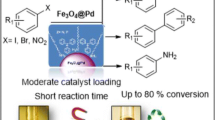
Abstract
The activity of palladium nanoparticles supported on poly (N-vinylpyrrolidone) grafted Fe3O4@SiO2 was investigated in the cross-coupling reactions. We have applied this catalyst under low loading of the supported palladium nanoparticles for the coupling of aryl halides with alkenes (Mizoroki–Heck reaction) and organoboronic acids (Suzuki–Miyaura reaction) in the absence of phosphorous ligands. Short reaction times and excellent yields of the products express the effectiveness of this catalyst. The nanocatalyst can be separated from the reaction mixture by applying a permanent magnet externally and can be reused for six times without appreciable change in catalytic activity. Also, the amount of leaching of Pd nanoparticles has been determined by ICP analysis and results showed low leaching of the metal into solution from the supported catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Palladium-catalyzed carbon–carbon cross-coupling reactions exemplify one of the important processes in organic chemistry [1–4]. Recent progress in palladium catalysis has revealed that Pd in nanoparticle forms can catalyze numerous reactions such as Sonogashira–Hagihara [5–7], Mizoroki–Heck [8, 9], Suzuki–Miyaura [10] and Stille [11] reactions. Coupling of aryl halides with alkenes (Mizoroki–Heck reaction) and organoboronic acids (Suzuki–Miyaura reaction) has significant importance, and is a well-established methodology in modern organic synthesis. These Pd-catalyzed coupling reactions are ranked today among the most general transformations in organic synthesis, and have great industrial potential for the synthesis of bioactive compounds, natural products, chemicals and advanced materials [12].
The Heck reaction is a powerful and modern palladium-catalyzed method for generation of carbon–carbon bonds between unsaturated entities, and it has been extensively utilized in arylation and vinylation of olefins [13–15].
The reaction has been applied to many areas, including natural products, bioactive compounds, UV absorbers, drug intermediates, antioxidants, fine chemicals syntheses, and industrial applications [16].
Also, the palladium catalyzed cross-coupling of aryl halides with arylboronic acids, the Suzuki reaction [17–20]. Among the traditional protocols, aryl iodides, bromides have been found to be the more reactive and are therefore commonly employed [21]. However, the more economic aryl chlorides have been rarely employed as coupling partners in palladium catalyzed Suzuki coupling because the oxidative addition of C–Cl bond to Pd(0) species is usually difficult [21]. In past decades, significant research effort has been focused on the preparation and use of catalysts capable of activating aryl chloride substrates. Complexes containing palladacyclic complexes [22] or N-heterocyclic carbenes [23] and bulky phosphines [24], have proved to be unique, highly efficient catalysts for Suzuki cross-coupling reactions of aryl chlorides.
Unfortunately, most of these catalysts are expensive and difficult to synthesize, which significantly limited their industrial applications.
Phosphines and phosphinites as important phosphorous-based ligands in organometallic chemistry are of current interest and their metal complexes have been used in many catalytic reactions [25]. However, Phosphorus ligands are very often moisture and air sensitive, which creates problems in the catalytic system [26].
Phosphine ligands are comparatively difficult to make or rather more expensive [27] and often lead to competitive degradation of the Pd catalyst through the P–C bond cleavage of a coordinated phosphine ligand [15]. In addition, there is a strong tendency to avoid application of these ligands because of their possibly negative impact on the natural environment [25]. Consequently, the development of economical, accessible phosphine-free palladium catalytic systems to overcome these difficulties is still of desirable goal.
Nanomagnetism is a vivid and highly interesting topic of modern solid state magnetism and nanotechnology [28–30]. This is not only due to the ever-increasing demand for miniaturization, but also due to novel phenomena and effects which appear only on the nanoscale: e.g., spin structures, superparamagnetism, coupling phenomena, new types of magnetic domain walls and interactions between electrical current and magnetism [31]. Fe3O4 magnetic nanoparticles have been used in various fields such as sealing, oscillation damping, information storage and electronic devices [32–34]. One of the rapidly developing applications of Fe3O4 magnetic nanoparticles in recent years is in biomedical areas, including rapid biologic separation and drug delivery and such as catalysts [35–37].
Recently, magnetic core–shell nanostructures have attracted more attention due to their unique magnetic properties [37]. In contrast to the difficulty observed in recovering and reusing most solid catalysts, core–shell nanostructure magnetic catalysts can be easily retrieved under the influence of a magnetic field and used in subsequent reactions. Due to this property, using magnetic core–shell structure composites as catalysts has been recommended in the literature [38, 39].
Homogeneous catalysis is superior to heterogeneous catalysis, making possible highly active and selective organic transformations. However, the separation and recovery of homogeneous catalysts are not easy and so it is still significant to prepare more active heterogeneous catalysts and to find effective ways of heterogenizing homogeneous catalysts for industrial reaction processes [40]. To overcome this problem, great efforts have been devoted to develop heterogeneous catalysts.
Because of these reasons and also as a part of our ongoing research program on the application of magnetic catalysts for the development of useful new synthetic methodologies [41] herein, we report the use of polymer-imid-Pd functionalized Fe3O4@SiO2 nanoparticle (Fe3O4@SiO2-polymer-imid-Pd) without added phosphine ligands in Mizoroki–Heck and Suzuki–Miyaura cross-coupling reactions with.
Experimental
General
The chemicals were obtained from Fluka or Merck chemical companies and used without further purification. Transmission electron microscopy (TEM) image was obtained on a Philips EM208 TEM with an accelerating voltage of 100 kV. X-ray photoelectron spectroscopy (XPS) of catalyst was performed with XR3E2 (VG Microtech) twin anode X-ray source using AlKα = 1486.6 eV. The progress of the reactions was followed with TLC using silica gel SILG/UV 254 plates or GLC on a Shimadzu model GC-10A instrument. IR spectra were run on a Shimadzu FTIR-8300 spectrophotometer. The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance DPX 250 MHz Spectrometer in CDCl3 or DMSO-d6 solvents using TMS as an internal standard. Mass spectra were obtained at 70 eV. Pd loading and leaching test was carried out with an inductively coupled plasma (ICP) analyzer (Varian, vista-pro). Melting points were determined with a Buchi 510 instrument in open capillary tubes and are uncorrected. Evaporation of solvents was performed at reduced pressure, with a Buchi rotary evaporator. All products were identified by comparison of their spectral data and physical properties with those of the authentic sample and all yields refer to isolated products.
General procedure
Preparation of Fe3O4@SiO2 core–shell
The core–shell Fe3O4@SiO2 nanospheres were prepared by a modified Stober method in our previous work [41]. In a typical procedure, the mixture of FeCl3·6H2O (1.3 g, 4.8 mmol) in water (15 ml) was added to the solution of polyvinyl alcohol (PVA 15000) (1 g), as a surfactant, and FeCl2·4H2O (0.9 g, 4.5 mmol) in water (15 mL), which was prepared by completely dissolving PVA in water followed by addition of FeCl2·4H2O. The resultant solution was left to be stirred for 30 min in 80 °C. Then, hexamethylentetraamine (HMTA) (1.0 mol/l) was added dropwise with vigorous stirring to produce a black solid product when the reaction media reaches pH 10. The resultant mixture was heated on water bath for 2 h at 60 °C and the black magnetite solid product was filtered and washed with ethanol three times and was then dried at 80 °C for 10 h. Then, Fe3O4 nanoparticle (0.50 g, 2.1 mmol) was dispersed in the mixture of ethanol (50 mL), deionized water (5 mL) and tetraethoxysilane (TEOS) (0.20 mL), followed by the addition of 5.0 mL of NaOH (10 wt%). This solution was stirred mechanically for 30 min at room temperature. Then, the product, Fe3O4@SiO2, was separated by an external magnet, and was washed with deionized water and ethanol three times and dried at 80 °C for 10 h.
Synthesis of poly (N-vinylimidazole) functionalized Fe3O4@SiO2 nanoparticle (Fe3O4@SiO2-polymer-imid)
Fe3O4@SiO2 (1 g) was added to the solution of 3-aminopropyl (triethoxy) silane (1 mmol, 0.176 g) in ethanol (10 mL) and the resultant mixture was under reflux for 12 h. The solvent was removed and the resulting solid (Fe3O4@SiO2-NH2) was dried at 80 °C overnight. The product was washed with ethanol and water to remove unreacted species and dried at 80 °C for 6 h. Fe3O4@SiO2-NH2 (1 g) was suspended in dry THF (15 mL) and suspension cooled down to 0 °C. Triethylamine (0.151 g, 1.5 mmol) was added, followed by addition of acryloyl chloride (0.109 g, 1.2 mmol) over a period of 1 h. Then, the resultant mixture was stirred at 0 °C for a further 4 h and the modified Fe3O4@SiO2 was isolated by external magnetic field and washing with THF (10 mL), water (2 × 10 mL) and acetone (10 mL). The solid obtained was then dried at 80 °C for 12 h. To the resultant mixture (1.0 g), N-vinylimidazole (2 mL) and recrystallized benzoyl peroxide (0.025 g) were added and the mixture was heated at 80 °C for 12 h. The poly (N-vinylimidazole) functionalized Fe3O4@SiO2 nanoparticle (Fe3O4@SiO2-polymer-imid) was separated by an external magnet, and was washed with deionized water and ethanol three times and dried at 80 °C for 10 h [42].
Synthesis of polymer-imid-Pd functionalized Fe3O4@SiO2 nanoparticle (Fe3O4@SiO2-polymer-imid-Pd)
Fe3O4@SiO2-polymer-imid (1 g) was dispersed in the DMF solution (15 mL), ultrasonically for 15 min. Then, methyl iodide (2.5 mmol, 0.155 mL) was added and the mixture was stirred at 80 °C for 16 h and filtered by an external magnet field and the product was washed with DMF and ethanol to remove no reacted species and dried at 70 °C for 6 h. The resultant solid product was stirred in NaCl solution (5 %) (30 mL) at room temperature for 24 h. The mixture was filtered off, washed thoroughly with excess H2O and then dried in an oven under vacuum at 70 °C for 8 h (Fe3O4@SiO2-polymer-imid-S). The chloride ion capacity of imidazolium type Fe3O4@SiO2-polymer was found using argentimetric titration method (0.1 g of Fe3O4@SiO2-polymer-imid-S was suspended in 10 mL of 0.1 M HNO3). After adding 1 mL of 0.1 M AgNO3, the mixture was stirred for 10 h at room temperature. The chloride counter ions precipitated as AgCl. The amount of remaining Ag+ was back titrated using 0.1 M HCl. The permanent charge density of imidazole groups was calculated to be 1.31 mmol/g. The resulting Fe3O4@SiO2-polymer-imid-S (1.0 g) was reacted with PdCl2 (3 mmol) in the presence of Et3N (6 mmol) as a base and DMF (15 mL) as a solvent at 80 °C for 16 h. The mixture (Fe3O4@SiO2-polymer-imid-Pd) was filtered by an external magnetic field and washed thoroughly with DMF (2 × 5 mL) and H2O (2 × 5 mL) and dried at 70 °C for 8 h (Scheme 1) [42].
General procedure for Heck cross-coupling reactions using Fe3O4@ SiO2-polymer-imid-Pd magnetic nanocatalyst
Fe3O4@SiO2-polymer-imid-pd (0.03 g), K2CO3 (2.0 mmol), olefin (1.2 mmol), aryl halide (1.0 mmol) were placed in a 25-mL flask equipped with a magnetic stirring bar in NMP and heated at 110 °C. On completion of the reaction determined by TLC or GC, the catalyst was easily recovered using magnetic field and the filtrate was poured into water (50 mL) and extracted with EtOAc (3 × 10 mL). The combined organic phases were dried over Na2SO4, filtered and evaporated in vacuum. The mixture was then purified by column chromatography over silica gel or recrystallization to afford a product with high purity.
General procedure for Suzuki–Miyaura reactions using Fe3O4@SiO2-polymer-imid-Pd magnetic nanocatalyst
A suspension of aryl halide (1.0 mmol), K2CO3 (2.0 mmol), Fe3O4@SiO2-polymer-imid-pd nanocatalyst (0.025 g) and NMP (3 mL) were mixed in a reaction flask and phenylboronic acid (1.2 mmol) was added. The reaction mixture was stirred at 90 °C in the air for an appropriate time. The completion of the reaction was monitored by TLC. After completion of the reaction, the catalyst was easily recovered using magnetic field and the mixture was diluted with n-hexane and water. The organic layer was washed with brine, dried over Na2SO4, and concentrated under reduced pressure. Further purification, if it was necessary, was performed on a silica gel column eluted with n-hexane/ethyl acetate to give the pure biphenyl product in high to excellent yields.
Results and discussion
TEM image of Fe3O4@SiO2-polymer-imid-pd nanocatalyst is presented in Fig. 1a. According to this figure, Pd nanoparticle has a diameter around 10 nm.
The catalyst was characterized by XPS to ascertain the oxidation state of Pd species. As shown in Fig. 1b, the peaks located around 335.6 and 340.6 eV were assigned to the Pd 3d5/2 and Pd 3d3/2 level in the Pd (0) metallic form in agreement with the literature report [43]. The binding energy clearly showed the fact that the Pd nanoparticles in our catalyst contained only Pd (0) species.
Determination of the Pd content was carried out by ICP analysis on the digested catalyst in refluxing HCl (37 %). According to the ICP analysis, the Pd content in the magnetic Fe3O4@SiO2-polymer-imid-Pd catalyst was 0.33 mmol/g.
To show the merit of application of these nanoparticles in organic synthesis, we applied them as the catalysts in the Mizoroki–Heck reaction (Scheme 2).
Initial studies were performed upon the reaction of iodobenzene with n-butyl acrylate as a model reaction and the effects of amount of catalyst, different solvents, bases and temperature were studied for this reaction (Table 1). A controlled experiment indicated that no cross-coupling product was observed in the absence of the base or catalyst and the reaction did not proceed even at a high temperature (Table 1, entries 9, 16).

For such a purpose, firstly the influences of the catalytic amount on the yield were investigated. The reaction was performed with different amounts of Fe3O4@SiO2-polymer-imid-pd as catalyst. The results are listed in Table 1. The best result was achieved by carrying out the reaction with (0.03 g:1 mmol:1.2 mmol:2 mmol) ratio of catalyst, iodobenzene, n-butyl acrylate and K2CO3 in NMP at 110 °C (Table 1, entry 1). Decreasing the catalyst concentration resulted in lower yields under the same conditions (Table 1, entries 17–19). On the other hand, higher amounts of catalyst did not improve the yield or reaction rate (Table 1, entries 20 and 21). Furthermore, we examined different reaction conditions in common organic solvents such as DMF, DMSO, CH3CN, EtOH, H2O, Toluene and THF at 110 °C (Table 1, entries 2–8) and excellent yields of the product were observed in NMP (Table 1, entry 1). The effect of temperature was studied by carrying out the model reaction at different temperatures in NMP in the presence of K2CO3 (room temperature, 100, 110 and 120 °C) (Table 1, entries 1 and 22–24) and the best result was obtained at 110 °C (Table 1, entry 1). The yield of the product was negligible at room temperature, and only a trace amount of the product was obtained (Table 1, entry 22). Moreover, a high temperature appears to be essential for the Heck reaction to take place (Table 1, entries 1, 23 and 24). Also, the coupling of iodobenzene and n-butyl acrylate was carried out in NMP with the Fe3O4@SiO2-polymer-imid-pd catalyst at 110 °C in the presence of 2 equiv of different bases (Table 1). The results indicate that K2CO3 was the best base for this reaction (Table 1, entry 1). The coupling with triethylamine, sodium carbonate, cesium carbonate, sodium hydroxide, tripropylamine and potassium fluoride gave n-butyl cinnamate in 50, 81, 76, 63, 38 and 59 % yield, respectively (Table 1, entries 10–15). Therefore, the best results for the Heck reaction of iodobenzene with n-butyl acrylate were obtained using 0.03 g of the catalyst in NMP as the solvent and K2CO3 as the base at 110 °C.
The C–C cross coupling reaction of functional substituted aryl halides has much attention. Under the determined reaction conditions, a wide range of aryl halides bearing electron-donating and electron-withdrawing groups reacted with n-butyl acrylate and styrene efficiently to produce the corresponding cross-coupling products in good to excellent yields. Nevertheless, Fe3O4@SiO2-polymer-imid-pd nanocatalyst exhibited higher activity with electron-withdrawing substituents (Table 2, entries 4, 5, 9, 10, 16, 22, 23, 29) relative to electron-donating substituents on the aryl halides (Table 2, entries 2, 3, 8, 15, 18, 19, 21, 28). Also, the coupling reaction of n-butyl acrylate or styrene with both electron-releasing and electron-withdrawing aryl bromides afforded the desired products in high yields (Table 2, entries 7–13, 20–26).

The more easily accessible and cheaper aryl chlorides have not been employed much, in palladium-catalyzed coupling reactions, because the oxidative addition of C–Cl bond to Pd (0) species is usually difficult. Although aryl chlorides are not as reactive as aryl iodides and bromides and are less likely employed in coupling reactions, Heck reactions could be carried out by this catalyst in the presence of tetrabutylammonium bromide (TBAB) (Jeffery Catalyst) as an additive (Table 2, entries 14–16 and entries 27–29) which increased the conversion rate by formation and stabilization of Pd colloids.
To investigate the possibility of conversion of aryl chlorides with less Pd amount, a case reaction (n-butyl acrylate with chlorobenzene) was carried out with various amount of catalyst (0.01, 0.02, 0.025 and 0.30 g) in NMP and K2CO3 at 110 and progress of reaction was monitored by GC. The percent of conversion versus time of reaction for four amounts is presented in Fig. 2. As shown the best result attributed to 0.03 g of catalyst although the result of 0.025 g of catalyst is also significant.
Also, the efficiency of catalyst was evaluated in the Suzuki coupling reaction as a versatile method for C–C bond formation in organic synthesis. (Scheme 3).
We initially studied the coupling of iodobenzene with phenylboronic acid as a model reaction under different reaction conditions (Table 3). The optimized reaction conditions were: 1 mmol of aryl halides, 1.2 mmol of arylboronic acids, 0.025 g of Fe3O4@SiO2-polymer-imid-Pd, 2 mmol of K2CO3 and 3 mL of NMP at 90 °C (Table 3, entry 1).

Under our optimized reaction conditions, the desired products were obtained in good to excellent yields for a wide array of aryl halides. The results are shown in Table 4.

However, aryl iodides were reacted efficiently with phenylboronic acid in excellent isolated yields (Table 4). Since aryl iodides are more expensive than their corresponding bromides and chlorides, the use of iodides for large-scale reactions is not economically encouraged. Therefore, we applied this catalytic system for the reaction of aryl bromides and chlorides with phenylboronic acid (Table 4, entries 3–21). As it is evident from the results in Table 4, different aryl bromides were reacted with phenylboronic acid at 90 °C in appropriate reaction times. The desired biphenyl compounds were isolated in 80–95 % yields (Table 4, entries 3–15). Compared to Heck reaction described in the previous paragraph, Suzuki reaction was performed under milder reaction condition (90 °C) and less palladium catalyst. Moreover, similar to Heck reaction, aryl chlorides can also give the coupling products in the presence of TBAB (Table 4, entries 16–21).
To investigate the formation of biphenyl as side reaction product by oxidative coupling of phenyl boronic acid, the reaction of 4-methoxyiodobenzene with phenylboronic acid in test condition (NMP as solvent, 0.025 g catalyst, 90 °C) was carried out (Scheme 4). The reaction mixer was analyzed by GC during and after reaction. According to GC results, just substance A is produced and substance B does not form. So we can say that oxidative coupling of phenyl boronic acid will not occur because biphenyl B was not observed in GC result.
For great certainty, reaction of 4-metoxychlorobenzene with phenylboronic acid in such condition (NMP as solvent, 0.025 g catalyst, 90 °C) was examined (Scheme 4). The progress of reaction was monitored by GC. In this reaction, just substance A is produced also.
Fe3O4@SiO2-polymer-imid-pd magnetic catalyst dispersed in NMP can be easily separated by external magnetic field within several minutes without the need for a filtration step, and then can be readily redispersed with slight shake, indicating directly that the nanoparticles possess magnetic properties (Fig. 3). Such magnetic separation performance makes the nanoparticles more effective and convenient in application.
Afterward, to investigate the recycling of the catalyst, the reaction of iodobenzene and 1-chloro-4-nitrobenzene with n-butyl acrylate (Heck reaction) (Fig. 4a, c, respectively), and also iodobenzene and 1-chloro-4-nitrobenzene with phenylboronic acid (Suzuki reaction) (Fig 4b, d, respectively) in optimum condition was tested.
After the completion of reaction, the magnetic catalyst could be conveniently and efficiently recovered from the reaction mixture with an external magnet, it can be used in the next run after washing with ethanol and drying. The results revealed that the recovered catalyst had not significantly lost its activity after six cycles for two substrates (iodobenzene and 1-chloro-4-nitrobenzene) (Fig. 4).
The amount of leached Pd in the reaction solution was measured by the ICP technique. The amount of palladium leaching after the first run was determined by ICP analysis to be only 0.6 and 0.3 % for Mizoroki–Heck reaction and Suzuki–Miyaura reaction, and after 6 repeated recycling was 6 and 4 %, respectively.
Conclusions
Surface of Fe3O4@SiO2 was covered with polymeric shell and then Pd nanoparticles were loaded in polymeric chins. The polymeric layer caused Pd nanoparticle faraway from surface of Fe3O4@SiO2. Additionally Pd particles which supported in polymer chin have a distinct distance between themselves. Therefore Pd nanoparticles not aggregate and provide better accessibility for reactants. The formation of the carbene–Pd bond leads to leaching of Pd nanoparticles reduces. Polymer chains act as spacer between Fe3O4@SiO2 and Pd and lead to the increased catalytic activity of Pd.
Using Fe3O4@SiO2-polymer-imid-Pd nanoparticles as an efficient catalyst for Mizoroki–Heck and Suzuki–Miyaura coupling reaction of aryl iodides, aryl bromides and aryl chlorides in the absence of phosphorous ligands was reported. Heterogeneous nature and easy separation of the catalyst, easy preparation, short reaction times, easy purification, excellent yields and very low Pd leaching are the main characteristics of the process. These special features make this method a facile tool in direct coupling reactions. Also, the heterogeneous catalyst is easily separated from the reaction mixture by a magnetic field and has been recycled for six runs without appreciable loss of its catalytic activity significantly.
References
L. Yin, J. Liebsher, Chem. Rev. 107, 133 (2007)
R. Kore, M. Tumma, R. Srivastava, Catal. Today 198, 189 (2012)
B. Tamami, F. Farjadian, J. Iran. Chem. Soc. 8, 77 (2011)
N.T.S. Phan, M.V. Der Sluys, C.W. Jones, Adv. Synth. Catal. 348, 609 (2006)
S.G. Ryu, S.W. Kim, S.D. Oh, S.H. Choi, H.G. Park, Y.P. Zhang, Colloid. Surface A. 313, 224 (2008)
Kh Niknam, A. Deris, F. Panahi, M.R. Hormozi, Nezhad. J. Iran. Chem. Soc. 10, 1291 (2013)
M.A. Zolfigol, V. Khakyzadeh, A.R. Moosavi-Zare, A. Rostami, A. Zare, N. Iranpoor, M.H. Beyzavie, R. Luque, Green Chem. 15, 2132 (2013)
V. Polshettiwar, A. Molnar, Tetrahedron 63, 6949 (2007)
B. Tamami, F. Nowroozi, Dodeji. J. Iran. Chem. Soc. 9, 841 (2012)
A.J. Amali, R.K. Rana, Green Chem. 11, 1781 (2009)
V. Kogan, Z. Aizenshtat, R. Popovitz-Biro, R. Neumann, Org. Lett. 4, 3529 (2002)
L. Brandsma, S.F. Vasilersky, H.D. Verkruijsse, Applications of transition metal catalysts in organic synthesis (Springer, Berlin, 1988)
R.F. Heck, Org. React. 27, 345 (1982)
I.P. Beletskaya, A.V. Cheprakov, Chem. Rev. 100, 3009 (2000)
N.J. Whitcombe, K.K. Hii, S.E. Gibson, Tetrahedron 57, 7449 (2001)
M. Bagherzadeh, M. Amini, A. Ellern, L.K. Woo, Inorg. Chim. Acta 383, 46 (2012)
A. Suzuki, J. Organomet. Chem. 576, 147 (1999)
S. Kotha, K. Lahiri, D. Kashinath, Tetrahedron 58, 9633 (2002)
S.P. Stanforth, Tetrahedron 54, 263 (1998)
K.H. Niknam, Habibabad, A. Deris, F. Panahi, M.R.H. Nezhad, J. Iran. Chem. Soc. 10, 527 (2013)
G. Mengping, Zh Zhiyong, H. Hongwei, Zh Qiaochu, Catal. Commun. 10, 865 (2009)
R. Chinchilla, C. Najera, M. Yus, Chem. Rev. 104, 2667 (2004)
T. Mino, Y. Shirae, M. Sakamoto, T. Fujita, J. Org. Chem. 70, 2191 (2005)
T.J. Colacot, H.A. Shea, Org. Lett. 6, 3731 (2004)
N. Iranpoor, H. Firouzabadi, S. Motevalli, M. Talebi, J. Organomet. Chem. 708, 118 (2012)
E. Mieczynska, A. Gniewek, I. Pryjomska-Ray, A.M. Trzeciak, H. Grabowska, M. Zawadzki, Appl. Catal A-Gen. 393, 195 (2011)
B. Punji, C. Ganesamoorthy, M.S. Balakrishna, J. Mol. Catal. A-Chem. 259, 78 (2006)
S. Blügel, T. Brückel, C.M. Schneider, Magnetism goes nano: electron correlations, spin transport, molecular magnetism (2005)
H. Kronmüller, S. Parkin, Handbook of magnetism and advanced magnetic materials (Wiley, Hoboken, 2007)
B.D. Terris, T. Thomson, J. Phys. D: Appl. Phys. 38, 199 (2005)
O. Petracic, Superlattice. Microst. 47, 569 (2010)
D.K. Lee, Y.S. Kang, C.S. Lee, P. Stroeve, J. Phys. Chem. B. 106, 7267 (2002)
D.H. Zhang, Z.Q. Liu, S. Han, C. Li, B. Lei, M.P. Stewart, Nano. Lett. 4, 2151 (2004)
P.C. Wang, C.F. Lee, T.H. Young, D.T. Lin, W.Y. Chiu, J. Polym. Sci. Part. A. Polym. Chem. 43, 1342 (2005)
S.T. Tan, J.H. Wendorff, C. Pietzonka, Z.H. Jia, G.Q. Wang, Chem. Phys. Chem. 6, 1461 (2005)
I. Garcia, N.E. Zafeiropoulos, A. Janke, A. Tercjak, A. Eceiza, M. Stamm, J. Polym. Sci. Part. A. Polym. Chem. 45, 925 (2007)
A. Schatz, M. Hager, O. Reiser, Adv. Funct. Mater. 19, 2109 (2009)
D.D. Shao, K.K. Xu, X.J. Song, J.H. Hu, W.L. Yang, C.C. Wang, J. Colloid, Interface. Sci. 336, 526 (2009)
Y.H. Deng, D.W. Qi, C.H. Deng, X.M. Zhang, D.Y. Zhao, J. Am. Chem. Soc. 130, 28 (2008)
Y. Akiyama, X. Meng, S. Fujita, Y.C. Chen, N. Lu, H. Cheng, F. Zhao, M. Arai, J. Supercrit. Fluid. 51, 209 (2009)
M. Esmaeilpour, A.R. Sardarian, J. Javidi, Appl. Catal A-Gen. 445–446, 359 (2012)
M. Esmaeilpour, J. Javidi, M.M. Abarghoui, F.N. Dodeji, J. Iran. Chem. Soc. (2013). doi:10.1007/s13738-013-0323-4
Zh Li, J. Chen, W. Su, M. Hong, J. Mol. Catal A: Chem. 328, 93 (2010)
H. Firouzabadi, N. Iranpoor, A. Ghaderi, J. Mol. Catal. A: Chem. 347, 38 (2011)
H. Firouzabadi, N. Iranpoor, F. Kazemi, M. Gholinejad, J. Mol. Catal A: Chem. 357, 154 (2012)
N. Iranpoor, H. Firouzabadi, A. Tarassoli, M. Fereidoonnezhad, Tetrahedron 66, 2415 (2010)
N. Iranpoor, H. Firouzabadi, S. Motevalli, M. Talebi, J. Organomet. Chem. 708–709, 118 (2012)
B. Punji, C. Ganesamoorthy, M.S. Balakrishna, J. Mol. Catal A: Chem. 259, 78 (2006)
B. Tamami, S. Ghasemi, J. Mol. Catal A: Chem. 322, 98 (2010)
C. Pan, M. Liu, L. Zhang, H. Wu, J. Ding, J. Cheng, Catal. Commun. 9, 508 (2008)
H. Firouzabadi, N. Iranpoor, M. Gholinejad, J. Organomet. Chem. 695, 2093 (2010)
Acknowledgments
Authors are grateful to the council of Iran National Science Foundation and University of Shiraz for their unending effort to provide financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esmaeilpour, M., Javidi, J., Dodeji, F.N. et al. Fe3O4@SiO2-polymer-imid-Pd magnetic porous nanosphere as magnetically separable catalyst for Mizoroki–Heck and Suzuki–Miyaura coupling reactions. J IRAN CHEM SOC 11, 1703–1715 (2014). https://doi.org/10.1007/s13738-014-0443-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-014-0443-5