Abstract
Thermoluminescence (TL) materials are well known for a very large number of applications in various fields such as medical research, in vivo dosimetry, environmental dosimetry, personal dosimetry, etc. There are several TL materials available in the market such as fluoride, borate, phosphate, silicate, borosilicate glasses, etc. The TL properties of materials change with the doping of rare-earth and transition impurities in different hosts which are useful for different applications. These doped TL materials can be prepared by different techniques such as, the melt-quenching technique, combustion method, sol–gel method, and others. Radiations such as γ-rays, X-rays, β-rays, photon beam, electron beam, neutron beam, etc., can be used to irradiate these TL materials. In the present state of research, interest is being raised to develop new thermoluminescent materials for various applications in the field of material science and radiation therapy for in vivo dosimetry in view of the rise in the number of cancer patients across the globe. In the last few years, borate and phosphate-based TL dosimeters got more attention in radiation dosimetry. So, this review deals with the recent developments and advancements in borate- and phosphate-based TL materials for in vivo dosimetry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 History of glasses
Glasses can be from ordinary jewelry beads to expensive artifacts to decorative window panes. There are several applications of different glasses depending on the requirements, and hence can be tailor made materials. The archeological evidence suggests that the first glass was made in coastal north Syria, Mesopotamia, or ancient Egypt [1]. There was a rapid growth in glass-making technology during the late bronze age in Egypt and western Asia. The development of glasses in India has begun in 1730BC [2]; the first site in India to manufacture the glasses was in Kopia, Uttar Pradesh between 700BC and 200AD [3]. The Indian glasses in this period differed significantly in chemical composition when compared to Roman and Chinese glasses [4].
In South Asia, glasses are being used as ornaments and casings since 100AD [5]. Although glass-making developed in China later than in Egypt and India, it played a very important role in arts and crafts making [6, 7]. In this period, the production of the glasses involved barium oxide (BaO) and lead (Pb), but the traditional use of BaO and Pb disappeared at the end of the Han Dynasty (220AD). The glass industry went through rapid technological growth in 100AD by Romans in Alexandria [8]. Colored glass production started in the Islamic world in 800AD and by the end of the eleventh century, clear glass mirrors were being manufactured in Islamic Spain. After the collapse of the western Roman empire, glass-making technologies emerged in northern Europe. Around 1000AD, the glass is made by potash obtained from wood ashes which is a breakthrough in north Europe. In the eleventh century, there was the emergence of new ways of making glass sheets in Germany.
1.2 Borate glass properties and applications
The borate glasses have more complex action and major differences in their optical properties than the Silicate glasses [9, 10]. The most important application of the borate glass over silicate is its low melting temperature (Tm) and low glass transition temperatures (Tg) [11]. Pure borate glasses have a glass transition temperature of approximately 260 ℃ [12] and a melting temperature of approximately 450 ℃ [13], which are very low as compared to silicate glasses. The pure form of borate glasses is not useful in most applications since they have very low chemical durability which can be increased by the addition of other oxides such as SiO2, Al2O3, and alkali oxides. The addition of silica to the borate glasses leads to another type of glass which is known as borosilicate glass with a broad range of applications. These glasses have high chemical durability and electrical resistivity compared to the individual silicate and borate glasses. Borate glasses are preferred over silicate glasses in certain optical and photonic devices [14] due to their unique properties such as low melting temperatures, compatibility with rare-earth elements and transition metals, wide glass-forming range, and the second- and third-order nonlinearity [15,16,17,18,19,20,21,22]. Adding rare-earth oxides, such as La2O3, Pr6O11, Nd2O3, and Sm2O3 to the borate glasses, these glasses will show the Faraday effect [23] and this effect is a magneto-optical effect that involves the application of magnetic field; there is a rotation in the plane polarization plane of linearly polarized light.
The recent research on glasses is based on the advanced glasses for the use in insulation purposes, falcon for cosmetics and pharmaceuticals, renewable energy such as solar energy glasses, medical technology, optical glasses, biotechnology, and radiation protection such as X-ray and Gamma-ray protectors.
1.3 Radiation dosimetry
Radiation can be naturally occurring or man-made. The response of human being to radiation from different sources is subjected to the great scientific and promising field of study that can be understood. Radiation can be used in the medicinal field in the treatment of diseases such as cancer, in which even a small dose of radiation can cause damage to the living tissue or cell. Many factors affect the response of the human body to radiation such as the amount of radiation deposited in the tissue and the ability of the radiation to generate harm to the tissue. The value for the equivalent dose for a person by the international regulation is 1mSv/year [24, 25]. Therefore, it is very important to measure the absorbed dose for the treatment of cancer cells.
Thermoluminescence dosimetry (TLD) is one of the most useful techniques to measure the absorbed dose [26]. The TLD devices are used to monitor the radiation in different applications such as radiation therapy and the measurement of radiation leakage from isolated laboratories [27, 28]. There are many glass-based TLD devices are developed during the last few decades out of which borate-based TLDs are the most promising and useful thermoluminescence dosimeters. There is a continuous effort being made by the researchers to develop new materials for the advanced dosimetric properties to be used as efficient TLD materials over a wide range of radiation doses [29,30,31]. The borate-based glasses are also efficient in the field of radiation detection and thermoluminescence studies because of their near-human tissue absorption coefficient.
1.4 Developments in TLD materials
Thermoluminescence (TL) was first used in radiation dosimetry by Farrington Daniels in 1953 when introducing LiF as TL material [32] and that was patented as TLD-100 by Harshaw Chemical Company. Many new dosimetric glass materials have been reported over the last few decades that have different efficiencies for different dose ranges of radiation. Presently, there are many commercial dosimeters are available such as LiF: Mg, Cu, P (TLD-700H), Al2O3 (TLD-500), CaSO4:Dy (TLD-900), and CaF2:Dy(TLD-200) [33, 34]. Each of these dosimeters is not suitable for all ranges of doses as they depend on different factors such as linearity, reproducibility, dose rate, etc. Therefore, there is a need to develop a material that shows a TL response linearly over a wide range of doses and should satisfy all the requirements.
Over the last few decades, many new and dosimetrically useful TLD materials have been reported such as Li2B4O7:Mn in 1965 [35], CaF2:Dy in 1969 [36], Al2O3:Si,Ti in 1976 [37], CaF2:Tm in 1977 [38], LiF:Mg,Cu,P in 1978 [39], Li2B4O7:Cu in 1980 [40] and Al2O3:Cu in 1990 [41]. Recently, many research groups are involved in exploring new materials for advanced TLD applications. El-Adawy et al. studied the thermoluminescence characteristics of Ag-doped and undoped Li2B2O4 (LB) glasses exposed to γ-radiation and observed a good fading response and linearity for 105 ppm Ag-doped LB glasses over the studied dose range [42]. Santiago and his colleagues studied the thermoluminescence and radio-luminescence properties of Dy-doped SrB2O4 glasses prepared by the sol–gel technique irradiated with the β-radiation source and it has been found that the TL and RL efficiency is high in Dy-doped metaborate and mixed borate and poor in tetraborates [43]. Ekdal et al. have reported Mn-doped Li2B4O7 single crystal phosphor for TLD characteristics and found that the activation energy is about 1.21eV and the frequency factor of 3.71×1011S-1 [44]. Saidu et al. studied the thermoluminescence characteristics of Cu-doped ZnLi2B2O4 glasses with the radiation dose varying from 0.5 to 4Gy and they have reported that the time-based thermal fading of the dosimeter is stable and it is having high potential ability to use in radiation processing dosimetry [45]. The dosimetric properties of Dy-doped Li2B2O4 glasses irradiated by γ-radiation have been studied by different researchers, Ab Rashid et.al. [46], Bhaskar Sanyal et.al. [47], and they found that Dy-doped LB glasses are having good linearity of regression coefficient which was useful for finding the quantitative estimation of absorbed dose, high reproducibility and high sensitivity compared to undoped glasses.
The neutron and gamma response of undoped and Dy-doped MgB4O7 thermoluminescent dosimeter has been studied by Iflazoglu et al., using computer glow curve deconvolution (CGCD) method [48] and they reported that MgB4O7:Dy (1 Mol%) has high sensitivity which is approximately 1.90 and 1.47 times than TLD-600 and TLD-700, respectively, which could be a promising material for the thermoluminescent neutron and gamma dosimeter. In similar material, Salama et al., using repeated initial rise (RIR) method [49], found that the dosimeter has high thermal fading and light sensitivity. The realization of Dy-doped Li2MgB2O4 glasses as thermoluminescent solid detector and subjected to high dose dosimetry in the range 1–100Gy by irradiating to radio-active cobalt (Co-60) source was done by Hasim et al. [50, 51]. They reported that the dosimeter has minimal fading, high sensitivity, good linearity and reproducibility which indicates that the material is a good candidate for accurate radiation detection in photon beam. The neutron dosimetry of LiMgBO3:Dy3+ has been studied by Meghnath Sen et al., [52] and found that the TL sensitivity for neutron and gamma radiation is 0.6 and 0.3 times, respectively, than commercially available dosimeter TLD-100.
Further developed material for TLD applications is NaBaBO3:Ce3+ which was synthesized by the Combustion method by Mehmet Oglakci et al., [53] they concluded that the sample is a favorable material for the application in environmental dosimetry as one depicts good TL dose response with adequate linearity and sensitivity. Daisuke Nakauchi et al., have studied the optical and radio-induced luminescence properties of Ce3+- and Sn+3-doped MgAl2B2O7 glasses, in the X-ray dose irradiated over 1–10Gy [54, 55] and reported that the TL intensity increases monotonically after the irradiation of X-rays with a dose of 1–10000mGy. Saidu et al., studied the thermoluminescence characteristics of Cu-doped ZnLi2B2O5 and reported that the TL intensity had increased with the addition of Na within the dose range of 0.5–1000Gy [45, 56]. Ahmad et al., have reported the thermoluminescence dosimetric characteristics of Cu-doped MgLi2B2O5 glasses which showed good linearity with a linear regression coefficient of 0.9980 and 0.9949, with the dose range of 1–10Gy and 10–100Gy, respectively [57]. Ismail Rammadhan and his colleagues have studied the thermoluminescence characteristics of Cu2O-doped CaLi2B2O4 glasses which were irradiated with 6 ℃o gamma rays having a range of 0.5–4Gy, 5–10Gy, and 20–100Gy and found the effective atomic number (Zeff) as 8.84 which is nearer to the atomic number of soft tissues [58]. Salleh et al., have reported the effect of Strontium(Sr) concentration on the thermoluminescence glow curve of Cu-doped MgLi2B2O5 glasses [59] and found that 0.003 Mol% of Sr has shown optimum TL intensity of 3.6×105 nCg-1. Chopra et al., studied the TL properties of Li2B4O7: Cu material prepared by combustion method and irradiated with a 3MeV proton beam [60] and according to their study, the sample has simple glow curve structure, good linearity and low fading makes it a good candidate for effective proton beam TL dosimeter for the treatment of cancer. Hossain et al., addressed the characteristics of Cu-doped and undoped K2B4O7 glass for use as ionizing radiation dosimeters by studying the thermoluminescence response [61] and mentioned that the Cu-doped KB glasses have superiority in terms of linearity and sensitivity which is 6.75 times greater than undoped glasses. Prabhu et al., have investigated the structural and radiation shielding properties of Er+3-doped zinc bismuth borate glasses by irradiating them with 1.25MeV gamma rays [62, 63] and reported that the glass shown the linearity in the dose range of 5–50kGy which proved their suitability for dosimetric applications in the food irradiation zones and in red LEDs.
Vijeta Bhatia et al., have developed Sm+3 and Er+3-doped MnK2B2O5 glasses and studied the structural, optical, and thermoluminescence properties [64, 65] and reported the high TL intensity recorded for 0.8Mol% Sm+3 under all irradiation sources with a good linear correlation factor of 0.997 which proves it is a suitable material for dosimetric applications. Anjaiah et al., have reported the preparation of Sm+3 and Eu+3-doped MLi2B2O5 (where M: Zn, Ca and Cd) glasses to study their absorption and luminescence properties to understand their lasing potentialities [66, 67]; they concluded that CdBSm glasses can be used as radiation dosimeters effectives as they exhibit high TL light output at high temperatures. Vinod Hegde et al., have studied the effects of high dose gamma irradiation on optical properties of Eu+3-doped ZnNa2Bi2B2O8 glasses which shows TL linearity in the dose range 0.25–3kGy which proved their suitability for dosimetric application in the food irradiation zones in addition to the red LEDs (<1kGy) [68]. Sudhakar and his colleagues have studied the influence of modifier oxide (viz., PbO, CaO and ZnO) on spectroscopic and thermoluminescence characteristics of Sm+3-doped Sb2B2O6 glasses and found that ZnO mixed glasses has high non-radiative losses among three glasses [69]. Hayder Obayes et al., have reported the improved thermoluminescence and kinetic parameters of the new Sr/Cu-doped LB glass system in the dose range 0.5–100Gy; the material has excellent reproducibility and low fading useful for dosimetry applications [70].
Ono et al. have reported the preparation of Tb+3-doped CaAl2B2O7 glasses; studied the thermoluminescence properties by X-ray irradiation which found to show good linearity and sensitivity with dynamic range[71]. Ismail Mohammed et al., studied the effect of ZnO co-dopant on the thermoluminescence properties of Cu2O-doped lithium borate glass and found that the TL properties of the dosimeter has enhanced in terms of sensitivity by 4 times, TL intensity by 3 times, fading, reproducibility, and minimum detectable dose which confirms that the dosimeter has potential to be used in dosimetry.[72]. Eman Mohammed et al. have studied the effect of gamma rays on Zn/Cu-doped SrNa2B2O5 glass system for dosimetric applications in many fields such as food irradiation processing, irradiation applications, and medical sterilization [73]. Bahra Mohammed et al. have studied the thermoluminescence dosimetric properties and kinematic parameters of ZnSiB2O6 glass doped with Cu2O and then co-doped with SnO2 within the range 0.5–100Gy and concluded that 0.1Mol% SnO2 co-doped glass is found to be useful in thermoluminescence dosimetry as it has high linearity, reproducibility, low thermal fading and minimum detectable dose [74]. El-Bayoumi et al. observed a well-resolved thermoluminescence peak at 168 ℃ observed in W-doped CdB2O3 glasses which are irradiated by gamma radiation in the range from 0.025 to 2kGy and found that 1.5Mol% of W-doped glass system could be valuable for the radiation dosimetry of perishable food items as it exhibited a good linearity and sensitivity in the range of 25 – 150Gy [75].
Furthermore, there have been many developments in phosphate glasses for thermoluminescence dosimetric applications. Ivascu et al. have investigated the FTIR, Raman spectra, and thermoluminescence properties of the P2Li2BaO7 glasses in the high dose range <10Gy and concluded that the addition of Li2O to the base glass BaO–P2O5, has not shown satisfactory in terms of linearity and sensitivity but the base glass sample has shown good linearity in the dose range up to 100Gy [76]. Swamy and his colleagues have reported the influence of Cu and Mn on thermoluminescence properties of boron phosphate glasses by exposing different gamma-ray doses in the range 250–1000Gy and the dose response of MnO-doped glass sample revealed that it exhibit a good linearity and an adequate sensitivity for the measurement of high doses when compared to commercially available dosimeter CaSO4:Dy phosphor [77, 78]. Barna Biro et.al. investigated the thermoluminescence properties of yttrium-doped silica phosphate vitro ceramics and concluded that 30 Mol% Y2O3-doped vitro ceramic exhibited good reproducibility, low thermal fading, and acceptable homogeneity which recommends this material for dosimetry applications [79].
Anwar et al. have reported the improvement in thermoluminescence characteristics of Li2O–P2O5 glasses and observed linearity up to the dose 200Gy and concluded that the addition of appropriate transition metal to the base sample can enhance the TL properties such as linearity and fading [80]. Kalpana et.al. has studied the influence of alumina in photoluminescence and thermoluminescence characteristics of Gd+3- and Tb+3-doped boron phosphate glasses and observed that TL linearity obtained in the dose range of 0.5–4kGy which concluded that the glass material has potential use in dosimetric applications in this range [81, 82]. Tanaka et.al. has studied the radio photoluminescence (RPL) properties of Ag-doped mixed phosphate glasses and found that the peak intensity of RPL increase with exposure except in Bi/Na–Ag [83].
Vallejo and his colleagues have studied the photoluminescence and thermoluminescence properties of Dy+3-doped phosphate glasses containing silver nanoparticles which increase the TL intensity and can be used in solid-state illumination and retrospective dosimetry [84]. Sunil Thomas et.al. reported the thermoluminescence of β-irradiated K–Mg–Al–Zn fluorophosphate glasses and found that the glasses exhibit superlinear in the dose range of 1–190Gy, fading is 11% in 15h and the activation energy for higher temperature peak was evaluated as 1.31eV and that of lower temperature is 0.47eV [85]. Amany El-Kheshon and his colleagues have investigated La-doped phosphate glass and revealed that the glasses have high reproducibility and linearity in the wide range of radiation and found that the minimum detectable dose of 27.3μGy which confirms the use dosimetry as beta dosimeter [86, 87]. Anwar et al. have studied the thermoluminescence characteristics of a newly developed glass of composition 40P2O5-50BaO-2.5Na2O-2.5MgO-5TiO2 and concluded that it has minor sensitivity up to 10Gy, linearity in the dose range 10–500Gy which recommends a vital role in the environmental dosimetry at high radiation doses [88].
El Mesady and his colleague have studied the optical and luminescence properties of Si-doped alumino-phosphate-sodium glass system and concluded that APCNSi5 sample shows good linearity and sensitivity in the selected UV region and this type of glass is useful material for UV as well as γ-dosimetry [89]. Hirano et.al. has evaluated the photoluminescence (PL) and thermally stimulated luminescence (TSL) properties of Sn-doped zinc sodium Phosphate glasses and identified that 0.5% Sn-doped glass sample exhibited highest sensitivity with good linearity among all the other samples and TSL fading is also evaluated [90]. The thermoluminescence characteristics and dose response of Gd+3, Dy+3, and Tb+3-doped alumino-phosphate glasses was studied by Gesiorowski et al., and observed that 0.5Mol% Tb2O3-doped glass sample has shown high TL efficiency, good linearity and sensitivity which makes this as a demanding material for electron and gamma-ray dosimetry [91,92,93].
The feasibility of the Mg–phosphate glasses as a thermoluminescent dosimeter in a high dose has been investigated by Abdou et al. and observed that the dosimeter is having TL linearity in the dose range 5–1000Gy and no significant fading is observed after 60 days of normal storage [94]. Rubalajyothi and colleagues have studied the thermoluminescence characteristic studies and anti-bacterial activity of Ba1-xCaxSO4: Dy, Er glasses and found that Dy and Er-doped glasses have sensitivity in radiotherapy and medical environment from 10 to 500Gy and 900Gy to 2kGy, respectively [95]. Vinod Kumar et al. investigated the thermoluminescence properties of Pr+3-doped SrBPO5 glasses and the TL properties are enhanced by the addition of UO2+2 and the dose response was recorded in the range of 1mGy–1kGy and concluded that the dosimeter exhibited the linearity in the dose range 100mGy–100Gy [96]. Raja et al., have prepared a novel fluoroperovskite RbCaF3: Sm+3 phosphors for radiation dosimetry and orange–red LED applications and observed that 0.6Mol% Sm+3 phosphor exhibited good linearity and low fading of 10% of the TL signal up to 50 days and almost stable later [97].
Muniratnam et al. have studied the effect of Mn on thermoluminescence properties of Na3Y(PO4)2:Dy phosphors irradiated by gamma rays for dosimetry applications and observed that 0.07Dy-doped phosphor exhibited linearity in the dose range 50Gy–1.5kGy and the glow curves exhibited second-order dynamics which confirms that the phosphor is having potential applications in TL dosimetry to measure low irradiation [98]. Li et al. has reported the synthesis and optical properties of novel Gd+3-doped BaZn2(PO4)2 glass ceramics and found that 0.5 mol% Gd-doped glass sample exhibited good linearity in the dose range 0.3–500Gy, good reusability and the minimum detectable dose is 0.675 mGy [99]. The synthesis of Nd+3-doped NaMgBSi glasses has been reported by Kaur et al., [100] and observed that 0.08mol% Nd-doped dosimeter exhibited a good linearity and low fading. Alazab et al. have studied the thermoluminescence properties of bioglass and observed that the glass dosimeter is slightly sublinear in the dose range 25–1000Gy which indicates that the glass also is useful for high dose dosimetry [101]. Anil Kumar et al. have reported the preparation of ZrxCa30-xP70 bioglass and studied its thermoluminescent properties under different gamma radiation doses with and without immersing in a simulated body fluid(SBF) and observed that the TL intensity decreases when immersed in SBF which is useful to modulate bio-behavior in term of hydroxyapatite layer growth on the glass surface [102].
This overview demonstrates that many researchers worldwide are showing interest in the preparation of various materials for radiation dosimetric applications. At present, nanocrystalline phosphors are being used for TLD materials but as they are failing to hold the high dosage of radiation, there is a need to develop the nanomaterials as TL dosimeters. In the present review, the application of glass materials as thermoluminescence dosimeters in radiation dosimetry is presented.
2 Materials and methods used in glass preparation
Different methods can be employed for the synthesis of luminescent materials such as glasses in microcrystalline and nanocrystalline forms. Mostly, the melt-quenching method, combustion method, and sol–gel method have been used as described below.
2.1 Melt-quenching method
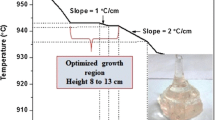
The melt-quenching technique is the most convenient method to prepare the glasses in which the molten form of the materials is cooled so quickly to avoid crystal formation. In this method, the raw materials are thoroughly mixed and are melted at the required temperature by placing them in a muffle furnace. The melt is then poured onto a stainless mould and pressed quickly with another plate which is at annealing temperature to avoid crystal formation. Therefore, transparent glass material is obtained. Different factors affect the color and formation of the glasses, such as the type of dopant added and the rate of quenching. The faster the rate of quenching, the better the formation of the glasses. Borosilicate glass with the composition (60-x) B2O3-20SiO2-xBi2O3-12ZnO-8BaO (x = 0,2, 4, 6, 8, 10 and 12 mol%) [103], Ge-doped calcium borate glasses with the composition (30-x) CaO-70B2O3: xGeO2 where x = 0.1,0.2,0.3, 0.4 and 0.5mol% [104] and lead borate and lead aluminum borate glasses with composition 4.5PbO-1.9H3BO3 and 11.1PbO-2.9H3BO3-0.2Al2O3 [105] have been synthesized using melt-quenching method Fig. 1.
2.2 Combustion method
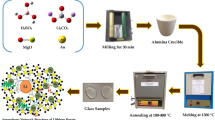
The combustion method is a low-cost and effective method for preparing glass nanocrystals. In this method, all the raw materials are taken in stoichiometric ratio, and fuel is taken to cause the combustion such as urea, citric acid, glycine, etc. By taking all the raw materials in a crucible and it is to be placed in a preheated furnace at a high temperature at which the combustion of the materials can take place. The phosphor with the composition Li2B4O7: Ag1%,La(x%) (x = 0.1, 0.5, 1 and 3 wt%) [106], NaBaBO3: Ce+3 [53] have been synthesized using combustion method Fig. 2.
2.3 Sol–gel method
The sol–gel method is a very simple and economical method for the preparation of glasses. In this method, first, we need to take the precursors of high purity in an aqueous solution form which is having a constant pH value (generally >7), and then add the solvent drop-wise till the gelatinous precipitate is formed Table 1. Filter this precipitate using a filter paper and transform this precipitate into a crucible which has to be put in the muffle furnace which is maintaining a high temperature for the formation of glasses Fig. 3. Dy-doped strontium borate glasses (SrB: Dy) have been prepared by the sol–gel method [43].
3 Few recently developed highly sensitive TLD glass materials
3.1 MgLi2B2Cu2O6: Sr
The MgLi2B2Cu2O6: Sr glass was synthesized by melt-quenching method and investigated the effect of different concentrations of Strontium (SrO) on TL characteristics at a dose of 50Gy and found that the optimum TL response with 0.003 Mol% of Sr concentration with TL intensity of 3.6×105nCg-1. The TL glow curve for 0.003Mol% Sr is shown in Fig. 4. The maximum peak temperature of glow curve for all the glass samples of different concentrations of Sr was observed between 170 and 200 ℃ [59].

(Reproduced from ref. [59], Open access under Common Creative Attribution Non-Commercial 4.0 International (CC-BY-NC 4.0) https://creativecommons.org/licenses/by-nc/4.0/)
TL glow curve of MgLi2B2Cu2O6: 0.003 Mol% Sr
3.2 BaZnSiB2O10: Bi2O3
The synthesis of BaZnSiB2O10: Bi2O3 was carried out by melt-quenching technique and has given the detailed studies of thermal, mechanical, radiation shielding, and thermoluminescence (TL) properties by D’Souza et al [103]. The TL study was done to assess the suitability of prepared glasses as a radiation dosimeter in the gamma dose range of 0.25–30kGy. Figure 5 shows the TL glow curve of powdered ZnBiB glass samples with gamma dose of 5kGy. It is observed that the addition of Bi2O3 decreases the TL intensity of the glass samples because, with the addition, Zn+2 ions reduced to Zn+ ions which do not form Si–O–Zn linkage to form glass network.

(Reproduced from ref. [104], Open access under Common Creative Attribution 4.0 International (CC-BY 4.0) https://creativecommons.org/licenses/by/4.0)
TL glow curve of BaZnSiB2O10: Bi2O3 of 0 and 2 Mol%
3.3 Ba1-xCaxSO4: Dy3+, Er3+
Barium calcium sulfate (Ba1-xCaxSO4: Dy3+, Er3+) phosphor material was prepared by combustion method for thermoluminescence characteristics [95]. In this study, the authors reported the TL properties of Dy3+- and Er3+-doped glass matrix for low and high dose response. The TL study was done for the glass samples in the gamma dose range of 10–500Gy for Dy3+ and 900Gy–2kGy for Er3+. Figure 6 shows the TL glow curves for 0.5Mol% Dy- and 1.0Mol% Er-doped glass sample with the gamma dose of 10Gy, 500Gy and 900Gy, 2kGy, respectively. From this study, it is also clear that the TL peaks have second-order active peaks and the dysprosium and erbium are found to be suitable for radiation therapy and medical environment in the range 10–500Gy and 900–2000Gy, respectively, and the frequency factors are found to be from 5.95±0.01 × 107 to 1.89±0.03 × 1018 and 0.5±0.05 × 109 to 1.43±0.01 × 1018, respectively.

(Reproduced from ref. [95], open access under Common Creative Attribution 4.0 International (CC-BY 4.0) https://creativecommons.org/licenses/by/4.0)
TL glow curve of (a) 0.5 Mol% Dy3+ and (b) 1.0 Mol% Er3+ doped Ba1-xCaxSO4
4 Future scope
There is a need to prepare high-efficient, high-precision, and high-quality materials for in vivo dosimetry applications which are biologically compatible and eco-friendly. Under reduce–reuse–recycle theme for ecological balance, one needs to prepare a glass material that is re-usable and should have high-level scalability and applications in many fields of science like space applications, nuclear testing, and shielding applications, solar testing, X-ray testing, and even as neutron detectors also. As the tailoring of the glass materials is possible, one can synthesize the materials in any geometrical structure according to the need of the application. For all the materials which are customized, there is a lot to explore in terms of characterization, physical and chemical properties, and especially in the field of in vivo dosimetry.
5 Conclusions
Different TLD materials in the form of glasses have been reviewed with a special focus on lithium borate glasses. It can be concluded that the studies on the TL dosimetry materials mainly focus on the development of more efficient glass materials, more consistency, easily preparable/reproduceable, high durability for radiation damage and biocompatible or 100% environment friendly, over the existing materials. The search for new materials should aim to produce the materials with simple and good linearity in the TL dose curves and low fading. Furthermore, it is very important to consider the TL mechanism before developing the new glass materials for the applications in in vivo dosimetry.
References
A. Shortland, E. Katherine, D. Patrick, K. Susanna, W. Marc, The origins of glasses: the near East or Egypt? Annual meeting of the American Schools of Oriental Research, (San Antonio, 2016)
J.A.J. Gowlett, High definition archaeology: threads through the past (Routledge, 1997)
A. Kanungo, B. Robert, Kopia, India’s first glassmaking site: dating and chemical analysis. J Glass Stud 51, 11–25 (2009)
D.M. Bose, A concise history of science in India (Indian National Science Academy, 1971), p.15. (ISBN 8173716196)
A. Ghosh, An encyclopaedia of Indian archaeology (BRILL, 1990). (ISBN 90-04-09262-5)
C. Braghin, “Introduction” pp. XI-XIV in Braghin, C. (ed) Chinese glass. Archaeological studies on the uses and social contest of glass artefacts from the Warring States to the Northern Song Period (fifth century B.C. to twelfth century A.D.). ISBN 8822251628(2002).
R. Pinder-Wilson, The Islamic lands and China, in Five thousand years of glass. ed. by H. Tait (University of Pennsylvania Press, 1991), p.140
J. P. Toner, Popular culture in ancient Rome. ISBN 0-7456-4310-8. (2009), p. 19
J. Krogh-Moe, The structure of vitreous and liquid boron oxide. J. Non-Cryst. Solids 1(4), 269–284 (1969)
C. Gautam, A.K. Yadav, A.K. Singh, A review on infrared spectroscopy of borate glasses with effects of different additives. ISRN Ceram. (2012). https://doi.org/10.5402/2012/428497
M. Bengisu, Borate glasses for scientific and industrial applications: a review. J. Mater. Sci. (2016). https://doi.org/10.1007/s10853-015-9537-4
J.E. Shelby, Introduction to glass science and technology (Royal Society of Chemistry, Cambridge, 2015)
N.M. Bobkova, Thermal expansion of binary borate glasses and their structure. Glass Phys. Chem. 29, 501–507 (2003)
M. Yamane, Glasses for photonics (Cambridge University Press, Port Chester, 2000)
K. Terashima, S. Tamura, S.H. Kim, T. Yoko, Structure and nonlinear optical properties of lanthanide borate glasses. J. Am. Ceram. Soc. 80, 2903–2909 (1997)
W. Nie, Optical nonlinearity: phenomena, applications, and materials. Adv. Mater. 5, 520–545 (1993)
O. Deparis, F.P. Mezzapesa, C. Corbari, P.G. Kazansky, K. Sakaguchi, Origin and enhancement of the second-order non-linear optical susceptibility induced in bismuth borate glasses by thermal poling. J. Non-Cryst. Solids 351, 2166–2177 (2005)
V. Nazabal, E. Fargin, B. Ferreira, G. Le Flem, B. Desbat, T. Buffeteau, M. Couzi, V. Rodriguez, S. Santran, L. Canioni, L. Sarger, Thermally poled new borate glasses for second harmonic generation. J. Non-Cryst. Solids 290, 73–85 (2001)
R.W. Boyd, G.L. Fischer, Nonlinear optical materials, in Encyclopedia of materials: science and technology. ed. by K.H.J. Buschow et al. (Elsevier, New York, 2001)
R.A. Myers, N. Mukherjee, S.R.J. Brueck, Large second order nonlinearity in poled fused silica. Opt. Lett. 16, 1732–1734 (1991)
C. Corbari, L.C. Ajitdoss, I.C.S. Carvalho, O. Deparis, F.P. Mezzapesa, P.G. Kazansky, K. Sakaguchi, The problem of achieving high second-order nonlinearities in glasses: the role of electronic conductivity in poling of high index glasses. J. Non- Cryst. Solids 356, 2742–2749 (2010)
X. Tiefeng, C. Feifei, D. Shixun, N. Qiuhua, S. Xiang, W. Xunsi, Third-order optical nonlinear characterizations of Bi2O3–B2O3–TiO2 ternary glasses. Phys. B 404, 2012–2015 (2009)
J. Qiu, N. Tanaka, N. Sugimoto, K. Hirao, Faraday effect in Tb3+-containing borate, fluoride and fluorophosphates glasses. J. Non-Cryst. Solids 213, 193–198 (1997)
C.K. Bomfork, I.H. Kunkler, J. Walter, Textbook of radiotherapy: radiation physics, therapy and oncology (Churchill Livingstone, China, 2002)
R. Chen, S.W.S. McKeever, Theory of thermoluminescence and related phenomenon (World Scientific Publishing, Singapore, 1997)
M.R. Ioan, Amorphous and crystalline optical materials used as instruments for high gamma radiation doses estimations. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam. Interact. Mater. Atoms (2016). https://doi.org/10.1016/j.nimb.2016.04.009
G.A. Alharshan, D.A. Aloraini, Thermoluminescence properties of slate relevant to radiation measurements. AIP Conf. Proc. 2043, 020004 (2018). https://doi.org/10.1063/1.5080023
S.B. Lochab, S.P. Pratik Kumar, CaSO4: Dy, Mn: a new and highly sensitive thermoluminescence phosphor for versatile dosimetry. Radiat. Phys. Chem. 119, 136–141 (2016). https://doi.org/10.1016/j.radphyschem.2015.10.004
R.L. Nyenge, H.C. Swart, D. Poelman, P.F. Smet, L.I.D.J. Martin, L.L. Noto, S. Som, O.M. Ntwaeaborwa, Thermal quenching, cathodoluminescence and thermoluminescence study of Eu2+ doped CaS powder. J. Alloy. Compd. (2015). https://doi.org/10.1016/j.jallcom.2015.10.143
N.J. Shivaramu, B.N. Lakshminarasappa, K.R. Nagabhushana, F. Singh, Thermoluminescence of sol–gel derived Y2O3: Nd3+ nanophosphor exposed to 100MeV Si8+ ions and gamma rays. J. Alloys Compd. 637, 564–573 (2015). https://doi.org/10.1016/j.jallcom.2015.02.218. (ISSN 0925-8388)
K.K. Gupta, R.M. Kadam, N.S. Dhoble, S.P. Lochab, V. Singh, S.J. Dhoble, Photoluminescence, thermoluminescence and evaluation of some parameters of Dy3+ activated Sr5(PO4)3F phosphor synthesized by sol-gel method. J. Alloys Compd. 688, 982–993 (2016). https://doi.org/10.1016/j.jallcom.2016.07.114. (ISSN 0925-8388)
F. Daniels, C.A. Boyd, D.F. Saunders, Thermoluminescence as a research tool. Science 117(3040), 343–349 (1953). https://doi.org/10.1126/science.117.3040.343
P.J. Fox, R.A. Akber, J.R. Prescott, Spectral characteristics of six phosphors used in thermoluminescence dosimetry. J. Phys. D: Appl Phys. 21(1), 189–193 (1988). https://doi.org/10.1088/0022-3727/21/1/026
A. Kumar, A.K. Sharma, R. Dogra, M. Manhas, R. Kumar, Thermoluminescence studies of γ-irradiated LiF: Sm3+ nanophosphor, AIP Conference Proceedings [NATIONAL CONFERENCE ON RECENT ADVANCES IN EXPERIMENTAL AND THEORETICAL PHYSICS (RAETP-2018) - Jammu, India (17–18 April 2018)] - 2006, 030013, (2018). https://doi.org/10.1063/1.5051269.
J.H. Schulman, R.D. Kirk, E.J. West, Proc. 1st Int. Conf. on luminescence dosimetry (Standford, 1965), p.113
W. Binder, S. Disterhoft, J.R. Cameron, In: Proc. 2nd Conf. on Luminescence Dosimetry, Gatlinburg. Conf. 680920 (NTIS, Springfield, VA), 113 pp (1968).
S.K. Mehta, S. Sengupta, Al203 phosphor for thermoluminescence dosimetry. Health Phys. 31(2), 176–177 (1976)
A.C. Lucas, B. Kaspar, In: Proc 5th Conf. on luminescence dosimetry, Sau Paulo (I Phys. Inst. Univ. Giessen), 131 pp (1977).
T. Nakajima, Y. Murayama, T. Matsuzawa, Preparation and dosimetric properties of a highly sensitive LiF thermoluminescent dosimeter. Health Phys. 36(1), 79–82 (1979)
M. Takenga, O. Yamamoto, T. Yamashita, Nucl. Instum. Methods 175, 77 (1980)
M.S. Akselrod, V.S. Kortov, D.J. Kravetsky, V.I. Gotlib, Highly sensitive thermoluminescent anion-defective alpha-Al203: C single crystal detectors. Radiat. Prot. Dosim. 32(1), 15–20 (1990). https://doi.org/10.1093/oxfordjournals.rpd.a080715
A. El-Adawy, N.E. Khaled, A.R. El-Sersy, A. Hussein, H. Donya, TL dosimetric properties of Li2O–B2O3 glasses for gamma dosimetry. Appl. Radiat. Isot. 68(6), 1132–1136 (2010). https://doi.org/10.1016/j.apradiso.2010.01.017
M. Santiago, J. Marcazzó, C. Grasselli, A. Lavat, P. Molina, F. Spano, E. Caselli, Thermo- and radioluminescence of undoped and Dy-doped strontium borates prepared by sol-gel method. Radiat. Meas. 46(12), 1488–1491 (2011). https://doi.org/10.1016/j.radmeas.2011.01.006
E. Ekdal, T. Karalı, A. Kelemen, M. Ignatovych, V. Holovey, C. Harmansah, Thermoluminescence characteristics of Li2B4O7 single crystal dosimeters doped with Mn. Radiat. Phys. Chem. 96, 201–204 (2014). https://doi.org/10.1016/j.radphyschem.2013.10.009
A. Saidu, H. Wagiran, M.A. Saeed, Y.S.M. Alajerami, Thermoluminescence characteristics of zinc lithium borate glass activated with Cu+(ZnO–Li2O–B2O3:Cu+) for radiation dosimetry. J. Radioanal. Nucl. Chem. 304(2), 627–632 (2015). https://doi.org/10.1007/s10967-014-3846-y
A. Ab Rasid, H. Wagiran, S. Hashim, Z. Ibrahim, H. Ali, Dosimetric properties of dysprosium doped lithium borate glass irradiated by 6MV photons. Radiat. Phys. Chem. 112, 29–33 (2015). https://doi.org/10.1016/j.radphyschem.2015.02.003
B. Sanyal, M. Goswami, S. Shobha, V. Prakasan, S.P. Chawla, M. Krishnan, S.K. Ghosh, Synthesis and characterization of Dy 3+ doped lithium borate glass for thermoluminescence dosimetry. J. Non-Cryst. Solids 475, 184–189 (2017). https://doi.org/10.1016/j.jnoncrysol.2017.09.016
S. İflazoğlu, A. Yılmaz, V.E. Kafadar, M. Topaksu, A.N. Yazıcı, Neutron+Gamma response of undoped and Dy doped MgB4O7 thermoluminescence dosimeter. Appl. Radiat. Isot. (2019). https://doi.org/10.1016/j.apradiso.2019.02.014
E. Salama, H.A. Soliman, Thermoluminescence glow curve deconvolution and trapping parameters determination of dysprosium doped magnesium borate glass. Radiat. Phys. Chem. (2018). https://doi.org/10.1016/j.radphyschem.2018.03.003
S. Hashim, M.H.A. Mhareb, S.K. Ghoshal, Y.S.M. Alajerami, D.A. Bradley, M.I. Saripan, N. Tamchek, K. Alzimami, Luminescence characteristics of Li2O-MgO-B2O3 doped with Dy3+ as a solid TL detector. Radiat. Phys. Chem. (2015). https://doi.org/10.1016/j.radphyschem.2015.04.007
S. Hashim, R.S. Omar, S.K. Ghoshal, Realization of dysprosium doped lithium magnesium borate glass based TLD subjected to 1–100 Gy photon beam irradiations. Radiat. Phys. Chem. (2019). https://doi.org/10.1016/j.radphyschem.2019.05.016
S. Meghnath, S. Rakesh, V. Sathian, M.S. Kulkarni, A.K. Tyagi, Thermoluminescence based personnel neutron dosimetry study of LiMgBO3:Dy3+. Ceram. Int. (2020). https://doi.org/10.1016/j.ceramint.2020.05.105
M. Oglakci, M. Topaksu, N. Can, Thermoluminescence glow curves of beta irradiated NaBaBO3: Ce3+ phosphor synthesized by combustion method. Sens. Actuators, A 315, 112299 (2020). https://doi.org/10.1016/j.sna.2020.112299
D. Nakauchi, G. Okada, Y. Fujimoto, N. Kawano, K. Noriaki, Y. Takayuki, Optical and radiation-induced luminescence properties of Ce-doped magnesium aluminoborate glasses. Opt. Mater. 72, 190–194 (2017). https://doi.org/10.1016/j.optmat.2017.05.063
D. Nakauchi, G. Okada, Y. Fujimoto, N. Kawano, K. Noriaki, Y. Takayuki, Optical and radiation-induced luminescence properties of Sn-doped magnesium aluminoborate glasses. Phys. Chem. Glasses: Eur. J. Glass Sci. Technol. Part B 60, 10–14 (2019). https://doi.org/10.13036/17533562.60.1.029
A. Saidu, H. Wagiran, M.A. Saeed, Y.S.M. Alajerami, A.B.A. Kadir, Effect of co-doping of sodium on the thermoluminescence dosimetry properties of copper-doped zinc lithium borate glass system. Appl. Radiat. Isot. (2016). https://doi.org/10.1016/j.apradiso.2016.10.005
T. Ahamad, Z.A. Alothman, M. Naushad, K. Yusuf, Synthesis and characterization of CuO doped lithium magnesium borate glasses for thermoluminescence dosimetry. Optik 231, 166369 (2021). https://doi.org/10.1016/j.ijleo.2021.166369
I. Rammadhan, S. Taha, H. Wagiran, Thermoluminescence characteristics of Cu2O doped calcium lithium borate glass irradiated with the cobalt-60 gamma rays. J. Lumin. 186, 117–122 (2017). https://doi.org/10.1016/j.jlumin.2017.02.026
N. Salleh, T. Abd Rahman, M. Saeed, Effect of strontium concentration on thermoluminescence glow curve of copper doped lithium magnesium borate glass. Malays. J. Fundam. Appl. Sci. (2017). https://doi.org/10.11113/mjfas.v13n3.570
V. Chopra, S.J. Dhoble, K.K. Gupta, A. Singh, A. Pandey, Thermoluminescence of Li2B4O7: Cu phosphor exposed to proton beam for dosimetric application. Radiat. Meas. (2018). https://doi.org/10.1016/j.radmeas.2018.05.002
I. Hossain, N.K. Shekaili, H. Wagiran, Thermoluminescence response of copper-doped potassium borate glass subjected to 6 megavolt X-Ray irradiation. J. Appl. Spectrosc. 82(1), 149–152 (2015). https://doi.org/10.1007/s10812-015-0078-z
N.S. Prabhu, V. Hegde, M.I. Sayyed, O. Agar, S.D. Kamath, Investigations on structural and radiation shielding properties of Er3+ doped zinc bismuth borate glasses. Mater. Chem. Phys. (2019). https://doi.org/10.1016/j.matchemphys.2019.03.074
V. Hegde, N.S. Prabhu, W. Akshatha, M.I. Sayyed, O. Agar, S.D. Kamath, Influence of 125 MeV gamma rays on optical and luminescent features of Er3+ doped zinc bismuth borate glasses. Results Phys. 12(1), 1762–1769 (2019). https://doi.org/10.1016/j.rinp.2019.02.003
V. Bhatia, D. Kumar, H. Singh, N. Kaur, S.M. Rao, A. Kumar, V. Mehta, S.P. Singh, Structural, optical and thermoluminescence properties of newly developed MnKB: Er3+ glass system. J. Non-Cryst. Solids 543, 120113 (2020). https://doi.org/10.1016/j.jnoncrysol.2020.120113
V. Bhatia, D. Kumar, H. Singh, N. Kaur, S.M. Rao, A. Kumar, V. Mehta, S.P. Singh, Effects of Sm3+ ions on the structural, optical and thermoluminescence properties of MnKB glass system. J. Phys. Chem. Solids 161, 110408 (2022). https://doi.org/10.1016/j.jpcs.2021.110408
J. Anjaiah, C. Laxmikanth, N. Veeraiah, Spectroscopic properties and luminescence behaviour of europium doped lithium borate glasses. Phys. B: Condens Matter 454, 148–156 (2014). https://doi.org/10.1016/j.physb.2014.07.070
J. Anjaiah, C. Laxmikanth, N. Veeraiah, P. Kistaiah, Infrared luminescence and thermoluminescence of lithium borate glasses doped with Sm3+ ions. Mater. Science-Poland (2014). https://doi.org/10.1515/msp-2015-0028
V. Hegde, N. Chauhan, V. Kumar, C.D. Viswanath, K.K. Mahato, D.K. Sudha, Effects of high dose gamma irradiation on the optical properties of Eu3+ doped zinc sodium bismuth borate glasses for red LEDs. J. Lumin. (2018). https://doi.org/10.1016/j.jlumin.2018.11.023
K. Sudhakar, M. Srinivasa Reddy, L. Rao, N. Veeraiah, Influence of modifier oxide on spectroscopic and thermoluminescence characteristics of Sm3+ ion in antimony borate glass system. J. Lumin. 128, 1791–1798 (2008). https://doi.org/10.1016/j.jlumin.2008.04.010
H. Obayes, O. Obayes, Q. Kadhim, A. Saidu, M.H. Al-Maamori, Improved thermoluminescence and kinetic parameters of new strontium/ copper co-doped lithium borate glass system. Nucl. Inst. Methods Phys. Res. Sect. B: Beam Interact. Mater. Atoms. (2019). https://doi.org/10.1016/j.nimb.2019.06.028
H. Ono, Y. Fujimoto, T. Yahaba, T. Yanagida, M. Koshimizu, K. Asai, Thermoluminescence properties of Tb3+-doped CaO–Al2O3–B2O3-based glasses. Optic. Mater. (2018). https://doi.org/10.1016/j.optmat.2018.07.004
M. Ismail, A. Saddon, M. Fahmi, Impact of Zn2+ ions co-doping on the TL properties of Cu2+ ion-doped calcium lithium borate glass irradiated by various radiation sources. J. Lumin. 236, 118091 (2021). https://doi.org/10.1016/j.jlumin.2021.118091
E. M. Abou Hussein, S.M. Gafar, Effect of gamma rays on Zn/Cu doped strontium borate glass system for dosimetric applications. Radiochimica Acta (2022). https://doi.org/10.1515/ract-2022-0029.
M. Bahra, M. Jaafar, H. Wagiran, Thermoluminescence dosimetry properties and kinetic parameters of zinc borate silica glass doped with Cu2O and co-doped with SnO2. J. Lumin. (2018). https://doi.org/10.1016/j.jlumin.2018.08.020
A.S. El-Bayoumi, H. Alazab, F. Ezz-Eldin, The impact of γ-irradiation on Cd-B2O3 glass doped WO3: new evidences by TL and ESR spectroscopy. J. Non-Cryst. Solids 551, 120459 (2021). https://doi.org/10.1016/j.jnoncrysol.2020.120459
C. Ivascu, A. Timar Gabor, O. Cozar, L. Daraban, I. Ardelean, FT-IR, Raman and thermoluminescence investigation of P2O5–BaO–Li2O glass system. J. Mol. Struct. 993(1–3), 249–253 (2011)
R. Swamy, S. Bhaskar, Y. Gandhi, R. Kadam, N. Venkatarman, P. Raghava, N. Veeraiah, Thermoluminescence study of MnO doped borophosphate glass samples for radiation dosimetry. J. Non-Cryst. Solids 368, 40–44 (2013). https://doi.org/10.1016/j.jnoncrysol.2013.02.020
B.J.R. Swamy, S. Bhaskar, R. Vijay, P. Ramesh Babu, D. Krishna Rao, N. Veeraiah, Influence of copper ions on thermoluminescence characteristics of CaF2–B2O3–P2O5 glass system. Ceram. Int. 40, 3707–3713 (2014)
B. Biró, A. Pascu, A. Timar-Gabor, V. Simon, Thermoluminescence investigations on xY2O3 (60–x)P2O5·40SiO2 vitroceramics. Appl. Radiat. Isot. 98, 49–53 (2015). https://doi.org/10.1016/j.apradiso.2015.01.019
M.A.K. Abdelhalim, B.M. Al-Shamrani, Improvement of the thermoluminescence properties of the P2O5-Li2O glass system by using nanoparticles. J. Lumin. (2016). https://doi.org/10.1016/j.jlumin.2016.08.028
T. Kalpana, Y. Gandhi, S. Bhaskar, V. Sudarsan, P. Bragiel, M. Piasecki, V. Ravi Kumar, N. Veeraiah, Influence of alumina on photoluminescence and thermoluminescence characteristics of Gd3+ doped barium borophosphate glasses. J. Lumin. (2016). https://doi.org/10.1016/j.jlumin.2016.06.053
T. Kalpana, S. Bhaskar, Y. Gandhi, V. Ravi Kumar, G.S. Baskaran, P. Bragiel, M. Piasecki, N. Veeraiah, Thermoluminescence features of alumina-mixed borophosphate glasses with Tb3+ ions for dosimetric applications. Int. J. Appl. Glass Sci. 8(2), 188–195 (2017)
H. Tanaka, Y. Fujimoto, K. Saeki, M. Koshimizu, T. Yanagida, K. Asai, Radiophotoluminescence properties of Ag-doped mixed phosphate glasses. Radiat. Meas. (2017). https://doi.org/10.1016/j.radmeas.2017.01.010
M.A. Vallejo, M. Perez, P.V. Ceron, R. Navarro, C. Villaseñor, T. Cordova, M. Sosa, Photoluminescence and thermoluminescence of phosphate glasses doped with Dy3+ and containing silver nanoparticles. Nano (2017). https://doi.org/10.1142/S1793292017501454
T. Sunil, M.L. Chithambo, Thermoluminescence of K-Mg-Al-Zn fluorophosphate glass. Optic. Mater. 64, 302–309 (2017). https://doi.org/10.1016/j.optmat.2016.12.035
A. El-Kheshen, C. Woda, M. Discher, N. El-Faramawy, Investigation of phosphate glass doped lanthanum as beta dosimeter. J. Lumin. (2018). https://doi.org/10.1016/j.jlumin.2018.04.001
H.F. El-Nashar, M. El-Kinawy, N.A. El-Faramawy, Investigations of the kinetic energy parameters of irradiated (La)-doped phosphate glass. Lumin. J. Biol. Chem. Lumin. (2019). https://doi.org/10.1002/bio.3703
M.A.K. Abdelhalim, M.S. Al-Ayed, B.M. Al-Shamrani, Synthesizing new glass 40P2O5 – 50BaO - 25Na2O – 25MgO - 5TiO2 for the application in high radiation environmental dosimetry. AIP Adv. 8, 095212 (2018). https://doi.org/10.1063/1.5030338
I. El Mesady, S. Alawsh, Optical and luminescence properties of silicon doped alumino-phosphate-sodium glass system. J. Non-Cryst. Solids 482, 236–242 (2018). https://doi.org/10.1016/j.jnoncrysol.2017.12.054
S. Hirano, N. Kawano, G. Okada, N. Kawaguchi, T. Yanagida, PL and TSL properties of tin-doped zinc sodium phosphate glasses. Radiat. Meas. (2018). https://doi.org/10.1016/j.radmeas.2018.03.002
A. Gasiorowski, P. Szajerski, Thermoluminescence characteristics and dose-response of electron beam and gamma rays irradiated alumino-phosphate glasses doped with Gd2O3 and Tb2O3. J. Lumin. 214, 116519 (2019). https://doi.org/10.1016/j.jlumin.2019.116519
A. Gasiorowski, P. Szajerski, Particles size increase assisted enhancement of thermoluminescence emission in gadolinium and dysprosium oxide doped phosphate glasses. J. Alloys Compds. 839, 155479 (2020). https://doi.org/10.1016/j.jallcom.2020.155479
A. Gasiorowski, P. Szajerski, J.F.B. Cuevas, Use of terbium doped phosphate glasses for high dose radiation dosimetry—thermoluminescence characteristics, dose response and optimization of readout method. Appl. Sci. 11, 7221 (2021). https://doi.org/10.3390/app11167221
N.Y. Abdou, M.M. Farag, W.M. Abd-Allah, Thermoluminescent properties of nano-magnesium phosphate ceramic for radiation dosimetry. Eur. Phys. J. Plus 135, 317 (2020). https://doi.org/10.1140/epjp/s13360-020-00310-1
P. Rubalajyothi, A. Rajendran, Thermoluminescence characteristics studies of phosphor material with anti-bacterial activity. J. Crit. Rev. 7(1), 2020 (2019)
P. Vinodkumar, S. Panda, U. Madhusoodanan, B.S. Panigrahi, Thermoluminescence properties of strontium borophosphate doped with praseodymium and enhancement with uranyl cooping. Radiat. Phys. Chem. (2020). https://doi.org/10.1016/j.radphyschem.2020.108914
A. Raja, R. Nagaraj, K. Ramachandran, V. Sivasubramani, G. Annadurai, D.J. Daniel, P. Ramasamy, A facile synthesis, structural and triple-luminescence properties of a novel fluoroperovskite RbCaF3: Sm3+ phosphor for radiation dosimetry and orange-red LED applications. Mater. Sci. Eng. B. 255, 114531 (2020). https://doi.org/10.1016/j.mseb.2020.114531
K. Munirathnam, P.C. Nagajyothi, K. Hareesh, M.M. Kumar, S.D. Dhole, Effect of Mn codopant on thermoluminescence properties of γ-rays irradiated Na3Y(PO4)2: Dy phosphors for dosimetry applications. Appl. Phys. A, Mater. Sci. Process. 127(1), 1–9 (2021). https://doi.org/10.1007/s00339-020-04202-0
W. Li, Z. Chen, J. Xu, P. Zhao, Y. Fan, C. He, Synthesis and optical properties of novel Gd3+-doped BaZn2(PO4)2 glass-ceramics for radiation detection applications. J. Rare Earths (2021). https://doi.org/10.1016/j.jre.2021.11.010
R. Kaur, R.B. Rakesh, S.G. Mhatre, V. Bhatia, D. Kumar, H. Singh, S.P. Singh, A. Kumar, Physical, optical, structural and thermoluminescence behaviour of borosilicate glasses doped with trivalent neodymium ions. Optic. Mater. 121, 111109 (2021). https://doi.org/10.1016/j.optmat.2021.111109
H.A. Alazab, N.Y. Abdou, H.A. Saudi et al., Thermoluminescence properties of bioglass for radiation dosimetry. Silicon (2021). https://doi.org/10.1007/s12633-021-01364-1
G.A. Kumar, Y. Rambabu, R.K. Guntu, K. Sivaram, M.S. Reddy, C.S. Rao, V. Venkatramu, V.R. Kumar, N.C.S.N. Iyengar, ZrxCa30-xP70 thermoluminescent bio glass, structure and elasticity. J. Mech. Behav. Biomed. Mater. 119, 104517 (2021). https://doi.org/10.1016/j.jmbbm.2021.104517
A.N. D’Souza, K. Sharmila, D.K. Gaikwad, M.I. Sayyed, H.M. Somashekarappa, H. Al-Ghamdi, A.H. Almuqrin, S.D. Kamath, Evaluation of bismuth added HMO glasses in terms of thermal, mechanical, gamma radiation shielding and thermoluminescence properties. Mater. Res. (2021). https://doi.org/10.1590/1980-5373-MR-2021-0243
T.N.H. Tengku KamarulBahri, H. Wagiran, R. Hussin, I. Hossain, T. Kadni, Thermoluminescence properties of CaO–B2O3 glass system doped with GeO2. Radiat. Phys. Chem. 102, 103–107 (2014). https://doi.org/10.1016/j.radphyschem.2014.03.029
G.R. Barrera, L.F. Souza, A.L.F. Novais, L.V.E. Caldas, C.M. Abreu, R. Machado, E.M. Sussuchi, D.N. Souza, Thermoluminescence and optically stimulated luminescence of PbO–H3BO3 and PbO–H3BO3– Al2O3 glasses. Radiat. Phys. Chem. (2018). https://doi.org/10.1016/j.radphyschem.2018.02.005
A. Ozdemir, Investigation of dosimetric properties of newly-developed Li2B4 O7:Ag+, La3+ using thermoluminescence (TL) technique. J. Alloy. Compd. (2020). https://doi.org/10.1016/j.jallcom.2020.153722
T. Sunil, C. Makaiko, Kinetic analysis and general features of thermoluminescence of B2O3-Li2O-ZnF2 glass. Radiat. Meas. 100, 1–8 (2017). https://doi.org/10.1016/j.radmeas.2017.03.038
H.A. Tajuddin, W.M.S. Wanhassan, S.A. Sani, N.S. Shaharin, Thermoluminescent properties of Dy doped calcium borate based glass for dose measurement subjected to photon irradiation. EPJ Web Conf. 156, 00002 (2017). https://doi.org/10.1051/epjconf/201715600002
Y. Fujimoto, T. Yanagida, M. Koshimizu, K. Asai, Optical and dosimeter properties of Li2O–Al2O3–B2O3 based glasses. J. Ceram. Soc. Jpn. 125, 728–731 (2017). https://doi.org/10.2109/jcersj2.17076
H. Vinod, C.S. Viswanath, M. Krishna, S. Sudha, Photoluminescence and thermally stimulated luminescence properties of Pr3+-doped zinc sodium bismuth borate glasses. Optic. Mater. (2018). https://doi.org/10.1016/j.optmat.2018.06.064
N.S. Prabhu, K. Sharmila, H.M. Somashekarappa, G. Lakshminarayana, S. Mandal, M.I. Sayyed, S.D. Kamath, Thermoluminescence features of Er3+ doped BaO-ZnO-LiFB2O3 glass system for high-dose gamma dosimetry. Ceram. Int. (2020). https://doi.org/10.1016/j.ceramint.2020.04.276
R. Kaur, R.B. Rakesh, S.G. Mhatre, V. Bhatia, D. Kumar, H. Singh, S.P. Singh, A. Kumar, Thermoluminescence, structural and optical properties of Ce3+ doped borosilicate doped glasses. J. Mater. Sci. Mater. Electron. (2021). https://doi.org/10.1007/s10854-021-06382-8
Funding
This study is not funded by any source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, B.A., Bindu, P.H. Advances in borate- and phosphate-based TL materials for in vivo dosimetry. J. Korean Ceram. Soc. 59, 537–550 (2022). https://doi.org/10.1007/s43207-022-00240-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-022-00240-x