Abstract
Purpose
Skin injuries are a worldwide health issue that affects millions of people each year. Tissue engineering has the potential to provide an opportunity to resolve this challenge. For more than 40 decades, researchers have focused on different aspects of skin tissue engineering. The purpose of the present study was to provide a comprehensive overview of the critical factors in skin tissue engineering.
Methods
Recent studies were investigated to gather relevant studies about the basic aspects of skin tissue engineering.
Results
In the present review, the nature of the native skin and natural repair of the skin injuries is described. The knowledge of this part is a foundation for producing a tissue or organ that mimics the actual one. Then, the different essential elements in skin tissue engineering are underlined. In this regard, a variety of cells and scaffolds that create the main structure of the engineered constructs are emphasized. On the other hand, the application of critical signaling factors including biochemical, physicochemical, and physical factors in skin tissue engineering are reviewed.
Conclusion
A comprehensive review of these components clarifies critical points in the regenerative medicine of the skin and guides researchers to adopt optimized approaches to achieve a viable and functional construct.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Skin injuries including lacerations, wounds, and burns can lead to the death of skin cells and the destruction of extracellular matrix (ECM). Skin injuries range from minor to extensive tissue loss. For optimal management of skin injuries, understanding the native structure of the skin and its repair process is crucial.
1.1 Skin Structure
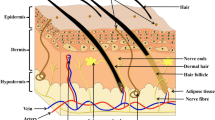
Skin is the largest organ in the human body. The skin has a multilayer structure, including the epidermis, dermis, and hypodermis (subcutaneous fascia). The interface between the epidermis and the dermis is the basement membrane zone which is composed of connective tissue. On the other hand, hypodermis functions to connect the dermis and epidermis to the underlying structures. Each layer of the skin has special characteristics (Table 1).
The first layer of the skin, the epidermis, consists of five layers stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale. The thickness of the epidermis layer is between 75 and 150 μm (up to 600 μm on palms/soles) [1]. Keratinocytes make up the majority of epidermal cells, almost 95%. But, melanocytes, Langerhans cells, and Merkel cells are also present in this layer [2]. The keratinocytes produce keratin and the cells' pattern varies depending on the layer in the epidermis. The innermost layer of the epidermis, the basal layer, involves the cells that divide. As the cells move upward, their morphology changes to larger, thinner, and flatter cells. Finally, in the outermost layer of the epidermis, stratum corneum, anucleated dead cells are present that are filled with keratin.
Dermis, as the second layer of the skin, consists of two layers of papillary dermis and reticular dermis. There is a slight demarcation between these two layers, which is the fiber morphology. In the papillary dermis, the fibers are thin, whereas, in another, the fibers are thick and rough. Fibroblasts are the main cells in the dermis. Moreover, dermal dendrocytes, mast cells, and histiocytes represent another population of resident cells in this layer. The extracellular matrix of the dermis consists of collagen, elastic fibers, and ground substance including glycosaminoglycans and water [3].
Hypodermis or subcutaneous layer is the deepest layer of the skin. It is comprised of adipocytes lobules. Collagen and elastin fibers attach to the dermis. It provides the storage site of fat in the body and acts as a thermal insulator.
Accessory structures of the skin, including nails, hair, sebaceous glands, and sweet glands embryologically originate from the epidermis. The nails lie in the epidermis and are made of hard keratin. The hair follicles are spread throughout the body, except the palms and soles. Sebaceous glands are generally attached to the hair follicle in the dermis layer and secrete sebum. Sweet glands lie in the dermis and act as exocrine glands that produce sweat.
The blood vessels are presented in the dermis and hypodermis. But the epidermis has no blood vessels; it obtains its nutrients from the blood vessels of the dermis that diffuse via the dermo-epidermal junction. Tissue nerves are distributed in all skin layers [3]. The nerves in the skin include Meissner receptors to detect gentle touch, Pacinian corpuscles to sense profound pressure and vibratory changes, Ruffini endings to detect deep pressure and stretching of collagen fibers in the skin, and free nerve endings located in the epidermis to respond to pain, light touch, and temperature changes [4].
The structure of the skin supplies the intended function of this organ. Skin functions as a barrier against loss of water and electrolytes. Moreover, skin protects the body against thermal and physical damage, as well as pathogens, radiations, and harmful substances. The skin also creates different sensations and allows movement of the body. Furthermore, this organ participates in the absorption, excretion, and secretion of specific substances [3].
1.2 Skin Repair
To aid understanding of normal skin injury healing and its challenging issues to design an appropriately engineered skin construct, in this part of the present review article, various phases of injury healing are investigated (Fig. 1). The complex process of skin repair takes place as the sequence of three phases: inflammation, proliferation, and remodeling phases. Though these phases overlap during the cutaneous repair process [5].
In the inflammation phase, after the formation of a fibrin clot due to platelet activation, chemotactic agents are released. A variety of cells, including endothelial cells, keratinocytes, fibroblasts, and some immune cells, secrete chemokines. Chemokines play a key role in the recruitment of inflammatory cells, such as macrophages and neutrophils. These cells cleanse the wound from debris before the reorganization of the tissue [6].
The second stage of skin injury healing is the proliferation phase, during which various damaged parts of the skin are restored. In this regard, the injured epidermis undergoes re-epithelialization. The re-epithelialization phase noticeably overlaps with the previous phase. On the other hand, the damaged dermis and other structures present in the skin layers also enter the renewal process [5]. The appearance of fibroblasts and synthesized collagen is the most significant phenomenon in this phase [7].
In the last stage, the remodeling phase, all the processes that have been activated so far will be gradually shut down. Moreover, the reconstruction and maturation of the tissue appear to design the structure as close as possible to the native one [5].
Although the skin healing process takes place after the injuries, this process can result in a disorganized structure and scar. Furthermore, the healing process is time-consuming, especially in deep and extensive wounds. Delays in the wound closure and healing process lead to infection, damage to surrounding tissues, and finally death. Skin tissue engineering is a rapidly evolving field that aims to create functional skin substitutes for patients with skin conditions such as burns and chronic wounds. Although there have been significant advances in skin tissue engineering, there are still several challenges and gaps in the studies that need to be addressed to achieve an ideal construction.
The progress in skin tissue engineering has produced remarkable results to overcome the current problems. Current skin substitutes have shown the potential to improve wound healing, reduce scarring, and decrease the risk of infection. In addition, skin tissue engineering offers the opportunity to develop a personalized treatment by using the patient's cells to produce a skin substitute. Despite advances in the field of skin tissue engineering, some challenges and problems remain unsolved. Lack of vascularization, limited longevity, limited availability, and cost are the main obstacles to this. In this review, we aim to take a step toward providing an accurate overview of skin structure engineering by addressing several important aspects of skin tissue engineering.
2 Tissue Engineering Applied for Skin Regeneration
The first studies in the production of engineered skin were started in the late 1970s and early 1980s. Rheinwald and Green designed a viable epithelial sheet and made a milestone in this field [8]. Until now, extensive studies have been done on different aspects of skin tissue engineering [9,10,11]. However, to create an optimal structure more effort is needed. In this part, we focused on the critical factors, including cells, scaffolds, and signaling factors to make a guide for researchers to design an ideal engineered skin structure (Fig. 2).
2.1 Cell Sources
Several Acellular scaffolds have been commercially produced. Although these constructs are more cost-effective and do not cause immune responses against allogeneic cells [12], multiple in vivo studies [13] and clinical reports [14] indicated that scaffolds containing cells are superior to the acellular ones. Different cells have been employed in skin tissue engineering including, keratinocytes [15,16,17], fibroblasts [15, 16, 18,19,20], endothelial cells [11, 15, 16, 19], endometrial stem cells (EnSCs) [21], adipose-derived stem cells (AD-SCs) [22, 23], umbilical cord-derived mesenchymal stem cells (UC-MSCs) [24], and bone marrow-derived stem cells (BM-MSCs) [25] (Table 2).
It should be noted that keratinocytes and fibroblasts are the cells mostly involved in skin tissue engineering studies. Keratinocytes are the main cells in the epidermis, therefore most interest arises from the application of these cells [26, 27]. Epidermal keratinocytes are highly proliferating cells that form a multilayer structure to achieve the skin barrier feature against external factors. One layer of keratinocytes was often used in the studies, but Barros et al. conducted a protocol of seeding human keratinocytes with gelatin-coating to create a multilayer structure simulating a native epidermis [16].
Human dermal fibroblasts are principal cells in the dermis which participate in the healing process and produce collagen and elastin fibers [28]. Multiple sources of fibroblasts have been applied in skin substitutes. Previous findings show that fibroblasts from different regions display distinct sizes, shapes, gene expression patterns, growth kinetic as well as the ability to contract collagen network [29]. The phenotypic diversity is evident in different ways such as extracellular matrix components production and growth factors and cytokine secretion [28, 30].
Human umbilical vein endothelial cells (HUVECs) were applied for the fabrication of the vascular network of the skin [15, 16]. These cells form the internal layer of the blood vessels and involve in the remodeling of the vasculature. The incorporation of human endothelial cells and pericytes into the dermal layer of a 3-dimensional (3D) bio-printed skin resulted in the endothelial network formation such that the pericytes are directly associated with a vessel-like structure, formed by the endothelial cells. Moreover, the findings indicated that the bio-printed grafts without endothelial cells show significant contraction [31].
The surveys found that mesenchymal stem cells (MSCs) may have all characteristics of an opportune cell population for skin tissue engineering. The application of mesenchymal stem cells in the context of wound healing relies on their features including differentiation potential and self-renewal capacity. It is predicted that this cell population, in addition to replacing the main skin cells, can also form skin appendages.
The application of EnSCs, as a new source of stem cells, demonstrated that these cells can express fibroblast cell markers like CK18 and involucrin [21]. Recently a widely used cell in tissue engineering is AD-MSCs. The wounds treated by these cells indicated the improvement of collagen fibrils alignment which is important for tissue maturation. On the other hand, AD-MSCs altered the collagen content that resulted in wound contraction [22]. BM-MSCs are another cell type that can participate in wound healing. It is because of the potential of BM-MSCs to differentiate toward the various cell types [32]. The findings demonstrated that these cells can progress toward epidermal lineage on electrospun nanofibers [33]. Moreover, the presence of BM-MSCs in the scaffold led to more conducive conditions for wound healing compared to the scaffold alone [27].
The incorporation of multiple cell types may improve the quality of the engineered skin tissue. Because in a native skin tissue, different cell types are presented to perform various functions and make an ideal structure. Barros and colleagues applied layers of endothelial cells, dermal fibroblasts, and multilayered keratinocytes in their construct. They proposed that their structure provided a multifunctional platform that could enable skin reconstruction in vitro [16].
Literature review indicates that using adult cells including keratinocytes and fibroblasts involves challenging issues like varying yield and quality of cells obtained from biopsies as well as immunological reactions in allogeneic or xenogeneic sources. The correct organization of the keratinocytes and fibroblasts in the skin substitute is crucial for its function and its integration into the host tissue. Therefore, finding the right cell ratio and spatial organization can be complicated, especially when using 3D scaffolds. Overall, the use of MSCs in skin tissue engineering shows promise for solving some of the challenges presented by cell sources such as keratinocytes and fibroblasts. In this regard, more research is needed to optimize the best MSCs sources and preparation of them, the appropriate ratio of MSCs to other cell types, and the long-term safety and efficacy of MSCs.
All in all, there are many ambiguous issues regarding the use of cells in skin tissue engineering that need to be clarified. The first is the designation of the best cell type, the second is the optimal cell count, and the last is the application of one or a combination of cell types.
2.2 Design and Production of the Scaffold
To construct an optimal scaffold two fundamental aspects should be considered; the applied biomaterials and the production methods. In this part, different synthetic and natural materials used in skin tissue engineering will be summarized, and the production methods to achieve a suitable platform explained.
2.2.1 Selection of the Materials
To date, a variety of biomaterials, including natural and synthetic have been investigated in skin tissue engineering. Each type of material involves different characteristics which, in correspondence with the production method, determine the final properties of the scaffold.
Fabrication of the scaffold for wound healing purposes has been carried out using natural biomaterials including collagen [34,35,36,37,38,39], gelatin [40,41,42,43,44], keratin [45], fibrin [34], chitosan [34, 36, 37, 41, 42, 44, 46,47,48], elastin [38], Cellulose [49], hyaluronic acid [50], fibroin [45], and alginate [48, 51] as well as synthetic biomaterials such as poly(ethylene glycol-terephthalate) (PEGT) [52], poly (butylenes terephthalate (PBT) [52], poly (lactic acid-co-glycolic acid) (PLGA) [35, 53, 54], poly (caprolactone) (PCL) [40, 41, 47], hydroxyethyl cellulose (HEC) [39, 55], and poly (dl-lactide) [56].
Natural materials involve easy availability, high bio-activity, poor mechanical properties, high bio-degradability, difficult reproducibility, and possible immunogenicity [57]. These types of biomaterials are divided into two subgroups, proteins and polysaccharides. Collagen is one of the most widely used natural-protein biomaterials in skin tissue engineering. There are innumerable sources from which collagen could be extracted with slight differences in characteristics. Collagen is the main component of ECM in the skin. The properties of collagen make it an ideal option for skin alternatives. Collagen has great biocompatibility, low antigenicity, and changeable functional groups [58]. Experimental studies demonstrated that collagen improves the attachment and proliferation of cells [21, 59]. Previous reports drastically indicated the bold role of collagen nanofibers in the impact on the viability and cytoskeletal organization [60]. Moreover, application of the collagen in a 3D structure could provide intercellular as well as cell–matrix interactions [61]. To overcome the low biostability of collagen, it has been proposed crosslinking or blending with appropriate materials [37]. In this regard, Ma et al. suggested that the composite of collagen and chitosan crosslinked by glutaraldehyde decreases the biodegradability of pure collagen [37]. The combination of collagen and elastin has been investigated in multiple studies of skin tissue remodeling [38, 62]. The combination of elastin in the collagen scaffold resulted in the improvement of mechanical and biological characteristics of dermal constructs [62, 63].
Hydrolysis of collagen resulted in the formation of gelatin with a triple helix structure. There is a preference for using gelatin over collagen in tissue engineering. Gelatin shares extremely close molecular properties and functions with collagen. On the other hand, it is more cost-effective than collagen. Therefore, it is possible to substitute collagen for biomedical purposes. The application of gelatin/chitosan blended scaffolds showed that gelatin increases cellular activity [44].
One more protein biomaterial utilized in skin tissue engineering is keratin. This structural protein is found as an intermediate filament inside the keratinocytes and produces a thick layer of keratin on the top surface of the skin. To form a skin barrier, keratin binds the cells together [64]. Because keratin contains binding motifs for cells. It has been found that the nanocomposite of keratin and bacterial cellulose improved the attachment and proliferation behavior of the keratinocytes compared to only bacterial cellulose [49]. Due to the similar properties of cellulose to natural tissues, its application has been focused especially in the last decade. The surveys demonstrated that ulvan cellulose improves tissue regeneration through accelerating fibroblast growth as well as improving angiogenesis [65]. Cellulose was also used in composite with silk fibroin in skin tissue engineering [66]. Research demonstrated that silk fibroin has privileged mechanical properties [67]. The potential of silk fibroin to support re-epithelialization [68] and promotion of cell anchorage [69] has been well documented.
In addition to the application of biomaterials that is present in the native skin tissue, there is a great potential to use materials forming during wound healing like fibrin. After the skin injury, fibrinogen in blood plasma converts to an insoluble protein fibrin which forms a network to trap platelets and immune cells. Previous findings indicated that fibrin promoted adhesion, proliferation, and functionality of the dermal fibroblasts [70, 71]. Recently, researchers functionalized the Hcel®NaT wound dressing with fibrin mesh and fibrin coating. The found fibrin, especially in the form of a mesh, can improve the wound healing process by supporting human dermal cell adhesion, migration, and spreading [72].
Polysaccharide-based biomaterials are another subgroup of natural materials. Chitosan is a glycosaminoglycan-like biodegradable polymer that is widely used in this field. Cytocompatibility and anti-microbial activities of chitosan make it a noteworthy candidate for skin tissue substitutes [73, 74]. The low mechanical integrity of chitosan motivated the researchers to apply crosslinking reagents like glutaraldehyde and dimethyl 3-3, dithio bis’ propionamide [46]. Chitosan has been extensively applied in combination with other biomaterials [37, 44, 75]. A study on the chitosan–gelatin-hyaluronic acid scaffold showed that this platform is suitable for preparing a bilayer skin construct. The cell characterization study demonstrated that hyaluronic acid improved fibroblast attachment and proliferation [76]. Hyaluronic acid is one of the main components of ECM that affect cell attachment, migration, and proliferation [77]. Hyaluronic acid act through binding to biochemical factors [78] and mechanosensor molecules, integrins [31].
Alginate is another polysaccharide-based biomaterial in tissue engineering. The application of alginate in skin tissue engineering has been well described [79, 80]. Previous findings demonstrated that alginate can play an important role in the healing process via the stimulation of IL-6 and TNF-α production from the monocytes [81, 82]. Brittle behavior is a major limitation in using alginate. The blending of alginate with other biomaterials can promote the physical and mechanical properties of it.
The second type of biomaterials is synthetic which have distinct properties; difficult availability, poor bio-activity, high mechanical properties, poor bio-degradability, high reproducibility, and probability of immunogenicity [57]. Among synthetic biomaterials PGA, PLA, PLGA, AND PCL are FDA approved.
PLGA is a biocompatible synthetic copolymer of PLA and PGA with tunable degradability. The culture of human skin keratinocytes on the PLGA nanofibrous scaffold showed the proliferation and infiltration of the cells [54]. Furthermore, to improve the bioactivity of PLGA, collagen has been an appropriate candidate. Collagen coating on the PLGA scaffold indicated a higher proliferation rate of dermal fibroblasts and epidermal cells [83]. PCL can also be used as synthetic FDA material for skin substitute production. Hydrophobicity and slow degradation rate are the main characteristics of PCL [84]. To promote the bioactivity of the PCL, the application of natural biomaterials like collagen [21] and gelatin [40] illustrated promising results.
To create a living dermal substitute, the usage of PEGT/PBT copolymer scaffolds was also investigated [52, 85]. Ghalbzouri and colleagues cultured fully differentiated epidermal sheets onto fibroblast-populated PEGT/PBT scaffolds. Through this process, Human skin equivalents formed [52]. Another studied synthetic biomaterial is PEG. In the form of gel, PEG simulated some properties of native ECM. The inoculation of PEG into electrospun poly (dl-lactide) mitigated the dimensional shrinkage, which affect the release of the embedded bioactive substances or proper placement of the cells in the scaffold [56].
Finally, biocompatibility, mechanical properties, degradation, scalability, and cost-effectiveness are critical issues in the application of special biomaterials in skin tissue engineering. A comparison of the main characteristics of the natural and synthetic materials indicates that the combination of different biomaterials, as a composite, is more advantageous to establish the best approach.
2.2.2 The Scaffold Production Methods
The technique used to fabricate the scaffold is also a fundamental issue in the production of skin tissue equivalents. The combination of the technologies to attain a unique structure mimicking native skin is the purpose of future studies. Electrospinning [68, 86], freeze-drying [48, 87], hydrogel [88, 89], and bioprinting [90, 91] methods are widely used for scaffold development in skin tissue engineering.
Electrospinning is a technique based on the production of nanofibrous structures through an electrohydrodynamic process. In this technique, the liquid is electrified to generate nanofibers [92]. Adjustment of different parameters including, voltage, working distance, flow rate, polymer concentration, solvent properties, temperature, and humidity are fundamental to succeed in producing a proper scaffold. Engineered 2D and 3D electrospun scaffolds have been widely studied in skin tissue regeneration. Although 2D electrospun structures have been widely investigated, there are preferences for 3D platforms over 2D ones. In 3D constructs, the porosity, cell-to-cell contact, cell migration, and mass transfer can be better mimicked in the natural microenvironment [93].
Park and colleagues developed a 3D electrospun silk-fibroin nanofiber for skin tissue engineering. They incorporated NaCl crystals into the nanofibers to overcome their small pore size and facilitated the penetration of the cells. They articulated that high proliferative fibroblasts in the deep layer and more differentiated keratinocytes in the superficial layer of this construct were established [94]. Recently, the composite of collagen and elastin was successfully electrospun. This experiment revealed the adhesion and proliferation of the cells in this microenvironment [38].
Freeze drying is another promising method in skin tissue engineering. Optimization of the parameters results in the creation of a uniform architecture throughout the scaffold. Fabrication of a bilayer scaffold containing cellulose nanofiber/poly (vinyl) alcohol using a novel one-step freeze-drying technique was conducted for skin tissue engineering applications. The designed constructs showed good biocompatibility and tunable pore size with polymer concentration [87]. Preparation of a 3D porous scaffold of natural biomaterials, including alginate and chitosan, using a facile freeze-drying method combined with amidation proposed a desirable skin tissue-engineered construct. This platform supplied an appropriate biocompatibility and anti-inflammatory environment [48].
Among the 3D-engineered structures, hydrogel scaffolds play an important role to achieve an ideal construct for use in skin tissue engineering. The application of Agarose-polydopamine hydrogel led to a high cell migration rate to the inside of the scaffold, promotion of matrix deposition, and facilitation of angiogenesis [89]. Biological and mechanical evaluation of super-absorbent tailor-made collagen–pullulan hydrogel revealed the biocompatibility and mechanical stability of the structure. Moreover, in vivo studies demonstrated better re-epithelialization and wound closure in the treated groups [95]. Therefore, hydrogels can simulate the ECM and provide a microenvironment for vital cell behaviors.
In recent years, 3D bioprinting has attracted the attention of researchers. 3D bioprinting achieves the fabrication of tissues in a layer-by-layer assembly. On the other hand, this technique has the potential to construct complex tissue architecture involving skin appendages, vascular and neuronal networks. All in all, due to the unique features of 3D bioprinting in tissue engineering, this method is an evolving research field. A fully 3D bio-printed skin equivalent structure was established through the printing of the dermis, basal layer, and epidermis using gelatin, collagen I, laminin, and entactin. The results illustrated the formation of human-like skin equivalent with minimal lateral tissue contraction [96]. In a recent study, full-thickness skin models were printed from methacrylated silk fibroin (Silk-GMA) and gelatin (Gel-GMA) seeded with keratinocytes, fibroblasts, and vascular endothelial cells using a digital light processing (DLP) 3D printer. The engineered skin simulated the structure of the human skin. In this structure cell viability was supported and wound healing was enhanced [97].
The engineered structure must make an appropriate microenvironment by providing suitable pore size, porosity percent, the interconnection between pores, and physical and chemical cues to support tissue formation. Recently, the combination of different techniques has been advised to obtain more complex scaffolds mimicking the original tissue. But, it needs to be taken into consideration that with the increase in the number of techniques, optimization, and expertise are the main complication.
2.3 Signaling Factors
In addition to the cells and scaffolds, signaling factors have a profound influence on the production of a bio-functional platform in regenerative medicine. Three general components are involved in signaling factors including, biochemical, physicochemical, and physical cues.
During the formation of natural skin, various biochemical factors such as serum proteins, growth factors, and cytokines that fulfill the expected functions of the tissue are involved. The application of these bioactive substances can provide the condition to direct cells and the structure toward a simulated tissue. The evaluated biochemical signaling factors in the recent studies have been summarized in Table 3.
Epidermal growth factor (EGF) has been introduced as one of the most important molecules in cell growth, differentiation, and migration [98]. Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is expressed in the keratinocytes, fibroblasts, and endothelial cells. It should be noted that EGF induces secretion of ECM and granulation tissue formation [99].
Another important growth factor is fibroblast growth factor (FGF). FGF is a family of cell signaling proteins that mediate a broad range of biological activities [100]. Skin is a specific target of FGF. This is one of the main reasons why this growth factor has been applied in skin tissue engineering. Findings indicated that FGF plays an important role in cell proliferation [101] and angiogenesis [102]. In this regard, researchers have focused on keratinocyte growth factor (KGF) for wound re-epithelialization. KGF is a member of the heparin-binding fibroblast growth factor family (FGF-7) with a distinctive role in epithelial cell proliferation. A significant increase in cell migration was also reported in ex vivo skin explant migration assay in the presence of KGF [103].
Vascular endothelial growth factor (VEGF) is among other factors used in tissue engineering. VEGF has been considered a strong signaling factor in angiogenesis and vasculogenesis. The surveys demonstrated the potential of this biochemical factor in directing stem cells toward endothelial cells [104]. Moreover, VEGF showed improvement in the early stages of wound healing through the promotion of TGF-β and CD31 expression [105]. Furthermore, another important molecule involved in the formation of functional blood vessels is platelet-derived growth factor (PDGF). PDGF can stimulate tubulogenesis and angiogenesis [106]. Previous findings elucidated the potential of PDGF in the induction of endogenous growth factors production at the wound site that resulted in the reduction of wound closure time [107].
Overall, achieving the desired effect in using signaling molecules in skin tissue engineering requires attention to several issues, including optimal concentration, delivery system, safety, stability, and specificity of the bioactive molecules. Addressing these challenges can improve the development of skin substitutes that can be used for wound healing.
Other groups of signaling cues involved in skin tissue engineering are physicochemical and physical factors. Physicochemical factors consist of pH, O2 level, and CO2 concentration. The maintenance of these factors in balance has a dominant influence on cell and tissue behavior. On the other hand, physical factors include mechanical stimuli, electromagnetic force, and temperature. Findings elucidated that physical stimuli trigger signaling pathways through the integrins, ionic channels, and cell–cell adhesion molecules which leads to a special cell behavior. In this regard, bioreactors have been introduced to provide a biologically active microenvironment for the development of new tissue.
Studies on multiple types of bioreactors have been performed in skin tissue engineering [108,109,110]. A dynamic bioreactor that applied cyclic biaxial tension to collagen hydrogels resulted in an increase in fibroblast proliferation and a decrease in human skin substitute production time. This study showed that cyclic deformation is the main physical factor affecting fibroblast proliferation. Moreover, the expression of dermal ECM proteins was promoted in this situation [110]. An in-house novel bioreactor that was able to supply automatic mass transport of nutrients and removal of waste was designed to make possible upscaling of a composite cultured skin. After transplantation of the composite in a porcine model, wound closure and complete epithelialization were observed [108]. Sun and colleagues developed a closed bioreactor system for the culture of tissue-engineered skin at an air–liquid interface. They validate the device in monoculture and coculture systems and different platforms. All in all, their results proposed a low-cost bioreactor for the culture of skin tissue substitutes [109]. Finally, the mentioned results indicated that the accurate recruitment of physical factors can eventuate in guiding cell and tissue behavior toward the appropriate epidermal and dermal formation.
In addition to the use of bioreactors in tissue culture, it is possible to use this system in culturing cells before transferring them to the scaffold [111, 112]. The application of the Kerator bioreactor did not demonstrate beneficial effects on cell viability, proliferation, and wound healing after transplantation. But, this device provided an opportunity for large-scale production of the cells as well as reduced cost in regenerative medicine [111, 113]. Liu and colleagues also developed a novel bioreactor microcarrier cell culture framework to produce autologous human keratinocytes on a large scale. The culture of the keratinocytes on the microbeads in this system led to rapid attachment and proliferation of the cells. This method suggested the possibility of using autologous keratinocyte microbeads on different skin defects [112].
Optimizing physicochemical and physical factors have a close relationship with developing biomaterials and scaffold production techniques. Therefore, all conditions adopted in tissue culture and whole characteristics of biomaterials and final engineered scaffold including mechanical strength and degradation rate can affect the mentioned factors.
3 Conclusion
Different commercial products are available for the management of skin injuries, while skin tissue engineering has the potential to revolutionize the treatment of skin conditions, the high cost and lack of the unique characteristics of natural skin in these products, limit the wide application of this technique. Addressing these challenges will be critical in advancing the field and improving patient outcomes.
For the recapitulation of native skin, tissue engineering seems to be a tremendous promise. In this regard, an orchestrated combination of a variety of cues such as cell sources, biomaterials, fabrication techniques, and signaling factors are fundamental to achieving astonishing accomplishments. In the present review, we studied the recent advancement in skin tissue engineering, with a particular focus on the application of different cell sources and biomaterials. The review found that while cellular scaffold offers advantages over acellular scaffolds in terms of faster and more efficient tissue regeneration, cellular scaffolds carry the risk of immune rejection and the potential for uncontrolled cell proliferation or unintended differentiation.
The review also highlighted the main biomaterials and techniques to create constructs. Lack of standardization in the methods of biomaterials purification and scaffold production is the main issue. Another matter is the limited understanding of the interactions between cells and biomaterials. Therefore, more research is required to evaluate the effects of biomaterials on cell behavior. In this regard, the development of biomaterials capable of simulating the native extracellular matrix of skin tissue is needed. Additionally, long-term studies are essential to establish the complete safety and efficacy of biomaterials in humans.
Beyond cells and scaffolds, signaling factors including biochemical, physicochemical, and physical cues play important roles in the development of bio-functional platforms in the regenerative medicine of skin. These bioactive molecules can support cell proliferation, differentiation, extracellular formation, and angiogenesis. Despite the advancements in this field, there are still gaps in terms of a comprehensive conception of the complex interactions between signaling factors and cells. The development of more targeted and specific signaling factors can promote tissue regeneration without promoting unwanted cell behaviors.
The results and criticisms of this review can serve as a basis for future research and can serve as a guide for the development and optimization of skin replacement products with improved functionality and clinical outcomes.
References
Wong, R., Geyer, S., Weninger, W., Guimberteau, J. C., & Wong, J. K. (2016). The dynamic anatomy and patterning of skin. Experimental Dermatology, 25(2), 92–98. https://doi.org/10.1111/exd.12832
White, S. D., & Yager, J. A. (1995). Resident dendritic cells in the epidermis: Langerhans cells, Merkel cells and melanocytes. Veterinary Dermatology, 6(1), 1–8. https://doi.org/10.1111/j.1365-3164.1995.tb00034.x
Zaidi, Z., & Lanigan, S. W. (2010). Skin: Structure and function. Dermatology in clinical practice. London: Springer.
Macefield, V. G. (2005). Physiological characteristics of low-threshold mechanoreceptors in joints, muscle and skin in human subjects. Clinical and Experimental Pharmacology and Physiology, 32(1–2), 135–144. https://doi.org/10.1111/j.1440-1681.2005.04143.x
Rittié, L. (2016). Cellular mechanisms of skin repair in humans and other mammals. Journal of Cell Communication and Signaling, 10(2), 103–120. https://doi.org/10.1007/s12079-016-0330-1
Ridiandries, A., Tan, J. T. M., & Bursill, C. A. (2018). The role of chemokines in wound healing. International Journal of Molecular Sciences, 19(10), 3217. https://doi.org/10.3390/ijms19103217
Clark, R. (1994). Wound repair. In R. A. F. Clark (Ed.), The molecular and cellular biology of wound repair. Boston: Springer.
Rheinwald, J., & Green, H. (1975). Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell, 6(3), 331–343.
Farhadihosseinabadi, B., Farahani, M., Tayebi, T., Jafari, A., Biniazan, F., Modaresifar, K., Moravvej, H., Bahrami, S., Redl, H., & Tayebi, L. (2018). Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artificial Cells, Nanomedicine, and Biotechnology, 46(sup2), 431–440. https://doi.org/10.1080/21691401.2018.1458730
Keirouz, A., Zakharova, M., Kwon, J., Robert, C., Koutsos, V., Callanan, A., Chen, X., Fortunato, G., & Radacsi, N. (2020). High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Materials Science and Engineering: C, 112, 110939. https://doi.org/10.1016/j.msec.2020.110939
Rad, Z. P., Mokhtari, J., & Abbasi, M. (2018). Fabrication and characterization of PCL/zein/gum Arabic electrospun nanocomposite scaffold for skin tissue engineering. Materials Science and Engineering: C, 93, 356–366. https://doi.org/10.1016/j.msec.2018.08.010
Zelen, C. M., Serena, T. E., Gould, L., Le, L., Carter, M. J., Keller, J., & Li, W. W. (2016). Treatment of chronic diabetic lower extremity ulcers with advanced therapies: A prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. International Wound Journal, 13(2), 272–282. https://doi.org/10.1111/iwj.12566
Nicholas, M. N., Jeschke, M. G., & Amini-Nik, S. (2016). Cellularized bilayer pullulan-gelatin hydrogel for skin regeneration. Tissue Engineering Part A, 22(9–10), 754–764. https://doi.org/10.1089/ten.TEA.2015.0536
Sabolinski, M. L., & Gibbons, G. (2018). Comparative effectiveness of a bilayered living cellular construct and an acellular fetal bovine collagen dressing in the treatment of venous leg ulcers. Journal of Comparative Effectiveness Research, 7(8), 797–805. https://doi.org/10.2217/cer-2018-0031
Baltazar, T., Merola, J., Catarino, C., Xie, C. B., Kirkiles-Smith, N. C., Lee, V., Hotta, S., Dai, G., Xu, X., Ferreira, F. C., Saltzman, W. M., Pober, J. S., & Karande, P. (2020). Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells. Tissue Engineering Part A, 26(5–6), 227–238. https://doi.org/10.1089/ten.TEA.2019.0201
Barros, N. R., Kim, H. J., Gouidie, M. J., Lee, K., Bandaru, P., Banton, E. A., Sarikhani, E., Sun, W., Zhang, S., Cho, H. J., Hartel, M. C., Ostrovidov, S., Ahadian, S., Hussain, S. M., Ashammakhi, N., Dokmeci, M. R., Herculano, R. D., Lee, J., & Khademhosseini, A. (2021). Biofabrication of endothelial cell, dermal fibroblast, and multilayered keratinocyte layers for skin tissue engineering. Biofabrication, 13(3), 035030. https://doi.org/10.1088/1758-5090/aba503
Zhong, J., Wang, H., Yang, K., Wang, H., Duan, C., Ni, N., An, L., Luo, Y., Zhao, P., Gou, Y., Sheng, S., Shi, D., Chen, C., Wagstaff, W., Hendren-Santiago, B., Haydon, R. C., Luu, H. H., Reid, R. R., Ho, S. H., … Fan, J. (2022). Reversibly immortalized keratinocytes (iKera) facilitate re-epithelization and skin wound healing: Potential applications in cell-based skin tissue engineering. Bioactive Material, 9, 523–540. https://doi.org/10.1016/j.bioactmat.2021.07.022
Hashemi, S. S., Mohammadi, A. A., Moshirabadi, K., & Zardosht, M. (2021). Effect of dermal fibroblasts and mesenchymal stem cells seeded on an amniotic membrane scaffold in skin regeneration: A case series. Journal of Cosmetic Dermatology, 20(12), 4040–4047. https://doi.org/10.1111/jocd.14043
Loh, E. Y. X., Fauzi, M. B., Ng, M. H., Ng, P. Y., Ng, S. F., & Amin, M. C. I. M. (2020). Insight into delivery of dermal fibroblast by non-biodegradable bacterial nanocellulose composite hydrogel on wound healing. International Journal of Biological Macromolecules, 159, 497–509. https://doi.org/10.1016/j.ijbiomac.2020.05.011
Shera, S. S., & Banik, R. M. (2021). Development of tunable silk fibroin/xanthan biopolymeric scaffold for skin tissue engineering using L929 fibroblast cells. Journal of Bionic Engineering, 18(1), 103–117. https://doi.org/10.1007/s42235-021-0004-4
Sharif, S., Ai, J., Azami, M., Verdi, J., Atlasi, M. A., Shirian, S., & Samadikuchaksaraei, A. (2018). Collagen-coated nano-electrospun PCL seeded with human endometrial stem cells for skin tissue engineering applications. Journal of Biomedical Materials Research Part B, Applied Biomaterials, 106(4), 1578–1586. https://doi.org/10.1002/jbm.b.33966
Oryan, A., Alemzadeh, E., Mohammadi, A. A., & Moshiri, A. (2019). Healing potential of injectable Aloe vera hydrogel loaded by adipose-derived stem cell in skin tissue-engineering in a rat burn wound model. Cell and Tissue Research, 377(2), 215–227. https://doi.org/10.1007/s00441-019-03015-9
Ranjbarvan, P., Golchin, A., Azari, A., & Niknam, Z. (2021). The bilayer skin substitute based on human adipose-derived mesenchymal stem cells and neonate keratinocytes on the 3D nanofibrous PCL-platelet gel scaffold. Polymer Bulletin, 79, 4013–4030. https://doi.org/10.1007/s00289-021-03702-0
Zhang, Z., Li, Z., Li, Y., Wang, Y., Yao, M., Zhang, K., Chen, Z., Yue, H., Shi, J., Guan, F., & Ma, S. (2021). Sodium alginate/collagen hydrogel loaded with human umbilical cord mesenchymal stem cells promotes wound healing and skin remodeling. Cell and Tissue Research, 383(2), 809–821. https://doi.org/10.1007/s00441-020-03321-7
Cui, B., Zhang, C., Gan, B., Liu, W., Liang, J., Fan, Z., Wen, Y., Yang, Y., Peng, X., & Zhou, Y. (2020). Collagen-tussah silk fibroin hybrid scaffolds loaded with bone mesenchymal stem cells promote skin wound repair in rats. Materials Science & Engineering, C: Materials for Biological Applications, 109, 110611. https://doi.org/10.1016/j.msec.2019.110611
Kaviani, M., Geramizadeh, B., Rahsaz, M., & Marzban, S. (2015). Considerations in the improvement of human epidermal keratinocyte culture in vitro. Experimental and Clinical Transplantation, 13(Suppl 1), 366–370. https://doi.org/10.6002/ect.mesot2014.L4
Rahsaz, M., Geramizadeh, B., Kaviani, M., & Marzban, S. (2015). Gelatin for purification and proliferation of primary keratinocyte culture for use in chronic wounds and burns. Experimental and Clinical Transplantation, 13(1), 361–365. https://doi.org/10.6002/ect.mesot2014.L4
Luckett, L. R., & Gallucci, R. M. (2007). Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice. British Journal of Dermatology, 156(6), 1163–1171. https://doi.org/10.1111/j.1365-2133.2007.07867.x
Nolte, S. V., Xu, W., Rennekampff, H.-O., & Rodemann, H. P. (2008). Diversity of fibroblasts–a review on implications for skin tissue engineering. Cells Tissues Organs, 187(3), 165–176. https://doi.org/10.1159/000111805
Zhao, X., Psarianos, P., Ghoraie, L. S., Yip, K., Goldstein, D., Gilbert, R., Witterick, I., Pang, H., Hussain, A., Lee, J. H., Williams, J., Bratman, S. V., Ailles, L., Haibe-Kains, B., & Liu, F. F. (2019). Metabolic regulation of dermal fibroblasts contributes to skin extracellular matrix homeostasis and fibrosis. Nature Metabolism, 1(1), 147–157. https://doi.org/10.1038/s42255-018-0008-5
Chopra, A., Murray, M. E., Byfield, F. J., Mendez, M. G., Halleluyan, R., Restle, D. J., Raz-Ben Aroush, D., Galie, P. A., Pogoda, K., Bucki, R., Marcinkiewicz, C., Prestwich, G. D., Zarembinski, T. I., Chen, C. S., Pure, E., Kresh, J. Y., & Janmey, P. A. (2014). Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials, 35(1), 71–82. https://doi.org/10.1016/j.biomaterials.2013.09.066
Sasaki, M., Abe, R., Fujita, Y., Ando, S., Inokuma, D., & Shimizu, H. (2008). Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. The Journal of Immunology, 180(4), 2581–2587. https://doi.org/10.4049/jimmunol.180.4.2581
Jin, G., Prabhakaran, M. P., & Ramakrishna, S. (2011). Stem cell differentiation to epidermal lineages on electrospun nanofibrous substrates for skin tissue engineering. Acta Biomaterialia, 7(8), 3113–3122. https://doi.org/10.1016/j.actbio.2011.04.017
Han, C. M., Zhang, L. P., Sun, J. Z., Shi, H. F., Zhou, J., & Gao, C. Y. (2010). Application of collagen-chitosan/fibrin glue asymmetric scaffolds in skin tissue engineering. Journal of Zhejiang University Science B, 11(7), 524–530. https://doi.org/10.1631/jzus.B0900400
Lee, J. J., Lee, S.-G., Park, J. C., Yang, Y. I., & Kim, J. K. (2007). Investigation on biodegradable PLGA scaffold with various pore size structure for skin tissue engineering. Current Applied Physics, 7, e37–e40. https://doi.org/10.1016/j.cap.2006.11.011
Liu, Y., Ma, L., & Gao, C. (2012). Facile fabrication of the glutaraldehyde cross-linked collagen/chitosan porous scaffold for skin tissue engineering. Materials Science and Engineering C, 32(8), 2361–2366. https://doi.org/10.1016/j.msec.2012.07.008
Ma, L., Gao, C., Mao, Z., Zhou, J., Shen, J., Hu, X., & Han, C. (2003). Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials, 24(26), 4833–4841. https://doi.org/10.1016/s0142-9612(03)00374-0
Vázquez, J. J., & Martínez, E. S. M. (2019). Collagen and elastin scaffold by electrospinning for skin tissue engineering applications. Journal of Materials Research, 34(16), 2819–2827. https://doi.org/10.1557/jmr.2019.233
Zulkifli, F. H., Hussain, F. S. J., Rasad, M. S. B. A., & Yusoff, M. M. (2014). In vitro degradation study of novel HEC/PVA/collagen nanofibrous scaffold for skin tissue engineering applications. Polymer Degradation and Stability, 110, 473–481. https://doi.org/10.1016/j.polymdegradstab.2014.10.017
Gautam, S., Chou, C. F., Dinda, A. K., Potdar, P. D., & Mishra, N. C. (2014). Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Materials Science & Engineering, C: Materials for Biological Applications, 34, 402–409. https://doi.org/10.1016/j.msec.2013.09.043
Gomes, S., Rodrigues, G., Martins, G., Henriques, C., & Silva, J. C. (2017). Evaluation of nanofibrous scaffolds obtained from blends of chitosan, gelatin and polycaprolactone for skin tissue engineering. International Journal of Biological Macromolecules, 102, 1174–1185. https://doi.org/10.1016/j.ijbiomac.2017.05.004
Han, F., Dong, Y., Su, Z., Yin, R., Song, A., & Li, S. (2014). Preparation, characteristics and assessment of a novel gelatin–chitosan sponge scaffold as skin tissue engineering material. International Journal of Pharmaceutics, 476(1–2), 124–133. https://doi.org/10.1016/j.ijpharm.2014.09.036
Pezeshki-Modaress, M., Mirzadeh, H., & Zandi, M. (2015). Gelatin–GAG electrospun nanofibrous scaffold for skin tissue engineering: fabrication and modeling of process parameters. Materials Science and Engineering: C, 48, 704–712. https://doi.org/10.1016/j.msec.2014.12.023
Pezeshki-Modaress, M., Zandi, M., & Rajabi, S. (2018). Tailoring the gelatin/chitosan electrospun scaffold for application in skin tissue engineering: An in vitro study. Progress in Biomaterials, 7(3), 207–218. https://doi.org/10.1007/s40204-018-0094-1
Bhardwaj, N., Sow, W. T., Devi, D., Ng, K. W., Mandal, B. B., & Cho, N.-J. (2015). Silk fibroin–keratin based 3D scaffolds as a dermal substitute for skin tissue engineering. Integrative Biology, 7(1), 53–63. https://doi.org/10.1039/C4IB00208C
Adekogbe, I., & Ghanem, A. (2005). Fabrication and characterization of DTBP-crosslinked chitosan scaffolds for skin tissue engineering. Biomaterials, 26(35), 7241–7250. https://doi.org/10.1016/j.biomaterials.2005.05.043
Shalumon, K. T., Anulekha, K. H., Chennazhi, K. P., Tamura, H., Nair, S. V., & Jayakumar, R. (2011). Fabrication of chitosan/poly(caprolactone) nanofibrous scaffold for bone and skin tissue engineering. International Journal of Biological Macromolecules, 48(4), 571–576. https://doi.org/10.1016/j.ijbiomac.2011.01.020
Zhu, T., Jiang, J., Zhao, J., Chen, S., & Yan, X. (2019). Regulating preparation of functional alginate-chitosan three-dimensional scaffold for skin tissue engineering. International Journal of Nanomedicine, 14, 8891–8903. https://doi.org/10.2147/IJN.S210329
Keskin, Z., Sendemir Urkmez, A., & Hames, E. E. (2017). Novel keratin modified bacterial cellulose nanocomposite production and characterization for skin tissue engineering. Materials Science & Engineering, C: Materials for Biological Applications, 75, 1144–1153. https://doi.org/10.1016/j.msec.2017.03.035
Monteiro, I. P., Shukla, A., Marques, A. P., Reis, R. L., & Hammond, P. T. (2015). Spray-assisted layer-by-layer assembly on hyaluronic acid scaffolds for skin tissue engineering. Journal of Biomedical Materials Research. Part A, 103(1), 330–340. https://doi.org/10.1002/jbm.a.35178
Solovieva, E. V., Fedotov, A. Y., Mamonov, V. E., Komlev, V. S., & Panteleyev, A. A. (2018). Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering. Biomedical Materials, 13(2), 025007. https://doi.org/10.1088/1748-605X/aa9089
El Ghalbzouri, A., Lamme, E. N., van Blitterswijk, C., Koopman, J., & Ponec, M. (2004). The use of PEGT/PBT as a dermal scaffold for skin tissue engineering. Biomaterials, 25(15), 2987–2996. https://doi.org/10.1016/j.biomaterials.2003.09.098
Kumbar, S. G., Nukavarapu, S. P., James, R., Nair, L. S., & Laurencin, C. T. (2008). Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials, 29(30), 4100–4107. https://doi.org/10.1016/j.biomaterials.2008.06.028
Ru, C., Wang, F., Pang, M., Sun, L., Chen, R., & Sun, Y. (2015). Suspended, shrinkage-free, electrospun PLGA nanofibrous scaffold for skin tissue engineering. ACS Applied Materials & Interfaces, 7(20), 10872–10877. https://doi.org/10.1021/acsami.5b01953
Zulkifli, F. H., Hussain, F. S. J., Zeyohannes, S. S., Rasad, M., & Yusuff, M. M. (2017). A facile synthesis method of hydroxyethyl cellulose-silver nanoparticle scaffolds for skin tissue engineering applications. Materials Science & Engineering, C: Materials for Biological Applications, 79, 151–160. https://doi.org/10.1016/j.msec.2017.05.028
Cui, W., Zhu, X., Yang, Y., Li, X., & Jin, Y. (2009). Evaluation of electrospun fibrous scaffolds of poly (dl-lactide) and poly (ethylene glycol) for skin tissue engineering. Materials Science and Engineering: C, 29(6), 1869–1876. https://doi.org/10.1016/j.msec.2009.02.013
Wolfe, P. S., Sell, S. A., & Bowlin, G. L. (2011). Natural and synthetic scaffolds. In N. Pallua & C. Suscheck (Eds.), Tissue engineering (pp. 41–67). Berlin: Springer.
Sundar, G., Joseph, J., John, A., & Abraham, A. (2021). Natural collagen bioscaffolds for skin tissue engineering strategies in burns: A critical review. International Journal of Polymeric Materials and Polymeric Biomaterials, 70(9), 593–604. https://doi.org/10.1080/00914037.2020.1740991
Willard, J. J., Drexler, J. W., Das, A., Roy, S., Shilo, S., Shoseyov, O., & Powell, H. M. (2013). Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Engineering Part A, 19(13–14), 1507–1518. https://doi.org/10.1089/ten.TEA.2012.0338
Sarkar, S. D., Farrugia, B. L., Dargaville, T. R., & Dhara, S. (2013). Chitosan–collagen scaffolds with nano/microfibrous architecture for skin tissue engineering. Journal of Biomedical Materials Research Part A, 101(12), 3482–3492. https://doi.org/10.1002/jbm.a.34660
Ramanathan, G., Singaravelu, S., Muthukumar, T., Thyagarajan, S., Perumal, P. T., & Sivagnanam, U. T. (2017). Design and characterization of 3D hybrid collagen matrixes as a dermal substitute in skin tissue engineering. Materials Science and Engineering: C, 72, 359–370. https://doi.org/10.1016/j.msec.2016.11.095
Rnjak-Kovacina, J., Wise, S. G., Li, Z., Maitz, P. K., Young, C. J., Wang, Y., & Weiss, A. S. (2012). Electrospun synthetic human elastin: Collagen composite scaffolds for dermal tissue engineering. Acta Biomaterialia, 8(10), 3714–3722. https://doi.org/10.1016/j.actbio.2012.06.032
Buttafoco, L., Kolkman, N. G., Engbers-Buijtenhuijs, P., Poot, A. A., Dijkstra, P. J., Vermes, I., & Feijen, J. (2006). Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials, 27(5), 724–734. https://doi.org/10.1016/j.biomaterials.2005.06.024
Gu, L. H., & Coulombe, P. A. (2007). Keratin function in skin epithelia: A broadening palette with surprising shades. Current Opinion in Cell Biology, 19(1), 13–23. https://doi.org/10.1016/j.ceb.2006.12.007
Madub, K., Goonoo, N., Gimie, F., Ait Arsa, I., Schonherr, H., & Bhaw-Luximon, A. (2021). Green seaweeds ulvan-cellulose scaffolds enhance in vitro cell growth and in vivo angiogenesis for skin tissue engineering. Carbohydrate Polymers, 251, 117025. https://doi.org/10.1016/j.carbpol.2020.117025
Shefa, A. A., Amirian, J., Kang, H. J., Bae, S. H., Jung, H. I., Choi, H. J., Lee, S. Y., & Lee, B. T. (2017). In vitro and in vivo evaluation of effectiveness of a novel TEMPO-oxidized cellulose nanofiber-silk fibroin scaffold in wound healing. Carbohydrate Polymers, 177, 284–296. https://doi.org/10.1016/j.carbpol.2017.08.130
Koh, L.-D., Cheng, Y., Teng, C.-P., Khin, Y.-W., Loh, X.-J., Tee, S.-Y., Low, M., Ye, E., Yu, H.-D., & Zhang, Y.-W. (2015). Structures, mechanical properties and applications of silk fibroin materials. Progress in Polymer Science, 46, 86–110. https://doi.org/10.1016/j.progpolymsci.2015.02.001
Hodgkinson, T., Yuan, X. F., & Bayat, A. (2014). Electrospun silk fibroin fiber diameter influences in vitro dermal fibroblast behavior and promotes healing of ex vivo wound models. Journal of Tissue Engineering, 5, 2041731414551661. https://doi.org/10.1177/2041731414551661
Chlapanidas, T., Tosca, M., Farago, S., Perteghella, S., Galuzzi, M., Lucconi, G., Antonioli, B., Ciancio, F., Rapisarda, V., & Vigo, D. (2013). Formulation and characterization of silk fibroin films as a scaffold for adipose-derived stem cells in skin tissue engineering. International Journal of Immunopathology and Pharmacology, 26(1_suppl), 43–49. https://doi.org/10.1177/03946320130260S106
Bacakova, M., Musilkova, J., Riedel, T., Stranska, D., Brynda, E., Zaloudkova, M., & Bacakova, L. (2016). The potential applications of fibrin-coated electrospun polylactide nanofibers in skin tissue engineering. International Journal of Nanomedicine, 11, 771–789. https://doi.org/10.2147/IJN.S99317
Bacakova, M., Pajorova, J., Stranska, D., Hadraba, D., Lopot, F., Riedel, T., Brynda, E., Zaloudkova, M., & Bacakova, L. (2017). Protein nanocoatings on synthetic polymeric nanofibrous membranes designed as carriers for skin cells. International Journal of Nanomedicine, 12, 1143. https://doi.org/10.2147/IJN.S121299
Bacakova, M., Pajorova, J., Sopuch, T., & Bacakova, L. (2018). Fibrin-modified cellulose as a promising dressing for accelerated wound healing. Materials, 11(11), 2314. https://doi.org/10.3390/ma11112314
Kenawy, E.-R., Abdel-Hay, F. I., El-Magd, A. A., & Mahmoud, Y. (2005). Biologically active polymers: Modification and anti-microbial activity of chitosan derivatives. Journal of Bioactive and Compatible Polymers, 20(1), 95–111. https://doi.org/10.1177/0883911505049655
Madni, A., Kousar, R., Naeem, N., & Wahid, F. (2021). Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. Journal of Bioresources and Bioproducts, 6(1), 11–25. https://doi.org/10.1016/j.jobab.2021.01.002
Asghari, F., Rabiei Faradonbeh, D., Malekshahi, Z. V., Nekounam, H., Ghaemi, B., Yousefpoor, Y., Ghanbari, H., & Faridi-Majidi, R. (2022). Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydrate Polymers, 278, 118926. https://doi.org/10.1016/j.carbpol.2021.118926
Liu, H., Mao, J., Yao, K., Yang, G., Cui, L., & Cao, Y. (2004). A study on a chitosan-gelatin-hyaluronic acid scaffold as artificial skin in vitro and its tissue engineering applications. Journal of Biomaterials Science, Polymer Edition, 15(1), 25–40. https://doi.org/10.1163/156856204322752219
Abatangelo, G., Vindigni, V., Avruscio, G., Pandis, L., & Brun, P. (2020). Hyaluronic acid: Redefining its role. Cells, 9(7), 1743. https://doi.org/10.3390/cells9071743
Wolf, K. J., & Kumar, S. (2019). Hyaluronic acid: incorporating the bio into the material. ACS Biomaterials Science & Engineering, 5(8), 3753–3765. https://doi.org/10.1021/acsbiomaterials.8b01268
Ehterami, A., Salehi, M., Farzamfar, S., Samadian, H., Vaez, A., Ghorbani, S., Ai, J., & Sahrapeyma, H. (2019). Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. Journal of Drug Delivery Science and Technology, 51, 204–213. https://doi.org/10.1016/j.jddst.2019.02.032
Shi, L., Xiong, L., Hu, Y., Li, W., Chen, Z., Liu, K., & Zhang, X. (2018). Three-dimensional printing alginate/gelatin scaffolds as dermal substitutes for skin tissue engineering. Polymer Engineering & Science, 58(10), 1782–1790. https://doi.org/10.1002/pen.24779
Aderibigbe, B. A., & Buyana, B. (2018). Alginate in wound dressings. Pharmaceutics, 10(2), 42. https://doi.org/10.3390/pharmaceutics10020042
Yang, D., & Jones, K. S. (2009). Effect of alginate on innate immune activation of macrophages. Journal of Biomedical Materials Research. Part A, 90(2), 411–418. https://doi.org/10.1002/jbm.a.32096
Sadeghi, A., Nokhasteh, S., Molavi, A., Khorsand-Ghayeni, M., Naderi-Meshkin, H., & Mahdizadeh, A. (2016). Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Materials Science and Engineering: C, 66, 130–137. https://doi.org/10.1016/j.msec.2016.04.073
Cipitria, A., Skelton, A., Dargaville, T., Dalton, P., & Hutmacher, D. (2011). Design, fabrication and characterization of PCL electrospun scaffolds—a review. Journal of Materials Chemistry, 21(26), 9419–9453. https://doi.org/10.1039/C0JM04502K
Wang, H. J., Bertrand-De Haas, M., Riesle, J., Lamme, E., & Van Blitterswijk, C. A. (2003). Tissue engineering of dermal substitutes based on porous PEGT/PBT copolymer scaffolds: Comparison of culture conditions. Journal of Materials Science. Materials in Medicine, 14(3), 235–240. https://doi.org/10.1023/a:1022880623151
Law, J. X., Liau, L. L., Saim, A., Yang, Y., & Idrus, R. (2017). Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Engineering and Regenerative Medicine, 14(6), 699–718. https://doi.org/10.1007/s13770-017-0075-9
Ghafari, R., Jonoobi, M., Amirabad, L. M., Oksman, K., & Taheri, A. R. (2019). Fabrication and characterization of novel bilayer scaffold from nanocellulose based aerogel for skin tissue engineering applications. International Journal of Biological Macromolecules, 136, 796–803. https://doi.org/10.1016/j.ijbiomac.2019.06.104
Pereira, R. F., Barrias, C. C., Bartolo, P. J., & Granja, P. L. (2018). Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomaterialia, 66, 282–293. https://doi.org/10.1016/j.actbio.2017.11.016
Su, T., Zhang, M., Zeng, Q., Pan, W., Huang, Y., Qian, Y., Dong, W., Qi, X., & Shen, J. (2021). Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioactive Materials, 6(3), 579–588. https://doi.org/10.1016/j.bioactmat.2020.09.004
Afghah, F., Ullah, M., Seyyed Monfared Zanjani, J., Akkus Sut, P., Sen, O., Emanet, M., Saner Okan, B., Culha, M., Menceloglu, Y., Yildiz, M., & Koc, B. (2020). 3D printing of silver-doped polycaprolactone-poly(propylene succinate) composite scaffolds for skin tissue engineering. Biomedical Materials, 15(3), 035015. https://doi.org/10.1088/1748-605X/ab7417
Daikuara, L. Y., Yue, Z., Skropeta, D., & Wallace, G. G. (2021). In vitro characterisation of 3D printed platelet lysate-based bioink for potential application in skin tissue engineering. Acta Biomaterialia, 123, 286–297. https://doi.org/10.1016/j.actbio.2021.01.021
Xue, J., Wu, T., Dai, Y., & Xia, Y. (2019). Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chemical Reviews, 119(8), 5298–5415. https://doi.org/10.1021/acs.chemrev.8b00593
Keirouz, A., Chung, M., Kwon, J., Fortunato, G., & Radacsi, N. (2020). 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. Wiley Interdisciplinary Reviews Nanomedicine and Nanobiotechnology, 12(4), e1626. https://doi.org/10.1002/wnan.1626
Park, Y. R., Ju, H. W., Lee, J. M., Kim, D. K., Lee, O. J., Moon, B. M., Park, H. J., Jeong, J. Y., Yeon, Y. K., & Park, C. H. (2016). Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. International Journal of Biological Macromolecules, 93(Pt B), 1567–1574. https://doi.org/10.1016/j.ijbiomac.2016.07.047
Iswariya, S., Bhanukeerthi, A., Velswamy, P., Uma, T., & Perumal, P. T. (2016). Design and development of a piscine collagen blended pullulan hydrogel for skin tissue engineering. RSC Advances, 6(63), 57863–57871. https://doi.org/10.1039/C6RA03578G
Derr, K., Zou, J., Luo, K., Song, M. J., Sittampalam, G. S., Zhou, C., Michael, S., Ferrer, M., & Derr, P. (2019). Fully three-dimensional bioprinted skin equivalent constructs with validated morphology and barrier function. Tissue Engineering. Part C, Methods, 25(6), 334–343. https://doi.org/10.1089/ten.TEC.2018.0318
Choi, K. Y., Ajiteru, O., Hong, H., Suh, Y. J., Sultan, M. T., Lee, H., Lee, J. S., Lee, Y. J., Lee, O. J., Kim, S. H., & Park, C. H. (2023). A digital light processing 3D-printed artificial skin model and full-thickness wound models using silk fibroin bioink. Acta Biomaterialia, 164, 159–174. https://doi.org/10.1016/j.actbio.2023.04.034
Park, U., & Kim, K. (2017). Multiple growth factor delivery for skin tissue engineering applications. Biotechnology and Bioprocess Engineering, 22(6), 659–670. https://doi.org/10.1007/s12257-017-0436-1
Colige, A., Nusgens, B., & Lapiere, C. (1988). Effect of EGF on human skin fibroblasts is modulated by the extracellular matrix. Archives of Dermatological Research, 280, S42–S46.
Yun, Y. R., Won, J. E., Jeon, E., Lee, S., Kang, W., Jo, H., Jang, J. H., Shin, U. S., & Kim, H. W. (2010). Fibroblast growth factors: Biology, function, and application for tissue regeneration. Journal of Tissue Engineering, 2010(1), 218142. https://doi.org/10.4061/2010/218142
Grazul-Bilska, A. T., Luthra, G., Reynolds, L. P., Bilski, J. J., Johnson, M. L., Adbullah, S. A., Redmer, D. A., & Abdullah, K. M. (2002). Effects of basic fibroblast growth factor (FGF-2) on proliferation of human skin fibroblasts in type II diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes, 110(4), 176–181. https://doi.org/10.1055/s-2002-32149
Rinsch, C., Quinodoz, P., Pittet, B., Alizadeh, N., Baetens, D., Montandon, D., Aebischer, P., & Pepper, M. S. (2001). Delivery of FGF-2 but not VEGF by encapsulated genetically engineered myoblasts improves survival and vascularization in a model of acute skin flap ischemia. Gene Therapy, 8(7), 523–533. https://doi.org/10.1038/sj.gt.3301436
Qu, Y., Cao, C., Wu, Q., Huang, A., Song, Y., Li, H., Zuo, Y., Chu, C., Li, J., & Man, Y. (2018). The dual delivery of KGF and b FGF by collagen membrane to promote skin wound healing. Journal of Tissue Engineering and Regenerative Medicine, 12(6), 1508–1518. https://doi.org/10.1002/term.2691
Tan, J., Li, L., Wang, H., Wei, L., Gao, X., Zeng, Z., Liu, S., Fan, Y., Liu, T., & Chen, J. (2021). Biofunctionalized fibrin gel co-embedded with BMSCs and VEGF for accelerating skin injury repair. Materials Science & Engineering, C: Materials for Biological Applications, 121, 111749. https://doi.org/10.1016/j.msec.2020.111749
Li, P., Ruan, L., Wang, R., Liu, T., Song, G., Gao, X., Jiang, G., & Liu, X. (2021). Electrospun scaffold of collagen and polycaprolactone containing ZnO quantum dots for skin wound regeneration. Journal of Bionic Engineering, 18(6), 1378–1390. https://doi.org/10.1007/s42235-021-00115-7
Saik, J. E., Gould, D. J., Watkins, E. M., Dickinson, M. E., & West, J. L. (2011). Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol) hydrogels. Acta Biomaterialia, 7(1), 133–143. https://doi.org/10.1016/j.actbio.2010.08.018
Judith, R., Nithya, M., Rose, C., & Mandal, A. B. (2010). Application of a PDGF-containing novel gel for cutaneous wound healing. Life Sciences, 87(1–2), 1–8. https://doi.org/10.1016/j.lfs.2010.05.003
Dearman, B. L., & Greenwood, J. E. (2021). Scale-up of a composite cultured skin using a novel bioreactor device in a porcine wound model. Journal of Burn Care & Research, 42(6), 1199–1209. https://doi.org/10.1093/jbcr/irab034
Sun, T., Norton, D., Haycock, J. W., Ryan, A. J., & MacNeil, S. (2005). Development of a closed bioreactor system for culture of tissue-engineered skin at an air–liquid interface. Tissue Engineering, 11(11–12), 1824–1831. https://doi.org/10.1089/ten.2005.11.1824
Wahlsten, A., Rutsche, D., Nanni, M., Giampietro, C., Biedermann, T., Reichmann, E., & Mazza, E. (2021). Mechanical stimulation induces rapid fibroblast proliferation and accelerates the early maturation of human skin substitutes. Biomaterials, 273, 120779. https://doi.org/10.1016/j.biomaterials.2021.120779
Kalyanaraman, B., & Boyce, S. T. (2009). Wound healing on athymic mice with engineered skin substitutes fabricated with keratinocytes harvested from an automated bioreactor. Journal of Surgical Research, 152(2), 296–302. https://doi.org/10.1016/j.jss.2008.04.001
Liu, J. Y., Hafner, J., Dragieva, G., & Burg, G. (2006). A novel bioreactor microcarrier cell culture system for high yields of proliferating autologous human keratinocytes. Cell Transplantation, 15(5), 435–443. https://doi.org/10.3727/000000006783981828
Kalyanaraman, B., & Boyce, S. (2007). Assessment of an automated bioreactor to propagate and harvest keratinocytes for fabrication of engineered skin substitutes. Tissue Engineering, 13(5), 983–993. https://doi.org/10.1089/ten.2006.0338
Norouzi, M., Shabani, I., Atyabi, F., & Soleimani, M. (2015). EGF-loaded nanofibrous scaffold for skin tissue engineering applications. Fibers and Polymers, 16(4), 782–787. https://doi.org/10.1007/s12221-015-0782-6
Golchin, A., & Nourani, M. R. (2020). Effects of bilayer nanofibrillar scaffolds containing epidermal growth factor on full-thickness wound healing. Polymers for Advanced Technologies, 31(11), 2443–2452. https://doi.org/10.1002/pat.4960
Mirdailami, O., Soleimani, M., Dinarvand, R., Khoshayand, M. R., Norouzi, M., Hajarizadeh, A., Dodel, M., & Atyabi, F. (2015). Controlled release of rh EGF and rhb FGF from electrospun scaffolds for skin regeneration. Journal of Biomedical Materials Research Part A, 103(10), 3374–3385. https://doi.org/10.1002/jbm.a.35479
Zhang, Z., Li, Z., Wang, Y., Wang, Q., Yao, M., Zhao, L., Shi, J., Guan, F., & Ma, S. (2021). PDGF-BB/SA/Dex injectable hydrogels accelerate BMSC-mediated functional full thickness skin wound repair by promoting angiogenesis. Journal of Materials Chemistry B, 9(31), 6176–6189. https://doi.org/10.1039/d1tb00952d
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MK and BG were major contributors in the writing and critical edition of the manuscript. All authors contributed to the study conception and design. The first draft of the manuscript was written by [Maryam Kaviani] and Dr. Bita Geramizadeh commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaviani, M., Geramizadeh, B. Basic Aspects of Skin Tissue Engineering: Cells, Biomaterials, Scaffold Fabrication Techniques, and Signaling Factors. J. Med. Biol. Eng. 43, 508–521 (2023). https://doi.org/10.1007/s40846-023-00822-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-023-00822-y