Abstract
This paper presents the main dosimetric characteristics and kinetic parameters of a newly proposed borate glass dosimeter modified with lithium and magnesium oxide (LMB) and co-doped with Dy2O3 and P2O5. Dosimetric features include thermoluminescence (TL) glow-curve, annealing procedure, TL sensitivity, photon dose response, fading, reproducibility, minimum detectable dose, kinetics order, activation energy (E) and frequency factor (s). The proposed dosimeter exhibits simple glow curves, good linearity from 0.01 up to 4 Gy, easy annealing, excellent reproducibility and minimal fading. These attractive features will pave the way to its use in radiation dosimetry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Definitely, many thermoluminescence (TL) parameters are essential to accurately evaluate the efficiency of any dosimeter. These parameters include the simple glow curve, the effective atomic number (Z eff), dose linearity, good sensitivity, fading and stability in varying climatic conditions. Despite the usage of several materials as TL phosphors, novel functional materials with superior dosimetric performance are far from being achieved. In this regard, borates are attractive due to their tissue equivalent, good linearity, high sensitivity to external dose, low cost and easy preparation [1]. Conversely, the hygroscopic nature of borate glass negatively affects its performance. Intensive research has been dedicated in improving the stability and enhancing the sensitivity by adding different types of metals (alkali/alkaline earth) as modifiers, transition metals, and rare earth(s) as dopants (co-dopants) [2–5]. In spite of many efforts, the effects of phosphorus (P) concentration as co-dopant on the thermoluminescent properties of LMB:Dy glass are far from being understood.
Previous studies showed the efficiency of lithium as modifier, which created large number of non-bridging oxygen sites (vacancies) and improved the strength of the host. In contrast, the lithium ions do not have a direct effect on luminescence activation because of their closed structure with no energy levels within 10 eV [6, 7]. It is established that the addition of magnesium oxide to lithium borate increases the strength of the glass and enhances the electron emission. MgO (alkaline earth oxide) incorporated lithium borate glasses are considered as good materials for dosimetry applications since this composition is relatively moisture resistant as compared to pure borate glasses [6]. Several studies reported the properties of borate glass doped with rare earths [4, 5, 8–13]. Dysprosium oxide being one of the most efficient rare earths is exploited to improve the TL properties of borate glasses [12].
The current study aims to explore different TL features and kinetic parameters of dysprosium and phosphor oxides co-doped borate glass that is modified by lithium and magnesium oxide (LMB). These features include glow curve, reproducibility, linearity, sensitivity, fading, effective atomic number and kinetic parameters.
Materials and methods
The current samples were prepared by mixing a stoichiometric amount of boron oxide with LMB. Dysprosium and phosphorus oxide were added to the admixture as a dopant and co-dopant, respectively. All chosen reagents were distinguished with high purity (at least 99.99 %, Sigma Aldrich Co., USA). The composition was ground and thoroughly stirred mechanically for 30 min to get high uniformity before placing the admixture (in an alumina crucible) inside an electrical furnace operated at 1200 °C for 60 min for complete melting. The selection of this composition is based on previous researchers [14] who used optimization at 30 Li2O–70 B2O3 to obtain the best TL response. In present work, we optimized the best concentration of LMB by replacing Li2O with MgO at various concentration based on their TL response. The nominal compositions of the glasses are:
-
I.
10 MgO–20 Li2O–(70−x) B2O3–x Dy2O3, 0.3 ≤ x ≤ 1 and
-
II.
10 MgO–20 Li2O–(69.5−y) B2O3–0.5 Dy2O3–y P2O5, 0.5 ≤ y ≤ 1.5
The chemical compositions and glass codes are summarized in Table 1.
Irradiation and reading process
The irradiation and reading processes were performed in the Secondary Standard Dosimeter Laboratory (SSDL) at the Malaysian Nuclear Agency. The 60Co source was used in irradiation process with a dose rate of 36.864 mGy min−1. The reading process was conducted by using the TLD reader 4,500 from Harshaw. Readings were obtained after 24 h of irradiation to reduce the spurious TL signals with heating rate of 5 °C s−1. The samples were stored at the room temperature in dark place to evade any effect of background light. Three samples were used for obtaining each experimental data point, where the average and standard deviation were calculated.
Results and discussion
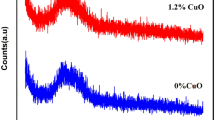
The X-ray diffraction as shown in Fig. 1a, b presented a broad peaks confirmed the amorphous nature of the prepared glasses samples. The complete absence of any sharp peaks further verified their disordered nature.
TL glow curve
The effects of Dy2O3 and P2O5 on the TL intensity of LMB were investigated in three stages. The first stage was executed to explore the TL of a pure sample (LMB). Figure 2a displays the glow curve of the pure sample that show a prominent peak placed at 180 °C after 3 Gy irradiation. This TL emission may be attributed to recombination between the excited electrons from the valence band and the defects produced after irradiation in the material [15]. The second stage exhibits the effect of different concentration of Dy2O3 on the TL glow curve as shown in Fig. 2b. The addition of Dy2O3 on LMB enhances the intensity by 98 times more than that of pure LMB. This enhancement synchronized with shifting of T m toward high temperature (190 °C) with optimum intensity at 0.5 % of Dy2O3. The reduction in the TL glow curve intensity beyond 0.5 mol% is majorly ascribed to the concentration quenching theory [16]. Lastly, the effect of the co-dopant P2O5 on the TL glow curve intensity was determined as depicted in Fig. 2c. The TL intensity gradually enhances by a factor of 2.5 with the increase of P2O5 concentration up to 1 mol% and then quenched beyond this value, accompanied with shifting in maximum temperature (T m) to 220 °C. However, no significant influence was reported in general shape of the glow curve with the increase of Dy3+ and P concentration. This behavior is ascribed to a concentration-independent character where the P impurities are cross linked with the Dy2O3 defect [17]. Meanwhile, the position of T m was shifted towards higher temperature with the addition of P. This observation is attributed to the ability of P ions to create deeper traps in the host [3].
Following Porwal [11] and Annalakshmi [18], the TL mechanism in the proposed dosimeters are explained. They identified the defect centers such as borate radical (BO3)2− and oxygen vacancy (\( {\text{O}}_{\text{V}}^{{}} \)) which are formed during gamma irradiation and established a mechanism for the TL process. Based on this assumption, the current TL peaks can be explained via the process of:
-
(i)
Irradiation yields, \( {\text{LMB:}}\;{\text{Dy}}^{{{\text{3 + }}}} \to {\text{(BO}}_{{\text{3}}} {\text{)}}^{{2 - }} {\text{,}}\;{\text{(O}}_{{\text{v}}}^{ - } {\text{)}} \) and
-
(ii)
Heating causes,
$$ \left( {{\text{BO}}_{3} } \right)^{{2 - }} \, \to \,\left( {{\text{BO}}_{3} } \right)^{{3 - }} \, + \,h, $$$$ \left[ {{\text{O}}_{{\text{v}}} } \right]^{ - } \, + \,h\, \to \,\left[ {{\text{O}}_{{\text{v}}} } \right]^{*} \to \,{\text{Dy}}^{{3 + *}} \to {\text{Dy}}^{{3 + }} \, + \,hv. $$
After gamma ray irradiation in \( {\text{LMB:}}\,{\text{Dy}},\, {\text{(BO}}_{{\text{3}}} {\text{)}}^{{2 - }} {\text{,}}\,{\text{(O}}_{{\text{v}}}^{ - } {\text{)}} \) radicals were formed. In the reading process (heating treatment), the borate radical released the hole which in turn recombined with the trapped electron at the oxygen vacancy resulting in the release of recombination energy. Then the energy released from Dy3+ recombination essentially excited the Dy ions and originates TL glow curve. The TL features of the Dy3+ dopant in LMB phosphors can be understood in terms of the non-radiative energy transfer from the excited oxygen vacancy-related defects to the nearby Dy3+ ions. The addition of P2O5 as co-dopant acts as activator for dysprosium oxide sensitization. There are two explanations that were drawn in the quest to understand the behavior of P2O5 as a co-dopant with Dy2O3. Firstly, the energy transferred from the Dy3+ ions caused the phosphor to be in an excited state, upon which, the energy may be transferred from the P ions to the Dy3+ ions, that way bringing it to the ground state. Secondly, the continuous increase of phosphorus created a reverse effect on the intensity of the peaks which results from the effect of the saturation quenching [19].
Annealing procedure
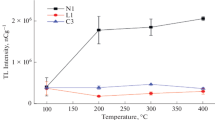
The estimation of pre-irradiation heat treatment of the LMB:Dy,P dosimeters was performed. This procedure was used to synchronize a appropriate temperature and time to remove the influence of prior exposure without producing damage or thermal strain on the dosimeter. The appropriate annealing will improve the TLD sensitivity by re-stabilizing the hole and mixture traps. In the current research, the annealing procedure was executed by using 20 samples for different annealing temperature and then gamma irradiated with 3 Gy by performing fixed time as shown in Fig. 3. The highest TL response with the lowest standard deviation was attained at 300 °C. After that, the temperature was fixed at 300 °C and the annealing time was varied as demonstrated in Fig. 4. The best pre-irradiation annealing for the new proposed dosimeter was 300 °C for 30 min, and it appears suitable and adequate to recover the original TL sensitivity.
TL sensitivity
McKeever [20] reported two formulas to express the sensitivity of TL materials: (i) TL yield to the mass of a dosimeter or (ii) TL yield to the mass and absorbed dose of a dosimeter. In the current study, the TL sensitivity was expressed as glow curve area per unit of mass dosimeter and per unit of γ 60Co (TL g−1 Gy−1). The acquired results were benchmarked relative to the sensitivity of TLD-100. The TL sensitivity of LMB:Dy,P was around 2.5 times greater than that of LMB:Dy and 18 times lesser than the TLD-100 sensitivity. Table 2 displays the results of TL sensitivity from previous studies where the glass was prepared by using melt-quenching method.
Photon dose response
Figure 5 shows dose dependence of LMB:Dy,P within the dose range of 0.01–4 Gy. The obtained results display excellent dose linearity with a linear correlation coefficient of 0.998. The y-axis represents the averages of mass-normalized TL yield readings. Where the error bars represent propagated errors corresponding to 1σ for three different samples. This feature supports the current dosimeter to candidate in the personal radiation monitoring.
The normalized dose response (linearity index) is defined as:
where TL(D) is the dose response at a dose D and D 1 is the lowest dose at which the dose response is linear. Figure 6 shows the linearity index of current dosimeters. The ideal TLD material has f(D) equal to 1 in a wide dose range. Our results clearly confirm the linear behavior.
Reproducibility
The reproducibility of the LMB:Dy,P dosimeters was achieved by repeating the reading cycle at two different doses (1 and 4 Gy). The main objective of this process was to demonstrate the significance of oven annealing at high doses. This process is carried out via two steps. Firstly, samples were annealed with external oven annealing, irradiated, and then reading as follows: oven annealing → irradiation → readout. To confirm the stability of TL intensity, the procedure was repeated ten times. Secondly, the annealing was carried out by using an external oven only one time (before the first irradiation) and then the TL intensity was determined as follows: irradiation → readout (without annealing between the first and the second readout). The procedure was repeated ten times.
Figure 7a, b illustrate the reproducibility of 10 repeated cycles at irradiated doses of 1 Gy, with and without oven annealing, respectively. At lower doses (1 Gy), it is evident that the readout annealing is sufficient to eliminate all previous signals and restore the sensitivity of the dosimeter with 2.2 % readings variation and 3.0 % without oven annealing. Figure 8a, b show the reproducibility at high dose (4 Gy), with and without oven annealing, respectively. The readout annealing is inadequate to eliminate all previous signals and restore the sensitivity of the dosimeter, hence the oven annealing is recommended and shows little variation of 2 %. From our findings, we suggest to anneal the glass dosimeters with dose of 4 Gy at 300 °C for 30 min prior to use.
Minimum detectable dose (MDD)
It is also known as the lowest level of detection. According to Furetta [22], the MDD can be calculated from following Eq. (2):
where B * is the mean TL background signal obtained from the samples annealed without irradiating, \( \sigma_{B} \) is the standard deviation of the mean background and F is the calibration factor (Gy TL−1). Regarding LMB:Dy,P, the B* is 2.01 nC, \( \sigma_{B} \) is 0.33 nC and F is 0.0000198 Gy nC−1. The MDD will be:
Fading
To determine the thermal fading features of LMB:Dy,P, a number of proposed samples were annealed and irradiated with a gamma dose of 3 Gy. The proposed samples were stored in dark conditions at room temperature to reduce the effect of background light. The readouts were started after 24 h of exposure and continued up to 90 days of irradiation. All measurements were performed under the same conditions. The obtained results confirmed a very small reduction of the TL response during the elapsed period of time. As displayed on Fig. 9, the thermal fading features of the LMB:Dy,P are less than 19 % after 3 months. The fading of the proposed dosimeter was compared with previous studies and it showed minimal fading as the one obtained in references [2, 21].
The findings regarding the fading effects from other researchers are listed in Table 3.
Effective atomic number (Z eff)
The interaction of radiation with matter divides into three types (Photoelectric effect, Compton scattering and pair production). These interactions depend on energy range. In low energy range (20–100 keV), the photoelectric is dominant and it depends on the third power of the atomic number. Thus, the response for high atomic number materials will be higher in that region of energies [20]. The Z eff is very significant in radiotherapy for calculating the treatment dose in cancer patients, and to assist dose evaluation during personal monitoring. Faiz suggested to use Eq. 3 to calculate the Z eff [23]:
where a 1 , a 2 …, are the fractional contents of electrons corresponding to the elements Z 1 , Z 2 ,…, respectively. The new proposed material exhibits a nearly tissue equivalent ~9.05, which is highly recommended for medical application purposes [24].
Kinetic parameters
The trapping levels are responsible for the position, shape, and intensities of the glow peaks [25]. The TL dosimetric properties mostly rely on the kinetic parameters of the glow peak. Any dosimetric studies of TL material should have enough information about its kinetic parameters. The kinetic parameters provide worthy information about TL mechanism that is responsible for glow curve formation. The most important of kinetic parameter are activation energy (E) or trap depth, frequency factor (s) and the kinetic order (b). In this study, the activation energy and frequency factor were determined by Peak Shape (PS) and Initial Rise (IR) methods.
Peak shape method (PS)
The peak shape method is generally known as Chen’s method [26], which is utilized to evaluate the kinetic parameters i.e. (order of kinetic, activation energy and frequency factor) of the created glow peak. This method depends on the temperatures T m, T 1 and T 2, where T m represents the peak temperature, T 1 and T 2 represent temperatures at full width half maximum of the glow curve peak [27]. Figure 10 shows the glow curve and obligatory values for PS of LMB: Dy,P. The PS is primarily utilized to calculate the geometric factor (μ g ) and the order of kinetics (b). The geometric factor can be calculated by using two formula:
Chen formula 1969 [26]:
Balarin formula 1975 [28]:
The μ g value for first order is between 0.7 and 0.8 and for the second order it is approximately 1.05–1.20. The μ g for dosimeters LMB:Dy,P is 1.37. These results give an indication that the current compositions obey the second order kinetic. To confirm the kinetic order, Balarin [29] derived the following formula (6):
where b is 1.75 for LMB:Dy,P, this value confirmed second kinetic order.
The activation energy was acquired by Chen’s Eq. (7) [26]:
where γ represents value τ, δ and ω as shown in Fig. 10, the C γ and b γ values can be obtained from following Eqs. (8–10):
From this value, the frequency factor (s) can be calculated of following Eq. (11) [26]:
where ‘b’ and β represents order of kinetics and the linear heating rate, respectively. The activation energies and frequency factors are listed in Table 4.
Initial rise method
In 1948, Garlick and Gibson proposed a simple method to evaluate activation energy (E) and frequency factor (s) in TL material [27]. The initial rise method is based on the hypothesis that in the initial part of the TL glow curve T << Tm, where T m is the maximum peak temperature, the rate of variation of the trapped carrier population is tiny. This hypothesis is applicable for temperatures up to a cut-off temperature, corresponding to an intensity that is smaller than 10–15 % of the maximum intensity [20]. This method is valid when the glow curve is a well defined and obviously separated peak.
According to Eq. (12), TL intensity I is proportional to exp(−E/kT) and the plot of ln(I TL) versus 1/kT gives a straight line. From the slope, the activation energy E can be acquired. This method depends on temperature. Figure 11 shows the relation between ln(I TL) versus 1/kT.
According to Rawat [30], the frequency factor can be calculate by using the intercept I obtained from the Fig. 11 and substitution it in Eq. (13):
where b represents the order of kinetic, A represents the area under glow curve, N represents the total concentration of traps and n 0 represents the initial concentration of trapped electrons. The equation can solve by assumption the ratio of n 0 /N = 1 (in the saturation case). Hence the term for s will be:
The kinetic parameter values obtained for LMB: Dy,P are given in Table 4. The activation energies of prepared dosimeters are determined from the slope of the Fig. 11 and the frequency factor s values are determined from Eq. (14).
Conclusion
Significant dosimetric features for a newly proposed TL dosimeter were determined. The current study exhibited promising results for borate glass modified by lithium and magnesium oxide and co-doped with dysprosium and phosphorous oxide. The incorporation of P2O5 as a co-dopant enhanced the TL intensity by a factor of 2.5 with shifting of T m toward a high temperature (220 °C). This enhancement is ascribed to the ability of P2O5 to create new electron traps and activate the ground state of the luminescence centers by raising the energy levels of the surrounding oxygen ions to the top of the valence band. It is envisaged that the proposed dosimeter demonstrates a simple glow curve with a single prominent peak at 220 °C, an easy annealing process, good dose linearity and excellent reproducibility. The dose response is observed to be linear up to 4 Gy subjected to photon irradiation. The effective atomic number (Z eff ≈ 9.05) obtained was nearly tissue equivalent. The TL sensitivity of LMB:Dy,P was approximately eighteen times less than that of LiF:Mg,Ti (TLD-100). The phosphor LMB:Dy,P obeyed the second-order kinetics in TL studies. The trapping parameters of LMB:Dy,P phosphor were calculated by two methods i.e. PS and initial raise method. A small variation between the activation energies and the frequency factors calculated by different methods are evidenced. Consequently, these methods depend on different factors. From the simple band model of luminescence, it follows that fading is small if the electron traps, which produce luminescence excitation centers, have a sufficiently large energy depth. The activation energy values obtained by two method that possess a adequately large energy depth [31]. It was concluded that the proposed dosimeter has great potential to be used in radiation dosimetry.
References
Elkholy MM (2010) J Lumin 130(10):1880–1892
Aboud H, Wagiran H, Hussin R, Ali H, Alajerami Y, Saeed MA (2014) Appl Radiat Isot 90:35–39
Alajerami YSM, Hashim S, Ramli AT, Saleh MA, Kadni T (2012) Radiat Prot Dosim 155(1):1–10
Liu L, Zhang Y, Hao J, Li C, Wang S, Su Q (2007) J Phys Chem Solids 68(9):1745–1748
Jiang LH, Zhang YL, Li CY, Hao JQ, Su Q (2010) Appl Radiat Isot 68(1):196–200
Balaji R, Rosario A, Veeraiah Gerhardt N (2008) J Phys Chem Solids 69(11):2813
Alajerami YSM, Hashim S, Ghoshal SK, Saleh MA, Kadni T, Saripan MI, Bradley DA (2013) J Phys Chem Solids 74(12):1816–1822
Furetta C, Sanipoli C, Scacco A, Somaiah K (1996) Radiat Prot Dosim 65(1–4):339–342
Furetta C, Prokic M, Salamon R, Kitis G (2000) Appl Radiat Isot 52(2):243–250
Jubera V, Chaminade JP, Garcia A, Guillen F, Fouassier C (2003) J Lumin 101(1):1–10
Porwal NK, Kadam RM, Seshagiri TK, Natarajan V, Dhobale AR, Page AG (2005) Radiat Meas 40(1):69–75
Anishia SR, Jose MT, Annalakshmi O, Ramasamy V (2011) J Lumin 131(12):2492–2498
Kawashima YS, Gugliotti CF, Yee M, Tatumi SH, Mittani JCR (2014) Radiat Phys Chem 95:91–93
El-Adawy A, Khaled NE, El-Sersy AR, Hussein A, Donya H (2010) Appl Radiat Isot 68(6):1132–1136
Alajerami YSM, Hashim S, Ramli AT, Saleh MA, Kadir ABBA, Saripan MI (2013) Chin Phys Lett 30(1):017801
Puppalwar SP, Dhoble SJ, Dhoble NS, Animesh K (2012) Nucl Instr Meth B 274:167–171
Elkholy MM (2003) Mater Chem Phys 77(2):321–330
Annalakshmi O, Jose MT, Madhusoodanan U, Sridevi J, Venkatraman B, Amarendra G, Mandal AB (2014) Radiat Eff Defects Solids 2014:1–10
Hashim S, Alajerami YSM, Ghoshal SK, Saleh MA, Saripan MI, Kadir ABA, Alzimami K (2014) Radiat Phys Chem 104:36–39
McKeever SW (1988) Thermoluminescence of solids, vol 3. Cambridge University Press
Saidu A, Wagiran H, Saeed MA, Alajerami YSM (2014) J Radioanal Nucl Chem 2014:1–6. doi:10.1007/s10967-014-3846-y
Furetta C, Prokic M, Salamon R, Prokic V, Kitis G (2001) Nucl Instr Meth 456(3):411–417
Faiz MK (1994) The physics of radiation therapy. Williams and Wilkins, Baltimore
Burkhardt B, Piesh E (1980) Nucl Instr Meth 175:159–161
Azorin J (1986) Rev Nucl Tracks 11(3):159–166
Chen R (1969) Electrochem J Soc 116:1254–1257
Garlick GFJ, Gibson AF (1948) Proc Phys Soc Lond 60:574–590
Balarin M (1975) Phys Status Solidi (a) 31:K111–K114
Balarin M (1979) Therm Anal 17:319
Rawat NS, Kulkarni MS, Mishra DR, Bhatt BC, Sunta CM, Gupta SK, Sharma DN (2009) Nucl Instr Meth B 267:3475–3479
Kortov V (2007) Radiat Meas 42(4):576–581
Acknowledgments
The authors are grateful to the Ministry of Education-Malaysia and UTM for providing financial assistance through the Fundamental Research Grant Scheme (FRGS), Vote number (R.J130000.7826.4F168) and GUP/RU (10J37).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mhareb, M.H.A., Hashim, S., Ghoshal, S.K. et al. Influences of dysprosium and phosphorous oxides co-doping on thermoluminescence features and kinetic parameters of lithium magnesium borate glass. J Radioanal Nucl Chem 305, 469–477 (2015). https://doi.org/10.1007/s10967-015-3984-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-3984-x