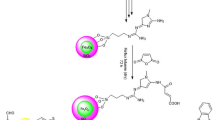
Abstract
The modification of Fe3O4 nanomagnetic particles with inorganic and organic compounds was accomplished. The Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 as Brønsted acid was synthesized by functionalization of Fe3O4 in several steps and characterized by various techniques. Domino reaction of dimedone, anilines, ninhydrin, and malononitrile was investigated by a magnetic catalyst without using solvent at 80 °C. This method has many advantages including short reaction time, green conditions, ecofriendly, excellent yields of products, and easy separation by an external magnet.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, using nanoparticles as catalysts in organic reactions has attracted many researchers. Among them, magnetic nanoparticles such as Fe3O4 are so important [1,2,3], because of their applications in many fields including drug delivery [4, 5], enzyme stabilization [6, 7], food industry [8], water refinery [9], hyperthermia [10, 11], and magnetic nanocatalyst [12,13,14,15,16].
Fe3O4 nanoparticles have various advantages including high stability, simple synthesis, low toxicity, high surface era, easy separation, and good magnetic behavior [17,18,19].
Propellanes have tricyclic systems, which are joined together by a carbon–carbon single bond [20]. Propellane derivatives exist in the natural compound structures such as modhephene [21], aspidohylline [22], and periglaucine A [23, 24] (Scheme 1).
The propellanes have various properties such as anticancer [25], selective T-cell cytotoxicity [26], antibiotics [27], and antibacterial [28]. Different types of propellanes include thioxo[3.3.3]propellane, oxa[3.3.3]propellane, oxathiaza[3.3.3]propellane, and oxaza[3.3.3]propellane [29, 30]. Propellanes are inert toward nucleophiles, but in the presence of free radical compounds, they have high activities. Also, the [3.3.3]propellanes are found in the natural product structures [31,32,33].
Multicomponent reactions (MCRs) have attracted much attention due to their different advantages such as easy work-up, reduction in reaction time, simple extraction, and purification in organic chemistry [34, 35]. In the past decades, they have been applied in the synthesis of various heterocycles. Therefore, various procedures for the synthesis of propellane compounds were published by many researchers [29, 36,37,38]. In continuation of our previous works [39,40,41,42,43], the surface of Fe3O4 core was improved by organic reagents for the synthesis of Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 as Brønsted acid. Afterward, the Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 was used as a catalyst to give [3.3.3]propellanes via the domino reaction of anilines, dimedone, ninhydrin, and malononitrile under the solvent-free condition.
Experimental
Synthesis of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2@Pr-NH2
It is reported in supporting information [44, 45].
Synthesis of Fe3O4@SiO2@Pr-Oxime
First, Fe3O4@SiO2@Pr-NH2 (1 g) in dry toluene (25 mL) was sonicated for 1 hour to give Fe3O4@SiO2@Pr-Oxime nanoparticles. Then, 1,4-diacetyl-monooxime-one (5 mmol, 0.5 g) was added to the colloidal mix and refluxed for 36 h. Finally, by an external magnet, the magnetic nanoparticles were separated, washed with EtOH, and dried.
Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 synthesis
First, Fe3O4@SiO2@Pr-Oxime (1 g) in dry toluene (25 mL) was sonicated for 1 h. Then, 1,4‐butane sultone (5 mmol, 5 mL) was added to the colloidal mix and refluxed for 36 h. Finally, the magnetic nanocatalyst was separated by an external magnet, washed with CH2Cl2, and dried.
Synthesis of [3.3.3]propellane
The reaction of dimedone (1.0 mmol, 0.14 g) and 4-bromoaniline (1 mmol, 0.16 g) by Fe3O4@SiO2@Pr-Oxim-(BuSO3H)3 (0.01 g) at 80 °C after ten minutes gave enaminone, which was reacted with ninhydrin (1 mmol, 0.16 g) and malononitrile (1 mmol, 0.66 g) and stirred under the same conditions. The reaction progress was monitored by TLC in EtOAc/n-hexane (8:2). The reaction mixture was dissolved in hot EtOH, and the nanomagnetic catalyst was separated using an external magnet. The pure product was obtained by cooling alcoholic filtrate. The products were investigated using melting point and FTIR spectra. The NMR and mass spectra of the new compound were characterized.
(4bR,9bS)-11-((l2-azaneylidene)-l3-methyl)-12-amino-5-(3,4-dimethylphenyl)-7,8-dihydro-5H,10H-4b,9b-(epoxyetheno)indeno[1,2-b]indole-9,10(6H)-dione (5k)
Yellow powder; m.p. 233-235 ˚C; IR (KBr, cm-1): 3418, 3342, 3229, 3184, 2962, 2942, 2920, 2191, 1717, 1636, 1596, 1502, 1435, 1391, 1323, 1271, 1218, 1188, 1017, 799. 1H NMR (DMSO, 500MHz): 1.84-1.90 (m, 2H, CH2), 2.05-2.10 (d.t, 1H, CH2), 2.20-2.22 (t, 2H, CH2), 2.24 (s, 3H, Me), 2.29 (s, 3H, Me), 2.42-2.49 (d.t, 1H, CH2), 6.75-6.77 (m, 1H, Ar), 7.04-7.05 (d, 1H, Ar), 7.12 (s, 1H, Ar), 7.27-7.28 (d, 1H, Ar), 7.48 (s, 2H, NH2), 7.63-7.66 (m, 2H, Ar), 7.84-7.86 (m, 1H, Ar). Mass (m/z)= 423 (M+), 395, 381, 340, 350, 323, 309, 270, 254, 240, 227, 190, 177, 164, 139, 128, 103, 90, 77.
Results and discussion
Preparation of Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3
First, Fe3O4 nanomagnetic particles were synthesized via the reaction of FeCl3.6H2O and FeCl2.4H2O in an ammonia solution (25%)/H2O at 100 °C under a nitrogen atmosphere. Then, nanomagnetic particles were coated with tetraethyl orthosilicate at room temperature to provide Fe3O4@SiO2 nanoparticles, which were modified by 3-(triethoxysilyl)propan-1-amine to provide Fe3O4@SiO2@Pr-NH2 nanoparticles, followed by the modification with 1,4-diacetyl-monoxime-one via Schiff base reaction. Finally, the Fe3O4@SiO2@Pr-Oxime nanoparticles were functionalized with acidic groups of BuSO3H via the reaction of the surface amine group with 1,4-butane sultone to obtain Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 (Scheme 2) [46].
Characterization of Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3
The FTIR spectra of the Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2@Pr-NH2, Fe3O4@SiO2@Pr-Oxime, and Fe3O4@SiO2Pr-Oxime-(BuSO3H)3 are shown in Figure 1. The Fe–O, SiO4, and Si–O–Si group vibrations were shown at around 574 cm-1, 800 cm-1, and 1080 cm-1, respectively. The C–H stretching vibrations of alkyl groups, acidic group (SO3H), and OH agent in the silica shell were indicated in the area 2931 and 3442 cm-1, respectively.
The XRD of (a) Fe3O4 and (b) Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 is shown in Fig. 2, which demonstrated the specific peaks at 2θ= 30.12°, 35°, 43.17°, 53.58°, 57.10°, 62.65°, and 74.28° corresponding to the (220), (311), (400), (422), (511), (440), and (533) planes.
The EDX analysis of Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 is shown in Fig. 3, which demonstrated the different elements such as C, N, O, Si, S, and Fe in the structure of nanocatalyst. The SEM image of Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 nanoparticles is shown in Fig. 4, which demonstrated its spherical structure with an average size of 80–100 nm.
The thermal stability of magnetic nanoparticles including Fe3O4@SiO2@Pr-NH2, Fe3O4@SiO2@Pr-Oxime, and Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 was characterized in the range of 25 to 1000 °C as shown in Fig. 5. The mass loss of 2% and 5% was related to the desorption of water on the surface of nanoparticles, which occurred below 200 °C. Also, the decomposition of organic parts occurred in the range of 200–700 °C, which was around 11 %, 21 %, and 26% for Fe3O4@SiO2@Pr-NH2, Fe3O4@SiO2@Pr-Oxime, and Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3, respectively. The obtained amount of the organic compound on the nanoparticle surface was about 0.40 mmol g-1.
The magnetic properties of nanoparticles of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 were studied by VSM. According to Fig. 6, the magnetization properties of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 were obtained at about 60 emu/g, 48 emu/g, and 20 emu/g, respectively.
Application of Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 in the synthesis of [3.3.3]propellanes
The catalyst activity of Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 was investigated for the synthesis of [3.3.3]propellane 5a as a reaction model (Scheme 3). According to Table 1, the effects of catalyst, solvent, and temperature were studied in the reaction model. Different conditions such as solvent-free system, reflux in EtOH, H2O, H2O/EtOH (1:1), and without a catalyst were tested. Therefore, among the examined conditions, the best result was provided via the domino reaction under the solvent-free condition at 80 °C using Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 as nanocatalyst.
The synthesis of [3.3.3]propellane derivatives by Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 under the solvent-free conditions at 80°C was studied (Scheme 4). According to Table 2, electron-donor groups such as methyl on the aniline structure led to the increase in the reaction velocity. Therefore, different derivatives of [3.3.3]propellanes were obtained in a high yield and a short reaction time. The new structure was characterized and confirmed by melting point, IR, and 1H NMR spectra.
The possible mechanism for the synthesis of [3.3.3]propellane 5a by Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 is reported in Scheme 2. Initially, carbonyl groups of dimedone 2a and ninhydrin 3 were protonated in the presence of Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 as Brønsted acid. Then, the nucleophilic addition of 4-bromoaniline 1a to protonated dimedone 2a gave an intermediate 6. Besides, the Knoevenagel reaction of malononitrile 4 with ninhydrin 3 created intermediate 7, which was reacted with enamine 6 through the Michael addition to provide intermediate 8. In the next stage, via the proton transfer, intermediate 8 was cyclized, to provide the compound 9, which was transferred to [3.3.3]propellane 5a via intramolecular O-cyclization and tautomerization reaction (Scheme 5).
Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 nanoparticles were washed several times with hot EtOH, distilled water, and diluted sulfonic acid solution and dried. Then, it was applied in the reaction model for four times as given in Table 3.
According to Table 4, the catalyst activity of Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 toward other reported conditions was compared. This work has advantages including excellent yields, simple work-up, ecofriendly, purity products, short reaction time, and green condition. Also, the nanomagnetic catalyst was separated from the mixture by the magnet.
Conclusions
In summary, Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 as a nanomagnetic catalyst was synthesized and characterized by different techniques such as EDS, VSM, SEM, and XRD. In the following, its catalytic activity was investigated in the synthesis of [3.33]propellanes in good yield and short reaction time.
References
G. Mohammadi Ziarani, M. Khademi, F. Mohajer, M. Anafcheh, A. Badiei, J.B. Ghasemi, Res. Chem. Intermed. 48, 2111 (2022)
B. Tahmasbi, A. Ghorbani-Choghamarani, Catal Lett. 147, 649 (2017)
M. Zarei, H. Sepehrmansourie, M.A. Zolfigol, R. Karamian, S.H.M. Farida, New J Chem. 42, 14308 (2018)
L. Zhao, X. Song, X. Ouyang, J. Zhou, J. Li, D. Deng, ACS Appl. Mater. Interfaces 13, 49631 (2021)
G. Mohammadi Ziarani, P. Mofatehnia, F. Mohajer, A. Badiei, RSC Adv. 10, 30094 (2020)
S. Altaf, Z. Rabeea, Q.Z. Waqas, A. Shakil, Y. Khurram, S. Asad, J.K. Asim, B. Muhammad, A. Muhammad, Ecotoxicol. Environ. Saf. 226, 112826 (2021)
J. Lu, Y. Li, H. Zhu, G. Shi, ACS Appl. Nano Mater. 4, 7856 (2021)
L. Xu, Q. Ding, Anal. Methods. 13, 5487 (2021)
H.R. Pouretedal, Z. Bashiri, M. Nasiri, A. Arab, Part. Sci. Technol. 39, 971 (2021)
M. Cohen-Erner, R. Khandadash, R. Hof, O. Shalev, A. Antebi, A. Cyjon, D. Kanakov, A. Nyska, G. Goss, J. Hilton, D. Peer, ACS Appl. Nano Mater. 4, 11187 (2021)
C. Caizer, M. Rai, Magnetic nanoparticles in alternative tumors therapy: biocompatibility, toxicity, and safety compared with classical methods magnetic nanoparticles in human health and medicine: current medical applications and alternative therapy of cancer (Wiley, 2021)
H. Karimi-Maleh, F. Karimi, Y. Orooji, G. Mansouri, A. Razmjou, A. Aygun, F. Sen, Sci. Rep. 10, 11699 (2020)
H. Karimi-Maleh, B.G. Kumar, S. Rajendran, J. Qin, S. Vadivel, D. Durgalakshmi, F. Gracia, M. Soto-Moscoso, Y. Orooji, F. Karimi, J. Mol. Liq. 314, 113588 (2020)
A. Lascu, ARKIVOC 2020, i, 272, (2020).
V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara, J.M. Basset, Chem Rev. 111, 3036 (2011)
D. Wang, D. Astruc, Chem Rev. 114, 6949 (2014)
J. Meurig Thomas, R. Raja, Rev. Mater. Res. 35, 315 (2005)
M.J. Yao, Z. Guan, Y.H. He, Synth. Commun. 43, 2073 (2013)
Z. Nezafat, M. Nasrollahzadeh, J. Mol. Struct. 1228, 129731 (2021)
R.W. Weber, J.M. Cook, Can. J. Chem. 56, 189 (1978)
L. Zu, B.W. Boal, N.K. Garg, J. Am. Chem. Soc. 133, 8877 (2011)
J.M. Huang, R. Yokoyama, C.S. Yang, Y. Fukuyama, A. Merrilactone, Tetrahedron Lett. 41, 6111 (2000)
Y. Sugimoto, H.A.A. Babiker, T. Saisho, T. Furumoto, S. Inanaga, M. Kato, J. Org. Chem. 66, 3299 (2001)
K. Weinges, P. Günther, W. Kasel, G. Hubertus, P. Güinther, Angew. Chem. 20, 960 (1981)
M. Konishi, H. Ohkuma, T. Tsuno, T. Oki, G.D. VanDuyne, J. Clardy, J. Am. Chem. Soc 112, 3715 (1990)
B.-W. Yu, J.-Y. Chen, Y.-P. Wang, K.-F. Cheng, X.-Y. Li, G.-W. Qin, Phytochemistry 61, 439 (2002)
G.A. Ellestad, M.P. Kunstmann, H.A. Whaley, E.L. Patterson, J. Am. Chem. Soc. 90, 1325 (1968)
J. Dugan, P. De Mayo, M. Nisbet, J. Robinson, M. Anchel, J. Am. Chem. Soc. 88, 2838 (1966)
A. Rezvanian, A. Alizadeh, L.-G. Zhu, Synlett 23, 2526 (2012)
A. Rezvanian, A. Alizadeh, Tetrahedron 68, 10164 (2012)
A.M. Dilmaç, T. Wezeman, R.M. Bär, S. Bräse, Nat. Prod. Rep. 37, 224 (2020)
A.M. Dilmaç, E. Spuling, A. de Meijere, S. Bräse, Chem. Int. Ed. 56, 5684 (2017)
J.C. Leung, A.A. Bedermann, J.T. Njardarson, D.A. Spiegel, G.K. Murphy, N. Hama, J.L. Wood, Angew. Chem. Int. Ed. 57, 1991 (2018)
K. Kumaravel, G. Vasuki, Curr. Org. Chem. 13, 1820 (2009)
V.K. Ahluwalia, R.S. Varma, Green solvents for organic synthesis (Alpha Science International, 2009)
L.J. Zhang, C.G. Yan, Tetrahedron 69, 4915 (2013)
A. Alizadeh, A. Rezvanian, L.G. Zhu, J. Org. Chem. 77, 4385 (2012)
I. Yavari, A. Malekafzali, S. Skoulika, Tetrahedron Lett. 55, 3154 (2014)
Z. Kheilkordi, G. Mohammadi Ziarani, A. Badiei, Polyhedron 178, 114343 (2020)
G. Mohammadi Ziarani, Z. Kheilkordi, F. Mohajer, A. Badiei, R. Luque, RSC Adv. 11, 17456 (2021)
Z. Kheilkordi, G. Mohammadi Ziarani, F. Mohajer, J. Iran. Chem. Soc. 17, 247 (2020)
Z. Kheilkordi, G. Mohammadi Ziarani, S. Bahar, A. Badiei, J. Iran. Chem. Soc. 16, 365 (2019)
Z. Kheilkordi, G. Mohammdi Ziarani, N. Lashgari, A. Badiei, Polyhedron 166, 203 (2019)
S. Mahmoudi-GomYek, D. Azarifar, M. Ghaemi, H. Keypour, M. Mahmoudabadi, Appl. Organomet. Chem. 33, e4918 (2019)
M. Rajabi-Salek, M.A. Zolfigol, M. Zarei, Res. Chem. Intermed. 44, 5255 (2018)
A.R. Hajipour, M. Karimzadeh, S. Jalilvand, H. Farrokhpour, A.N. Chermahini, Comput. Theor. Chem. 1045, 10 (2014)
L. Fu, W. Lin, Z.B. Huang, D.Q. Shi, J. Heterocycl. Chem. 52, 1075 (2015)
H. Naeimi, S. Lahouti, Appl. Organomet. Chem. 31, e3732 (2017)
Acknowledgements
We are grateful to the Alzahra University Research Council's support.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kheilkordi, Z., Mohammadi Ziarani, G., Badiei, A. et al. Fe3O4@SiO2@Pr-Oxime-(BuSO3H)3 synthesis and its application as magnetic nanocatalyst in the synthesis of heterocyclic [3.3.3]propellanes. J IRAN CHEM SOC 20, 591–597 (2023). https://doi.org/10.1007/s13738-022-02685-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02685-7