Abstract
A series of magnetically separable catalysts consisting of Schiff base complexes of metal ions (Zn, Mn, Cd, Co, Cu, Ni, Fe and Pd) supported on superparamagnetic Fe3O4@SiO2 nanoparticles has been prepared. The structural features of the catalysts were characterized by XRD, FTIR, TEM, FE-SEM, DLS, VSM, UV–Vis, TGA, AFM and N2 adsorption–desorption. Some of the Fe3O4@SiO2/Schiff base complexes were found to be efficient catalysts for the Cu-free and the phosphine free Sonogashira–Hagihara coupling reaction of aryl halides and phenylacetylene. The catalysts are superparamagnetic, and thus could be easily separated by the utilization of an external magnetic field, and could be reused for six cycles without significant loss of activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Sonogashira coupling reaction of aryl halides with terminal alkynes is an essential tool in the synthesis of organic intermediates and has been widely applied to various areas such as biologically active molecules, natural product synthesis, agrochemicals, fine chemicals and materials science [1]. Palladium-catalyzed coupling reactions have been continually developed and many reviews and books have already been published [2]. Despite their importance, many palladium catalysts utilized for Sonogashira–Hagihara reactions are homogeneous [3]; these catalysts, in spite of many advantages, cannot normally be recovered. Also, residual palladium in the product confines their use in bioactive molecules and large-scale synthesis. To be able to overcome these drawbacks, recently, ligand-free palladium nanoparticles [4], in addition to different ligands like palladacycles [5], dendrimers [6], Schiff bases [7] and N-heterocyclic carbenes [8] have been grafted on various inorganic and organic supports such as polymers [9], ionic liquids [10], and mesoporous silica [11] for the preparation of heterogeneous catalysts. In spite of a large number of methods reported for this transformation, the development of new procedures for the Pd-catalyzed Sonogashira–Hagihara reaction, in particular without copper or phosphorous, is a desirable goal.
Magnetite, Fe3O4 nanoparticles, has attracted increasing interest due to interesting properties including a large surface to volume ratio, low toxicity, biocompatibility, superparamagnetism, and potential applications in several fields [12, 13]. Nanoscale magnetite (Fe3O4) has potential applications in magnetic bioseparations [28], drug delivery [14], magnetic resonance imaging (MRI) contrast agents [15], treatment of cancer [16] and catalysis [17]. Hence, the use of magnetic catalysts has been widely advocated [18].
Schiff base transition metal complexes have been studied extensively as essential intermediates in the synthesis of various organic and inorganic compounds [19]. These complexes have been extensively employed for epoxidation of olefins [20], hydrogenation of organic substrates [21], asymmetric ring opening of terminal epoxides [22] conversion of epoxides into halohydrins [23], oxidation reactions [24], synthesis of pyridopyrazine and quinoxaline derivatives and cross-coupling reactions [25].
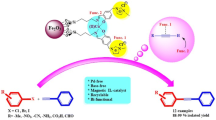
Following our interest in using silica coated iron oxide functionalized with Schiff base complexes [18], in this work we have prepared Schiff base complexes of metal ions immobilized on superparamagnetic Fe3O4 nanoparticles (Scheme 1) and investigated their application in the Cu-free and phosphine free Sonogashira–Hagihara coupling of aryl halides and phenylacetylene.
Experimental
General procedures
Solvents were purified by conventional methods. All reagents were purchased from Aldrich. The 1H and 13C NMR spectra were recorded on a Bruker Avance DPX 250 MHz spectrometer (using CDCl3 or DMSO-d6 with TMS as the standard). FTIR spectra were recorded on a Shimadzu FTIR 8300 spectrometer using pressed KBr pellets. X-ray powder diffraction (XRD) analysis was conducted on a Bruker AXS d8-advance X-ray diffractometer using Cu kα radiation (λ = 1.5418). A transmission electron microscope (Philips EM208) with an accelerating voltage of 100 kV was used to examine morphology, and size of the nanoparticles and field emission scanning electron microscopy (FE-SEM) images were obtained on a Hitachi S-4160 instrument. Magnetic characterization was carried out on a vibrating sample magnetometer (Meghnatis Daghigh Kavir Co., Iran) at room temperature, and dynamic light scattering (DLS) was recorded on a HORIBA-LB550 instrument. UV–Vis spectra were recorded on a PerkinElmer Lambda 25 UV/Vis spectrometer. The thermogravimetric analysis (TGA) curves were recorded using a Perkin Elmer device manufactured by Thermal Sciences. An atomic force microscope, AFM (ARA AFM (Model No: 0201/A), Ara research Co., Iran) was also used to obtain AFM images. BET surface area and porosity of catalysts were determined from nitrogen physisorption measured on a Micromeritics ASAP 2000 instrument at 196 °C. The amount of palladium nanoparticles supported on silica was measured by ICP analyzer (Varian, Vista-pro) and atomic absorption spectroscopy. All products were identified by comparison of their spectral data and physical properties with those of authentic samples, and all yields refer to isolated products. The progress of the reactions was monitored by TLC, and purification was achieved by silica gel column chromatography.
Preparation of Fe3O4 nanoparticles
Fe3O4 nanoparticles were prepared by chemical coprecipitation of Fe3+ and Fe2+ ions with a molar ratio of 2:1. Typically, a solution was prepared from FeCl2·4H2O (736 mg, 3.7 mmol) and FeCl3·6H2O (2 g, 7.4 mmol) in deionized H2O (30 ml) under an Ar atmosphere, and polystyrene (PS 35000) (400 mg) was then added. The resultant mixture was stirred for 30 min at 80 °C, then 25 % aqueous NH3 was added dropwise with vigorous stirring to generate a black solid product when the pH of reaction media reached 10. The dropping rate was controlled with a constant dropper at 1 ml/min.
The resultant mixture was heated for 2 h at 60 °C, and the black magnetite solid product was separated, washed with deionized H2O, dried and calcined at 600 °C for 8 h.
Preparation of Fe3O4@SiO2 core–shell
The core–shell Fe3O4@SiO2 nanospheres were prepared via hydrolysis of tetraethylorthosilicate (TEOS) in basic solution via Stöber’s method with minor modification [18]. A mixture of ethanol (40 ml), deionized water (10 ml) and ammonia (1.5 ml) was stirred for 10 min before addition of TEOS. After 30 min, 0.10 g of the freshly prepared iron oxide was added. After 30 min mixing, TEOS (2 ml) was added and stirring was continued for 8 h. The obtained particles were sequentially washed with water and ethanol and dried at 60 °C for 6 h.
Preparation of Fe3O4@SiO2 functionalized with 3-(triethoxysilyl)-propylamine (Fe3O4@SiO2–NH2)
Fe3O4@SiO2 (0.5 g) was added to a solution of 3-(triethoxysilyl)-propylamine (0.176 g, 1 mmol) in ethanol (5 ml), and the resultant mixture was boiled under reflux for 16 h under a nitrogen atmosphere. After refluxing, the mixture was cooled to room temperature, separated by an external magnet and the product was washed with ethanol and water then dried at 80 °C for 6 h.
Preparation of Schiff base functionalized magnetite@silica nanoparticles (Fe3O4@SiO2/Schiff base ligand)
The modification of Fe3O4@SiO2–NH2 with isatin was carried out via Schiff base formation between the amino group and the active carbonyl group of isatin, as presented in Scheme 1. A suspension of isatin (1 mmol, 0.147 g) and Fe3O4@SiO2–NH2 (0.5 g) in ethanol (10 ml) was refluxed with continuous stirring under an inert atmosphere for 24 h. The product was isolated by filtration and thoroughly washed several times with ethanol and distilled water, then finally dried in a vacuum oven at 70 °C.
Preparation of immobilized Schiff base complexes (Fe3O4@SiO2/Schiff base/M(II))
The Fe3O4@SiO2/Schiff base ligand (0.5 g) was added to a solution of the required metal acetate (1 mmol) in ethanol (10 ml), and the mixture was boiled under reflux for 6 h. The catalyst was separated from the crude product using an external magnet and washed with ethanol and water. Then, it was dried in vacuum at 70 °C for 6 h and stored in a desiccator.
General procedure for Sonogashira–Hagihara coupling
A mixture of aryl halide (1.0 mmol), phenylacetylene (122 mg, 1.2 mmol), K2CO3 (276 mg, 2.0 mmol), DMF (2 ml), and the catalyst (0.5 mol%) was stirred at 90 °C under air. The progress of the reaction was monitored by TLC. After completion of the reaction and separation of the nanocatalyst with an external magnetic field, water (20 ml) was added and the mixture was extracted with Et2O. The organic phase was washed with water (2 × 5 ml) and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure, and the resulting crude product was purified by flash chromatography to give the desired cross-coupling products.
Results and discussion
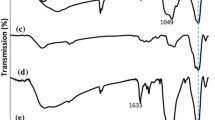
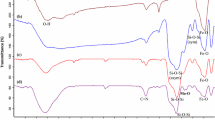
The binding of the Schiff base complexes on the surfaces of Fe3O4@SiO2 nanoparticles was confirmed by FTIR spectroscopy. Figure 1a–m shows the FTIR spectra of magnetic Fe3O4 nanoparticles (MNPs), Fe3O4@SiO2, Fe3O4@SiO2–NH2, Fe3O4@SiO2/Schiff base ligand and Fe3O4@SiO2/Schiff base/M(II) complexes in the 500–4,000 cm−1 wavenumber range. In Fig. 1a, characteristic peaks at around 3,400 and 1,620 cm−1 are related to the stretching vibration of OH originating from the MNPs, and an absorption band at 580 cm−1 is attributed to the Fe–O bond of Fe3O4. The surfaces of pure Fe3O4 nanoparticles were readily covered with SiO2 layers. The FTIR spectrum of Fe3O4@SiO2 showed absorption peaks at 578 cm−1 (Fe–O bond), 811 cm−1 (Si–O–Si symmetric stretching vibrations), 1,000–1,150 cm−1 (Si–O–Si asymmetric stretching vibrations), and 3,400 cm−1 (O–H bond) (Fig. 1b). In the FTIR spectrum of Fe3O4@SiO2–NH2 nanoparticles (Fig. 1c), obvious bands at 578, 809, 957, 1,000–1,150, and 1,551 cm−1 are attributed to Fe–O (stretching vibration), Si–O–Si (symmetric stretching), Si–O–H (stretching vibration), Si–O–Si (asymmetric stretching), and N–H (bending), respectively. Also, several bands of medium intensity at 3,100–3,390 cm−1 are assigned to the N–H stretching vibration [26]. The presence of the anchored propyl group is confirmed by C–H stretching vibrations at 2,870 and 3,090 cm−1 (Fig. 1c). In the FTIR spectrum of the Fe3O4@SiO2/Schiff base, a new band is observed at 1,651 cm−1 due to the C=N stretch of the azomethine group and another new band around 1,497 cm−1 is assigned to the aromatic C=C stretch (Fig. 1d). These bands prove that isatin has been successfully grafted onto the Fe3O4@SiO2–NH2 nanoparticles. The C=O stretch of the amide group occurs at 1,712 cm−1, and O–H stretching band is found at 3,400 cm−1 (Fig. 1d). The free ligand exhibits a ν (C=N) stretch at 1,651 cm−1 while in the complexes, this band shifts to reduced frequency, appearing at 1,623–1,642 cm−1 as a result of coordination of the nitrogen with the metal (Fig. 1e–m). From the IR spectra presented in Fig. 1e–m, the absorption peaks at 575–585 cm−1 can be assigned to the stretching vibrations of the Fe–O bonds in Fe3O4, while the absorption peak at 1,000–1150 cm−1 is due to the stretching vibration of the silica framework and terminal Si–O–Si groups. Also, bands at 3,400 (O–H stretching), 2,870–3,080 (C–H stretching), 1,696–1,704 (C=O amide), 1,623–1,642 (C=N), 1,480–1,600 (C=C aromatic ring stretching) and 1,470–1,390 cm−1 (CH2 bending) demonstrate the presence of the Schiff base complexes on the Fe3O4@SiO2 nanoparticles (Fig. 1e–m).
FT-IR spectra of a Fe3O4, b Fe3O4@SiO2 c Fe3O4@SiO2–NH2, d Fe3O4@SiO2/Schiff base, e Fe3O4@SiO2/Schiff base/Pd(II), f Fe3O4@SiO2/Schiff base/Ni(II), g Fe3O4@SiO2/Schiff base/Fe(II), h Fe3O4@SiO2/Schiff base/Mn(II), i Fe3O4@SiO2/Schiff base/Cu(II), j Fe3O4@SiO2/Schiff base/Co(II), k Fe3O4@SiO2/Schiff base/Zn(II), l Fe3O4@SiO2/Schiff base/Cd(II), and m Fe3O4@SiO2/Schiff base/Mg(II)
The crystalline structures of the prepared materials were characterized with XRD. The XRD patterns of Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2/Schiff base/Pd(II) are shown in Fig. 2. In Fig. 2a, the relatively strong diffraction peaks at 2θ: 30.1°, 35.4°, 43.1°, 53.4°, 57° and 62.6° could be assigned to the (220), (311), (400), (422), (511) and (440) crystallographic faces of magnetite (reference JCPDS card no. 19-629), which agreed well with the crystallographic planes of Fe3O4 and indicated the presence of the Fe3O4 core with a highly crystalline cubic spinel structure [27]. As shown in Fig. 2b, c, XRD patterns of the synthesized Fe3O4@SiO2 and Fe3O4@SiO2/Schiff base/Pd(II) display several relatively strong reflection peaks in the 2θ region of 20°–70°, which are quite similar to those of the parent Fe3O4 nanoparticles. This confirmed that the surface modification and conjugation of the Fe3O4 nanoparticles do not lead to a phase change. In contrast with Fe3O4 NPs (Fig. 2a), the Fe3O4@SiO2 NPs (Fig. 2b) also presented a wide featureless peak at 2θ = 20°–25°, corresponding to the noncrystalline state SiO2 shell. Hence, the attachment of silica nanolayers onto the magnetite is confirmed. Also, the XRD pattern of the Fe3O4@SiO2/Schiff base/Pd(II) nanocatalyst, showing the peaks of both Fe3O4 and Pd, and the diffraction peaks at 39.8° and 46.9° show that the Pd particles are in a metallic state (Fig. 2c). Since the concentration of palladium is very low, its characteristic XRD peaks (311), (220), (200) and (111) could not observed in the XRD pattern of the catalyst. However, broadened (111) and (220) diffraction peaks could be still identified in the XRD pattern. The average crystallite size could be estimated from the XRD pattern, utilizing the Scherrer equation:
where λ is the X-ray wavelength (λ = 1.5418 nm), d is the average diameter in A°, β is the broadening of the diffraction line measured at half of its maximum intensity in radians and θ is the Bragg angle in degrees [28]. The mean crystallite size of the superparamagnetic nanocatalyst was found to be around 40 nm.
TEM, SEM and DLS images of the as-prepared nanoparticles are shown in Fig. 3. In the TEM image of Fe3O4 nanoparticles shown in Fig. 3a, the illustrated particle size of the magnetic particles is in excellent agreement with the DLS and SEM results. Figure 3d, g shows a representative SEM and size histogram of the Fe3O4 nanoparticles; the nanoparticles have nearly spherical morphology and 14 nm diameter. After coating the Fe3O4 nanoparticles with a silica shell, the size and morphology of the obtained powder was investigated by TEM, SEM and DLS (Fig. 3b, e, h). The mesoporous silica shell on the surface of Fe3O4 has a thickness of about 12 nm. Figure 3c, f and i shows TEM, SEM and DLS images of Fe3O4@SiO2/Schiff base/Pd(II) nanoparticles. As illustrated, surface functionalization of Fe3O4@SiO2 with the Schiff base complex of Pd(II) caused nanoparticle aggregation, such that the size increased to 38 nm. In the TEM image of these particles (Fig. 3c), Pd nanoparticles are clearly seen.
In order to obtain additional information about the topography of the surface of the prepared catalysts, we have also obtained AFM images (Fig. 4A).
A 3D AFM image of the Fe3O4@SiO2/Schiff base/Pd(II) nanoparticles. B Nitrogen adsorption–desorption isotherms for a Fe3O4, b Fe3O4@SiO2 and c Fe3O4@SiO2/Schiff base/Pd(II). C UV–Vis spectra of a Fe3O4@SiO2/Schiff base ligand and b Fe3O4@SiO2/Schiff base/Pd(II) and D TEM image of catalyst after six reused
N2 adsorption–desorption isotherms were conducted to investigate the porous structure and surface area of the bare Fe3O4 NPs, Fe3O4@SiO2 and the Fe3O4@SiO2/Schiff base/Pd(II) (Fig. 4B). All of the samples exhibited type IV isotherms and a very steep capillary condensation step accompanied by relative pressures P/P 0 ranging from 0.3 to 0.95. However, the amount of N2 adsorbed on all of the anchored samples is lower compared to the parent Fe3O4 NPs, reflected in the surface areas. The BET surface area of parent Fe3O4 NPs and Fe3O4@SiO2 is 438.0 and 430.3 m2/g, respectively. When the Schiff base complex was added to Fe3O4@SiO2, the surface area markedly decreased to 382.1 m2/g, suggesting that the blockage of some pores may have occurred at this stage (Fig. 4Bc).
The decrease in surface area could be explained by the surface functionalization of parent Fe3O4 NPs with the silicate and the Schiff base complex of Pd(II) (Fig. 4Bc). The high surface area and narrow size distribution of the prepared catalyst should be advantageous for improving their catalytic performance toward cross-coupling reactions.
Further evidence for metal ion complexation was obtained from the electronic spectra of the Schiff base ligand and its corresponding palladium complex (Fig. 4C). The UV–Vis spectrum of the Schiff base complex of palladium supported on Fe3O4@SiO2 nanoparticles exhibits one broad band at 420 nm that could be assigned either to a ligand-to-metal charge-transfer (LMCT) band or to a π → π* transition corresponding to the azomethine chromophore. The charge-transfer confirms the presence of anchored Pd(II) in the Schiff base complex (Fig. 4Cb) [29].
The thermal stability of the Fe3O4@SiO2 and Fe3O4@SiO2/Schiff base/Pd(II) nanoparticles was analyzed by TGA, and the resulting thermograms are presented in Fig. 5Aa, b respectively. Fe3O4@SiO2 shows two-step weight loss behavior. Its initial weight loss up to 125 °C is attributed to the loss of physically adsorbed solvent and surface hydroxyl groups (Fig. 5Aa) [30].
The weight reduction above 200 °C was tentatively ascribed to the loss of structural water within amorphous SiO2 (Fig. 5Aa). In this way, the gradual slope in the temperature range of 150–650 °C in the TGA curve of Fe3O4@SiO2 could be explained. As shown in Fig. 5Ab, the TGA curve of Fe3O4@SiO2/Schiff base/Pd(II) shows an initial weight loss of 5.1 % below 130 °C, which may be due to the loss of adsorbed water. The mass loss of about 26.92 % in the range of 150–530 °C is attributed to decomposition of the Schiff base and organic volatile compounds, and the temperature of the maximum weight loss is 532 °C (Fig. 5Ab). On the basis of these results, grafting of the Schiff base onto the Fe3O4@SiO2 is verified.
The magnetization hysteresis loops were measured with a vibrating sample magnetometer at room temperature and gave magnetization saturation values for Fe3O4 and Fe3O4@SiO2/Schiff base/Pd(II) of 64.9 and 31.8 emu/g, respectively (Fig. 5Ba, b). The magnetization and demagnetization curves are coincident; no hysteresis is observed and the remanent magnetization and coercivity is both equal to zero. Hence, the magnetization of Fe3O4 decreased considerably with the attachment of SiO2 and the Schiff base complex (Fig. 5Bb). Nevertheless, these nanoparticles with paramagnetic characteristics and high magnetization values can quickly respond to an external magnetic field and quickly redisperse when the external magnetic field is removed. This supports their potential application for targeting and separation.
The amount of palladium content supported on Fe3O4@SiO2/Schiff base ligand was determined by ICP and atomic absorption techniques as 0.19 mmol/g, showing has a high ability to accommodate palladium nanoparticles.
To explore the catalytic activity, the Sonogashira–Hagihara cross-coupling of aryl halides with phenyl acetylene was conducted with these magnetic nanoparticles as catalyst (Scheme 2).
For optimization of the reaction conditions, we chose the cross-coupling of iodobenzene with phenylacetylene as a model reaction and the effects of different amounts of Fe3O4@SiO2/Schiff base/Pd(II) magnetic nanoparticles were studied (Fig. 6a).
The best result was found to be (0.5 mol%: 1: 1.2 mmol) ratio of magnetic Fe3O4@SiO2/Schiff base/Pd(II) nanoparticles, iodobenzene and phenylacetylene in DMF at 90 °C. Next, various bases, solvent, and temperatures were screened. Different organic and inorganic bases were studied. The conversion was reduced when organic bases such as Et3N were employed (Fig. 6b), but a substantial increase in conversion was observed in the presence of K2CO3; hence, the cheap K2CO3 was chosen as a base for these coupling reactions. Solvents such as toluene, H2O and CH3CN gave low conversions, ranging from 15 to 45 % (Fig. 6c). Of the tested solvents, DMF was the best choice, giving the desired product in shorter reaction time (60 min) and higher conversion (93 %) (Fig. 6c).
The effect of different temperatures on the reaction of iodobenzene (204 mg, 1 mmol) with phenylacetylene (122 mg, 1.2 mmol) in the presence of Fe3O4@SiO2/Schiff base/Pd(II) magnetic nanoparticles (0.5 mol%) in DMF (2 ml) was studied. The results of this study, presented in Fig. 6d, suggests that 90 °C is the optimum temperature for this reaction. Also, we examined the effect of molar ratios of phenylacetylene:iodobenzene in the coupling reaction (Fig. 6e). The best result was obtained with a molar ratio of 1.2:1 (phenylacetylene:iodobenzene).
The utilization of transition metals such as co [31], Fe [32], Ni [33], In [34] and Ru [35] has been also reported for the Sonogashira coupling of terminal alkynes with aryl halides. Thus, we investigated the reaction using a variety of transition metals under the optimized conditions. The results are shown in Fig. 7.
Various Fe3O4@SiO2/Schiff base of metal ions were employed in the Sonogashira coupling of 4-iodoanisole and phenylacetylene in DMFa,b. a Reaction condition for Fe3O4@SiO2/Schiff base/Pd(II) (0.5 mol%): iodobenzene (1 mmol), phenylacetylene (1.2 mmol), and K2CO3 (2 mmol) in 2.0 ml of DMF at 90 °C for 1 h. b Reaction condition for Fe3O4@SiO2/Schiff base/M(II) (M = Fe, Ni, Cu, Co, Cd, Mn, Zn) (0.6 mol%): iodobenzene (1 mmol), phenylacetylene (1.5 mmol), and K2CO3 (2 mmol) in 2.0 ml of DMF at 110 °C for 4 h
The coupling of iodobenzene (204 mg, 1 mmol) with phenylacetylene (150 mg, 1.5 mmol) proceeded smoothly in the presence of two equivalents of K2CO3 and 0.6 mol% of Fe3O4@SiO2/Schiff base complex of these metal(II) ions (Fe, Ni, Cu, Co, Cd, Mn, Zn) in DMF at 110 °C. From Fig. 7, it can be seen that although palladium showed highest efficiency, other metals such as iron, nickel, copper, and cobalt also showed promising catalytic activities.
To check the versatility of the Fe3O4@SiO2/Schiff base/Pd(II) magnetic nanoparticles, they were used in the coupling of various aryl iodides, bromides and chlorides with phenyl acetylene under the optimized conditions. The results are shown in Table 1. The electron-rich and electron-poor aryl iodides and bromides reacted with alkynes very well (Table 1, entries 1–17). Also, 5-bromopyrimidine and 2-thio-phenyl bromide gave the corresponding arylated alkynes in good yields (Table 1, entries 16–17). Few heterogeneous Pd catalysts have been found to convert activated aryl chlorides at high temperatures [36]. In our catalytic system, the reactions with chlorides had to be carried out without the presence of tetrabutylammonium bromide (TBAB, Jeffery’s catalyst) (Table 1, entries 18–23).
The magnetically supported catalyst could be recovered with an external magnet due to the paramagnetic character of the support, resulting in excellent catalyst recovery without the necessity for a filtration step (Scheme 3). To probe the issue of the reusability and stability of the magnetic nanocatalyst, it was separated from the reaction mixture after its first use in the Sonogashira–Hagihara reaction, and then, the activity of the recycled catalyst was examined under the optimized conditions. The desired products were obtained in high yields after six runs without significant deterioration of the catalytic activity (Table 2).
To confirm if any aggregation of the palladium nanoparticles occurs, TEM analysis of the reused catalyst was performed (Fig. 4D). After six consecutive reaction cycles, no significant change in the morphology of the catalyst was observed. Nevertheless, some Pd particles might have aggregated onto the surfaces of the matrix, through the leaching/re-deposition processes during the catalytic reactions.
To investigate the heterogeneity of the catalyst, we conducted a hot filtration test for the Sonogashira–Hagihara coupling reaction between iodobenzene and phenylacetylene using Fe3O4@SiO2/Schiff base/Pd(II) nanocatalyst. The reaction was allowed to proceed for 30 min (GC yield: 84 %), and then, the catalyst was separated. There was no further yield of the desired product when the reaction was continued for a further 2 h after the catalyst was magnetically separated, confirming the heterogeneous character of the catalyst.
Conclusion
In summary, Fe3O4 was prepared by co-precipitation with FeCl2 and FeCl3 as reaction substrate, polystyrene as surfactant and aqueous NH3 as precipitant. The core–shell Fe3O4@SiO2 nanospheres were prepared by a modified Stober method. The surface of Fe3O4@SiO2 was then functionalized with Schiff base complexes of various transition metals. Both TEM and SEM revealed a very fine layer of SiO2 and Schiff base complex on the Fe3O4. The characterization results showed that the diameter of Fe3O4 was about 14 nm, and the size of the Fe3O4@SiO2/Schiff base/Pd(II) microspheres was about 40 nm. The crystallite size obtained from X-ray line profile fitting was comparable with the particle size obtained from TEM. The Fe3O4@SiO2/Schiff base complexes possess a high saturation magnetization, and are recoverable by magnetic decantation. They could be reused several times without significant loss in catalytic activity for ligand and copper-free Sonogashira–Hagihara coupling reactions. This catalyst shows notable advantages including simplicity of operation, excellent yields, short reaction times, heterogeneous nature, easy separation and recyclability.
References
Ren T (2008) Chem Rev 108:4185–4207
Lyons TW, Sanford MS (2010) Chem Rev 110:1147–1169
Doucet H, Hierso JC (2007) Angew Chem Int Ed 46:834–871
Patil AB, Patil DS, Bhanage BM (2012) J Mol Catal A Chem 365:146–153
Dupont J, Pfeffer M (2008) Palladacycles: synthesis characterization and applications. Wiley-Vch, Weinheim
Garcia-Martinez JC, Scott RWJ, Crooks RM (2003) J Am Chem Soc 125:11190–11191
Li Y, Fu X, Gong B, Zou X, Tu X, Chen J (2010) J Mol Catal A Chem 322:55–62
Kim JH, Lee DH, Jun BH, Lee YS (2007) Tetrahedron Lett 48:7079–7084
Bakherad M, Jajarmi S (2013) J Mol Catal A Chem 370:152–159
Kawasaki I, Tsunoda K, Tsuji T, Yamaguchi T, Shibuta H, Uchida N, Yamashita M, Ohta S (2005) Chem Commun 2134–2136. doi:10.1039/b500320b
Huang J, Zhu F, He W, Zhang F, Wang W, Li H (2010) J Am Chem Soc 132:1494–1495
Sevilla M, Valdés-Solis T, Tartaj P, Fuertes AB (2009) J Colloid Interface Sci 340:230–236
Bruce IJ, Sen T (2005) Langmuir 21:7029–7035
Alexiou C, Jurgons R, Schmid R, Hilpert A, Bergemann C, Parak F, Iro H (2005) J Magn Magn Mater 293:389–393
Huh YM, Lee ES, Lee JH, Jun YW, Kim PH, Yun CO, Kim JH, Suh JS (2007) Adv Mater 19:3109–3111
Kircher MF, Mahmood U, King RS, Weissleder R, Josephson LA (2003) Cancer Res 63:8122–8125
Wong EW, Bronikowski MJ, Hoenk ME, Kowalczyk RS, Hunt BD (2005) Chem Mater 17:237–241
Esmaeilpour M, Sardarian AR, Javidi J (2012) Appl Catal A Gen 445–446:359–367
Urus S, Purtas S, Ceyhan G, Tumer F (2013) Chem Eng J 220:420–430
Lane BS, Burgess K (2003) Chem Rev 103:2457–2474
Skoda-Földes R, Kollár L, Arcadi A (1995) J Mol Catal 101:37–43
Nielson LPC, Stevenson CP, Backmond DG, Jacobsen EN (2004) J Am Chem Soc 126:1360–1362
Righi G, Bonini C (1994) Synthesis 3:225–238
Lopez J, Liang S, Bu XR (1998) Tetrahedron Lett 39:4199–4202
He Y, Cai C (2011) Catal Commun 12:678–683
Xu Z, Liu Q, Finch JA (1997) Appl Surf Sci 120:269–278
Yu K, Zhang X, Tong H, Yan X, Liu S (2013) Mater Lett 106:151–154
Baby TT, Ramaprabhu S (2010) Talanta 80:2016–2022
Firouzabadi H, Iranpoor N, Kazemi F, Gholinejad M (2012) J Mol Catal A Chem 357:154–161
Polshettiwar V, Cha D, Zhang X, Basset JM (2010) Angew Chem Int Ed 49:9652–9656
Feng L, Liu F, Sun P, Bao J (2008) Synlett 10:1415–1417
Xie X, Xu X, Li H, Xu X, Yang J, Li Y (2009) Adv Synth Catal 351:1263–1267
Bakherad M, Keivanloo A, Mihanparast S (2010) Synth Commun 40:179–185
Borah HN, Prajapati D, Boruah RC (2005) Synlett 655:2823–2825
Park S, Kim M, Koo DH, Chang S (2004) Adv Synth Catal 346:1638–1640
Prockl S, Kleist W, Gruber MA, Kohler K (2004) Angew Chem Int Ed 43:1881–1882
Acknowledgments
Authors are grateful to the council of Iran National Science Foundation and University of Shiraz and Isfahan for their unending effort to provide financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esmaeilpour, M., Javidi, J., Dodeji, F.N. et al. M(II) Schiff base complexes (M = zinc, manganese, cadmium, cobalt, copper, nickel, iron, and palladium) supported on superparamagnetic Fe3O4@SiO2 nanoparticles: synthesis, characterization and catalytic activity for Sonogashira–Hagihara coupling reactions. Transition Met Chem 39, 797–809 (2014). https://doi.org/10.1007/s11243-014-9862-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-014-9862-5