Abstract
Denak (Oliveria decumbens Vent.) is an endemic aromatic plant belonging to Apiaceae family. This study evaluated the antifungal activity of denak essential oil (EO) nano-emulsions formulated by ultrasonic emulsification on Penicillium digitatum, the main causal agent of postharvest decay of citrus fruit. EO chemical compositions detected by gas chromatography demonstrated that thymol (28.5%), carvacrol (26.2%), myristicin (17.5%) and elemicin (9.9%) were the dominant components. Denak EO nano-emulsions with droplet diameter of 26.9 nm formulated by ultrasonic emulsification. Inhibition of mycelial growth was observed at EO concentrations higher than 0.5 µL mL−1. The antifungal activity of the EO increased with increasing the concentration of EO. The minimal inhibitory concentration of EO nano-emulsion was found to be 0.5 µL mL−1. These results demonstrate the potential of ultrasonication for preparing denak EO nano-emulsion which could be used as a suitable treatment to control postharvest diseases caused by P. digitatum and possibly other pathogens. This newly formulated denak EO nano-emulsion can be applied as a useful treatment for prevention of citrus losses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Citrus fruits are among the most important economic products in the international fruit market. However, it is very sensitive to decay, especially due to Penicillium digitatum and Penicillium italicum pathogens, causing green and blue molds [1]. Fungal infections are the main causes of postharvest decay of fresh fruits and vegetables during different stages of the postharvest chain, thereby leading to important economic losses and waste [2]. The use of antifungal agents including a number of synthetic fungicides such as imazalil to control fungal spoilage has been a common practice for the last few decades [3]. However, the continuous use of fungicides to control postharvest diseases produce resistant isolates of fungi with multiple fungicide resistance. In addition, there is as increasing public concern regarding the contamination of fruit and vegetables with fungicidal residues [4]. Considering the public awareness about harmful effects of chemical compounds on both human health and the environment, and increasing consumers demand to use natural and healthy food products [5], the current research was conducted to develop safer and eco-friendly alternative strategies for replacing synthetic chemicals with natural substances in the food industry.
In previous research, it was found that coating ʻThomson navelʼ oranges with shellac enriched with cinnamon EO improved fruit storability [6]. Biological approaches such as the application of nanocompounds and plant bioactive derivatives in essential oils (EO) are promising techniques to ensure human health by replacing synthetic fungicides for the control of citrus postharvest diseases. Some plant products have been recognized and used for food protection [5, 7, 8]. EOs are natural complex compounds derived from aromatic plants, containing many compounds at different concentrations with potent antifungal, antiviral, and antibacterial activities, which are widely used for food preservation [9,10,11]. They can be used directly or in other forms such as edible coatings [12].
Nanotechnology has made significant advances in various fields of science and product innovation, including nano-emulsions or nanometric scale emulsions for their potential applications in the food, agriculture, cosmetic, and pharmaceutical industries [9]. Nano-emulsions or nanometric scale emulsions are metastable submicron oil-in-water dispersions, which have a droplet diameter of 1–100 nm [10]. They can penetrate into or be absorbed by cells efficiently [11]. Nano-emulsions have also received attention as antimicrobial agents, given their particular physicochemical properties such as their nanometric droplet size and high physical stability [12].
EO can be used as oil cores in oil-in-water nano-emulsions [13]. Nano-emulsions of EO as active agents have shown efficient antimicrobial properties against different pathogens [14]. Denak (Oliveria decumbens Vent.) is an anuual herbaceous plant indigenous to subtropical regions of Iran, Iraq, Syria and Turkey. It has been used traditionally as a medicinal plant against some diseases [15]. There is scarce literature about the beneficial effects of this plant EO. So, this study was carried out in order to investigate the in vitro activity of denak essential oil (DEO) nano-emulsion prepared by ultrasonication against P. digitatum (Pers.) Sacc.
Materials and methods
Plant material and essential oil preparation
The denak (Oliveria decumbens) flowers were collected at the full-bloom stage from their natural habitat in the city of Khonj, Fars province, Iran (53° 24′ N, 27° 57′ E) from June to September, 2017 (Fig. 1). The flowers were air dried at 25–30 °C and packed in dark high density polyethylene bags and kept in a cool place (22 °C, RH = 40%) for further experiments. For each experiment, required quantity of the flowers were crushed. The hydro-distillation was carried out using a Clevenger apparatus, according to the description of British Pharmacopoeia (1980), with a magnetic heater stirrer (220 V, 1 kW) as heating source. One hundred g of powder was extracted with 1 L distilled water until no measurable essential oil was extracted (about 3 h). The essential oil was collected, dried under anhydrous sulfate and stored in dark air-tight vials at 4 °C for further analysis and preparation of nano-emulsion. Each extraction was performed at least three times. The yield (%) was evaluated by dividing the volume of essential oil extracted (mL) into the weight of dry flowers (g), multiplied by 100 [16].
Essential oil components identification and quantification
The DEO chemical composition determination was carried out using a gas chromatograph (Agilent 7890A, Santa Clara, CA, USA) coupled with mass spectrometer (Agilent 5975C, USA) and a mass fused silica capillary column (HP-5 MS, 30 m length × 0.25 mm internal diameter × 0.25 μm film thickness, J & W Scientific, Folsom). The samples were injected at split ratio of 1:50. Helium was used as a carrier gas at a flow rate of 1 mL min−1 at temperatures of 230, 280, and 280 °C for ion-source, injector, and detector, respectively. The oven temperature program initiated at 60 °C and linearly increased to 210 °C at 3 °C min−1 followed by a 20 °C min−1 rise to 240 °C and a 8.5 min isothermal time. Mass spectra were recorded from 50 to 480 atomic mass unit (amu) at 70 eV in the electronic ionization (EI) mode, as described by Damyeh and Niakousari [17]. Individual components were identified by comparison of mass spectra with those in the NIST and Wiley libraries.
Nano-emulsion preparation
The oil-in-water nano-emulsions were prepared as described by Salvia-Trujillo et al. [18] with some modifications. Briefly, water (94% v/v) was added to a mixture of DEO and tween 80 (HLB 15) (1:1 v/v) at room temperature under gentle magnetic stirring (60 rpm) for 15 min. The coarse emulsion was then sonicated for 10 min using a 20 kHz ultrasonic processor with a 750 W output (Bandelin, Sonopuls HD 4200, Bandelin Elec., Berlin, Germany). Sonication of nano-emulsion was accomplished using a 13 mm ultrasonic horn, which kept in about 20 mm below the surface while keeping the content cooled using an ice bath.
Nano-emulsion characterization
The hydrodynamic diameter (Dh) and polydispersity index (PDI) of DEO nano-emulsions were determined by Dynamic Light Scattering (DLS) using a Horiba SZ-100 nanoparticle analyzer (Horiba, Kyoto, Japan) equipped with a DPSS laser (λ = 532 nm) at a scattering angle of 90° at 25 °C. The particle size and size distribution of the nano-emulsion were also monitored after being diluted with an aqueous solution of tween 80 (3% v/v).
Antifungal activity
Mold strain
The fungal strain, Penicillium digitatum IR1037c, was provided by the Iranian Research Institute of Plant Protection (IRIPP). After 2 min of immersion in ethanol, ʻValenciaʼ oranges (Citrus sinensis cv. Valencia) as a commercial cultivar were dried and artificially wounded at two sites around the stem end using a 5 mm cork-borer. The wounds were then inoculated with mycelial plugs cut from the periphery of a 7-day old culture of P. digitatum on potato dextrose agar (PDA, Sigma-Aldrich, St. Louis, MO). The oranges were stored at room temperature (22 °C, RH = 40%) for 5 days until visual mold growth was observed. The grayish olive-green mold was isolated from fruit skin by washing with sterile water and then plating on PDA. The mold isolate was then purified by the single-spore method [19], and grown on PDA at 25 °C for 7 days. The identity of the isolate was confirmed by macroscopic observations and microscopic examination (grayish olive colonies, reverse yellowish-brown, unequal growth and branching, short conidiophores and conidial chains, cylindrical to oval, smooth-walled conidia) [20] (Fig. 2).
Screening for antifungal activity
The antifungal activity of DEO nano-emulsion against P. digitatum was assessed using a disc diffusion assay [21]. The spore suspension was prepared by washing a 7-day old PDA culture of the isolate with 2 mL of normal saline (9 g L−1 NaCl) and adjusted to 106 conidia mL−1 using a Neubauer’s chamber. Molten PDA was then seeded with conidia inoculum to give a final concentration of 104 conidia mL−1. Solutions with lower concentrations of essential oil (0.05, 0.10, 0.20, 0.25, 0.30, 0.50, 1.0, 1.5, 2.0, 2.5 µL mL−1) were obtained by diluting the stock DEO nano-emulsion with an aqueous solution of tween 80 (3% v/v). 6 mm-diameter blank disks were placed on the agar surface and inoculated with 10 µL aliquots of various dilutions of nano-emulsion. Distilled water, tween 80, and DEO were used as controls. After 72 h incubation at 25 °C, the plates were examined for growth inhibition zones around the disks.
Determination of fungistatic activities
Minimal inhibitory and fungicidal concentrations of DEO nano-emulsion against P. digitatum were determined by the method of Puškárová et al. [22]. The stock solution of DEO nano-emulsion in tween 80 was added to molten PDA to give final concentrations of essential oil of 0.20, 0.25, 0.30, 0.50, 1.0, 1.5, 2.0, and 2.5 µL mL−1. The plates were then inoculated at the center with 5 mm fungal plugs from the outer margin of 7-day old cultures of P. digitatum and incubated at 25 °C for 7 days. The controls used included PDA without any added dispersant and the culture medium mixed with tween 80. The radial growth of the mycelial plugs was measured at 24-h time intervals. At the end of the incubation time, the plugs with no visible growth were re-inoculated on fresh PDA and incubated at the same temperature for an additional 7 days to assess their viability. Minimal inhibitory concentration (MIC) was defined as the lowest concentration allowing growth revival of the inhibited mycelial plugs.
Statistical analysis
Data were analyzed using IBM SPSS 21.0 statistical software (IBM Corp., Armonk, NY, USA) and comparisons were performed through Mann–Whitney U test. The significant differences were evaluated at P < 0.05.
Results and discussion
Essential oil components
The production yield of DEO was 2% (v/w) which was well comparable to the values reported by Esmaeili et al. [23] (2.8%). The flowering stage of denak has been reported as the phenological stage with the highest essential oil yield.
A total of 37 compounds were identified using GC–MS, representing 99.97% of the DEO (Table 1). Thymol was the most abundant compound accounting for 28.53% of the total peak area, followed by carvacrol (26.22%), myristicin (17.49%), and elemicin (9.95%). These findings are in line with previous studies reporting thymol and carvacrol as the major components of the DEO [23, 24]. Differences in EO composition can be attributed to several factors such as geographical area and the time and stage of plant collection. The antimicrobial effect of a given EO depends on its chemical composition, possibly its phenolic components [25].
Nano-emulsion characterization
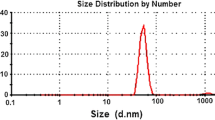
The mean droplet size and PDI of DEO nano-emulsion were 26.9 nm and 0.36, respectively. The droplet size decreased with increasing the dilution of the nano-emulsion, while the PDI values remained < 0.57.
The average drop size (DS) of DEO nano-emulsion was 26.9 nm (Fig. 3). Tween 80 effectively minimized the droplet diameter; much more efficient than other polymers [26]. Indeed, surface-active agents acted as emulsifiers and reduced the free energy needed to prepare nano-emulsions by reducing the tension between the faces in the oil/water interface [27]. With an increase in surfactant concentration (by dilution), the mean droplet diameter of the emulsion was decreased.
These results are in line with those of Gutierrez et al. [28] indicating that the minimum droplet size can be obtained with a low oil surfactant ratio. Mean DS (Z-averages) and PDI are indeed very important indicators that describe the quality, uniformity and dispersibility of the nano-emulsions [29]. Ultrasonication results in smaller dispersed water droplets and DS in emulsion. Polydispersity shows the range of size or the homogeneity of the particle, thereby the smaller the value, the more homogenous the emulsion. Polydispersity index (PDI) is the ratio of molecular weight averages, which is used to show molecular weight distributions (MWD) or the heterogeneity ratio [30]. Polydispersity, in turn, shows the extent of consistency and cohesion of the DS in the emulsion [31]. The PDI measured with a range of 0.06–0.574 indicated that all nano-emulsions had a relatively narrow range of size distribution. Likewise, the DS increased with an increase in the concentration of the oil phase in the formulations, while it decreased with dilution (which in turn increased surfactant concentration). The DS of the nano-emulsions is a function of oil surfactant weight ratio [32].
Antifungal activity
In the disk diffusion assay, the DEO nano-emulsion showed antifungal activity against P. digitatum at concentrations higher than 0.5µL mL−1 (Fig. 4A). The lower concentrations of DEO nano-emulsion (0.20, 0.25, 0.30 µL mL−1) were found to reduce the mycelium radial growth of P. digitatum on the culture medium. By contrast, there was no growth in the presence of concentrations higher than 0.50 µL mL−1 (Figs. 4B and 5). The inhibitory activity of DEO nano-emulsion against P. digitatum increased in a concentration-dependent manner, as inhibition percentage values of 24.1, 34.3, 56.7% were obtained at the concentrations of 0.20, 0.25, and 0.30 µL mL−1, respectively. Full growth inhibition was observed at concentrations equal to or above 0.50 µL mL−1. Tween 80 did not significantly affect the growth rate (Fig. 6). All the mycelial plugs with no evident growth (at DEO nano-emulsion concentrations of 0.50 µL mL−1 or higher) showed recovered activity following transfer to non-treated PDA. The concentration of 0.50 µL mL−1 was, therefore, recorded as MIC of DEO nano-emulsion against P. digitatum. In line with our results, the incorporation of basil EO to chitosan improved antimicrobial activity [33].
A Disk diffusion assay showing antifungal activity of denak essential oil (DEO) nano-emulsion against Penicillium digitatum IR1037c at different concentrations: (a) 0.5 µL mL−1, (b) 1.0 µL mL−1, (c) 1.5 µL mL−1, (d) 2.0 µL mL−1, (e) 2.5 µL mL−1, (f) tween 80. B Growth inhibition of Penicillium digitatum IR1037c by different concentrations of DEO nano-emulsion [0.0 (control), 0.20, 0.25, 0.30, 0.50, 1.0, 1.5, and 2.0 µL mL−1] after 7 days of incubation at 25 °C
Radial growth of Penicillium digitatum IR1037c on potato dextrose agar plates containing different concentrations (µL mL−1) of denak essential oil nano-emulsion [0.0, filled square; 0.20 1, filled triangle; 0.25, open square; 0.30, open circle; ≥ 0.50 (0.50, 1.0, 1.5, 2.0, and 2.5), open triangle; tween 80, filled circle] after 7 days at 25 °C. Values are the mean of six replicates. Error bars indicate standard error of the mean
Growth inhibition of Penicillium digitatum IR1037c by different concentrations of denak essential oil (DEO) nano-emulsion [0.20, 0.25, 0.30, and ≥ 0.50 (0.50, 1.0, 1.5, 2.0, and 2.5) µL mL−1] in potato dextrose agar during 7 days of incubation at 25 °C. No growth was observed on days 0 and 1 for all treatments including the control (without DEO). Values are given as mean of six replicates. Error bars indicate standard error of the mean. *P < 0.05; **P < 0.01 indicate statistically signifcant growth diferences compared to the control on the same day (Mann–Whitney U test)
To the best of our knowledge, this is the first report on the use of DEO nano-emulsion in the medium against P. digitatum. It has been reported that addition of 250 µL L−1 of oregano and thyme EO to the PDA medium inhibits the mycelium growth and spore production of P. digitatum [34]. Likewise, Hall [35] reported that the growth of P. digitatum was significantly reduced in the medium containing cinnamon EO. Similarly, the antimicrobial activity of potato starch film increased following fennel (Foeniculum vulgare L.) EO incorporation [36]. The antibacterial effect of films containing EO constituents have been reported [37]. Besides, DEO with high ratios of thymol and carvacrol could be an effective source of active antimicrobial agents which can be, in turn, attributable to oxygenated monoterpenes such as thymol and carvacrol [38]. The beneficial effects of thymol and carvacrol have noted in some researches [38, 39].
However, the antifungal mode of action of DEO is not sufficiently understood. Although, conidia and/or hyphae membrane and cell wall scratch with morphological changes have been hypothesized [40]. Habibian et al. [41] reported that thyme EOs were associated with degeneration of fungal hyphae. It has been proposed that the antimicrobial activity of phenolic compounds such as thymol and carvacrol is attributable to the hydroxyl group and the presence of electrons delocalized system [42].
The most important active ingredients of DEO are thymol and carvacrol, which may induce the antifungal effect of this EO. It has been reported that thymol has a strong antifungal effect on Penicillium species with a low MIC of about 0.1% [43].
Conclusions
The DEO contains bioactive compounds with antimicrobial characteristics with a potential use as an antimicrobial agent to control postharvest decay, preserve quality and prolong the postharvest life of fruits and vegetables. DEO with high ratios of thymol and carvacrol could be an effective source of active antimicrobial agents. Sonication was shown to be an effective technique to produce stable nano-emulsions containing DEO. Nano-emulsion of DEO at a drop diameter of 29.6 nm produced by 10 min ultrasonication process exhibited efficient antifungal activity. The present work indicates that nano-emulsion containing DEO is an effective mean for postharvest treatment of citrus fruit to control diseases caused by P. digitatum. Full growth inhibition was observed at concentrations equal to or above 0.50 µL mL−1. This nano formulated EO is a promising treatment to use in citrus postharvest industry instead of chemical fungicides.
References
M. Cháfer, L. Sánchez-González, Ch. González-Martínez, A. Chiralt, J. Food Sci. 77, E182 (2012)
M.A. Gatto, A. Ippolito, V. Linsalata, N.A. Cascarano, F. Nigro, S. Vanadia, D. Di Venere, Postharvest Biol. Technol. 61, 72 (2011)
J.L. Smilanick, I.F. Michael, M.F. Mansour, B.E. Mackey, D.A. Margosan, D. Flores, C.F. Weist, Plant Dis. 81, 1299 (1997)
P. Tripathi, N.K. Dubey, Postharvest Biol. Technol. 32, 235 (2004)
A. Rodriguez-Lafuente, C.N. de la Puerta, R. Batlle, Anal. Bioanal. Chem. 395, 203 (2009)
F. Khorram, A. Ramezanian, J. Food Sci. Technol. 58, 2963 (2020)
S. Paidari, H. Ahari, J. Food Meas. Charact. 15, 3195 (2021)
Z. Ghorbani, N. Zamindar, S. Baghersad, S. Paidari, S.M. Jafari, L. Jafari, J. Food Meas. Charact. 15, 3770 (2021)
G. Guglielmini, Clin. Dermatol. 26, 341 (2008)
D.J. McClements, Soft Matter 7, 2297 (2011)
C.-C. Lin, H.-Y. Lin, H.-C. Chen, M.-W. Yu, M.-H. Lee, Food Chem. 116, 923 (2009)
J. Feng, Y. Shi, Q. Yu, C. Sun, G. Yang, Colloids Surf. A Physicochem. Eng. Asp. 497, 286 (2016)
G.K. Zorzi, E.L.S. Carvalho, G.L. von Poser, H.F. Teixeira, Rev. Bras. Farmacogn. 25, 426 (2015)
F. Donsì, M. Annunziata, M. Sessa, G. Ferrari, LWT-Food Sci. Technol. 44, 1908 (2011)
A. Karami, T. Khoshbakht, H. Esmaeili, F. Maggi, Plants 9, 680 (2020)
M.S. Damyeh, M. Niakousari, M.J. Saharkhiz, Ind. Crops Prod. 87, 105 (2016)
M.S. Damyeh, M. Niakousari, Ind. Crops Prod. 98, 100 (2017)
L. Salvia-Trujillo, M.A. Rojas-Graü, R. Soliva-Fortuny, O. Martín-Belloso, Food Hydrocoll. 30, 401 (2013)
J.B. Sinclair, O.D. Dhingra, Basic Plant Pathology Methods (CRC Press, 2017)
J.I. Pitt, A.D. Hocking et al., Fungi and Food Spoilage (Springer, Dordrecht, 2009)
G. Nikaeen, S. Yousefinejad, S. Rahmdel, F. Samari, S. Mahdavinia, Sci. Rep. 10, 1 (2020)
A. Puškárová, M. Bučková, L. Kraková, D. Pangallo, K. Kozics, Sci. Rep. 7, 1 (2017)
H. Esmaeili, A. Karami, F. Maggi, J. Clean. Prod. 198, 91 (2018)
G. Amin, M.H.S. Sourmaghi, M. Zahedi, M. Khanavi, N. Samadi, Fitoterapia 76, 704 (2005)
F. Bakkali, S. Averbeck, D. Averbeck, M. Idaomar, Food Chem. Toxicol. 46, 446 (2008)
C. Qian, D.J. McClements, Food Hydrocoll. 25, 1000 (2011)
T. Tadros, P. Izquierdo, J. Esquena, C. Solans, Adv. Colloid Interface Sci. 108, 303 (2004)
J.M. Gutiérrez, C. González, A. Maestro, I. Solè, C.M. Pey, J. Nolla, Curr. Opin. Colloid Interface Sci. 13, 245 (2008)
T.G. Mason, J.N. Wilking, K. Meleson, C.B. Chang, S.M. Graves, J. Phys. Condens. Matter 18, R635 (2006)
M. Rogošić, H.J. Mencer, Z. Gomzi, Eur. Polym. J. 32, 1337 (1996)
S. Sugumar, V. Ghosh, M.J. Nirmala, A. Mukherjee, N. Chandrasekaran, Ultrason. Sonochem. 21, 1044 (2014)
M.E.I. Badawy, A.-F.S.A. Saad, E.-S.H.M. Tayeb, S.A. Mohammed, A.D. Abd-Elnabi, J. Environ. Sci. Health Part B 52, 896 (2017)
T. Hemalatha, T. UmaMaheswari, R. Senthil, G. Krithiga, K. Anbukkarasi, J. Food Meas. Charact. 11, 2160 (2017)
D.J. Daferera, B.N. Ziogas, M.G. Polissiou, J. Agric. Food Chem. 48, 2576 (2000)
D.J. Hall, Y.J. Fernandez, in Proceedings of the Florida State Horticultural Society (2004), pp. 377–379
H. Babapour, H. Jalali, A.M. Nafchi, Food Sci. Nutr. 9, 3893 (2021)
D. Mousavian, A. Mohammadi Nafchi, L. Nouri, A. Abedinia, J. Food Meas. Charact. 15, 883 (2021)
N.P. Didry, L. Dubreuil, M. Pinkas, Pharmazie 48, 301 (1993)
M. Saki, B. ValizadehKaji, A. Abbasifar, I. Shahrjerdi, J. Food Meas. Charact. 13, 1147 (2019)
F. Neri, M. Mari, S. Brigati, Plant Pathol. 55, 100 (2006)
S. Habibian, H. Sadeghi, R. Rahimi, A. Ebrahimi, Veterinary J. 115, 147 (2017)
A. Ben Arfa, S. Combes, L. Preziosi-Belloy, N. Gontard, P. Chalier, Lett. Appl. Microbiol. 43, 149 (2006)
L. Boudine, B. Louaste, N. Eloutassi, N. Chami, F. Chami, A. Remmal, Int. J. Innov. Appl. Stud. 17, 1120 (2016)
Acknowledgements
Authors acknowledge the staff of Shiraz University for comprehensively supporting this study.
Funding
This research financially supported by the Research Affairs Office at Shiraz University (Grant # 97GCU1M153030).
Author information
Authors and Affiliations
Contributions
Conceptualization, AR; methodology, AR, MJS, MN, RM, EY; writing—original draft preparation, SR; review and editing, AR, MJS, MN, RM, EY; supervision, AR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rafiee, S., Ramezanian, A., Mostowfizadeh-Ghalamfarsa, R. et al. Nano-emulsion of denak (Oliveria decumbens Vent.) essential oil: ultrasonic synthesis and antifungal activity against Penicillium digitatum. Food Measure 16, 324–331 (2022). https://doi.org/10.1007/s11694-021-01163-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-01163-7