fungicides (Panja et al. 2013). Therefore, it is not unusual to observe such low level of sensitivity of the two Colletotrichum spp. to antifungal properties of mustard oil NE. Till date little information is available regarding the sensitivity of Colletotrichum spp. to mustard oil formulation and nano-formulation. Therefore, scope remains to explore the application of NE of mustard oil for protection of crops against pre- and postharvest plant pathogens.
Conclusions
In the present study, development and use of environmentally compatible, non-phytotoxic mustard oil-based nanoemulsions in the control of food borne pathogen fungi C. musae and C. capsici was green concept. Generally, use of mustard oil in water nanoemulsions as a nanofungicide has great potential for the replacement of the traditional emulsified oil. Mustard oil nanoemulsion is highly a stable product for long duration, improvement of the biological efficacy. Chemical characterization showed a significant variety of antifungal compounds are present in mustard oil. In the earlier several research works, crude mustard oil exhibited prominent antifungal efficacy was reported. In the present bioassay studies of mustard oil nanoemulsion against C. musae and C. capsici, percent inhibitions were marginal or less but it was significant at 5.0% label. This study can be useful in understanding the potential of mustard oil nanoemulsion in food processing against pre and post harvest plant pathogens. Industries also may have a great commercial value for the uses of edible oil based biofungicide as a green preservative agent in broad spectrum in near future.
References
Aguilar-Gonzalez AE, Palou E, Lopez-Malo A (2015) Antifungal activity of essential oils of clove (Syzygium aromaticum) and/ or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov Food Sci Emerg Technol 32:181–185. https://doi.org/10.1016/j.ifset.2015.09.003
Aider FA, Kellouche A, Fellag H, Debras JF (2016) Evaluation of the bio-insecticidal effects of the main fatty acids of olive oil on Callosobruchus maculatus F. (Coleoptera-Bruchidae) in cowpea (Vigna unguiculata L.). J Plant Dis Protect 123:235–245. https://doi.org/10.1007/s41348-016-0034-z
Azeem A, Rizwan M, Ahmad FJ, Iqbal Z, Khar RK, Aqil M, Talegaonkar S (2009) Nanoemulsion components screening and selection: a technical note. AAPS Pharm Sci Tech 10:69–76. https://doi.org/10.1208/s12249-008-9178-x
Bazie S, Ayalew A, Woldetsadik K (2014) Integrated management of postharvest banana anthracnose (Colletotrichum musae) through plant extracts and hot water treatment. Crop Prot 66:14–18. https://doi.org/10.1016/j.cropro.2014.08.011
Bhargava K, Conti DS, Rocha SRPD, Zhang Y (2015) Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol 47:69–73. https://doi.org/10.1016/j.fm.2014.11.007
BIS Specification (1997) Indian standard methods of test for pesticides and their formulations, IS: 6940-1982, Reaffirmed, pp 21–26
Booth C, Sutton BC (1984) Fusarium pallidoroseum, the correct name for F. semitectum. Trans Br Mycol Soc 83:702–704. https://doi.org/10.1016/S0007-1536(84)80193-X
Chang Y, McLandsborough L, McClements DJ (2012) Physical properties and antimicrobial efficacy of thyme oil nanoemulsions: influence of ripening inhibitors. J Agric Food Chem 60:12056–12063. https://doi.org/10.1021/jf304045a
CIPAC MT 18 (1995) Preparation of Standard waters A and D. In: Dobrat W, Martijn A (eds) CIPAC handbook F. Physico-chemical methods for technical and formulated pesticides. Collaborative International Pesticides Analytical Council Ltd., Harpenden, pp 59–62
CIPAC MT 36.3 (2003) Emulsion stability and re-emulsification. In: Dobrat W, Martijn A (eds) CIPAC handbook K. Physico-chemical methods for technical and formulated pesticides. Collaborative International Pesticides Analytical Council Ltd., Harpenden, p 137
CIPAC MT 46.3 (2000) Accelerated storage procedure. In: Dobrat W, Martijn A (eds) CIPAC handbook J. Physico-chemical methods for technical and formulated pesticides. Collaborative International Pesticides Analytical Council Ltd., Harpenden, p 128
CIPAC MT 47.2 (1995) Persistent foaming. In: Dobrat W, Martijn A (eds) CIPAC handbook F. Physico-chemical methods for technical and formulated pesticides. Collaborative International Pesticides Analytical Council Ltd., Harpenden, pp 152–153
Clemente I, Aznar M, Silva F, Nerin C (2016) Antimicrobial properties and mode of action of mustard and cinnamon essential oils and their combination against foodborne bacteria. Innov Food Sci Emerg Technol 36:26–33. https://doi.org/10.1016/j.ifset.2016.05.013
Clemente I, Aznar M, Nerin C (2019) Synergistic properties of mustard and cinnamon essential oils for the inactivation of foodborne moulds in-vitro and on Spanish bread. Int J Food Microbiol. https://doi.org/10.1016/j.ijfoodmicro.2019.03.012
Coleman RD (2002) Fungicide compositions, patent no: US 7741244B2. Available via DIALOG. https://patentimages.storage.googleapis.com/1d/74/c1/589a81fcf3b2fc/US7741244.pd. Accessed 2 Feb 2020
Danaei M, Dehghankhold M, Ataei S, Davarani FH, Javanmard R, Dokhani A, Khorasani S, Mozafari M (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10:57. https://doi.org/10.3390/pharmaceutics10020057
Dean R, Kan JALV, Pretorius ZA, Hammond-Kosack KE, Pietro AD, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430. https://doi.org/10.1111/j.1364-3703.2011.00783.x
Diao YZ, Zhang C, Liu F, Wang WZ, Liu L, Cai L, Liu XL (2017) Colletotrichum species causing anthracnose disease of chilli in China. Persoonia 38:20–37. https://doi.org/10.3767/003158517x692788
Dutta S, Woo EE, Yu SM, Nagendran R, Yun BS, Lee YH (2019) Control of anthracnose and gray mold in pepper plants using culture extract of white-rot fungus and active compound schizostatin. Mycobiology 47:87–96. https://doi.org/10.1080/12298093.2018.1551833
Farr DF, Rossman AY, Palm ME, McCray EB (2016) Fungal databases, systematic botany and mycology laboratory, ARS, USDA. Available via DIALOG. https://nt.ars-grin.gov/fungaldatabases/. Accessed 2 Feb 2020
Fernandes CP, Mascarenhas MP, Zibetti FM, Lima BG, Oliveira RPRF, Rocha L, Falcao DQ (2013) HLB value, an important parameter for the development of essential oil phytopharmaceuticals. Braz J Pharmacogn 23:108–114. https://doi.org/10.1590/S0102-695X2012005000127
Fortunati E, Rescignano N, Botticella E, Fiandra DL, Renzi M, Mazzaglia A, Torre L, Kenny JM, Balestra GM (2016) Effect of poly(dl-lactide-co-glycolide) nanoparticles or cellulose nanocrystals-based formulations on Pseudomonas syringae pv. tomato (Pst) and tomato plant development. J Plant Dis Protect 123:301–310. https://doi.org/10.1007/s41348-016-0036-x
Galvez A, Lopez-Galindo A, Pena A (2018) Effect of different surfactants on germination and root elongation of two horticultural crops: implications for seed coating. N Zeal J Crop Hortic. https://doi.org/10.1080/01140671.2018.1538051
Ghosh V, Mukherjee A, Chandrasekaran N (2012) Mustard oil microemulsion formulation and evaluation of bactericidal activity. Int J Pharm Pharm Sci 4:497–500
Gupta A, Eral HB, Hatton TA, Doyle PS (2016) Nanoemulsions: formation, properties and applications. Soft Matter 12:2826–2841. https://doi.org/10.1039/c5sm02958a
Hu Q, Gerhard H, Upadhyaya I, Venkitanarayananb K, Luo Y (2016) Antimicrobial eugenol nanoemulsion prepared by gum arabic and lecithin and evaluation of drying technologies. Int J Biol Macromol 87:130–140. https://doi.org/10.1016/j.ijbiomac.2016.02.051
Jang SJ, Kuk YI (2018) Effects of different fractions of Rheum palmatum root extract and anthraquinone compounds on fungicidal, insecticidal, and herbicidal activities. J Plant Dis Protect 125:5. https://doi.org/10.1007/s41348-018-0179-z
Jumina J, Mutmainah M, Purwono B, Kurniawan YS, Syah YM (2019) Antibacterial and antifungal activity of three monosaccharide monomyristate derivatives. Molecules 24(20):3692. https://doi.org/10.3390/molecules24203692
Kim HY, Gornsawun G, Shin II-S (2015) Antibacterial Activities of isothiocyanates (itcs) extracted from horseradish (Armoracia rusticana) root in liquid and vapor phases against 5 dominant bacteria isolated from low-salt jeotgal, a Korean salted and fermented seafood. Food Sci Biotechnol 24:1405–1412. https://doi.org/10.1007/s10068-015-0180-2
Kumar A, Kudachikar VB (2017) Antifungal properties of essential oils against anthracnose disease: a critical appraisal. J Plant Dis Protect 125:133–144. https://doi.org/10.1007/s41348-017-0128-2
Lechuga M, Fernandez-Serrano M, Jurado E, Nunez-Olea J, Rios F (2016) Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotox Environ Safe 125:1–8. https://doi.org/10.1016/j.ecoenv.2015.11.027
Luesakul U, Puthong S, Sansanaphongpricha K, Muangsin N (2019) Quaternized chitosan-coated nanoemulsions: a novel platform for improving the stability, anti-inflammatory, anti-cancer and transdermal properties of Plai extract. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115625
Mohamed AIA, Sultan AS, Hussein IA, Al-Muntasheri GA (2017) Influence of surfactant structure on the stability of water-in-oil emulsions under high-temperature high-salinity conditions. J Chem. https://doi.org/10.1155/2017/5471376
Mohy El-Din SM, Mohyeldin MM (2018) Component analysis and antifungal activity of the compounds extracted from four brown seaweeds with different solvents at different seasons. J Ocean Univ China 17:1178–1188. https://doi.org/10.1007/s11802-018-3538-2
Ostertag F, Weiss J, McClements DJ (2012) Low-energy formation of edible nanoemulsions: factors influencing droplet size produced by emulsion phase inversion. J Colloid Interface Sci 388:95–102. https://doi.org/10.1016/j.jcis.2012.07.089
Palou L (2018) Postharvest treatments with GRAS salts to control fresh fruit decay. Hortic 4:46. https://doi.org/10.3390/horticulturae4040046
Panja BN, Das A, Saha J (2013) Fungicide tolerance management of isolates of rice sheath blight pathogen (Rhizoctonia solani Kuhn). J Mycopathol Res 51:163–168
Parera-Valadez Y, Yam-Puc A, Lopez-Aguiar LK, Borges-Argaez R, Figueroa-Saldivar MA, Caceres-Farfan M, Marquez-Velazquez NA, Prieto-Dava A (2019) Ecological strategies behind the selection of cultivable actinomycete strains from the yucatan peninsula for the discovery of secondary metabolites with antibiotic activity. Microb Ecol. https://doi.org/10.1007/s00248-019-01329-3
Pawignya H, Prasetyaningrum A, Dyartanti ER, Kusworo TD, Pramudono B (2016) Estimation hydrophilic–lipophilic balance number of surfactants. AIP Conf Proc 1710:030055. https://doi.org/10.1063/1.4941521
Peng C, Zhao SQ, Zhang J, Huang GY, Chen LY, Zhao FY (2014) Chemical composition, antimicrobial property and microencapsulation of Mustard (Sinapis alba) seed essential oil by complex coacervation. Food Chem 165:560–568. https://doi.org/10.1016/j.foodchem.2014.05.126
Peters RJB, Bouwmeester H, Gottardo S, Amenta V, Arena M, Brandhoff P, Marvin HJP, Agnieszka M, Moniz FB, Pesudo LQ, Rauscher H, Schoonjans R, Undas AK, Vettori M, Weigel S, Aschberger K (2016) Nanomaterials for products and application in agriculture, feed and Food. Trends Food Sci Technol 54:155–164. https://doi.org/10.1016/j.tifs.2016.06.008
Pinto MEA, Araujo SG, Morais MI, Sa NP, Lima CM, Rosa CA, Siqueira EP, Johann S, Lima LARS (2017) Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. Ann Braz Acad Sci 89:1671–1681. https://doi.org/10.1590/0001-3765201720160908
Prittesh P, Amaresan N, Rushabh S, Krishnamurthy R, Bhasker VV (2016) Isolation and pathogenic variability of Colletotrichum falcatum causing red rot in sugarcane. J Plant Dis Protect 123:273–277. https://doi.org/10.1007/s41348-016-0044-x
Pubchem NIH US (2019) National Library of Medicine, National central for biotechnology information. Retrieved on December 09, 2019. Available via DIALOG. https://pubchem.ncbi.nlm.nih.gov/. Accessed 2 Feb 2020
Purkait A, Biswas S, Saha S, Hazra DK, Roy K, Biswas PK, Ghosh SK, Kole RK (2019) Formulation of plant based insecticides, their bio-efficacy evaluation and chemical characterization. Crop Prot 125:104907. https://doi.org/10.1016/j.cropro.2019.104907
Rashid MM, Kabir MH, Hossain MM, Bhuiyan MR, Khan MAI (2015) Eco-friendly management of chilli anthracnose (Colletotrichum capsici). Int J Plant Pathol 6:1–11. https://doi.org/10.3923/ijpp.2015.1.11
Reyes-Jurado F, Cervantes-Rincon T, Bach H, Lopez-Malo A, Palou E (2019) Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind Crop Prod 131:90–95. https://doi.org/10.1016/j.indcrop.2019.01.036
Riquelme N, Zuniga RN, Arancibia C (2019) Physical stability of nanoemulsions with emulsifier mixtures: replacement of tween 80 with quillaja saponin. LWT Food Sci Technol 111:760–766. https://doi.org/10.1016/j.lwt.2019.05.067
Rizwana H (2018) Postharvest control of anthracnose lesions and its causative agent, Colletotrichum musae by some oils. Cell Mol Biol (Noisy-le-grand) 64:52–58. https://doi.org/10.14715/cmb/2018.64.4.9
Roy A, Guha P (2018) Formulation and characterization of betel leaf (Piper betle L.) essential oil based nanoemulsion and its in-vitro antibacterial efficacy against selected food pathogens. J Food Process Preserv 42:e13617. https://doi.org/10.1111/jfpp.13617
Savage SD, Evans SL, Havgood RA, Zomer PS (1991) Fatty acid based compositions for the control of established plant infections, patent no: US 6136856A. Retrieved on December 09, 2019. Available via DIALOG. https://patentimages.storage.googleapis.com/4c/e1/cb/07bdf0891f4215/WO1992019104A2.pdf. Accessed 2 Feb 2020
Sole I, Solans C, Maestro A, Gonzalez C, Gutierrez JM (2012) Study of nanoemulsion formation by dilution of microemulsions. J Colloid Interface Sci 376:133–139. https://doi.org/10.1016/j.jcis.2012.02.063
Vilaplana R, Pazmino L, Valencia-Chamorro S (2018) Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biol Technol 138:56–63. https://doi.org/10.1016/j.postharvbio.2017.12.008
Zhang L, Zhang F, Fan Z, Liu B, Liu C, Meng X (2019) DHA and EPA nanoemulsions prepared by the low-energy emulsification method: process factors influencing droplet size and physicochemical stability. Int Food Res 121:359–366. https://doi.org/10.1016/j.foodres.2019.03.059
Khan SA, Shahid S, Jameel M, Ahmad A (2016) In vitro antibacterial, antifungal and GC-MS analysis of seeds of mustard brown. Int J Pharm Chem. 6(4):107–115. https://doi.org/10.7439/ijpc.v6i4.3185
Acknowledgements
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We are grateful to Bidhan Chandra Krishi Viswavidyalaya (BCKV) for providing infrastructural facilities.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RKK and BNP were conceived and designed the experiments; AP and REW were contributed reagents, materials, performed the experiments, analyzed and interpreted the data and wrote the paper; DKH and DB were analyzed and interpreted the data; PKB was wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Purkait, A., Worede, R.E., Baral, D. et al. Development of nanoemulsion formulation of mustard oil, its chemical characterization and evaluation against post harvest anthracnose pathogens. Indian Phytopathology 73, 449–460 (2020). https://doi.org/10.1007/s42360-020-00237-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-020-00237-8