Abstract
The thermoluminescence (TL) behavior of a new type of glass system made up of lithium borate doped with magnesium oxide and gold, known as LB: Mg/Au, was examined. The Mg/Au co-doped LB glasses can contribute to the development of high-performance thermoluminescent dosimetry (TLD) materials for ionizing radiation dose detection and quantification. The composite shows excellent properties compared to other TLD materials. The concentrations of MgO and Au varied from 0.4 to 1 mol% and 0.01–0.07 mol%, respectively. We produced the glasses through the melt-quenching process and then characterized them to evaluate the influence of dopant concentration variation on the TL properties. All samples exhibited a single broad peak ranging between 175 and 196 °C, with the sample made up of 15 mol% Li, 0.03 mol% Au, and 0.8 mol% Mg displaying an optimum TL response. Moreover, the glass that contained 0.03 mol% of Au had the highest TL intensity. The glass showed minimum fading, best linearity, and excellent reproducibility; the XRD profiles of the samples showed their true amorphous nature, while the FESEM morphology displayed their surface homogeneity and excellent transmittance. The DTA curve of the samples revealed that their glass transition, crystallization, and melting peaks were at 500–600, and 700 °C, respectively. The proposed glass composition could prove useful as radiation dosimeters.
Graphical Abstract

Highlights
Dosmetric features of LB glass is notably modified vie Magnesium /Gold Co-doping.
For the first time LBMg and LBMgAu synthesis.
Affecting of annealing and heating rate on glow carve.
This glass shows excellent sensitivity, great linearity and extremely low fading over dose range.
Excellent reproducibility, suggesting their potential a radiation dosimeter material.
These new glass compositions are prospective for ionizing radiation measurements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Commercially, the TL dosimeters are expensive at the present time, complex in terms of their reuse, suffer from permanent radiation damages [1], and sensitive to the temperature of heat treatment [2]. To overcome these drawbacks many studies have been performed to produce borate glass-based efficient TL materials. Borates show high chemical stability and compatible with diverse TL sensitizers like trivalent rare earth elements, copper, and manganese. In addition, borate glasses display excellent sensitivity, linearity, and storage by overcoming the shortcomings related to fading, light, humidity, and so on. Amongst all borate-based glass system lithium borate (LB) glasses with various dopants reveal superior physical and TL characteristics effective for radiation dosimeter applications [3]. However, there is a need to improve the TL dosimetric performance of such doped and co-doped LB glass system.
Recently, properties of lithium borate [LiB3O5] thermoluminescent have also attracted many scientists and researchers attentions for the purposes of medical applications, due to their effective atomic number, which is very close to the biological tissue [4]. The present study focuses to identify some TL properties of lithium borate glass prepared with different compositions of Li2CO3 and H3BO3 [5]. First studied the TL dosimeter (TLD) traits of LB glass, wherein such glass was made from Li2CO3 and H3BO3 mixture at 950 °C by melt-quenching approach. Then, the obtained glasses were crystallized via heating at 650 °C and the dopants were incorporated [1, 6]. The TLD features of LB glasses in which the sample made with 15 mol% of Li2BO3 and 85 mol% of H3CO3 displayed the best TL response, tissue equivalence, excellent sensitivity and linearity under 10 Gy gamma ray irradiation [7]. The optical absorbance of rare earths doped lithium tetra-borate glasses. Melt-quenched glass system with 15 mol% of Li2CO3 and 85 mol% of H3BO3 were doped using 0.1 mol% of nano-gold to examine their TLD properties. Both undoped and Au-doped glasses were irradiated with Co-60 gamma ray at 1.25 MeV. The results showed a remarkable increase in the TL intensity of these glasses due to Au inclusion [8]. Li2B4O7:Sr phosphors were synthesized [9], melt quenching method to determine their TLD properties. Wall et al. also reported the TL response of various lithium borate glasses. Various studied showed that Li2B4O7:Cu glasses have excellent TL characteristics and linearity up to 103 Gy thereafter sub-linearity and supra-linearity in response [10,11,12]. To fulfill the growing demand of high-performance glass-based TLD materials, LB glasses with improved properties are needed.
Researchers have shown that the physicochemical properties of semiconductor materials, such as their size, shape, porosity, and recombination rate suppression efficiency, can control the possibility of removing toxic substances [13]. Currently, metals of transition are becoming developed for the sake of producing glass because of their technological applications potentiality in diverse fields of life including optic, electro, electrochemical, and electronic devices [14,15,16]. Due to its unique materials structures, borate glass was targeted by various researchers as subjected core in numerous infrared studies.
Some studies indicated that the structural and physical properties of LB glass system can be improved via Au doping wherein white powder of LB can be melted at 917 °C to produce such amorphous structure [17]. LB has density of 2.4 g/cm3 and solubility is in the range of 1–10%. A simple glow curve and annealing process makes LB glass system attractive for TLD and piezoelectric devices [18]. However, many characteristic parameters (like glow curve, dose linearity and sensitivity) of LB glass system must be refined to apply them as efficient and reliable dosimeters [19]. Based on these factors, we used the standard melt-quenching approach to prepare some LB glasses without Au (coded as LB: Mg) and with Au (coded as LB: Mg/Au) and characterized by various analytical instruments. The role of Mg/Au co-doping one the improved TL properties were determined to explore their possibility for radiation dosimeter fabrication. Results related to the kinetic parameters, fading, linearity, reproducibility, glow curve, and sensitivity were analyzed and presented.
2 Materials and Methods
2.1 Preparation of Glasses
A total of eight glass samples 4 doped with Mg and without Au (LB: Mg) of the form (100-x) H3BO3 + 15Li2CO3 a + xMgO (× = 0.4, 0.6, 0.8 and 1 mol%) and 4 co-doped Au (LB: Mg/Au) of the form (85-y) H3BO3 + 15Li2CO3 + 0.8MgO + yAu (y = 0.01, 0.03, 0.05, 0 and 0.7 mol% Au) were prepared following the melt-quenching method and characterized at room temperature. LB: Mg glass containing 0.8 mol% of MgO was selected as the optimum sample for further Au doping at different contents. Results revealed that the sample containing 0.03 mol% of Au was optimum. In the process of glass making, pure powders (purchased from Sigma Aldrich) of Li2CO3 (99% purity), H3BO3 (99.98% purity), MgO (99.9% purity) and Au (98% purity) as raw constituents of the glass were weighed and mixed homogeneously (using milling technique). For each glass batch, the resultant mix was placed in an alumina crucible and melted at \(1300 ^\circ C\) in a furnace (NabGmbHat) for one hour. Once the mixture was completely melted, it was spilt and quenched in a steel mold that was already pre-heated, following that annealing at 400 °C for 3 h, thus an eliminating of the mechanical stress that can cause glass cracking was resulted. Table 1 details how each prepared sample was composed and named.
2.2 Characterizations of Glasses
The XRD analyses (in the 2θ range of 5°-90° and scanning rate of 0.05 deg./sec) of the samples were conducted using a Siemens Diffractometer (D5000) to confirm their amorphous phase. The diffractometer used Cu Kα line of wavelength \(\left( \lambda \right)\, \approx \,1.54\,\mathop A\limits^ \circ\), and operated with \(40 kV\) and \(30 mA\). FESEM micrographs of the samples were recorded to determine their surface morphology, transmittance, phase purity, and uniformity. The DTA was conducted (Perkin Elmer Pyris Diamond Analyzer worked at 40 kV and 30 mA) to evaluated the temperature of glass transition (Tg), melting (Tm) and crystallization (Tc). The measurement was carried out in the range of 50–1000 °C with a heating rate of 10 °C/min at resolution of ± 0.1 °C. A Har-shaw4500 TLD-reader equipped with A source of gamma rays containing the isotope Co-60 was used to record the samples TL response.
3 Results and Discussions
3.1 XRD Profiles
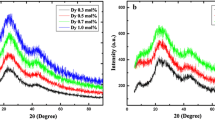
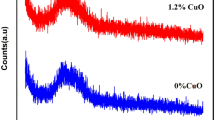
Figure 1 shows the XRD profiles of the selected two annealed (at 400 °C) samples, wherein the complete absence of any crystalline Bragg peak confirmed their amorphous nature [20, 21]. Besides, the presence of two broad hump around 20–30° and 40–50° corresponded to the weak micro-crystalline phases of LiBO3 and MgBO3.
3.2 FESEM Micrographs
Figures 2 and 3 show the FESEM micrographs of the optimum samples LBMg0.8 and LBMg0.8Au0.03, respectively. The surface morphology of the studied glasses did not display the presence of any granular structures [22, 23], indicating their homogeneous textures. In addition, the glass network structures were appreciably modified due to Au doping.
3.3 DTA Curves
The DTA provided the information of glass thermal properties including Tg, Tm and Tc. The GFA, or glass forming ability, was calculated using the following equation:
The GFA (Trg) of a glass is considered to be good when \(0.5\le{T}_{rg}\le 0.66\). The obtained excellent values of \({T}_{rg}\) of the proposed glasses indicated the fulfillment of Kauzmann assumption.
The thermal stability of the glasses were verified by estimating the Hurby parameter [24]:
Glasses with \({H}_{g}\le 0.1\) and\({H}_{g}\ge 0.5\) the corresponding thermal stability is considered to be very poor and superior [25]. Table 2 shows the values of glass forming ability and stability of the studied compositions.
Figure 4 depicts DTA results of the studied samples (LBMg0.8 and LBMg0.8Au0.3) which exhibited Tg at 515 °C and 523 °C (endothermic peaks). The values of Tc (exothermic peaks) were probed at 581 °C and 659 °C. In addition, the values of Tm (endothermic peaks) were observed at 628 °C and 761 °C. The value of Trg and Hg of LBMg0.8 and LBMg0.8Au0.3 glass was 0.8 and 0.6, respectively.
3.4 Annealing Process
The annealing of the samples was performed to remove all residual TL signal, thus re-establishing the TL sensitivity by eliminating all unstable glow peaks occur at low temperature. Generally, different TL materials show different annealing regime [26, 27]. Before Co-60 gamma radiation exposure of 50 Gy, the proposed glasses were annealed for (15–60) min ranging in 100–400 °C (an optimization process for pre-irradiation annealing based on temperature and duration). Figures 5 and 6 show how the TL-intensity for LBMg0.8 and LBMg0.8Au0.03 changes with the annealing temperature, respectively. The glasses annealed at 400 °C displayed the minimum level of standard error and the maximum degree of TL-intensity [28, 29], selecting the value for pre-irradiation annealing of both samples.
Figures 7 and 8 show the dependence of TL intensity and STD for LBMg0.8 and LBMg0.8Au0.03 glass on annealing temperatures, respectively. Maximum reproducibility (low STD) and TL intensity for LBMg0.8 and LBMg0.8Au0.03 glasses were correspondingly observed at 60 min and 45 min. For the whole investigation, these pre-irradiation annealing procedures were applied.
3.5 Heating Rate Optimization
Figures 9 and 10 show the heating rate dependence of the TL glow curve for LBMg0.8 and LBMg0.8Au0.03 glasses, respectively under the exposure of 50 Gy Co-60 gamma radiation. The peak intensity was amplified with the raise of heating rate. In short, the approved heating rate of the TLD reader decided the TL intensity of the glow curve.
Figures 11 and 12 show the TL glow peak intensity and STD corresponding to LBMg0.8 and LBMg0.8Au0.03 measured at various heating rates. Each measurement was performed 5 times and the mean value was estimated to get the STD. Ideally, a best heating rate setting in a minimum standard deviation (STD) of the dosimeter output data and an optimal TL response are required from the TL measurement. In the present glass, the maximum TL response and minimum STD LBMg0.8 and LBMg0.8Au0.03 were found at \(5 ^\circ C.{s}^{-1}\), and \(3 ^\circ C.{s}^{-1}\), respectively [30].
3.6 TL Glow Curve
Both peak position and shape of the glow curve are important to determine the nature of trap states and fading attributes of a material used for dosimeter fabrication, thus providing an insight regarding the TL mechanism. Figure 13 depicts the TL glow curves of the LBMg glasses under 50 Gy Co-60 gamma ray exposures, which displayed a broad peak at 205 °C. Figure 14 displays the TL peak intensity and STD of the LBMg glasses as a function of Mg ions contents. The LBMg0.8 glass exhibited the highest TL peak intensity. This can be attributed to the recombination of excited electrons, which originated from the valence band, with the defects induced by irradiation [31].
Figure 15 shows the TL glow curves of the LBMg0.8Au glasses under 50 Gy Co-60 gamma ray exposures, which revealed an intense peak at 196 °C. Sample LBMg0.8Au0.03 showed the maximum TL peak intensity. Figure 16 TL peak intensity and STD as a function of Au contents.
Figure 17 compares the TL glow curve of LBMg0.8 glass with that of LBMg0.8Au0.03. The TL peak intensity was improved by a factor of 1.3 due to the inclusion Au into the LBMg0.8 glass. The LBMg0.8Au0.03 glass showed the optimum TL peak intensity. In addition, the observed decrease of the TL peak intensity at Au contents above 0.03 mol% is due to concentration dependent quenching mechanism [32].
3.7 Kinetic Parameters
The observed alteration in the TL glow curves shapes, peak positions, and peak intensities are determined by the trap states in the material [33]. Thus, the kinetic parameters of the dosimeter material like activation energy (E) or trap depth, frequency factor (s), geometric factor (µg) and kinetic order (b) strongly depend on the TL mechanisms that decide glow curve characteristics. In this work, the peak shape (also called Chen’s method) and initial rise approach was applied to determine the values of E and s for the proposed glasses [34]. The precision of these approaches depend on the characteristics TL glow curve peak temperature (\({T}_{m}\)), and the temperatures T1 and T2 connected to the FWHM of the TL peak [35].
3.7.1 Peak Shape Method
Figures 18 and 19 display the peak shape method to estimate the kinetic parameters of LBMg0.8 and LBMg0.8Au0.03 glass, respectively. The following relations were used to compute the geometric factors [35]:
The ideal values of\({\mu }_{g}\)for the first order and the second kinetics ones are correspondingly ≈ 0.42 and ≈ 0.52. The estimated values of \({\mu }_{g}\) for LBMg0.8 and LBMg0.8Au0.03 glasses corresponded to 0.69, and 0.62. Also, Balarin equation [36] was used to evaluate the value to kinetic order:
The obtained values of b for LBMg0.8 and LBMg0.8Au0.03 glasses corresponded to 3.96, and 2.46, confirming that the proposed glasses could conform to the second order kinetics. The values of \(E\) of the studied glasses were estimated using [37]:
where \({k}_{B}\) is the Boltzmann constant, α signifies the τ, δ and ω values as appeared in Figs. 18 and 19. The values of \({C}_{\alpha }\) and \(b\alpha\) were determined via [37]:
Table 3 enlists the values of all relevant constants for LBMg0.8 and LBMg0.8Au0.03 glasses obtained from the Chen’s peak shape method. The values of s for the proposed glasses were evaluated using:
where \(b\) and \(\beta\) corresponds to the of kinetics order and rate of linear heating. Table 4 shows the estimated values of frequency factor and thermal activation energy of LBMg0.8 and LBMg0.8Au0.03 glasses [38].
The observed values of the kinetics parameters of the glasses at various absorbed doses show the continuous energy distribution of the trap states in the material. Herein, Eτ, Eδ and Eω are the activation energy based on the first half width on the rising side, second half width on the falling side and total width of the glow curve, respectively. It is known that higher energy and higher temperature is needed to de-trap the electrons from deeper traps [39].
3.7.2 Initial Rise Method
The values of \(E\) and s of the obtained glasses were computed using Garlick and Gibson initial rise method [40]. This method hypothesized that the change of the trapped carrier population rate is small at the initial part of the TL glow curve (T < < Tm), The validity of the temperature cut-off is determined by an intensity level that is below 10–15% of the maximum intensity [41]. This method is suitable when the glow curve has clear peak separation. The formula for the intensity of TL radiation \(\left(I\right)\) is:
Figures 20 and 21 shows the \(ln\left(ITL\right)\) linear variation as a function of \((1/kT)\) for LBMg0.8 and LBMg0.8Au0.03 glasses, respectively.
The values of \(s\) for the glasses were estimated using [42]:
where A, \(N\), and \({n}_{0}\) is the area under the glow curve, total concentration of traps and initial concentration of the trapped electrons. Assuming the ratio of \({n}_{0}/N=1\) (saturation case) one gets:
Table 5 shows the computed kinetic parameters (E and S) of LBMg0.8 and LBMg0.8Au0.03 glass obtained using two different methods. The slopes of the initial rise plots (Figs. 20 and 21) were used to estimate the values of E and S of the glasses.
3.8 Minimum Detectable Dose
The lowest detection level or minimum detectable dose (MDD) was evaluated using [43]:
The variable \({B}^{*}\) represents the average background signal of TL resulting from the annealing of samples without irradiation. \({\sigma }_{B }\) represents the average standard error of the background signal, and \(F\) represents the calibration factor in units of (Gy TL− 1) [44, 45].
The estimated values of \(MDD\), \({B}^{*}\), \({\sigma }_{B}\), and \(F\) for LBMg0.8 glass corresponded to \(2.15\times {10}^{3} \mu Gy\), \(2.12 nC,\)\(0.0141 nC\), and \(1.199\times {10}^{3} mGy .n{C}^{-1}\). The obtained values of \(MDD\), \({B}^{*}\), \({\sigma }_{B}\), and \(F\) for LBMg0.8Au0.03 glass was \(2.32\times {10}^{5} \mu Gy\), \(2.255 nC\), \(0.0.021213 nC\), and \(2.81\times {10}^{5} Gy .n{C}^{1}\), respectively. The value of \(MDD\) for LBMg0.8Au0.03 glass was lower than LBMg0.8 glass, indicating its potential in radiation dosimetry [46]. The values of the MDD and calibration factor for the optimal glasses that were studied as dosimeter materials are shown in Table 6.
3.9 Dose Response
Figure 22 shows the TL intensity variation of the LBMg0.8 and LBMg0.8Au0.03 glasses as a function of 0.5 to 4 Gy Co-60 gamma ray exposures, wherein a linear dose response was observed as desired for the accurate dosimetric applications. In addition, the TL response of LBMg0.8Au0.03 glass was superior compared to the LBMg0.8 glass, indicating an improvement in the TL features of the glasses due to Au codoping.
The normalized dose response (linearity index) of the synthesized glasses was obtained using:
The variable \(TL\left(D\right)\) reflects the dosage response at a specific dose \(D\), and \({D}_{0}\) represents the lowest dose at which the dose response exhibits a linear form. The linearity index of the suggested glass-based dosimeters is shown in Figs. 23 and 24 [47, 48]. The optimal top-level domain (TLD) material exhibits a \(f\left(D\right)\) value of 1 across a broad range of doses. The current results confirmed the linear response of the examined TLD composed of LBMg0.8 and LBMg0.8Au0.03 glasses when subjected to Co-60 gamma radiation within the dose range of 0.5–4 Gy.
3.10 TL Sensitivity
A dosimeter’s TL sensitivity is the area of its glow curve divided by the mass and dose (\(nC {g}^{-1}.G{y}^{1}\)) [49]. The values of the sensitivity for the studied LBMg0.8 and LBMg0.8Au0.03 glasses (calculated from the slope of Fig. 22) were 0.834 µC g−1Gy−1 and 33.51 µC g−1Gy−1, respectively. In the studied dose range, the sensitivity of LBMg0.8Au0.03 glass was approximately 40 times higher than that of LBMg0.8 glass.
3.11 Thermal Fading
Figures 25 and 26 depict the thermal fading characteristics corresponding to LBMg0.8 and LBMg0.8Au0.03 glass when irradiated with 4 Gy gamma ray dose. The thermal fading of LBMg0.8 and LBMg0.8Au0.03glasses was determined after annealing and irradiation process at a constant dose of 4 Gy followed by storage in the dark conditions at room temperature, reducing the background illumination. After one day of the dose exposure, the readout was started and sustained till 2 months. Identical conditions were maintained to conduct all measurements (several samples), during which the TL response showed a tiny drop while the measurement was underway. Ten days later, the thermal fading for LBMg0.8 glass was 15%, one month later it was 21%, and two months later it was 28%. On the other side, LBMg0.8Au0.03 glass exhibited 10% thermal fading after 10 days, 16% after one month, and 21% after two months. The observed slight better fading of the co-doped glass may be due to the irradiation-induced creation of defects on phosphor and generation of new trap states [50].
3.12 Reproducibility
Ten specimens of each LBMg0.8 and LBMg0.8Au0.03 glass were repetitively irradiated with 4Gy gamma ray with appropriate preliminary annealing, obtaining their TL signals after every irradiation cycle [51]. Figure 27 show the normalized TL intensity as a function of number of measurements. The results showed a decrease in the reproducibility by approximately \(1.25\%\) per cycle for LBMg0.8 glass and \(1.07\%\) per cycle for LBMg0.8Au0.03 glass with STD value below \(2\%\), indicating the reusability LBMg0.8Au0.03 glass as dosimetric material [52].
4 Conclusion
The TL characteristics of two types of LB glasses with MgO doping and MgO/Au co-doping at various contents were studied to determine their potential application in reusable dosimeters. It was shown that the TL features of LB glasses can be improved appreciably by tuning the concentrations of Au/MgO co-doping. All the produced glasses displayed good dose response linearity, reproducibility of the signals, simple glow curve show very slow fading of the signals. suggesting their potential a radiation dosimeter material. The structural and morphological characteristics of the glasses verified their suitability as reusable dosimeter. The DTA results confirmed an excellent thermal stability of the glasses. The inclusion of Au found to induce many new trap states and luminescence centers for recombination by raising the energy levels of the surrounding oxygen ions to the top of the valence band, thus enhancing the TL properties of the glasses. In brief, the Mg/Au co-doped LB glasses can contribute to the development of high performance TLD material for ionizing of detection and quantification of radiation dose.
Data Availability
Yes, the data and material are available.
References
H.K. Obayes, O. Obayes, Q.S. Kadhim, A. Saidu, M. Almaamori, Improved thermoluminescence and kinetic parameters of new strontium/copper co-doped lithium borate glass system. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 455, 74–82 (2019). https://doi.org/10.1016/j.nimb.2019.06.028
G. Kitis, V. Pagonis, On the need for Deconvolution Analysis of Experimental and simulated thermoluminescence glow curves. Mater. (Basel). 16, 871 (2023). https://doi.org/10.3390/ma16020871
V. Chopra, N. El-Faramawy, S.J. Dhoble, Thermoluminescent study of borates for dosimetric applications. In: Phosphor Handbook. 393–415. Elsevier (2023). https://doi.org/10.1016/B978-0-323-90539-8.00013-9
G.I. Efenji, I.S. Mustafa, O.O. Ogunleye, T.H. Khazaalah, N.S. Ezra, H.S. Naeem, H.M. Shariff, M. Jamil, M.F.I.A. Malik, Description and dosimetric features of lithium borate glass doped with transition metals for thermoluminesce, a re-evaluation. Phys. Scr. 98, 52001 (2023). https://doi.org/10.1088/1402-4896/acc23c
P. Murugasen, M. Dhavamurthy, P. Anthoniammal, A.A. Suresh, M. Mohapatra, Structural, optical, and thermoluminescence characterizations of 1 mol% Dy3 + ion-activated fluro boro-phosphate glass for photonic devices. Spectrochim Acta Part. Mol. Biomol. Spectrosc. 308, 123757 (2024). https://doi.org/10.1016/j.saa.2023.123757
A. Saidu, H. Wagiran, M.A. Saeed, H.K. Obayes, A. Bala, F. Usman, Thermoluminescence response of rare earth activated zinc lithium borate glass. Radiat. Phys. Chem. 144, 413–418 (2018). https://doi.org/10.1016/j.radphyschem.2017.10.004
B.V. Padlyak, I.I. Kindrat, V.T. Adamiv, Y.O. Kulyk, I.M. Teslyuk, A. Drzewiecki, I. Stefaniuk, Local structure, spectroscopy and luminescence of the Li2B4O7: Cu, Eu glass. Mater. Res. Bull. 167, 112432 (2023). https://doi.org/10.1016/j.materresbull.2023.112432
H.K. Obayes, H. Wagiran, R. Hussin, M.A. Saeed, Structural and optical properties of strontium/copper co-doped lithium borate glass system. Mater. Des. 94, 121–131 (2016). https://doi.org/10.1016/j.matdes.2016.01.018
P.A. Nagpure, N.S. Bajaj, R.P. Sonekar, S.K. Omanwar, Synthesis and luminescence studies of novel rare earth activated lanthanum pentaborate. Indian J. Pure Appl. Phy. 49, 799–802 (2011)
H.K. Obayes, R. Hussin, H. Wagiran, M.A. Saeed, Strontium ion concentration effects on structural and spectral properties of Li4Sr (BO3) 3 glass. J. Non Cryst. Solids. 427, 83–90 (2015). https://doi.org/10.1016/j.jnoncrysol.2015.07.026
B.A. Doull, L.C. Oliveira, D.Y. Wang, E.D. Milliken, E.G. Yukihara, Thermoluminescent properties of lithium borate, magnesium borate and calcium sulfate developed for temperature sensing. J. Lumin. 146, 408–417 (2014). https://doi.org/10.1016/j.jlumin.2013.10.022
B. Sanyal, M. Goswami, S. Shobha, V. Prakasan, S.P. Chawla, M. Krishnan, S.K. Ghosh, Synthesis and characterization of Dy3 + doped lithium borate glass for thermoluminescence dosimetry. J. Non Cryst. Solids. 475, 184–189 (2017). https://doi.org/10.1016/j.jnoncrysol.2017.09.016
Z.K. Amirhosseini, R. Monsef, S.A. Ehsanizadeh, W.K.Y. Albahadly, H.S. Majdi, A.A. Amir, A.H. Dawood, M. Salavati-Niasari, Tailoring the photocatalytic activity of novel magnetically separable ZnFe12O19-chitosan bionanocomposites: a green preparation, structural characterization and comparative study. Int. J. Hydrogen Energy. 48, 37286–37301 (2023). https://doi.org/10.1016/j.ijhydene.2023.06.130
X. Zeng, G. Li, J. Zhu, M. Sain, R. Jian, NBR/CR-Based high‐Damping Rubber composites Containing Multiscale structures for Tailoring Sound Insulation. Macromol. Mater. Eng. 308, 2200464 (2023). https://doi.org/10.1002/mame.202200464
F.S. Olise, S.B. Ola, F.A. Balogun, Characterisation of clay samples from minerals–rich deposits for thermoluminescence applications. J. King Saud Univ. 30, 158–167 (2018). https://doi.org/10.1016/j.jksus.2017.01.004
A.J.J. Bos, Thermoluminescence as a research tool to investigate luminescence mechanisms. Mater. (Basel). 10, 1357 (2017). https://doi.org/10.3390/ma10121357
O.B. Aljewaw, M.K.A. Karim, N. Effendy, H.M. Kamari, M.H.M. Zaid, N.M. Noor, A.A. Salim, N.M. Isa, A.B.A. Kadir, M.T. Chew, A.I. Abokridiga, Physical, optical and thermoluminescence properties of lithium aluminum borate glass co-doped with Dy2O3. Radiat. Phys. Chem. 209, 111004 (2023). https://doi.org/10.1016/j.radphyschem.2023.111004
Z. Jiang, Y. Zhang, L. Zhang, B. Cheng, L. Wang, Effect of calcination temperatures on photocatalytic H2O2-production activity of ZnO nanorods. Chin. J. Catal. 43, 226–233 (2022). https://doi.org/10.1016/S1872-2067(21)63832-9
B.A. Kumar, P.H. Bindu, Advances in borate- and phosphate-based TL materials for in vivo dosimetry. J. Korean Ceram. Soc. 59, 537–550 (2022). https://doi.org/10.1007/s43207-022-00240-x
M.M. Abdelhamied, W.M. Abd-Allah, A.M. Abdelreheem, The effect of nickel additive and ion irradiation on the structural and optical properties of lithium borate glasses. Inorg. Chem. Commun. 163, 112309 (2024). https://doi.org/10.1016/j.inoche.2024.112309
K.S. Shaaban, A.M. Al-Baradi, Z.A. Alrowaili, A.M. Ali, M.S. Al-Buriahi, E.A.A. Wahab, Structural, thermal, and mechanical characteristics of yttrium lithium borate glasses and glass–ceramics. J. Mater. Sci. Mater. Electron. 32, 28065–28075 (2021). https://doi.org/10.1007/s10854-021-07158-w
A. Saidu, H. Wagiran, M.A. Saeed, Y.S.M. Alajerami, Structural properties of zinc lithium borate glass. Opt. Spectrosc. 117, 396–400 (2014). https://doi.org/10.1134/S0030400X14090239
A. Askary, N.S. El, Awwad, H.A. Ibrahium, M.E. Moustapha, A.A. Menazea, Structural, thermal, morphological and antibacterial activities of hybrid bio-nanocomposite Chitosan/Au/Bi2O3 for biomedical applications. J. Polym. Res. 29, 269 (2022). https://doi.org/10.1007/s10965-022-03116-0
R. Sharma, A. Kumar, A. Dawar, S. Ojha, A. Mishra, A. Goyal, R. Laishram, V.G. Sathe, R. Srivastava, O.P. Sinha, Liquid phase exfoliation and characterization of few Layer MoS2 and WS2 nanosheets as Channel Material in Field Effect Transistor. Trans. Electr. Electron. Mater. 24, 140–148 (2023). https://doi.org/10.1007/s42341-023-00429-9
O.I. Sallam, R.M. Ahmed, Gamma-irradiated fluorophosphate glasses doped with various transition metals: a spectroscopic study. Opt. Mater. (Amst). 147, 114624 (2024). https://doi.org/10.1016/j.optmat.2023.114624
A.J.J. Bos, Theory of thermoluminescence. Radiat. Meas. 41, S45–S56 (2006). https://doi.org/10.1016/j.radmeas.2007.01.003
V. Thakur, A. Singh, R. Punia, M. Kaur, L. Singh, Effect of BaTiO3 on the structural and optical properties of lithium borate glasses. Ceram. Int. 41, 10957–10965 (2015). https://doi.org/10.1016/j.ceramint.2015.05.039
J.M. Kalita, M.L. Chithambo, The effect of annealing and beta irradiation on thermoluminescence spectra of α-Al2O3: C, mg. J. Lumin. 196, 195–200 (2018). https://doi.org/10.1016/j.jlumin.2017.12.036
R.R. Dawam, M.L. Chithambo, Thermoluminescence of annealed synthetic quartz: the influence of annealing on kinetic parameters and thermal quenching. Radiat. Meas. 120, 47–52 (2018). https://doi.org/10.1016/j.radmeas.2018.06.004
S. Saha, H.J. Kim, A. Khan, D.J. Daniel, R. Absar, R. Barman, P. Aryal, J. Kaewkhao, S. Kothan, Luminescence and Scintillation properties of Dy3 + doped Li6Y(BO3)3 crystal. Opt. Mater. (Amst). 106, 109973 (2020). https://doi.org/10.1016/j.optmat.2020.109973
J.M. Kalita, M.L. Chithambo, Thermoluminescence of α-Al2O3: C, mg: kinetic analysis of the main glow peak. J. Lumin. 182, 177–182 (2017). https://doi.org/10.1016/j.jlumin.2016.10.031
C.M. Sunta, Kinetics analysis of TL Glow curves. Unraveling Thermoluminescence. Springer Series in Materials Science, 202. Springer, New Delhi. (2015). https://doi.org/10.1007/978-81-322-1940-8_4
R. Cao, Y. Ye, Q. Peng, G. Zheng, H. Ao, J. Fu, Y. Guo, B. Guo, Synthesis and luminescence characteristics of novel red-emitting Ba2TiGe2O8: Mn4 + phosphor. Dye Pigment. 146, 14–19 (2017). https://doi.org/10.1016/j.dyepig.2017.06.061
A.M. Sadek, G. Kitis, A critical look at the kinetic parameter values used in simulating the thermoluminescence glow-curve. J. Lumin. 183, 533–541 (2017). https://doi.org/10.1016/j.jlumin.2016.12.002
R. Chen, Glow curves with general order kinetics. J. Electrochem. Soc. 116, 1254 (1969). https://doi.org/10.1149/1.2412291
R. Chen, J.L. Lawless, V. Pagonis, Thermoluminescence associated with two-electron traps. Radiat. Meas. 99, 10–17 (2017). https://doi.org/10.1016/j.radmeas.2017.03.002
J.J.M. Órfão, Review and evaluation of the approximations to the temperature integral. AIChE J. 53, 2905–2915 (2007). https://doi.org/10.1002/aic.11296
C. Manjunath, M.S. Rudresha, K.R. Nagabhushana, R.H. Krishna, B.M. Nagabhushana, B.M. Walsh, Thermoluminescence glow curve analysis of gamma irradiated Sr2SiO4:Dy3 + nanophosphor. Phys. B Condens. Matter. 585, 412113 (2020). https://doi.org/10.1016/j.physb.2020.412113
A.M. Sadek, H.M. Eissa, A.M. Basha, G. Kitis, Resolving the limitation of the peak fitting and peak shape methods in the determination of the activation energy of thermoluminescence glow peaks. J. Lumin. 146, 418–423 (2014). https://doi.org/10.1016/j.jlumin.2013.10.031
A.M. Sadek, H.M. Eissa, A.M. Basha, G. Kitis, Development of the peak fitting and peak shape methods to analyze the thermoluminescence glow-curves generated with exponential heating function. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 330, 103–107 (2014). https://doi.org/10.1016/j.nimb.2014.04.006
N.S. Rawat, M.S. Kulkarni, D.R. Mishra, B.C. Bhatt, C.M. Sunta, S.K. Gupta, D.N. Sharma, Use of initial rise method to analyze a general-order kinetic thermoluminescence glow curve. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 267, 3475–3479 (2009). https://doi.org/10.1016/j.nimb.2009.08.002
C. Furetta, M. Prokic, R. Salamon, V. Prokic, G. Kitis, Dosimetric characteristics of tissue equivalent thermoluminescent solid TL detectors based on lithium borate. Nucl. Instruments Methods Phys. Res. Sect. Accel Spectrometers Detect. Assoc. Equip. 456, 411–417 (2001). https://doi.org/10.1016/S0168-9002(00)00585-4
G. Kitis, A. Sadek, G. Polymeris, V. Pagonis, Simulation of the effect of resolution between thermoluminescence peaks on the initial rise method of analysis. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 524, 1–7 (2022). https://doi.org/10.1016/j.nimb.2022.05.004
M.M. Mair, M. Kattwinkel, O. Jakoby, F. Hartig, The minimum detectable difference (MDD) concept for establishing trust in nonsignificant results: a critical review. Environ. Toxicol. Chem. 39, 2109–2123 (2020). https://doi.org/10.1002/etc.4847
H.K. Obayes, H. Wagiran, R. Hussin, M.A. Saeed, A new strontium/copper co-doped lithium borate glass composition with improved dosimetric features. J. Lumin. 176, 202–211 (2016). https://doi.org/10.1016/j.jlumin.2016.03.024
J.A. Harvey, K.J. Kearfott, M. Rafique, Dose response linearity and practical factors influencing minimum detectable dose for various thermoluminescent detector types. J. Radioanal Nucl. Chem. 303, 1711–1718 (2015). https://doi.org/10.1007/s10967-014-3794-6
R. Kaur, R.B. Rakesh, S.G. Mhatre, V. Bhatia, D. Kumar, H. Singh, S.P. Singh, A. Kumar, Physical, optical, structural and thermoluminescence behaviour of borosilicate glasses doped with trivalent neodymium ions. Opt. Mater. (Amst). 117, 111109 (2021). https://doi.org/10.1016/j.optmat.2021.111109
A. Soni, D.R. Mishra, B.C. Bhatt, S.K. Gupta, N.S. Rawat, M.S. Kulkarni, D.N. Sharma, Thermally assisted OSL: a potent tool for improvement in minimum detectable dose and extension of dose range of Al 2 O 3: C. Geochronometria. 40, 258–265 (2013). https://doi.org/10.2478/s13386-013-0133-6
V. Correcher, J. Garcia-Guinea, T. Rivera, Thermoluminescence sensitivity of daily-use materials. Radiat. Eff. Defects Solids. 164, 232–239 (2009). https://doi.org/10.1080/10420150902734064
J.A. Harvey, N.P. Haverland, K.J. Kearfott, Characterization of the glow-peak fading properties of six common thermoluminescent materials. Appl. Radiat. Isot. 68, 1988–2000 (2010). https://doi.org/10.1016/j.apradiso.2010.04.028
A.O. Elsheikh, A. El-Hafez, M.M. Ahmed, Y.A.-A. Abdul-Razek, M.E. El-Nagdy, Quantification of various factors influencing repeatability and reproducibility of TLD-600 detector. Arab. J. Nucl. Sci. Appl. 53, 58–66 (2020). https://doi.org/10.21608/AJNSA.2019.13313.1219
A. Amarlou, K. Mohammadi, N. Banaee, H.A. Nedaei, Synthesis and evaluation of thermoluminescence properties of ZrO2:mg for radiotherapy dosimetry. Radiat. Environ. Biophys. 60, 647–652 (2021). https://doi.org/10.1007/s00411-021-00928-4
Acknowledgements
The authors express their gratitude to Universiti Teknologi Malaysia and the University of Babylon for their financial assistance and facilities in accordance with Grant Nos. R.J130000.7826.4F490 and 4F736. Appreciation is expressed by the authors for UTFR grant 21H78.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Hayder. K. Obayes, Mohanad H. Meteab, Bairaq Abd Al-Kareem.
Corresponding author
Ethics declarations
Consent for Publication
Consent for Publication.
Human and Animal Rights
The authors assert that the paper does not involve any human study participants, and therefore, no permission is necessary. The authors further confirm that they have not previously submitted this original paper in any other form or language.
Conflict of Interest
No conflict of interest.
Consent to Participate
Consent to participate.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Obayes, H.K., Meteab, M.H. & Al-Kareem, B.A. Modified Dosimetric Features of New Type of Lithium Borate Glass System: Role of Magnesium and Gold Co-doping. Trans. Electr. Electron. Mater. (2024). https://doi.org/10.1007/s42341-024-00551-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42341-024-00551-2