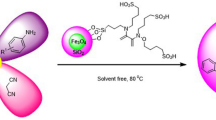
Abstract
Fe3O4@SiO2-imid-PMAn efficiently catalyzed the conversion of aromatic, heteroaromatic, and aliphatic aldehydes to 1,1-diacetates under solvent-free conditions at room temperature. Deprotection of the resulting 1,1-diacetates can also be achieved using the same catalyst in methanol. The acylation of aldehydes was highly chemoselective, and no ketone was acylated, which provided a method for the synthesis of acylals from aldehydes in the presence of ketones. Therefore, this method gives notable advantages such as excellent chemoselectivity, mild reaction condition, short reaction times and excellent yield. Also, nanocatalyst can be easily recovered by a magnetic field and is reusable without efficient loss of its catalytic activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The selective protection and deprotection of carbonyl groups are often necessary during a multistep organic synthesis. Aldehydes are often present in organic molecules so development of economical, efficient and mild procedures for the protection of them is very important for synthetic organic chemistry. The protection of aldehydes as acetals, acylals, oxathioacetals or dithioacetals is a common practice for manipulation of other functional groups during multi-step syntheses (Kochhar et al. 1983). Among various procedures for protection of aldehyde, acylals (1,1-diacetates) are appropriate candidates to this aim due to their stability in basic and neutral reaction media as well as in aqueous acids (Kochhar et al. 1983). 1,1-Diacetates are also important for the preparation of other compounds, for example, in reaction with appropriate nucleophiles they can be converted to other useful functional groups (Shirini et al. 2013).
Meanwhile, gem-diacetates derived from α,β-unsaturated aldehydes are useful as dienes for Diels–Alder cycloaddition reactions (Frick and Harper 1984). Moreover, acylals are used as crosslinking reagents for cellulose in cotton (Frick and Harper 1984). Numerous methods for the preparation of 1,1-diacetates from aldehydes and acetic anhydride have been reported (Borikar and Daniel 2011; Gao et al. 2009; Moosavifar et al. 2011; Romanelli et al. 2010; Shelke et al. 2009). Although some of these methods afford good to high yields of the corresponding diacetates, the majority suffer from one or more of the following disadvantages: reactions under oxidizing conditions, use of strong acids, high temperatures, long reaction times, poor selectivity, use of unrecyclable catalysts, moisture sensitivity as well as high cost and high toxicity of the reagents. In addition, a few of the above mentioned catalysts are claimed to give protection as well as deprotection. Thus, the development of simple, convenient, and environmentally benign methods for the protection of aldehydes as 1,1-diacetates and their deprotection is still required.
In recent years, heteropoly acids (HPAs), especially Keggin type have attracted increasing interest due to their high acidity, low toxicity and tunable redox properties (Javidi et al. 2014). Although HPAs are versatile compounds in their acidic form, their main disadvantages are high solubility in polar solvents and low surface area (<10 m2/g). Therefore, in a homogeneous reaction the isolation of the products and the reuse of the catalyst after reaction become difficult. So, to overcome this problem, these materials disperse on supports (such as silica, acidic ion-exchange resins, active carbon, etc.) which possess large surface area (Zhang et al. 2011).
In the field of catalysis, superparamagnetic nanoparticles have been utilized as catalyst supports for organic transformations such as alcohol hydrogenation (Polshettiwar et al. 2009), olefin hydrogenation (Lu et al. 2004), olefin hydroformylation (Yoon et al. 2003), Sonogashira and Carbonylative Sonogashira reactions (Esmaeilpour et al. 2014a), Suzuki and Heck cross-coupling reactions (Esmaeilpour et al. 2014b), etc.
Development of catalysts working under mild reaction conditions is desirable. Recently, our group has reported the catalytic potentiality of Fe3O4@SiO2@PMA as a nanocatalyst in the synthesis of 1-amidoalkyl-2-naphthols (Esmaeilpour et al. 2014c). In this article, we report a new and efficient method for protection and deprotection of aromatic and aliphatic aldehydes at room temperature in the presence of Fe3O4@SiO2-imid-PMAn as an effective as well as highly chemoselective heterogeneous catalyst (Scheme 1).
2 Experimental
2.1 General Methods
All chemicals were commercially available and used without further purification. The NMR spectra were recorded on a Bruker avance DPX 250 MHz spectrometer in chloroform (CDCl3) using tetramethylsilane (TMS) as an internal reference. Fourier transform infrared (FT-IR) spectra were obtained using a Shimadzu FT-IR 8300 spectrophotometer. Melting points were obtained in open capillary tubes and were measured on an electrothermal 9200 apparatus. Mass spectra were obtained at 70 eV. All yields refer to the isolated products. The products were characterized by comparison of their spectral and physical data with previously reported data or with the authentic samples.
2.2 General Procedure
2.2.1 Preparation of Fe3O4@SiO2 Core–Shell
The core–shell Fe3O4@SiO2 nanospheres were prepared by a modified Stober method in our previous work (Mohsen Esmaeilpour et al. 2012). In a typical procedure, the mixture of FeCl3.6H2O (1.3 g, 4.8 mmol) in water (15 mL) was added to the solution of polyvinyl alcohol (PVA 15,000), as a surfactant, and FeCl2·4H2O (0.9 g, 4.5 mmol) in water (15 mL), which was prepared by completely dissolving PVA in water followed by addition of FeCl2.4H2O. The resultant solution was left to be stirred for 30 min at 80 °C. Then, hexamethylenetetramine (HMTA) (1.0 mol/l) was added drop by drop with vigorous stirring to produce a black solid product when the reaction media reaches pH 10. The resultant mixture was heated on water bath for 2 h at 60 °C and the black magnetite solid product was filtered and washed with ethanol three times and was then dried at 80 °C for 10 h. Then Fe3O4 nanoparticle (0.50 g, 2.1 mmol) was dispersed in the mixture of ethanol (50 mL), deionized water (5 mL) and tetraethoxysilane (TEOS) (0.20 mL), followed by the addition of 5.0 mL of NaOH (10wt %). This solution was stirred mechanically for 30 min at room temperature. Then the product, Fe3O4@SiO2, was separated by an external magnet, and was washed with deionized water and ethanol three times and dried at 80 °C for 10 h.
2.2.2 Preparation of H3PW12O40 Nanoparticles (PMAn)
PMAn nanoparticles were prepared in our previous work (Jaber and Mohsen 2013). In a typical procedure, 5 mmol of bulk H3PMo12O40 (PMAb) was dispersed in 50 mL n-octane and the resulting dispersion was stirred vigorously for 30 min at room temperature to form a homogeneous dispersion. This dispersion was transferred into a Teflon-lined stainless autoclave filling 80 % of the total volume. The autoclave was sealed and maintained at 150 °C for 12 h. The autoclave was then cooled to room temperature. Finally, the resulted powder was filtered and washed several times by Octane, and dried in a vacuum at 80 °C for 12 h.
2.2.3 Preparation of Fe3O4@SiO2-imid-PMAn
Fe3O4@SiO2 (1 g) was added to the solution of 3-chlorotriethoxypropylsilane (1 mmol, 0.241 g) and imidazole (1 mmol, 0.0680 g) in p-xylene (20 mL) and the resultant mixture was under reflux for 24 h under nitrogen atmosphere. After refluxing for about 24 h, the mixture was cooled to room temperature, filtered by an external magnet and the product was washed with xylene to remove no reacted species and dried at 70 °C for 6 h. Fe3O4@SiO2-imid (1.0 g) was added to an acetonitrile solution of PMAn (1.0 mmol) in 20 mL that was taken in a round-bottom flask. The mixture was refluxed for 24 h under nitrogen atmosphere. After 24 h, the mixture was filtered by an external magnet, washed with acetonitrile and dichloromethane, and dried at 70 °C for 6 h. Also, the same method was used for the synthesis of Fe3O4@SiO2-imid-PMAb (PMAn = nano H3PMo12O40, PMAb = H3PMo12O40) (Esmaeilpour et al. 2014c).
2.2.4 General Procedure for the Preparation of 1,1-Diacetates
To a mixture of aldehyde (1 mmol) and acetic anhydride (2–3 mmol) 0.025 g Fe3O4@SiO2-imid-PMAn was added and the mixture was stirred at room temperature. The progress of the reaction was followed by thin-layer chromatography (TLC). After completion of the reaction, and separation of catalyst using magnetic field, the residue was extracted with CH2Cl2 (2 × 10 mL). The resulting solution was successively washed with 10 % NaHCO3 (2 × 10 mL) solution and water (3 × 5 mL) and then dried over anhydrous Na2SO4. After removal of the solvent in vacuum, the crude product was purified by column chromatography on silica gel (ethyl acetate/hexane, 1:8).
2.2.5 General Procedure for Deprotection of 1,1-Diacetates
A mixture of the substrate (1 mmol) and Fe3O4@SiO2-imid-PMAn (0.025 g) in methanol (2 mL) was stirred at room temperature for the specified time in Table 2. After completion of the reaction (monitored by TLC), the mixture was filtered using magnetic field to separate the catalyst. The combined filtrates were concentrated on a rotary evaporator to remove MeOH. Water (5 mL) was added and the product was extracted with Et2O (5 mL). The organic phase was washed with 10 % aqueous solution of sodium bicarbonate (2 × 10 mL) to remove excess of Ac2O and dried over Na2SO4. The solvent was evaporated under reduced pressure. The resultant product was passed through a short column of silica gel (n-hexane–EtOAc, 8:1) to afford the pure aldehyde.
3 Results and Discussion
First, the Fe3O4@SiO2 nanosphere core–shell was synthesized. Then, H3PMo12O40 nanoparticles were synthesized by the treatment of H3PMo12O40 with n-octane as solvent by a solvothermal method and this nano heteropolyacid immobilized onto imidazole functionalized Fe3O4@SiO2 nanoparticles (Scheme 2) (Jaber and Mohsen 2013).
Process for preparation of immobilization of H3PMo12O40 nanoparticles on imidazole functionalized Fe3O4@SiO2 nanoparticle (Jaber and Mohsen 2013)
To find the most appropriate reaction conditions and evaluate the catalytic efficiency of Fe3O4@SiO2-imid-PMAn catalyst on the protection of aldehydes as the corresponding 1,1-diacetates, initially the reaction of 4-chlorobenzaldehyde with acetic anhydride was chosen as a model reaction.
For optimization of the reaction media, 4-Chlorobenzaldehyde as model substrate and Fe3O4@SiO2@PMA as catalyst were conducted in different solvents. The results show that the efficiency and the yield of the reaction in solutions were much less than those observed under solvent-free conditions (Table 1, entries 1–9). According to the results, the proposed method (solvent-free conditions) is suitable for conversion of aldehydes to geminal diacetates (Table 1). Then the results showed that the best catalyst loading in solvent-free conditions was about 0.025 g. Lower amounts of catalyst resulted in lower yields, while higher amounts did not affect the reaction times and yields (Table 1, entries 13–18). The amount of acetic anhydride was also optimized and the best result was obtained with a 2:1 molar ratio of acetic anhydride: aldehyde (Table 1, entries 9–12).
Under the optimized reaction conditions, a wide range of aldehydes were reacted with acetic anhydride in the presence of Fe3O4@SiO2-imidazol-PMAn under solvent-free conditions at room temperature and the corresponding 1,1-diacetates were obtained in good to excellent yields (Scheme 1) (Table 2).
As shown in Table 2, all aromatic aldehydes carrying either electron-donating (Tables 2 entries 2–5) or electron- withdrawing (Tables 2, entries 6–15) substituents reacted very well, giving good to excellent yields. We investigated the reaction of 4-hydroxybenzaldehyde under the above conditions and observed that both carbonyl and phenolic –OH groups were acylated (Table 2, entry 16).
Other aldehydes such as benzaldehyde, 2-naphthaldehyde and anthracene-10-carbaldehyde produced acylals in good yields (Table 2, entries 1, 17–18). Heteroaromatic aldehydes such as thiophene-2-carbaldehyde and the acid sensitive substrate (furfural) are converted to 1,1-diacetate without the formation of any side products (Table 2, entries 19–20). Aliphatics and α,β-unsaturated aldehydes produced acylals in good yields (Table 2, entries 21–22). Attempted acetylation of benzenedicarbaldehydes, gave the tetracetylated products in good yields for phthaldialdehyde and tere-phthaldialdehyde. The tetracylated products were obtained in 94 and 91 % yields, respectively (Table 2, entries 14,15).
In accordance with the fact reported in the literature (Li et al. 1998), 4- dimethylamino benzaldehyde and indole-3-carbaldehyde (Table 2, entries 23 and 24) failed to give the corresponding 1,1-diacetates and the starting materials were quantitatively recovered after prolonged reaction times (Table 2, entries 23 and 24). This result may be due to the strong electron donation of dimethylamino and NH groups. Also, a degree of tautomerization may occur with formation of a quininoid structure, which decreases the reactivity of the aldehydic carbonyl group (Shirini and Jolodar 2012).
We have also found that the conversion of 1,1-diacetates to their corresponding aldehydes can be easily catalyzed in the presence of Fe3O4@SiO2-imid-PMAn in methanol. All reactions were performed at room temperature in good to high yields but need a longer time (Scheme 1 and Table 2).
To evaluate the selectivity of this method, we studied the competitive reaction for the acylation of aldehydes in the presence of ketones using Fe3O4@SiO2-imidazol-PMAn as the catalyst at room temperature (Table 3). With this catalytic system the highly selective conversion of aldehydes in the presence of ketones was observed (Table 3, entries 1–2). The acylation of 4-methoxybenzaldehyde versus 4-nitro-benzaldehyde also showed a high selectivity in the presence of this catalyst, which indicated the importance of electronic effects upon these reactions (Table 3, entry 3). Also, to examine the steric effects, 2-propylbenzaldehyde, 3-propylbenzaldehyde and 4-propylbenzaldehyde were allowed to react with acetic anhydride in the presence of the Fe3O4@SiO2-imid-PMAn as a catalyst. As shown, the catalyst was able to discriminate between the aldehydes with different steric effects (Table 3, entry 4). As we can see from Table 3, 1,1-diacetate was produced only from 4-propyl benzaldehyde in good yields.
To test the worth of the present work in comparison with results in the literature, we compared results of Fe3O4@SiO2-Imid-PMAn and Fe3O4@SiO2-Imid-PMAb with other applied Lewis and Bronsted acids in synthesis of 1, 1-diacetate. These data, which are shown in Table 4, revealed that Fe3O4@SiO2-Imid-PMAn and Fe3O4@SiO2-Imid-PMAb are better catalysts than most of the conventional catalysts mentioned with respect to reaction times and yields of the obtained products (Table 4, entries 14, 15).
After completion of the reaction, the catalyst was washed well with ethylacetate, and then dried at 80 °C prior to use and tested for its activity in subsequent run and fresh catalyst was not added. The recovered catalyst was found to be reusable for six cycles with a slight loss in their activities (Fig. 1).
Reusability of Fe3O4@SiO2-imid-PMAn in the conversion of 4-Chlorobenzaldehyde to its corresponding 1,1-diacetates (A) and deprotection of the obtained 1,1-diacetate (B). a Reaction conditions: 4-Chlorobenzaldehyde (1 mmol), acetic anhydride (2 mmol), catalyst (0.025 g), room temperature, solvent-free. b Reaction conditions: 1,1-Diacetoxy-1-(4-chlorophenyl) methane (1 mmol), catalyst (0.025 g), room temperature, MeOH (2 mL)
4 Conclusion
In conclusion, a simple, efficient and chemoselective protocol has been developed for the acylation of various aldehydes and deprotection of the obtained 1,1-diacetates using Fe3O4@SiO2-imid-PMAn as a heterogeneous magnetic Bronsted acid catalyst. This method is highly selective for the synthesis of acylals from aldehydes in the presence of ketones. The use of an inexpensive and relatively nontoxic catalyst and also green reagent is another advantage of this method. In addition, the catalyst can be reused several times with magnetic field without use of toxic solvents for it reusability.
References
Aggen DH, Arnold JN, Hayes PD, Smoter NJ, Mohan RS (2004) Bismuth compounds in organic synthesis. Bismuth nitrate catalyzed chemoselective synthesis of acylals from aromatic aldehydes. Tetrahedron 60:3675–3679
Azarifar D, Ghasemnejad H, Ramzanian-lehmali F, (2005) 1,3-Dibromo-5,5-dimethylhydantoin as catalyst for the conversion of aldehydes to their 1,1-diacetates (acetylals) under solvent-free and neutral conditions. Mendeleev Commun, pp 209–210
Borikar SP, Daniel T (2011) A convenient and efficient protocol for the synthesis of acylals catalyzed by Brønsted acidic ionic liquids under ultrasonic irradiation. Ultrasound Sonochemistry 18:928–931
Desai UV, Thopate TS, Pore DM, Wadgaonkar PP (2006) An efficient, solvent-free method for the chemoselective synthesis of acylals from aldehydes and their deprotection catalyzed by silica sulfuric acid as a reusable solid acid catalyst. Catalyst Communications 7:508–511
Esmaeilpour M, Sardarian AR, Javidi J (2012) Schiff base complex of metal ions supported on superparamagnetic Fe3O4@SiO2 nanoparticles: an efficient, selective and recyclable catalyst for synthesis of 1,1-diacetates from aldehydes under solvent-free conditions. Appl Catal A 445–446:359–367
Esmaeilpour M, Javidi J, Dodeji FN, Abarghoui MM (2014) M(II) Schiff base complexes (M = zinc, manganese, cadmium, cobalt, copper, nickel, iron, and palladium) supported on superparamagnetic Fe3O4@SiO2 nanoparticles: synthesis, characterization and catalytic activity for Sonogashira-Hagihara coupling reactions. Transit Metal Chem 39:797–809
Esmaeilpour M, Javidi J, Dodeji FN, Hassannezhad H (2014b) Fe3O4@SiO2-polymer-imid-Pd magnetic porous nanosphere as magnetically separable catalyst for Mizoroki-Heck and Suzuki-Miyaura coupling reactions. J Iran Chem Soc 11:1703–1715
Esmaeilpour M, Javidi J, Zandi M (2014c) Preparation and characterization of Fe3O4@SiO 2@PMA:AS an efficient and recyclable nanocatalyst for the synthesis of 1-amidoalkyl-2-naphthols. Mater Res Bull 55:78–87
Frick JG Jr, Harper RJ Jr (1984) Acetals as crosslinking reagents for cotton. J Appl Polym Sci 29:1433–1447
Gao ST, Zhao Y, Li C, Ma JJ, Wang C (2009) NbCl5 as an efficient catalyst for chemoselective synthesis of 1,1-diacetates under solvent-free conditions. Synth Commun 39:2221–2229
Hajipour AR, Zarei A, Ruoho AE (2007) P2O5/Al2O3 as an efficient heterogeneous catalyst for chemoselective synthesis of 1,1-diacetates under solvent-free conditions. Tetrahedron Lett 48:2881–2884
Heravi MM, Bakhtiari K, Bamoharram FF (2006) An efficient and chemoselective synthesis of acylals from aromatic aldehydes and their regeneration, catalyzed by 12-molybdophosphoric acid. Catal Commun 7:499–501
Jaber J, Mohsen E (2013) Synthesis of Fe3O4@silica/poly(N-isopropylacrylamide) as a novel thermo-responsive system for controlled release of H3PMo12O40 nano drug in AC magnetic field. Colloids Surf B 102:265–272
Javidi J, Esmaeilpour M, Rahiminezhad Z, Dodeji FN (2014) Synthesis and Characterization of H3PW12O40 and H3PMo12O40 Nanoparticles by a Simple Method. J Cluster Sci 25:1511–1524
Jin TS, Sun G, Li YW, Li TS (2002) An efficient and convenient procedure for the preparation of 1,1-diacetates from aldehydes catalyzed by H2NSO3H. Green Chem 4:255–256
Khan AT, Choudhury LH, Ghosh S (2006) Silica supported perchloric acid (HClO4-SiO2): a highly efficient and reusable catalyst for geminal diacylation of aldehydes under solvent-free conditions. J Mol Catal A Chem. 255:230–235
Kochhar KS, Bal BS, Deshpande RP, Rajadhyaksha SN, Pinnick HW (1983) Conversion of aldehydes into geminal diacetates. J Org Chem 48:1765–1767
Kumar P, Hegde VR, Pavan Kumar T (1995) An efficient synthesis of diacetates from aldehydes using beta zeolite. Tetrahedron Lett 36:601–602
Li TS, Zhang ZH, Gao YJ (1998) A rapid preparation of acylals of aldehydes catalysed by Fe3+—montmorillonite. Synth Commun 28:4665–4671
Lu AH, Schmidt W, Matoussevitch N, Bönnemann H, Spliethoff B, Tesche B, Bill E, Kiefer W, Schüth F (2004) Nanoengineering of a magnetically separable hydrogenation catalyst. Angew Chemie Int Ed 43:4303–4306
Moosavifar M, Tangestaninejad S, Moghadam M, Mirkhani V, Mohammadpoor-Baltork I (2011) Host (nanocavity of zeolite-Y)-guest (molybdophosphoric acid) nanocomposite materials: an efficient catalyst for solvent-free synthesis and deprotection of 1, l-diacetates. C R Chim 14:953–956
Nagy NM, Jakab MA, Kónya J, Antus S (2002) Convenient preparation of 1,1-diacetates from aromatic aldehydes catalysed by zinc-montmorillonite. Appl Clay Sci 21:213–216
Negrón GE, Palacios LN, Angeles D, Lomas L, Gaviño R (2005) A mild and efficient method for the chemoselective synthesis of acylals from aromatic aldehydes and their deprotections catalyzed by sulfated zirconia. J Braz Chem Soc 16:490–494
Niknam K, Saberi D, Sefat MN (2009) Silica-bonded S-sulfonic acid as a recyclable catalyst for chemoselective synthesis of 1,1-diacetates. Tetrahedron Lett 50:4058–4062
Palacios-Grijalva LN, Cruz-González DY, Lomas-Romero L, González-Zamora E, Ulibarri G, Negrón-Silva GE (2009) Sulphated zirconia as an eco-friendly catalyst in acylal preparation under solvent-free conditions, acylal deprotection assisted by microwaves, and the synthesis of anhydro-dimers of o-hydroxybenzaldehydes. Molecules 14:4065–4078
Polshettiwar V, Baruwati B, Varma RS (2009) Nanoparticle-supported and magnetically recoverable nickel catalyst: a robust and economic hydrogenation and transfer hydrogenation protocol. Green Chem 11:127–131
Romanelli G, Ruiz D, Vázquez P, Thomas H, Autino JC (2010) Preyssler heteropolyacid H14[NaP5W29MoO110]: a heterogeneous, green and recyclable catalyst used for the protection of functional groups in organic synthesis. Chem Eng J 161:355–362
Satam JR, Jayaram RV (2008) (NH4)3PW12O40 as an efficient and reusable catalyst for the synthesis and deprotection of 1,1-diacetates. Synth Commun 38:595–602
Shelke KF, Sapkal SB, Kakade GK, Shinde PV, Shingate BB, Shingare MS (2009) Boric acid as an efficient catalyst for the synthesis of 1,1-diacetate under solvent-free condition. Chin Chem Lett 20:1453–1456
Shirini F, Jolodar OG (2012) Introduction of N-sulfonic acid poly(4-vinylpyridinum) chloride as an efficient and reusable catalyst for the chemoselective 1,1-diacetate protection and deprotection of aldehydes. J Mol Catal A Chem. 356:61–69
Shirini F, Mamaghani M, Mostashari-Rad T, Abedini M (2010) Saccharin sulfonic acid as an efficient catalyst for the preparation and deprotection of 1,1-diacetates. Bull Korean Chem Soc 31:2399–2401
Shirini F, Mamaghani M, Seddighi M (2013) Sulfonated rice husk ash (RHA-SO3H): a highly powerful and efficient solid acid catalyst for the chemoselective preparation and deprotection of 1,1-diacetates. Catal Commun 36:31–37
Wang L, Cai C (2009) Preparation and characterization of chloroacetylated polystyrene-supported zinc complexes and evaluation of their catalytic activities for the synthesis of 1,1-diacetates. J Appl Polym Sci 112:2087–2093
Wang J, Yan L, Qian G, Yang K, Liu H, Wang X (2006) Heteropolyacid encapsulated into mesoporous silica framework for an efficient preparation of 1,1-diacetates from aldehydes under a solvent-free condition. Tetrahedron Lett 47:8309–8312
Wang QY, Sheng SR, Wei MH, Xie ZL, Liu XL (2007) PEG-supported sulfonic acid as an efficient and recyclable catalyst for the synthesis of 1,1-diacetates under solvent-free conditions. Synth Commun 37:1019–1026
Yoon TJ, Lee W, Oh YS, Lee JK (2003) Magnetic nanoparticles as a catalyst vehicle for simple and easy recycling. N J Chem 27:227–229
Zhang Z, Zhang F, Zhu Q, Zhao W, Ma B, Ding Y (2011) Magnetically separable polyoxometalate catalyst for the oxidation of dibenzothiophene with H2O2. J Colloid Interf Sci 360:189–194
Acknowledgments
Authors are grateful to the council of Iran National Science Foundation and University of Shiraz for their unending effort to provide financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mohsen, E., Jaber, J., Maryam, Z. et al. Fe3O4@SiO2-imid-PMAn as a Novel, Efficient and Reusable Magnetic Nanocatalyst for the Chemoselective Preparation and Deprotection of 1,1-Diacetates. Iran J Sci Technol Trans Sci 41, 343–353 (2017). https://doi.org/10.1007/s40995-016-0034-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-016-0034-7