Abstract
Healthy skin protects from pathogens, water loss, ultraviolet rays, and also maintains homeostasis conditions along with sensory perceptions in normal circumstances. Skin wound healing mechanism is a multi-phased biodynamic process that ultimately triggers intercellular and intracellular mechanisms. Failure to implement the normal and effective healing process may result in chronic injuries and aberrant scarring. Chronic wounds lead to substantial rising healthcare expenditure, and innovative methods to diagnose and control severe consequences are urgently needed. Skin tissue engineering (STE) has achieved several therapeutic accomplishments during the last few decades, demonstrating tremendous development. The engineered skin substitutes provide instant coverage for extensive wounds and facilitate the prevention of microbial infections and fluid loss; furthermore, they help in fighting inflammation and allow rapid neo-tissue formation. The current review primarily focused on the wound recovery and restoration process and the current conditions of STE with various advancements and complexities associated with different strategies such as cell sources, biopolymers, innovative fabrication techniques, and growth factors delivery systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
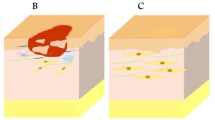
The human skin is the major and the most complex multilayered sensory organ, encompassing 1–1.5 m2 of total exposed surface area and also accounts for one-tenth of overall body mass (Moore and Chien 1988). It shields the body's internal vital organs from the hostile environment. Skin tissue is subdivided into three layers, called: the epidermis (upper layer), dermis (middle layer), and subcutaneous tissues or hypodermis (bottom layer), as shown in Fig. 1 (Kanitakis 2002; Abdo et al. 2020). The outermost waterproof epidermal layer is mainly composed of 90% of viable Keratinocyte cells along with melanocytes (containing skin color pigment-protein called melanin providing color to the skin and acts as a harmful UV ray barrier), Merkel cells (which mediate sensations and help in pressure detection on the skin), and Langerhans cells (provides immunity by recognizing antigens and presenting them to T-helper cells which further activates the immune responses) (Brohem et al. 2011; Yousef and Sharma 2018). In-between both the epidermis and dermis layers, a dermal–epidermal junction (DEJ) is found, called Rete ridges. It provides mechanical strength to the epidermis and also allows the diffusion of soluble molecules within the layers (Butcher and White 2005).
The dermis, or middle stratum of the skin, is positioned between the epidermal and subcutaneous fatty layers. It is an integrated connective tissue system comprising ECM components (including collagen, elastin, reticulin, polysaccharides), blood vessels, fibroblasts, mast cells, hair follicles, sweat glands, nerve endings, and lymphatic systems (Brohem et al. 2011; Roig-Rosello and Rousselle 2020). This layer provides elasticity, tensile strength, and pliability to the skin, thus protecting the body from abrasions and helping in wound healing (Roig-Rosello and Rousselle 2020).
The subcutaneous fatty layer or hypodermis is the bottom-most part of the skin tissue that acts as a storehouse of energy and exclusively comprises fat cells, fibroblasts, nerves, and blood vessels (Brohem et al. 2011). Its major role is to provide cushioning and heat to the body (Lawton 2019; Watt 2014). Overall, each layer of the skin has distinct functions and performs multiple active roles such as thermoregulation, body homeostasis, sensation, and body structure confinement. Additionally, it provides protection against physical, chemical, and biological influences caused by the external environment. Despite the above key roles, the skin also has a pivotal role in vitamin D synthesis and storage (Barry 1983; Park 2015; Lawton 2019).
Skin injuries can occur for various reasons, including thermal, mechanical, physiochemical, and biological damages. Depending on nature, type of damage, and its ability to cure, they fall into two categories: acute and chronic wounds (Boateng et al. 2008; Augustine et al. 2014). Acute wounds heal completely within 8–12 weeks by undergoing normal stages of wound healing. However, chronic wounds heal slowly, taking more than 12 weeks and leaving abnormal scars. It has been seen that chronic wounds reappear after recovery because of several factors such as poor blood circulation, edema, repetitive trauma, extensive wounding, infection, aging, obesity, and diseases such as diabetes and autoimmunity (Boateng et al. 2008; Cole-King and Harding 2001). Although precise observational studies on impaired chronic wounds are tough to obtain realistic data. However, research conducted in the United States proclaimed that chronic wound sickness affects 6–6.5 million individuals worldwide, costing $20 billion in the United States each year (Sen et al. 2009). As a result, wounds have a high incidence of severity and fatalities, as well as a significant psychological, psychosocial, and financial influence on individuals and substantial expenditures for the health system, making them a critical public health issue.
Skin wound healing is the inherent and highly complex process of the human body which involves several sequential phases, including hemostasis (blood clotting at the wound site), inflammation (immune cells infiltration that cleanses out the dead cell debris), re-epithelialization (keratinocyte's migration from the periphery to the center at a wound site), granular tissue formation (multiplication and migration of fibroblast cells and endothelial cells that secretes ECM components), vascular tissue regeneration and dermal remodeling (collagen remodeling and wound size reduction) (Demidova-Rice et al. 2012; Rousselle et al. 2019). Different external and internal factors significantly affect the tissue healing process, including body fluid, intrinsic and extrinsic factors, temperature, stress, oxygenation, medications, infections, diabetes, obesity, and nutrition. A healing process also varies with age; fetal wound healing happens rapidly, leading to scarless tissue formation, whereas the healing process slows down and leads to scarring in adults (Guo and Di Pietro 2010; Ding et al. 2021). The sequential regulated cycles that occur just after skin tissue damage and scar development are remarkably equivalent to those that occur after myocardial infarction and many other tissue injuries (Evans et al. 2013). In this respect, particularly owing to its availability, skin is among the greatest models for studying tissue regeneration mechanisms and developing innovative biomedical approaches.
Tissue engineering (TE), a crucial rising topic in therapeutic medical science and biological engineering, has shown tremendous promise in designing and constructing biosynthetic alternatives for cadaver tissues or organs, implants, and prosthetics to reduce patient suffering and mortality rates. Tissue-engineered skin substitutes (TESSs) provide an alternative therapeutic medication for major skin tissue injuries and burn wounds. Since the successful isolation and expansion of human epidermal cells and keratinocytes in 1975, TESSs have evolved from epidermal replacements to full-thickness grafts, including multiple skin seeded cells (Kim and Evans 2005). Recently, an incredibly innovative advancement in designing and constructing skin substitutes has been clinically reported in the domain of STE. Further, the skin tissue construct should meet the following attributes: clinical effectuality, biodegradability, durability, elasticity, biocompatibility, low toxicity, cytocompatibility, and medical stability. Although skin tissue engineering is one of the prominent therapies where translational investigations are at the frontline, the need to consider several important factors while designing and constructing a skin tissue scaffold, such as physicochemical properties, excellent inter-connectivity, standard pore size, permeability, etc., makes it much more challenging. For decades, split-thickness skin grafts (STSGs), blended and clinically grown skin autografts have been the global method of treatment for extensive burn wounds and are still the method of choice for wound closure (Liu et al. 2019). Commercially available acellular dermal substitutes like Integra®, Renoskin, Hyalomatrix, and Terudermis, comprising dermal and epidermal components, are extensively used by surgeons to achieve wound contraction and healing. However, these substitutes have certain limitations due to high economic costs and vulnerability to infection (Chocarro‐Wrona et al. 2019).
Various innovative advancements in designing and constructing skin substitutes have recently been clinically reported in the STE domain. Significant advancement has been achieved in designing and fabricating grafts for regenerative and biomedical purposes during the last few decades. However, a skin substitute that replaces or resembles the anatomy and physiology of the native healthy skin is yet to be achieved. The central purpose of this review article is to show the many approaches used in the research area of STE. It discusses the most up-to-date sophisticated techniques for creating perfect and effective skin replacements that can imitate the ECM structure of the skin tissue.
Overview of skin wound healing mechanism
The epidermis, dermis, and subcutaneous or fatty layers of skin serve as a defensive layer to shield pathogenic microbial infection and fluid loss (Yousef et al. 2017). A wound is developed due to the disturbance of layers of skin tissue. In superficial or minor wounds, the skin repairs to generate tissue nearly identical to the native skin. However, if the lesion is sufficiently deep or extensive, it will regenerate, leading to scarring. These injuries are regularly restored and regenerated in the human body through the inherent capability of the skin wound healing process. The complex and sequential skin repair process has been broadly grouped into four regulated overlapping stages: hemostasis (or blood clotting phase), inflammatory (activation and infiltration of immune cells phase), proliferative (multiplication and migration of skin cells), and tissue maturation/remodeling (extracellular matrix protein remodeling phase) (Zomer and Trentin 2018). The early stage in skin wound repair is hemostasis, and this phase lasts for seconds to hours. This phase includes many steps such as vasoconstriction, which decreases the flow of blood; platelet stimulation and recruitment at the site of damage which continues to produce numerous growth factors (GFs) that further promote cellular migration and multiplication; and lastly, the thrombus formation all around the blood clot (Rajendran et al. 2018).
After the clotting of blood at the injury site, within an hour, different inflammatory cells move into the injury and initiate the inflammation phase, which is marked by the successive infiltration of polymorphonuclear neutrophils (PMNs), monocyte differentiated macrophages (MΦ), and lymphocytes (Short et al. 2022). These inflammatory cells actively participate in phagocytosis, eliminating pathogenic microorganisms and cellular debris from the wound. Furthermore, they also secrete various GFs and cytokines, which facilitate the activation of different cells from nearby tissues, such as fibroblasts which further differentiate into myofibroblasts, endothelial cells, and keratinocytes during the proliferation phase (Wallace et al. 2017). These cells continue to migrate and proliferate, synthesizing new ECM, promoting neo-vascularization and forming granular tissue, progressively establishing an obstruction between the wounded region and the environment, permitting wound closure (Armstrong and Meyr 2022). The remodeling phase of the wound repair process, which lasts weeks to months, co-occurs with the development of neo-granular tissue. During this phase, recruited cells from the injured site (fibroblasts, myofibroblasts, macrophages, endothelial cells, and keratinocytes) remodel the skin's ECM to form collagenous networks. These cells release matrix metalloproteinases that degrade unorganized collagen Type III of the granular tissue and start replacing it with highly organized collagen Type I matrix, which closely resembles the intact skin tissue (Wilkinson and Hardman 2020).
The conventional approach to wound healing
The skin tissue itself has the capability to restore the damaged tissue naturally through the process of re-epithelialization and helps in maintaining its integrity and tensile strength (Barry 1983; Swaney and Kalan 2021). It is also observed that the self-healing properties of the skin get weakened in some circumstances, such as deep chronic wounds, diabetes, and non-healing ulcers, which further increases the chances of infections and also affects the patient's daily life. Thus these wounds require wound dressings that can assist and strengthen the wound healing process at the desired pace (Watt 2014; Lee and Koehler 2021). Wound patches, including natural or synthetic dressings, cotton wool, lint, and gauzes, were once used to keep injuries dry and sterile by aiding wound exudates to evaporate and preventing harmful microorganisms from infecting the wound (Boateng et al. 2008; Pereira and Bartolo 2016). Since pre-modern times, conventional techniques have been used to treat damaged tissue because of their low cost, feasibility, simplicity, and effectiveness in the wound healing process.
The skin grafts provide instant coverage for extensive wounds and protect against infections and fluid loss. The consolidated role of the graft further reduces inflammation and allows rapid wound healing. Surgeons generally use autografts to treat more profound dermal injuries due to their non-immunogenic nature (Koller 2005; Luo et al. 2019). Various drawbacks, including limited donor availability, are associated with autografts. The above limitations can be sorted out using allografts, genetically engineered xenografts, and amnion membranes (Koller 2005; Carter and Holmes 2016; Cooper et al. 2016; Liu et al. 2010). Clinically, it has been reported that most grafts may cause immune rejection and disease transmission during transplantation (Koller 2005; Liu et al. 2010). The placental amniotic layer in mammals is the thinnest, measuring up to 0.5 mm thick. (Murphy et al. 2017). Since the early twentieth century, amnion membranes have been employed extensively for wound and regenerative applications (John 2003). The human amniotic membrane is a safe, painless, and effective method to treat wounds and has been reported in numerous studies (Murphy et al. 2017; Farhadihosseinabadi et al. 2018). Irrespective of its benefits, it has significant drawbacks, such as low availability, quick biodegradation, handling issues, and high cost, all of which limit its use (Taghiabadi et al. 2015; Murphy et al. 2017). As a result, researchers in the discipline of tissue engineering and regenerative medicine (TERM) have been working to resolve the drawbacks of conventional established methods and develop a skin substitute that can mimic the functional skin tissue and be used to treat a broad range of skin problems, including acute and chronic wounds, as well as burns. Some of the current conventional graft implants that have been analyzed are tabulated below with their pros and cons as follows (Table 1).
Skin tissue engineering: principles
Tissue engineering, as described by Langer and Vacanti (Vacanti and Langer 1999), is an "inter-disciplinary field or a growing approach that uses the principles of basic biology, engineering and fundamental science for the restoration, improvement, and maintenance of damaged tissues, organ failure, and regeneration of the lost tissue. In recent times advancement in STE minimizes the need for organ transplantation, reducing graft rejection, pain, the need for solid immune suppressants, the transmission of diseases, complications, and the cost of processing (Hutmacher et al. 2001; Cao et al. 2020).
In skin tissue engineering, an ideal skin substitute should possess some desirable properties such that they should mimic the local skin environment, speed up the healing process of wounds, cytocompatibility, non-toxicity, allows regulated and sustained drug/growth factor release at the target site without losing its bioactivity and so on (Halim et al. 2010; Lord et al. 2017; Chen et al. 2022). A modern era of tissue engineering was started in the 1980s with the ultimate goal of maintaining, improving, strengthening, and recovering damaged or lost tissues (Alrubaiy and Al-Rubaiy 2009). The basic intellection behind tissue restoration includes 3Rs: tissue regeneration, repair, and replacement. Regeneration or resurrection is basically where the tissue is replaced with tissue itself, thus, initiating the regeneration where it is usually not seen. In tissue repair, drugs or growth factors are externally delivered to the targeted site to enhance the repair rate.
Furthermore, replacement is the possible alternative where tissue regeneration and repair are not possible. This approach replaces the damaged or lost tissue with a substitute. The 3R's concept can be applied to all types of tissues; however, the degree of complexity would differ among the targeted tissues, for example, kidney, heart, bone, liver, etc. The fundamental goal of this approach is to overcome the certain limitations associated with conventional methodologies and provide an alternative treatment to patients suffering from different disabilities and ailments (Theoret 2009).
The approach of tissue engineering triad
The tissue engineering strategies basically involve the concept of using the living cells (autogenic, allogenic, xenogenic, syngenic, stem cells, and genetically engineered cells), natural or synthetic scaffolds (must be biocompatible and biodegradable), and signals (growth factors, drugs, mechanical forces, and physiochemical signs) independently or in different combinations, to build a 3D construct. This concept further helps to regenerate the functional tissue-like structure under controlled laboratory conditions for other clinical applications (O'brien 2011; Almouemen et al. 2019). These three factors are collectively known as the "Tissue Engineering Triad," as shown in Fig. 2.
Currently, there are different ways to develop a functional tissue or an organ by using scaffolds, cells, or signaling molecules that can be accustomed separately or in various combinations in the form of a triad (O'brien 2011). Even though these factors have shown promising results when used independently, they can only help regenerate the target tissue to a certain extent. For instance, Cultured Epithelial Autografts (CEA) and Keratinocyte suspensions like Epicel, RECELL, and BioSeed have been used as clinical adjuncts with conventional treatment methods to accelerate wound re-epithelialization. However, they are still limited to minor injuries (Brockmann et al. 2018). Therefore, to achieve the comprehensive, a multi-functional skin substitute is required that can mechanically, micro-structurally, and functionally mimic the healthier tissue. The extraordinary advances in STE to recreate the functional skin substitute have been accomplished because of the developments in bio-fabrication methods, genetically engineered cell sources, and different ways of delivering the signaling molecules (Khademhosseini and Langer 2016; Bhardwaj et al. 2018). While all of these advancements are still being researched, there seems to be a substantial likelihood that many, not all but most, of the skin appendages, such as hair follicles, hair shafts, and sweat glands, will be included in future designs of skin replacements and medically accessible for the management of full-thickness or chronic injuries, including deep burns.
Clinically and commercially available TESSs for wound healing
Skin replacements are used to restore skin's integrity and functionality after burns or many other severe skin problems. In situations where conventional therapeutic approaches are not applicable, skin substitutes offer a solution to standard injury covering. All engineered skin replacements must meet some critical areas: patient safety, clinical efficacy, user-friendly, ease of availability, long shelf life, non-fragile, and cost-effective (MacNeil 2007; Wang et al. 2019; Kharaziha et al. 2021). In the broad sense, such therapeutic biomaterials should be non-cytotoxic, non-allergic, or induce no inflammation and pain. Furthermore, they should be biocompatible and allow complete wound healing without posing any scarring, fluid loss, infections, and disease transmission (Yu et al. 2018). There are not presently commonly available engineered skin substitutes that offer all of the aforementioned attributes, nor can they entirely replicate the morphological and functional features of native skin tissue. The majority of the presently commercialized and therapeutically accessible TESS products were addressed in this review study based on the different classifications discussed below.
-
1.
A layer of skin they restore:
-
2.
epidermal
-
3.
dermal
-
4.
dermo-epidermal (or composites)
-
5.
Duration of wound coverage:
-
6.
temporary (these replacements are often used as a wound dressing for a limited period to safeguard the wounded region from possible environmental risks such as microbial infection or physical damage and also offer safer as well as hydrated conditions to support and assist the healing process and are normally replaced with an autogenous graft after 3 to 4 weeks)
-
7.
semi-permanent (these are acellular replacements that may be left at the wounded location for many days and operate as secondary dressings to prevent fluid loss and microbial infections, as well as to heal and regenerate injured skin tissue, although they are ineffective for severely injured wounds)
-
8.
permanent (these TESSs have been used to replace and restore the overall equivalent depth of skin layers and to remain permanently at the injured site to enhance the overall integrity of the skin tissue, thus reducing the need for donor skin sites)
-
9.
Type and source of the biomaterial:
-
10.
biological (auto-, allo-, and xenografts)
-
11.
synthetic (biodegradable or non-biodegradable)
-
12.
Classification of skin replacement in terms of cellular components:
-
13.
cellular
-
14.
acellular
There are several distinct classes of commercially available TESS products that are now capable of helping with tissue healing and regeneration (Snyder et al. 2020) and are summarized in Table 2. Each has its own range of qualities that make it acceptable to be used in different ailments (burns or chronic wounds), but only a few can completely heal regenerative native skin tissue.
A patent overview for skin tissue engineering (STE)
STE is a novel approach that tends to bring combined multidisciplinary teams to facilitate effective healthcare translation. Severe deeper wounds, including burn wounds and other serious skin loss problems, are clinical reasons where tissue-engineered skin replacements are being researched as an alternative that can restore the epidermal and dermal structural integrity of the skin tissues. In comparison to existing standards of treatment, the TESSs could minimize fatalities and disability, thus improving the quality of people's lives and fully functioning results. In the STE discipline, it is sometimes neglected and difficult to understand how TE-related fundamental experimentation may be transferred to practical and translational work, eventually seeking to produce and commercialize TE products (Al-Himdani et al. 2017). However, technological advancements such as the advent of automated bioreactors and bio-printing for fully functional skin tissue development may make healthcare products more accessible (Zhu et al. 2022).
Furthermore, all TE products must be approved by governmental authorities (Food and Drug Administration, FDA in the United Nations, etc.) to assure excellent quality, reliability, health safety, and verified efficiency (Haddad et al. 2017). Over the last few decades, various trustworthy STE patents have been researched; for example, RenovaCare, Inc. has recently established patented innovative technologies such as the CellMist™ and SkinGun™ systems spraying self-donated stem cells for functional skin tissue and other organ regeneration (Kareem et al. 2021). Other patented TESSs include Kerecis's fish-skin products; dCELL (decellularized dermal skin allograft); ReCell (device for extraction of autologous skin cells), and many more, which are used for healing and regeneration of a wide variety of skin wounds (Dai et al. 2020; Kirsner et al. 2020). In one research, in vitro examinations of decellularized fish skin revealed improved structural and mechanical qualities comparable to normal human skin for regeneration, as well as improved cellular activities, indicating that it might be a viable choice for skin tissue regeneration (Kamalvand et al. 2021). Skin sprays are a potential approach for STE applications since they can transport cells and biocompatible hydrogels to extensive lesions with ease and safety. Several clinical studies are being conducted to investigate spray solutions for skin illnesses such as chronic wounds, diabetic foot ulcers (DFUs), psoriasis and others (Esteban-Vives et al. 2018; Chen et al. 2020). Decellularized ECM (dECM) derived from diverse human and animal tissues has been used for burn wound therapy and surgical restoration for the past few decades. Tissue decellularization is necessary for transplantation operations to minimize the activation of immunological responses and inflammatory responses that cells in the donor tissue may trigger, leading to transplant rejection. For instance, Singh et al. demonstrated that integrating curcumin into modified decellularized small intestine submucosa (SIS) membranes resulted in potent antimicrobial and free radical scavenging activity, making them effective in neutralizing the negative effect of oxidative stress and bacterial colony development in chronic skin wounds (Singh et al. 2022). Eventually, these approaches and advancements in STE will reduce or eliminate the requirement for skin autografts and allow for faster commercial translation of viable autologous engineered skin grafts ideal and safe for patient usage.
The current approaches in STE
An impactful TESSs, at its most fundamental aspect, optimally replicates the complexity of the original 3D anatomy and performs the mechanisms of biological skin tissue. Furthermore, these substitutes should promote pre-vascularization and offer supporting signals to cells in the surrounding environment. Finally, it should be able to successfully integrate into the recipient with minimum or no scarring while creating a minimal and regulated immune response if transplanted in vivo. Over the past few generations, a wide range of strategies has been developed and implemented in the STE field. The foundation of this area is the notion that efficient tissue growth requires the synergistic action of numerous cell types rather than the isolated implications of any one population. Currently, several approaches are investigated to design skin substitutes analogous to native healthy skin. Over the past three decades, TESS developed by Cincinnati, Ohio, researchers comprised of autologous cells, including keratinocytes and fibroblasts seeded in bovine-derived collagen-glycosaminoglycans (GAG) scaffold is currently considered to be the most clinically successful scaffold with skin structure analogy (Mohamad et al. 2019; Smiley et al. 2006). Yet another skin substitute based on bovine collagen is commercially available denovoSkin™, made up of collagen hydrogel, fibroblast, and keratinocytes. DenovoSkin™ has received FDA and EMA Orphan status to treat burns under Advanced therapy Medical Product (ATMP) (Schiestl et al. 2021). However, these skin tissue substitutes have certain limitations owing to their xenogenic components, raising the risk of an immune response and high cost of production. Few approaches have shown significant clinical effectiveness, using fibroblast alone or with a subsequent seeding density of keratinocytes along with fibroblasts. However, the constrain of the long production time (about 9 weeks) still remain a challenge. Furthermore, researchers use autologous, allogenic, xenogenic, and stem cells as a cell therapy to create skin analogs that closely resemble native tissue architecture (Sierra-Sánchez et al. 2021). The creation of stratified constructions matching the bilayered organization of the epidermis and dermis has dominated skin restoration in tissue regeneration for the most part. An ideal engineered skin construct must allow proper cell adherence, regulated and sustainable delivery of growth factors/drugs, and the re-growth of the tissue in the injured region after its implantation. Currently, three major approaches are being used to create the best possible skin substitutes: scaffold-based, cell-based, and drug/signals delivery-based approaches, as briefly discussed below and schematically represented in Fig. 3.
Diagrammatic representation illustrating different strategies of STE. STE Approach I: cells isolated from an individual (A1), isolated cells are further cultured under controlled in vitro conditions (A2) to differentiate and proliferate, eventually the cultured cells are genetically modified (A3 and A4) and expanded under specified lab conditions (A5) preceding to potentially being re-implanted into the same individual’s body (A6). STE Approach II: explanted genetically altered cells (A7) along with growth factors/drugs (B) could be added to the polymeric scaffolds (C1) before implantation onto the target site for tissue restoration (C2); Approach III: Pre-implantation, cell-seeded or GFs/drug-loaded constructs (D1) can be cultivated in a bioreactor to develop an artificial skin tissue (D2) and then transplanted at the target site of an individual (D3)
Scaffolds: different biomaterials and fabrication methods
The "Scaffolds" play a significant supportive role in the domain of TERM because they tend to reproduce the structural and functional properties of ECM, as well as aid in enhancing the cellular activities, growth factor delivery, and neo-vascularization in vivo to improve healing and regeneration of the worn-out tissue (Shafiee and Atala 2017; Dutta and Dutta 2009). The primary purpose of an ideal scaffold is to offer a three-dimensional habitat for cells to grow and synthesize their matrix, which will eventually replace the scaffold, resulting in a three-dimensional structure for the injured tissue (Yu et al. 2019). Its framework determines the final structural layout of newly formed soft or hard tissue. The synthesis of cellular matrix and breakdown of scaffold framework should be coordinated so that one process does not overtake the other (Naderi et al. 2011; Afjoul et al. 2020). In addition, a variety of factors determine the scaffold's selection and functionality (Auger et al. 2013; Atala 2012; Solovieva et al. 2018; Ghosal et al. 2017; Chang and Wang 2011; Mndlovu et al. 2020), as shown schematically in Fig. 4. The scaffold can be employed as a delivery system to supply growth factors/drugs/signals to the affected area to guide and promote cellular proliferation and differentiation for controlled tissue growth (Naderi et al. 2011; Chen et al. 2022; Sharma et al. 2020). A large variety of biomaterials with natural, synthetic sources and their blends, as well as innovative manufacturing processes, have been suggested in recent decades and are briefly outlined hereunder.
Scaffolding materials: natural, synthetic, and composites
Tissue-designed skin substitutes have been in use for decades, and the biomaterial used for scaffolding must be customized to the specific requirements of the target tissue. Numerous research work has demonstrated the positive impacts of biomaterial modification on skin tissue engineering scaffolds qualities such as pro-angiogenesis, excellent physiochemical properties, enhanced cellular responses, and many other superior attributes (Shi et al. 2018; Zhang et al. 2017b; Zehra et al. 2020; Yin et al. 2021; Eskandarinia et al. 2020; Olad and Hagh 2019; Martins et al. 2018; Chandra et al. 2020). The development of "smart or novel biomaterials" capable of regulating cellular activities and/or increasing tissue functioning is now the subject of study (Furth and Atala 2014; Govindharaj et al. 2019). The interaction between the scaffold and cells depends on the properties of the selected material, either natural or synthetic, applied for the specific tissue. In producing engineered skin substitutes, three distinct biomaterials are most often used, including synthetic polymers, natural polymers, and their blends, as summarized in Table 2.
Natural polymers, often known as biopolymers, are organic compounds produced by biological entities. They are further sub-divided into two categories, including protein-based materials (collagen, fibrin, silk fibroin, keratin, and gelatin) and polysaccharide-based materials (hyaluronan, cellulose, alginate, chondroitin, and chitosan) (Sahana and Rekha 2018). The freeze-drying approach was used to generate an entirely novel ciprofloxacin-loaded collagen–chitosan matrix for wound therapy (Tripathi et al. 2021b). In current history, 3D printed chitosan (Ch) structures with enhanced cytocompatibility and biocompatibility were already viewed as a useful and convenient biomaterial for wound repair (Intini et al. 2018). Several prior studies have shown that blending gelatin with a variety of other natural biopolymers promotes human dermal fibroblasts (HDF) growth and division, which further enhances the rate of wound healing (Vatankhah et al. 2014; Yuan et al. 2018). Multiple studies have shown that different natural polymers and their combinations could construct scaffolds for wound healing applications (Sajjad et al. 2020; Afjoul et al. 2020; Zhang et al. 2017a).
Synthetic polymers, such as polylactic acid (PLA), polyvinyl alcohol (PVA), polycaprolactone (PCL), polyglycolic acid (PGA), and their copolymers (e.g., PLGA, etc.) are extensively implemented. The FDA authorized biomedical skin applications because of their non-toxic by-products and tunable physiochemical characteristics, processability, and malleability (Pina et al. 2019). The biodegradability and biocompatibility of Ciprofloxacin-loaded electrospun PLGA nanofibrous mats treated with sodium alginate microparticles as skin replacements were studied by Liu et al. (2018b). In one research, composite scaffolds made of PCL, Zein, and Gum Arabic exhibited increased cellular activity, porosity, and hydrophilicity (Sharifi et al. 2020). In vitro and in vivo investigation exhibited cytocompatibility, remarkable tissue development, and neovascularization, confirming its promising applications for skin tissue engineering (Zhang et al. 2018). A dual system of PVA and sodium alginate (SA) hydrogel integrating bFGF-loaded PCL microspheres exhibited enhanced cell-induced wound healing in vitro and in vivo (Bahadoran et al. 2020).
Almost all of the above-discussed biomaterial has its own set of benefits and, unsurprisingly, drawbacks. Composite scaffolds derived from natural sources and/or synthetic materials combine the benefits of each polymer's specific chemical, mechanical and biological qualities to surpass each polymer's limits and allow the construction of an ideal as well as a functional skin substitute. Electrospun hybrid nanofibrous mats (PVA/Chitosan/Starch) were shown to be non-cytotoxic and antibacterial, allowing for faster wound healing (Adeli et al. 2019). The fibroblast-loaded composite nanofibrous scaffold (PCL/gelatin/collagen type I) could be a possible future strategy for repairing and regenerating skin tissues (Gomes et al. 2017). The recombinant collagen-hyaluronic acid composites had outstanding physical characteristics and bio-compatibility, suggesting that they might be utilized for wound repair or biomedical applications (He et al. 2020). In current history, the results of cross-linked nanofibrous bilayer scaffolds (Fish collagen-PCL) demonstrated improved healing of the target tissue (Chandika et al. 2021). In vitro and in vivo investigations with curcumin-loaded Cellulose-PLLA-nanosilica showed improved cell proliferation, granular tissue formation, neovascularization, wound healing, and full-thickness tissue restoration (Ramphul et al. 2020).
Scaffold fabrication strategies for STE
Modern TERM methods have primarily focused on 3D porous hydrogels or composite scaffolds generated in nanostructured frameworks featuring regulated breakdown efficiency and permeability for gas, nutrition, and growth regulatory component transfer. 3D biocompatible scaffolds in TE serve as cell and GF/drug mediators, providing a favorable environment for cell expansion and controlled GF release. The usage of TE constructs can alleviate the scarcity of tissues, including skin, bone, cartilage, and so forth, minimizing the need for animal models while increasing the dependability of observational data. Various fabricating procedures, as described in Fig. 5, are often used to create 3D polymeric constructs with excellent porosity, interconnectivity, uniform pore size, and surface area using biodegradable and biocompatible natural/synthetic materials. Traditional and advanced rapid prototyping are two methods for fabricating functionalized 3D constructs with suitable designs. Conventional fabrication methods (e.g., porogen leaching (Yin et al. 2016), phase separation (Mi et al. 2003), gas foaming (Ji et al. 2011), lyophilizer (Afjoul et al. 2020; Bahadoran et al. 2020; Niu et al. 2020), and many more), are inexpensive, easy to scale up and allow to construct of a scaffold of the desired shape. Still, they frequently fail to offer appropriate strength characteristics, and as a result, such scaffolds distort due to cellular movements. On the other hand, the prototypic method (e.g., electrospinning Atila et al. 2015; Fang et al. 2019; Lopresti et al. 2021), blow spinning (Singh et al. 2018, 2019), 3D bioprinting (Liu et al. 2018a; Seol et al. 2018; Michael et al. 2013; Kim et al. 2017), melt-blowing, etc.) seems to have no drawbacks and could offer additional necessary qualities, including higher porosity, physiochemical properties as well as enhanced cellular activity to TESS (Table 3).
The current advanced strategy to fabricate scaffolds includes 3D bioprinting. It's indeed a CAD-based approach in which live cells and structural material or hydrogel are deposited in a temporal and spatially structured way using a printer-based dispensing mechanism (Vijayavenkataraman et al. 2016). The polymeric solution containing cells (or bioinks) are printed in a 3D framework using a range of processes, including extrusion-, laser- and inkjet-based methods (Mahendiran et al. 2021; Liu et al. 2018a; Seol et al. 2018; Michael et al. 2013; Kim et al. 2017), and the entire procedure is illustrated in Fig. 6 (Augustine 2018). 3D bioprinting allows for accurate cell communication inside a 3-dimensional space by allowing the architecture to be controlled at both the micro-and nano-scale (Michael et al. 2013). Therefore, it is conceivable to create human skin, cartilage, blood vessels, and bone in their native form. A 3D printing approach combining two or more popular scaffold manufacturing technologies is currently being developed. Kim et al. demonstrated the effects of integrating the two 3D printing techniques on wound healing and regeneration (Kim et al. 2017). Despite the many advantages of 3D printing, fabricated scaffolds may well not encourage biologically important responses (Tamay et al. 2019). 4D bioprinting could be utilized to resolve the challenges associated with 3D printed technology in which 3D printed frameworks are designed to change over time in response to external or internal stimuli (physiochemical and biological responses) (Mota et al. 2020; Qasim et al. 2019). The same rapid prototyping processes and technologies used in three-dimensional printing are used in four-dimensional printing. Table 4 summarizes ongoing cellular and acellular research for STE that used various scaffolding methods.
“Steps in the fabrication of bioprinted skin. Various cells such as keratinocytes, fibroblasts and melanocytes would be collected from the patient and grow and multiply in cell culture system. A suitable biopolymer is mixed with the cells and the formed bioink is fed to the bioprinting system. Features of the wound are captured and a 3D structure is reconstructed using CAD/CAM approaches. According to the 3D pattern, wound tissue will be reconstructed, allowed for maturation in vitro and implanted back to the patient” by (Augustine 2018), licensed under CC by 4.0
Cell sources and their application in STE
Cells are key constituents of skin because they play a multitude of activities in maintaining natural skin homeostasis and physiological functions. Cells including keratinocytes, endothelial cells, melanocytes, and dermal fibroblasts are often used in skin injury and burn therapeutic products (Shevchenko et al. 2010; De Pieri et al. 2021). Tissue regeneration advancements have improved the potency and application of cell-based treatments. There are several methods for strengthening cellular skin therapies. This would include cell type selection, cell source (autologous vs. allogenic vs. xenogenic), and strategies for increasing cellular survivability and biological function after seeding. The first methodology is to use cells as a therapeutic agent for the restoration of functional tissue (De Pieri et al. 2021). This method entails isolating various types of skin cells, including autologous, allogenic, and xenogenic, growing them under suitable growth conditions, and then implanting them back to the target site for proper tissue functioning and restoration. Furthermore, the second approach involves isolating and seeding multiple types of skin cells onto scaffolds from diverse sources, subsequently implanting them back into the target site for appropriate tissue functioning and restoration (Sierra-Sánchez et al. 2021; Goyer et al. 2019).
The use of autologous cell sources in TE is of significant clinical interest because of their cost-effectiveness and non-immunogenic nature, reducing the intake of solid immunosuppressants and their ill effects. Still, their limited availability, long culturing periods, handling issues, and scarring limit their usage for clinical purposes (Cozzolino et al. 1999; Olender et al. 2011; Goyer et al. 2019; Sierra-Sánchez et al. 2021). The other cell sources, such as—allogenic and xenogenic, provide an alternative to autologous cell sources with the advantage of their ample availability, ease to use, painless grafting, and scar-free tissue formation (Olender et al. 2011; Salgado et al. 2017). However, autologous cells source is clinically preferred over allo- and xenogenic cell sources because later one has certain limitations such as their immunogenic nature, intake of solid immunosuppressants, high risk of disease transmission, and ethical issues (Lu et al. 2011; Tomford 1995; Haldar et al. 2019).
In the current scenario, a wide range of cell sources, including genetically modified stem cells as shown in Fig. 7 (Kwon et al. 2018), and cell lines have been explored and are manipulated, providing an alternative option to the above-discussed cell sources in the field of skin tissue regeneration and are extensively discussed in Table 5. Clinically, the most common and actively used stem cell sources for STE are discussed below.
“Stem cell engineering strategy” by (Kwon et al. 2018), licensed under CC by 4.0
Embryonic stem cells (ESCs), derived or extracted from the blastocyst's inner cell mass, are the most appropriate cell source that can be used for the origin of various differentiated cell populations under controlled culture conditions (Kitsberg 2007). Even though ESC have remarkable advantages such as abundancy, self-renewal potency, and pluripotency, their usage is limited at clinical levels because of their immunogenic nature, ethical issues, the transmission of inherited diseases or chromosomal aberrations (Kwon et al. 2018), and the formation of teratomas during the differentiation of ESCs into a particular lineage (Spits et al. 2008; Khademhosseini et al. 2020). The hESCs and iPSCs bank typed with human leukocyte antigen (HLA) have recently been tested in clinical trials to overcome the immunological rejection problem in allogenic patients associated with these stem cell therapies (Taylor et al. 2011).
Adipose-derived stem cells (ADSCs) are mesenchymal-derived adult stem cells that can be found in any white animal tissue, connective tissue, and omental fat. These stem cells are younger and have the potential to regenerate themselves with multipotential cellular differentiation (mostly cartilage, bone, tendon, and fat), and are easy to harvest in large quantities via the liposuction process under optimal culture conditions (Estes et al. 2006; Li et al. 2014). One study of an albino rat with a full-thickness defect treated with a bioscaffold composed of adipose-derived stem cells showed minimal wound contraction, ECM remodeling, epithelialization, and promoted angiogenesis (Ozpur et al. 2016). In another study, the 3D-printed ADSC-loaded scaffolds showed an enhanced wound healing and angiogenesis rate, thus providing an ultimately engineered skin graft (Roshangar et al. 2021). These cells are still in clinical trials to monitor their cellular properties and efficacy (Aso et al. 2016).
Mesenchymal stem cells (MSCs), similar to ADSCs, are adult stem cells with the capacity to distinguish between nearly all distinct types of cellular lineages, such as osteoblasts, chondrocytes, muscles, and adipocytes (Tong et al. 2015). A growing body of research indicates that MSCs (mesenchymal stromal cells) could be helpful in the treatment of cutaneous wound tissue regeneration. In a research of a pig partial-thickness severe burn wound model, it was discovered that MSCs could develop into dermal cells (Li et al. 2006), allowing for a faster wound healing process, keratinization, and wound contraction, as well as increased vascularization. In one survey, MSCs were found to facilitate the functionality of rejuvenated skin, regulate collagen accumulation, augment re-epithelization, significantly improve neo-angiogenesis, and encourage restoration of skin appendages by seeding them well within the scaffolding matrix (Formigli et al. 2015). Besides this, MSCs possess immune-modulatory, pro-angiogenic, and scarless wound healing properties (Li et al. 2019; Martinello et al. 2018). MSCs provide an ultimate auto- or allogenic source for the treatment of moderate to severe wounds/burns and relatively lacking ethical issues in comparison to ESCs (Pittenger et al. 1999; Maxson et al. 2012). Several MSC-based cellular therapeutics are clinically approved and available at the commercial level. However, their stability and efficacy are still in discussion for several reasons, including their limited proliferation rate, reduced secretion of growth factors, and the loss of cellular differentiation (Jossen et al. 2018; Zhang et al. 2012).
Another stem cell source known as induced pluripotent stem cells (iPSCs) has been managed to be produced from the differentiated adult stem cells by artificially reprogramming the main four transcription factors (Oct-4, Sox-2, Klf-4, and c-Myc) (Takahashi and Yamanaka 2006; Takahashi et al. 2007). The iPSCs, in their potency, are very similar to embryonic stem cells (ESCs) and are the inexhaustible and renewable resource of autologous cells (Park et al. 2008). One study showed that the potential proliferation and survival rate of iPSCs-derived MSCs is better than that of bone marrow-derived MSCs because of the higher expression of the KCNH1 ion channels. However, the functional role of these ion channels on the proliferation rate of iPSCs-MSCs is still not fully understood (Zhang et al. 2012). Furthermore, iPSCs-derived human melanocytes are being investigated to treat the skin discoloration disorders such as albinism, vitiligo, or melasma. They can also be used as a cell source for the development of TESSs (Ohta et al. 2011; Gledhill et al. 2015). According to a recent study, an organoid culture technique could be used to build multilayered skin from the human-induced pluripotent stem (Lee et al. 2020). Itoh and his co-workers have created in vitro 3-D skin analogs made entirely of hiPSC-derived skin cells, mainly keratinocytes and fibroblasts (Itoh et al. 2013). However, iPSCs are clinically prohibited because of their immunogenicity, tumorigenic nature (caused by genetic instability and active reprogramming factors), and heterogeneity (Garreta et al. 2018; Yamanaka 2020; Doss and Sachinidis 2019). As a consequence, researchers are concentrating their efforts on establishing alternative approaches to iPSC-related complexities. For example, tumorigenic testing kits are accessible to anticipate the presence of tumors, HLA-typed hiPSCs, as well as HLA or immunocloaking practices, are in clinical trials to decrease immunogenicity and the generation of naive human iPSCs, or the employment of diverse attributes to combat heterogeneity (Sato et al. 2019; Taylor et al. 2011; Gornalusse et al. 2017; Rohani et al. 2020; Kunitomi et al. 2016).
On the other hand, cell lines are a viable alternative to the previously mentioned cell sources due to their robustness, immortality, and ease of culture. HeCaT (human keratinocyte cell line) (Intini et al. 2018), HFF (human foreskin fibroblast cell line) (de Torre et al. 2018), L-929 (murine fibroblast cell line) (Bahadoran et al. 2020), NIH3T3 (fibroblast cell line) (Fang et al. 2019), and other cell lines are currently being utilized in clinical practices to develop a biocompatible skin substitute. Above mentioned cell lines can also be used as a feeder layer to nourish and support grown cells (Unger et al. 2009). Instead of being robust and immortal, they may become genetically modified or have significantly altered cellular properties, resulting in differences in the outcomes, and thus are not very dependable (Seo et al. 2012).
Growth factor/drug delivery strategy and their role in STE
Tissue restoration and re-growth are a crucial part of the healing process and are thus essential for all species' proper functioning and survivability. Growth factors/bioactive/drugs were already well understood as a crucial aspect of the wound recovery process, whose involvement begins when damage occurs and persists until the injury is entirely cured. Growth factors are either produced immediately by cells within the body or become incorporated into the matrix and delivered in a regulated way. Many composites can provide crucial structural reinforcement and adhesion sites, but they cannot influence cellular phenotype as effectively as signaling molecules. These factors are the primary source of chemical and biological signals in biomedical applications by guiding the ultimate destiny of cells and permitting the regeneration of target tissue (Ladewig 2011; Demidova-Rice et al. 2012; Atienza-Roca et al. 2018). The characteristics of the GFs/drugs and polymers utilized to enable effective restoration of wounds and tissue regeneration are just as essential as the delivery methods adopted. Targeted and sustained administration of wound healing agents has excellent promise for critical patient wound treatment, especially as the proportion of individuals diagnosed with incurable chronic infections grows across the world (Whittam et al. 2016).
Exogenous GF's/drugs are a viable therapeutic option for many chronic wound conditions. A variety of GFs, such as PDGF, VEGF, EGF, FGF, KGF, and TGF-α, β have been extensively established in studies for their ability to hasten wound healing (Atienza-Roca et al. 2018; Martí‐Carvajal et al. 2015). Growth factors or drug delivery methods have recently been studied in wound healing and skin re-growth and are summarized in Table 6. The potential of fabricating method and biomaterial framework to retain and allow for customized release kinetics (Wang et al. 2017) (e.g., burst, sustained, prolonged, mixed, and pre-programmed) of relevant dosages of GF's or drugs for an extended period well within the specified site is driving attention in their advancement towards their treatment delivery (Wang et al. 2015, 2016; Geer et al. 2005; Goh et al. 2016; Garcia-Orue et al. 2017; Choi et al. 2017; Değim et al. 2011; Gainza et al. 2014; Mizuno et al. 2003; Niiyama and Kuroyanagi 2014; Li et al. 2017; Xu et al. 2020; Cao et al. 2015; Shi et al. 2019; Tripathi et al. 2021b; Mo et al. 2017; Ghaseminezhad et al. 2020). For example, a regulated and prolonged release of EGF is necessary to boost cell proliferation along with wound healing and neo-tissue development (Johnson and Wang 2013). In addition, these delivery systems should preserve biostability, restrict burst release, reduce immune reactions, enhance cellular activity, favor neo-angiogenesis, permit effective chronic wound healing, and allow for neo-tissue regeneration (Ladewig 2011; Demidova-Rice et al. 2012; Atienza-Roca et al. 2018; Whittam et al. 2016; Wang et al. 2017; Martí‐Carvajal et al. 2015).
Conclusion and future direction
As stated earlier, distinct layers of skin tissue have prominent non-homogenous properties that must be considered while evaluating and developing constructs for skin tissue engineering purposes. The worldwide cost of skin wounds has significant public health care consequences, accounting for almost half of the globe's yearly medical expenditure. The rising area of skin tissue engineering (STE) offers hope to sufferers who urgently demand skin grafts in a period when tissues seem to be less available for transplantation, and there is a significant need for viable and functional alternatives. While assessing the skin regenerative characteristics of tissue-engineered implants, wider and complex injuries have a number of difficulties that must be taken into account. To present, potential therapeutic findings have been reported using TESSs based on cells and drug delivery systems in terms of effective transplant (60–90% in most investigations), durability (minor negative effects in certain case scenarios), re-epithelialization, and injury recovery rates.
In general, the translation of such TE skin grafts utilizing the innovative technologies discussed above demands model-based test techniques as well as relevant norms and standard safety guidelines in order to prove the transplants' durability, stability, and fully operational dependability. However, after achieving significant clinical achievements with widely accessible commercial skin substitutes, the desire for an ideal full-thickness graft that precisely replicates the host's native tissue in terms of multilayer complexity, thermoregulation, sensations, appendage regeneration, Ultraviolet rays shielding, pigmentation, impaired angiogenesis, and reduced scarring continues to be unfulfilled. Angiogenesis is critical for the effectiveness of engineered skin grafts, resulting in a longer life period and improved compatibility with the host's native skin. By integrating cells including endothelial cells, MSCs, and ADSCs into the scaffold framework as well as utilizing angiogenic active biomolecules, and modifying the microstructural and functional features of the engineered grafts, in vivo neo-angiogenesis of the grafts may be encouraged (Hashemi et al. 2021). It is feasible to promote the migration and recruitment of cells, either stem cells or progenitor cells, at the injury site by developing bioactive smart scaffolding materials in vitro with appropriate 3D frameworks. Additional strategies for preserving artificial tissues are required for effective transportation from the manufacturing location to the implantation site still has to be improved.
The following are some of the most significant challenges and the active areas of future research:
-
(i)
selection and designing of different composites that can stimulate cellular activities, promote pre-vascularization and tissue re-growth;
-
(ii)
choosing a manufacturing technique capable of creating a scaffold with suitable skin tissue-specific properties;
-
(iii)
identifying the type and origin of cell source, along with growth factors, in perspective of diversity throughout the layers of the skin tissue and;
-
(iv)
development and optimization of innovative GFs/drug delivery methods to maintain and strengthen the therapeutic effectiveness of GFs/drugs on wound healing by exerting temporal and spatial command throughout their delivery.
To summarize, these many techniques to develop advanced skin replacements, such as the utilization of stem cells, smart biomaterial, rapid prototyping fabrication techniques, and novel drug delivery systems, provide new optimism that the functional and ideal skin constructs may indeed be produced soon and will be easily accessible in adequate quantities and cost-effective. Latest advancements, particularly in the engineering of biomaterials allowing integration into skin replacements, along with stem cell therapy, suggest that more successful methods may be possible shortly.
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- 3D:
-
Three dimensional
- 3R's:
-
Regeneration, repair, and replace
- 3T3 fibroblasts:
-
3-Day transfer, inoculum 3 × 105 fibroblast cells
- ADSCs:
-
Adipose-derived stem cells
- AgSD:
-
Silver sulfadiazine
- ATMP:
-
Advanced therapy medical product
- BMSCs:
-
Bone marrow stem cells
- BC:
-
Bacterial cellulose
- CAM:
-
Computer-aided manufacturing
- CAD:
-
Computer-aided design
- CEA:
-
Cultured epithelial autografts
- Ceffe:
-
Cell-free fat extract
- Ch:
-
Chitosan
- CM:
-
Cortex Moutan
- CNCs:
-
Cellulose nanocrystals
- Col:
-
Collagen
- CS:
-
Chondroitin sulphate
- dECM:
-
Decellularized extracellular matrix
- DEJ:
-
Dermal–epidermal junction
- DFUs:
-
Diabetic foot ulcers
- ECM:
-
Extracellular matrix
- EE%:
-
Encapsulation efficiency percentage
- EMA:
-
European medicines agency
- EUP3:
-
Polysaccharide—Eucommia ulmoides
- FDA:
-
Food and Drug Administration
- GAGs:
-
Glycosaminoglycans
- GFs:
-
Growth factors
- hiPSCs:
-
Human-induced pluripotent stem cells
- hBMSCs:
-
Human bone marrow derived mesenchymal stem cells
- HLA:
-
Human leukocyte antigen
- HeCaT:
-
Human epidermal keratinocyte cell line
- HFF:
-
Human foreskin fibroblast
- HDF:
-
Human dermal fibroblasts
- HUVECs:
-
Human umbilical vein endothelial cells
- HEKs:
-
Human epidermal keratinocytes
- HA:
-
Hyaluronic acid
- hKCs:
-
Primary human keratinocytes
- hFBs:
-
Primary human fibroblasts
- IGF-1:
-
Insulin growth factor-1
- KCNH-1:
-
Potassium voltage-gated channel subfamily H member-1
- Kef:
-
Kefiran
- KGF:
-
Keratinocyte growth factor
- L-929:
-
NCTC clone 929 Clone of strain L fibroblast cell line
- MSCs:
-
Mesenchymal stem cells
- MDA-MB-231:
-
Epithelial, human breast cancer cell line
- MCF7:
-
Breast cancer cell lines
- MΦ:
-
Macrophages
- NIH3T3:
-
Mouse embryonic fibroblast cells
- NHDF-neo cells:
-
Human dermal fibroblasts-neonatal cells
- PLA:
-
Polylactic acid
- PCL:
-
Poly(caprolactone)
- PLGA:
-
Poly D,L-lactic-co-glycolic acid
- PDGF:
-
Platelet-derived growth factor
- PVA:
-
Polyvinyl acetate
- PGA:
-
Polyglycolic acid
- PEO:
-
Polyethylene oxide
- PU:
-
Polyurethane
- PEG:
-
Polyethylene glycol
- PMNs:
-
Polymorphonuclear neutrophils
- rhVEGF:
-
Recombinant human vesicular endothelial growth factor
- SIS:
-
Small intestine submucosa
- STE:
-
Skin tissue engineering
- STSGs:
-
Split-thickness skin grafts
- TE:
-
Tissue engineering
- TESS:
-
Tissue-engineered skin substitutes
- TERM:
-
Tissue engineering and regenerative medicine
- TGF- α,β:
-
Transforming growth factor- α,β
References
Abdo JM, Sopko NA, Milner SM (2020) The applied anatomy of human skin: a model for regeneration. Wound Med 28:100179
Adeli H, Khorasani MT, Parvazinia M (2019) Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int J Biol Macromol 122:238–254
Afjoul H, Shamloo A, Kamali A (2020) Freeze-gelled alginate/gelatin scaffolds for wound healing applications: an in vitro, in vivo study. Mater Sci Eng, C 113:110957
Al-Himdani S, Jessop ZM, Al-Sabah A, Combellack E, Ibrahim A, Doak SH, Hart AM, Archer CW, Thornton CA, Whitaker IS (2017) Tissue-engineered solutions in plastic and reconstructive surgery: principles and practice. Front Surg 4:4
Almouemen N, Kelly HM, O’leary C (2019) Tissue engineering: understanding the role of biomaterials and biophysical forces on cell functionality through computational and structural biotechnology analytical methods. Comput Struct Biotechnol J 17:591–598
Alrubaiy L, Al-Rubaiy KK (2009) Skin substitutes: a brief review of types and clinical applications. Oman Med J 24(1):4
Amani H, Dougherty WR, Blome-Eberwein S (2006) Use of Transcyte® and dermabrasion to treat burns reduces length of stay in burns of all size and etiology. Burns 32(7):828–832
Armstrong D, Meyr A (2022) Basic principles of wound healing. Uptodate[Internet] Waltham (MA) UpToDate Inc
Aso K, Tsuruhara A, Takagaki K, Oki K, Ota M, Nose Y, Tanemura H, Urushihata N, Sasanuma J, Sano M (2016) Adipose-derived mesenchymal stem cells restore impaired mucosal immune responses in aged mice. PLoS ONE 11(2):e0148185
Atala A (2012) Regenerative medicine strategies. J Pediatr Surg 47(1):17–28
Atienza-Roca P, Cui X, Hooper GJ, Woodfield TB, Lim KS (2018) Growth factor delivery systems for tissue engineering and regenerative medicine. Cutting-edge enabling technologies for regenerative medicine. Springer Singapore, pp 245–269
Atila D, Keskin D, Tezcaner A (2015) Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohyd Polym 133:251–261
Auger FA, Gibot L, Lacroix D (2013) The pivotal role of vascularization in tissue engineering. Annu Rev Biomed Eng 15:177–200
Augustine R (2018) Skin bioprinting: a novel approach for creating artificial skin from synthetic and natural building blocks. Prog Biomater 7(2):77–92
Augustine R, Kalarikkal N, Thomas S (2014) Advancement of wound care from grafts to bioengineered smart skin substitutes. Prog Biomater 3(2):103–113
Bahadoran M, Shamloo A, Nokoorani YD (2020) Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci Rep 10(1):1–18
Barry B (1983) Structure, function, diseases, and topical treatment of human skin. Marcel Dekker, New York
Bhardwaj N, Chouhan D, Mandal BB (2018) 3D functional scaffolds for skin tissue engineering. Functional 3D tissue engineering scaffolds. Elsevier, pp 345–365
Boateng JS, Matthews KH, Stevens HN, Eccleston GM (2008) Wound healing dressings and drug delivery systems: a review. J Pharm Sci 97(8):2892–2923
Brockmann I, Ehrenpfordt J, Sturmheit T, Brandenburger M, Kruse C, Zille M, Rose D, Boltze J (2018) Skin-derived stem cells for wound treatment using cultured epidermal autografts: clinical applications and challenges. Stem Cells Int 2018:1–9
Brohem CA, da Silva Cardeal LB, Tiago M, Soengas MS, de Moraes Barros SB, Maria-Engler SS (2011) Artificial skin in perspective: concepts and applications. Pigment Cell Melanoma Res 24(1):35–50
Brown-Etris M, Milne CT, Hodde JP (2019) An extracellular matrix graft (Oasis® wound matrix) for treating full-thickness pressure ulcers: a randomized clinical trial. J Tissue Viability 28(1):21–26
Butcher M, White R (2005) The structure and functions of the skin. Skin care in wound management: assessment, prevention and treatment. Aberdeen, Wounds UK, pp 1–16
Cao H, Chen M-M, Liu Y, Liu Y-Y, Huang Y-Q, Wang J-H, Chen J-D, Zhang Q-Q (2015) Fish collagen-based scaffold containing PLGA microspheres for controlled growth factor delivery in skin tissue engineering. Colloids Surf, B 136:1098–1106
Cao J, Wang P, Liu Y, Zhu C, Fan D (2020) Double crosslinked HLC-CCS hydrogel tissue engineering scaffold for skin wound healing. Int J Biol Macromol 155:625–635
Carsin H, Ainaud P, Le Bever H, Rives J-M, Lakhel A, Stephanazzi J, Lambert F, Perrot J (2000) Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns 26(4):379–387
Carter JE, Holmes JH, Albanna MZ, Holmes, JH (2016) Chapter 14—The surgical management of burn wounds.In: Albanna MZ, Holmes IV JH, (Eds) Skin tissue engineering and regenerative medicine. pp 289–298
Centanni JM, Straseski JA, Wicks A, Hank JA, Rasmussen CA, Lokuta MA, Schurr MJ, Foster KN, Faucher LD, Caruso DM (2011) StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds: results from a prospective, randomized, controlled dose escalation trial. Ann Surg 253(4):672
Chandika P, Oh G-W, Heo S-Y, Kim S-C, Kim T-H, Kim M-S, Jung W-K (2021) Electrospun porous bilayer nano-fibrous fish collagen/PCL bio-composite scaffolds with covalently cross-linked chitooligosaccharides for full-thickness wound-healing applications. Mater Sci Eng, C 121:111871
Chandra PK, Soker S, Atala A (2020) Tissue engineering: current status and future perspectives. Principles of tissue engineering. Elsevier, pp 1–35
Chang DK, Louis MR, Gimenez A, Reece EM (2019) The basics of integra dermal regeneration template and its expanding clinical applications. Seminars in plastic surgery, vol 3. Thieme Medical Publishers, pp 185–189
Chang H-I, Wang Y (2011) Cell responses to surface and architecture of tissue engineering scaffolds. InTechOpen
Chen J, Wang H, Mei L, Wang B, Huang Y, Quan G, Lu C, Peng T, Pan X, Wu C (2020) A pirfenidone loaded spray dressing based on lyotropic liquid crystals for deep partial thickness burn treatment: healing promotion and scar prophylaxis. J Mater Chem B 8(13):2573–2588
Chen J, Zhang G, Zhao Y, Zhou M, Zhong A, Sun J (2022) Promotion of skin regeneration through co-axial electrospun fibers loaded with basic fibroblast growth factor. Adv Composites Hybrid Mater 5(2):1111–1125
Chocarro-Wrona C, López-Ruiz E, Perán M, Gálvez-Martín P, Marchal J (2019) Therapeutic strategies for skin regeneration based on biomedical substitutes. J Eur Acad Dermatol Venereol 33(3):484–496
Choi JU, Lee SW, Pangeni R, Byun Y, Yoon I-S, Park JW (2017) Preparation and in vivo evaluation of cationic elastic liposomes comprising highly skin-permeable growth factors combined with hyaluronic acid for enhanced diabetic wound-healing therapy. Acta Biomater 57:197–215
Cole-King A, Harding KG (2001) Psychological factors and delayed healing in chronic wounds. Psychosom Med 63(2):216–220
Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D (2016) The role of genetically engineered pigs in xenotransplantation research. J Pathol 238(2):288–299
Cozzolino DJ, Cendron M, DeVore DP, Hoopes PJ (1999) The biological behavior of autologous collagen–based extracellular matrix injected into the rabbit bladder wall. Neurourol Urodyn 18(5):487–495
Cubo N, Garcia M, Del Canizo JF, Velasco D, Jorcano JL (2016) 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 9(1):015006
Dai C, Shih S, Khachemoune A (2020) Skin substitutes for acute and chronic wound healing: an updated review. J Dermatol Treat 31(6):639–648
De Pieri A, Rochev Y, Zeugolis DI (2021) Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast. NPJ Regener Med 6(1):1–15
de Torre IG, Ibáñez-Fonseca A, Quintanilla L, Alonso M, Rodríguez-Cabello J-C (2018) Random and oriented electrospun fibers based on a multicomponent, in situ clickable elastin-like recombinamer system for dermal tissue engineering. Acta Biomater 72:137–149
Değim Z, Çelebi N, Alemdaroğlu C, Deveci M, Öztürk S, Özoğul C (2011) Evaluation of chitosan gel containing liposome-loaded epidermal growth factor on burn wound healing. Int Wound J 8(4):343–354
Demidova-Rice TN, Hamblin MR, Herman IM (2012) Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 25(7):304
Ding X, Kakanj P, Leptin M, Eming SA (2021) Regulation of the wound healing response during aging. J Investig Dermatol 141(4):1063–1070
Doss MX, Sachinidis A (2019) Current challenges of iPSC-based disease modeling and therapeutic implications. Cells 8(5):403
Dutta RC, Dutta AK (2009) Cell-interactive 3D-scaffold; advances and applications. Biotechnol Adv 27(4):334–339
Edmonds M, European, Group AADFUS (2009) Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Lower Extremity Wounds 8(1):11–18
Erbatur S, Coban YK, Aydın EN (2012) Comparison of clinical and histopathological results of hyalomatrix usage in adult patients. Int J Burns Trauma 2(2):118
Eskandarinia A, Kefayat A, Agheb M, Rafienia M, Amini Baghbadorani M, Navid S, Ebrahimpour K, Khodabakhshi D, Ghahremani F (2020) A novel bilayer wound dressing composed of a dense polyurethane/propolis membrane and a biodegradable polycaprolactone/gelatin nanofibrous scaffold. Sci Rep 10(1):1–15
Esteban-Vives R, Corcos A, Choi MS, Young MT, Over P, Ziembicki J, Gerlach JC (2018) Cell-spray auto-grafting technology for deep partial-thickness burns: problems and solutions during clinical implementation. Burns 44(3):549–559
Estes BT, Wu AW, Guilak F (2006) Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum 54(4):1222–1232
Evans ND, Oreffo RO, Healy E, Thurner PJ, Man YH (2013) Epithelial mechanobiology, skin wound healing, and the stem cell niche. J Mech Behav Biomed Mater 28:397–409
Fang Y, Zhu X, Wang N, Zhang X, Yang D, Nie J, Ma G (2019) Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur Polymer J 116:30–37
Farhadihosseinabadi B, Farahani M, Tayebi T, Jafari A, Biniazan F, Modaresifar K, Moravvej H, Bahrami S, Redl H, Tayebi L (2018) Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif Cells Nanomed Biotechnol 46(suppl 2):431–440
Farroha A, Frew Q, El-Muttardi N, Philp B, Dziewulski P (2013) The use of Biobrane® to dress split-thickness skin graft in paediatric burns. Ann Burns Fire Disasters 26(2):94
Formigli L, Paternostro F, Tani A, Mirabella C, Quattrini Li A, Nosi D, D’Asta F, Saccardi R, Mazzanti B, Lo Russo G (2015) MSCs seeded on bioengineered scaffolds improve skin wound healing in rats. Wound Repair Regeneration 23(1):115–123
Furth ME, Atala A (2014) Tissue engineering: future perspectives. Principles of tissue engineering. Elsevier, pp 83–123
Gainza G, Pastor M, Aguirre JJ, Villullas S, Pedraz JL, Hernandez RM, Igartua M (2014) A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: in vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J Control Release 185:51–61
Garcia-Orue I, Gainza G, Gutierrez FB, Aguirre JJ, Evora C, Pedraz JL, Hernandez RM, Delgado A, Igartua M (2017) Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int J Pharm 523(2):556–566
Garreta E, Sanchez S, Lajara J, Montserrat N, Belmonte JCI (2018) Roadblocks in the path of iPSC to the clinic. Curr Transplant Rep 5(1):14–18
Geer DJ, Swartz DD, Andreadis ST (2005) Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. Am J Pathol 167(6):1575–1586
Ghaseminezhad K, Zare M, Lashkarara S, Yousefzadeh M, Aghazadeh Mohandesi J (2020) Fabrication of althea officinalis loaded electrospun nanofibrous scaffold for potential application of skin tissue engineering. J Appl Polym Sci 137(16):48587
Ghosal K, Manakhov A, Zajíčková L, Thomas S (2017) Structural and surface compatibility study of modified electrospun poly (ε-caprolactone)(PCL) composites for skin tissue engineering. AAPS PharmSciTech 18(1):72–81
Gledhill K, Guo Z, Umegaki-Arao N, Higgins CA, Itoh M, Christiano AM (2015) Melanin transfer in human 3D skin equivalents generated exclusively from induced pluripotent stem cells. PLoS ONE 10(8):e0136713
Goh M, Hwang Y, Tae G (2016) Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohyd Polym 147:251–260
Gomes S, Rodrigues G, Martins G, Henriques C, Silva JC (2017) Evaluation of nanofibrous scaffolds obtained from blends of chitosan, gelatin and polycaprolactone for skin tissue engineering. Int J Biol Macromol 102:1174–1185
Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, Prunkard D, Colunga AG, Hanafi L-A, Clegg DO (2017) HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol 35(8):765–772
Govindharaj M, Roopavath UK, Rath SN (2019) Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J Clean Prod 230:412–419
Goyer B, Larouche D, Kim DH, Veillette N, Pruneau V, Bernier V, Auger FA, Germain L (2019) Immune tolerance of tissue-engineered skin produced with allogeneic or xenogeneic fibroblasts and syngeneic keratinocytes grafted on mice. Acta Biomater 90:192–204
Günday C, Anand S, Gencer HB, Munafò S, Moroni L, Fusco A, Donnarumma G, Ricci C, Hatir PC, Türeli NG (2020) Ciprofloxacin-loaded polymeric nanoparticles incorporated electrospun fibers for drug delivery in tissue engineering applications. Drug Deliv Transl Res 10(3):706–720
Haddad AG, Giatsidis G, Orgill DP, Halvorson EG (2017) Skin substitutes and bioscaffolds: temporary and permanent coverage. Clin Plast Surg 44(3):627–634
Haldar S, Sharma A, Gupta S, Chauhan S, Roy P, Lahiri D (2019) Bioengineered smart trilayer skin tissue substitute for efficient deep wound healing. Mater Sci Eng, C 105:110140
Halim AS, Khoo TL, Yussof SJM (2010) Biologic and synthetic skin substitutes: an overview. Indian J Plast Surg 43(1):S23–S28
Hashemi SS, Mohammadi AA, Moshirabadi K, Zardosht M (2021) Effect of dermal fibroblasts and mesenchymal stem cells seeded on an amniotic membrane scaffold in skin regeneration: a case series. J Cosmet Dermatol 20(12):4040–4047
He Y, Hou Z, Wang J, Wang Z, Li X, Liu J, Liang Q, Zhao J (2020) Assessment of biological properties of recombinant collagen-hyaluronic acid composite scaffolds. Int J Biol Macromol 149:1275–1284
Hu S, Kirsner RS, Falanga V, Phillips T, Eaglstein WH (2006) Evaluation of Apligraf® persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regeneration 14(4):427–433
Hutmacher D, Goh J, Teoh S (2001) An introduction to biodegradable materials for tissue engineering applications. Ann-Acad Med Singapore 30(2):183–191
Intini C, Elviri L, Cabral J, Mros S, Bergonzi C, Bianchera A, Flammini L, Govoni P, Barocelli E, Bettini R (2018) 3D-printed chitosan-based scaffolds: an in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohyd Polym 199:593–602
Itoh M, Umegaki-Arao N, Guo Z, Liu L, Higgins CA, Christiano AM (2013) Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS ONE 8(10):e77673
Jangde R, Srivastava S, Singh MR, Singh D (2018) In vitro and In vivo characterization of quercetin loaded multiphase hydrogel for wound healing application. Int J Biol Macromol 115:1211–1217
Ji C, Annabi N, Khademhosseini A, Dehghani F (2011) Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater 7(4):1653–1664
John T (2003) Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am 16(1):43–65
Johnson NR, Wang Y (2013) Controlled delivery of heparin-binding EGF-like growth factor yields fast and comprehensive wound healing. J Control Release 166(2):124–129
Jossen V, van den Bos C, Eibl R, Eibl D (2018) Manufacturing human mesenchymal stem cells at clinical scale: process and regulatory challenges. Appl Microbiol Biotechnol 102(9):3981–3994
Kamalvand M, Biazar E, Daliri-Joupari M, Montazer F, Rezaei-Tavirani M, Heidari-Keshel S (2021) Design of a decellularized fish skin as a biological scaffold for skin tissue regeneration. Tissue Cell 71:101509
Kanitakis J (2002) Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol 12(4):390–401
Kareem NA, Aijaz A, Jeschke MG (2021) Stem cell therapy for burns: story so far. Biologics Targets Ther 15:379
Khademhosseini A, Ashammakhi N, Karp JM, Gerecht S, Ferreira L, Annabi N, Darabi MA, Sirabella D, Vunjak-Novakovic G, Langer R (2020) Embryonic stem cells as a cell source for tissue engineering. Principles of tissue engineering. Elsevier, pp 467–490
Khademhosseini A, Langer R (2016) A decade of progress in tissue engineering. Nat Protoc 11(10):1775–1781
Kharaziha M, Baidya A, Annabi N (2021) Rational design of immunomodulatory hydrogels for chronic wound healing. Adv Mater 33(39):2100176
Kilic Bektas C, Kimiz I, Sendemir A, Hasirci V, Hasirci N (2018) A bilayer scaffold prepared from collagen and carboxymethyl cellulose for skin tissue engineering applications. J Biomater Sci Polym Ed 29(14):1764–1784
Kim BS, Lee J-S, Gao G, Cho D-W (2017) Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 9(2):025034
Kim K, Evans G (2005) Tissue engineering: the future of stem cells. Top Tissue Eng 2:1–21
Kirsner RS, Margolis DJ, Baldursson BT, Petursdottir K, Davidsson OB, Weir D, Lantis JC (2020) Fish skin grafts compared to human amnion/chorion membrane allografts: a double-blind, prospective, randomized clinical trial of acute wound healing. Wound Repair Regeneration 28(1):75–80
Kitsberg D (2007) Human embryonic stem cells for tissue engineering. Tissue engineering. Humana Process, pp 33–65
Koller J (2005) Effects of radiation on the integrity and functionality of amnion and skin grafts. Sterilisation of tissues using ionising radiations. Elsevier, pp 197–220
Kouhbananinejad SM, Derakhshani A, Vahidi R, Dabiri S, Fatemi A, Armin F, Farsinejad A (2019) A fibrinous and allogeneic fibroblast-enriched membrane as a biocompatible material can improve diabetic wound healing. Biomater Sci 7(5):1949–1961
Kunitomi A, Yuasa S, Sugiyama F, Saito Y, Seki T, Kusumoto D, Kashimura S, Takei M, Tohyama S, Hashimoto H (2016) H1foo has a pivotal role in qualifying induced pluripotent stem cells. Stem Cell Rep 6(6):825–833
Kwon SG, Kwon YW, Lee TW, Park GT, Kim JH (2018) Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater Res 22(1):1–8
Ladewig K (2011) Drug delivery in soft tissue engineering. Expert Opin Drug Deliv 8(9):1175–1188
Lawton S (2019) Skin 1: the structure and functions of the skin. Nurs times 115:30–33
Lee J, Koehler KR (2021) Skin organoids: a new human model for developmental and translational research. Exp Dermatol 30(4):613–620
Lee J, Rabbani CC, Gao H, Steinhart MR, Woodruff BM, Pflum ZE, Kim A, Heller S, Liu Y, Shipchandler TZ (2020) Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582(7812):399–404
Li AI, Hokugo A, Jarrahy R, Zuk PA (2014) Human adipose tissue as a source of multipotent stem cells. Stem cells in aesthetic procedures. Springer, pp 67–83
Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T (2006) Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res 326(3):725–736
Li H, Shen S, Fu H, Wang Z, Li X, Sui X, Yuan M, Liu S, Wang G, Guo Q (2019) Immunomodulatory functions of mesenchymal stem cells in tissue engineering. Stem Cells Int 2019:1–18
Li Q, Niu Y, Diao H, Wang L, Chen X, Wang Y, Dong L, Wang C (2017) In situ sequestration of endogenous PDGF-BB with an ECM-mimetic sponge for accelerated wound healing. Biomaterials 148:54–68
Liu J, Sheha H, Fu Y, Liang L, Tseng SC (2010) Update on amniotic membrane transplantation. Expert Rev Ophthalmol 5(5):645–661
Liu T, Qiu C, Ben C, Li H, Zhu S (2019) One-step approach for full-thickness skin defect reconstruction in rats using minced split-thickness skin grafts with Pelnac overlay. Burns Trauma 7:19
Liu W, Zhong Z, Hu N, Zhou Y, Maggio L, Miri AK, Fragasso A, Jin X, Khademhosseini A, Zhang YS (2018a) Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 10(2):024102
Liu X, Nielsen LH, Kłodzińska SN, Nielsen HM, Qu H, Christensen LP, Rantanen J, Yang M (2018b) Ciprofloxacin-loaded sodium alginate/poly (lactic-co-glycolic acid) electrospun fibrous mats for wound healing. Eur J Pharm Biopharm 123:42–49
Lopresti F, Campora S, Tirri G, Capuana E, Pavia FC, Brucato V, Ghersi G, La Carrubba V (2021) Core-shell PLA/Kef hybrid scaffolds for skin tissue engineering applications prepared by direct kefiran coating on PLA electrospun fibers optimized via air-plasma treatment. Mater Sci Eng, C 127:112248
Lord MS, Ellis AL, Farrugia BL, Whitelock JM, Grenett H, Li C, O’Grady RL, DeCarlo AA (2017) Perlecan and vascular endothelial growth factor-encoding DNA-loaded chitosan scaffolds promote angiogenesis and wound healing. J Control Release 250:48–61
Lu H, Hoshiba T, Kawazoe N, Chen G (2011) Autologous extracellular matrix scaffolds for tissue engineering. Biomaterials 32(10):2489–2499
Luo Y, Yi X, Liang T, Jiang S, He R, Hu Y, Bai L, Wang C, Wang K, Zhu L (2019) Autograft microskin combined with adipose-derived stem cell enhances wound healing in a full-thickness skin defect mouse model. Stem Cell Res Ther 10(1):1–15
MacNeil S (2007) Progress and opportunities for tissue-engineered skin. Nature 445(7130):874–880
Mahendiran B, Muthusamy S, Sampath S, Jaisankar S, Popat KC, Selvakumar R, Krishnakumar GS (2021) Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: a review. Int J Biol Macromol 183:564–588
Marston WA, Hanft J, Norwood P, Pollak R, Group DDFUS (2003) The efficacy and safety of dermagraft in improving the Healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26(6):1701–1705
Martí-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, Cedeño-Taborda J (2015) Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD008548.pub2
Martinello T, Gomiero C, Perazzi A, Iacopetti I, Gemignani F, DeBenedictis G, Ferro S, Zuin M, Martines E, Brun P (2018) Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Vet Res 14(1):1–9
Martins JG, de Oliveira AC, Garcia PS, Kipper MJ, Martins AF (2018) Durable pectin/chitosan membranes with self-assembling, water resistance and enhanced mechanical properties. Carbohyd Polym 188:136–142
Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, LeRoux MA (2012) Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 1(2):142–149
Mi F-L, Wu Y-B, Shyu S-S, Chao A-C, Lai J-Y, Su C-C (2003) Asymmetric chitosan membranes prepared by dry/wet phase separation: a new type of wound dressing for controlled antibacterial release. J Membr Sci 212(1–2):237–254
Michael S, Sorg H, Peck C-T, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K (2013) Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 8(3):e57741
Min JH, Yun IS, Lew DH, Roh TS, Lee WJ (2014) The use of matriderm and autologous skin graft in the treatment of full thickness skin defects. Arch Plast Surg 41(4):330
Mizuno K, Yamamura K, Yano K, Osada T, Saeki S, Takimoto N, Sakurai T, Nimura Y (2003) Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res A 64(1):177–181
Mndlovu H, du Toit LC, Kumar P, Choonara YE, Marimuthu T, Kondiah PP, Pillay V (2020) Bioplatform fabrication approaches affecting chitosan-based interpolymer complex properties and performance as wound dressings. Molecules 25(1):222
Mo Y, Guo R, Zhang Y, Xue W, Cheng B, Zhang Y (2017) Controlled dual delivery of angiogenin and curcumin by electrospun nanofibers for skin regeneration. Tissue Eng Part A 23(13–14):597–608
Mohamad N, Loh EYX, Fauzi MB, Ng MH, Mohd Amin MCI (2019) In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv Transl Res 9(2):444–452
Moore L, Chien Y (1988) Transdermal drug delivery: a review of pharmaceutics, pharmacokinetics, and pharmacodynamics. Crit Rev Ther Drug Carrier Syst 4(4):285–349
Mota F, Braga L, Rocha L, Cabral B (2020) 3D and 4D bioprinted human model patenting and the future of drug development. Nature Publishing Group
Moustafa M, Bullock AJ, Creagh FM, Heller S, Jeffcoate W, Game F, Amery C, Tesfaye S, Ince Z, Haddow DB (2007) Randomized, controlled, single-blind study on use of autologous keratinocytes on a transfer dressing to treat nonhealing diabetic ulcers. Regener Med 2(6):887–902
Murphy SV, Skardal A, Song L, Sutton K, Haug R, Mack DL, Jackson J, Soker S, Atala A (2017) Solubilized amnion membrane hyaluronic acid hydrogel accelerates full-thickness wound healing. Stem Cells Transl Med 6(11):2020–2032
Naderi H, Matin MM, Bahrami AR (2011) Critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl 26(4):383–417
Nguyen DQ, Potokar TS, Price P (2010) An objective long-term evaluation of Integra (a dermal skin substitute) and split thickness skin grafts, in acute burns and reconstructive surgery. Burns 36(1):23–28
Niiyama H, Kuroyanagi Y (2014) Development of novel wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor and vitamin C derivative. J Artif Organs 17(1):81–87
Niu X, Wei Y, Liu Q, Yang B, Ma N, Li Z, Zhao L, Chen W, Huang D (2020) Silver-loaded microspheres reinforced chitosan scaffolds for skin tissue engineering. Eur Polymer J 134:109861
O’brien FJ (2011) Biomaterials & scaffolds for tissue engineering. Mater Today 14(3):88–95
Ohta S, Imaizumi Y, Okada Y, Akamatsu W, Kuwahara R, Ohyama M, Amagai M, Matsuzaki Y, Yamanaka S, Okano H (2011) Generation of human melanocytes from induced pluripotent stem cells. PLoS ONE 6(1):e16182
Olad A, Hagh HBK (2019) Graphene oxide and amin-modified graphene oxide incorporated chitosan-gelatin scaffolds as promising materials for tissue engineering. Compos B Eng 162:692–702
Olender E, Uhrynowska-Tyszkiewicz I, Kaminski A (2011) Revitalization of biostatic tissue allografts: new perspectives in tissue transplantology. Transplantation proceedings, vol 8. Elsevier, pp 3137–3141
Ozpur MA, Guneren E, Canter HI, Karaaltin MV, Ovali E, Yogun FN, Baygol EG, Kaplan S (2016) Generation of skin tissue using adipose tissue-derived stem cells. Plast Reconstr Surg 137(1):134–143
Park I-H, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451(7175):141–146
Park K-S, Lee W-S, Ji S-Y, Yang W-S (2018) The treatment of post-traumatic facial skin defect with artificial dermis. Arch Craniofac Surg 19(1):35
Park K (2015) Role of micronutrients in skin health and function. BiomolTherapeut 23(3):207
Pereira RF, Bartolo PJ (2016) Traditional therapies for skin wound healing. Adv Wound Care 5(5):208–229
Pina S, Ribeiro VP, Marques CF, Maia FR, Silva TH, Reis RL, Oliveira JM (2019) Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 12(11):1824
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Qasim M, Chae DS, Lee NY (2019) Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int J Nanomed 14:4333
Rajendran NK, Kumar SSD, Houreld NN, Abrahamse H (2018) A review on nanoparticle based treatment for wound healing. J Drug Deliv Sci Technol 44:421–430
Ramphul H, Gimié F, Andries J, Jhurry D, Bhaw-Luximon A (2020) Sugar-cane bagasse cellulose-based scaffolds promote multi-cellular interactions, angiogenesis and reduce inflammation for skin tissue regeneration. Int J Biol Macromol 157:296–310
Rohani L, Borys BS, Razian G, Naghsh P, Liu S, Johnson AA, Machiraju P, Holland H, Lewis IA, Groves RA (2020) Stirred suspension bioreactors maintain naïve pluripotency of human pluripotent stem cells. Commun Biol 3(1):1–22
Roig-Rosello E, Rousselle P (2020) The human epidermal basement membrane: a shaped and cell instructive platform that aging slowly alters. Biomolecules 10(12):1607
Roshangar L, Rad JS, Kheirjou R, Khosroshahi AF (2021) Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J Tissue Eng Regen Med 15(6):546–555
Rousselle P, Braye F, Dayan G (2019) Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev 146:344–365
Sa G, DiPietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229
Sahana T, Rekha P (2018) Biopolymers: applications in wound healing and skin tissue engineering. Mol Biol Rep 45(6):2857–2867
Sajjad W, He F, Ullah MW, Ikram M, Shah SM, Khan R, Khan T, Khalid A, Yang G, Wahid F (2020) Fabrication of bacterial cellulose-curcumin nanocomposite as a novel dressing for partial thickness skin burn. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.553037
Salgado G, Ng YZ, Koh LF, Goh CS, Common JE (2017) Human reconstructed skin xenografts on mice to model skin physiology. Differentiation 98:14–24
Sato Y, Bando H, Di Piazza M, Gowing G, Herberts C, Jackman S, Leoni G, Libertini S, MacLachlan T, McBlane J (2019) Tumorigenicity assessment of cell therapy products: the need for global consensus and points to consider. Cytotherapy 21(11):1095–1111
Schiestl C, Meuli M, Vojvodic M, Pontiggia L, Neuhaus D, Brotschi B, Reichmann E, Böttcher-Haberzeth S, Neuhaus K (2021) Expanding into the future: combining a novel dermal template with distinct variants of autologous cultured skin substitutes in massive burns. Burns Open 5(3):145–153
Schulz A, Depner C, Lefering R, Kricheldorff J, Kästner S, Fuchs PC, Demir E (2016) A prospective clinical trial comparing Biobrane® Dressilk® and PolyMem® dressings on partial-thickness skin graft donor sites. Burns 42(2):345–355
Sclafani AP, Romo T III, Jacono AA, McCormick S, Cocker R, Parker A (2000) Evaluation of acellular dermal graft in sheet (AlloDerm) and injectable (micronized AlloDerm) forms for soft tissue augmentation: clinical observations and histological analysis. Arch Facial Plast Surg 2(2):130–136
Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT (2009) Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regeneration 17(6):763–771
Seo M-D, Kang TJ, Lee CH, Lee A-Y, Noh M (2012) HaCaT keratinocytes and primary epidermal keratinocytes have different transcriptional profiles of cornified envelope-associated genes to T helper cell cytokines. Biomol Therapeut 20(2):171
Seol Y-J, Lee H, Copus JS, Kang H-W, Cho D-W, Atala A, Lee SJ, Yoo JJ (2018) 3D bioprinted biomask for facial skin reconstruction. Bioprinting 10:e00028
Shafiee A, Atala A (2017) Tissue engineering: toward a new era of medicine. Annu Rev Med 68:29–40
Sharifi M, Bahrami SH, Nejad NH, Milan PB (2020) Electrospun PCL and PLA hybrid nanofibrous scaffolds containing Nigella sativa herbal extract for effective wound healing. J Appl Polym Sci 137(46):49528
Sharma A, Mittal A, Puri V, Kumar P, Singh I (2020) Curcumin-loaded, alginate–gelatin composite fibers for wound healing applications. 3 Biotech 10(11):1–13
Shevchenko RV, James SL, James SE (2010) A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface 7(43):229–258
Shi M, Zhang H, Song T, Liu X, Gao Y, Zhou J, Li Y (2019) Sustainable dual release of antibiotic and growth factor from pH-responsive uniform alginate composite microparticles to enhance wound healing. ACS Appl Mater Interfaces 11(25):22730–22744
Shi Y, Xing T, Zhang H, Yin R, Yang S, Wei J, Zhang W (2018) Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed Mater 13(3):035008
Short WD, Wang X, Keswani SG (2022) The role of T lymphocytes in cutaneous scarring. Adv Wound Care 11(3):121–131
Sierra-Sánchez Á, Kim KH, Blasco-Morente G, Arias-Santiago S (2021) Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regener Med 6(1):1–23
Singh H, Purohit SD, Bhaskar R, Yadav I, Bhushan S, Gupta MK, Mishra NC (2022) Curcumin in decellularized goat small intestine submucosa for wound healing and skin tissue engineering. J Biomed Mater Res B Appl Biomater 110(1):210–219
Singh R, Ahmed F, Polley P, Giri J (2018) Fabrication and characterization of core–shell nanofibers using a next-generation airbrush for biomedical applications. ACS Appl Mater Interfaces 10(49):41924–41934
Singh R, Khan S, Basu SM, Chauhan M, Sarviya N, Giri J (2019) Fabrication, characterization, and biological evaluation of airbrushed gelatin nanofibers. ACS Appl Bio Mater 2(12):5340–5348
Smiley AK, Klingenberg JM, Boyce ST, Supp DM (2006) Keratin expression in cultured skin substitutes suggests that the hyperproliferative phenotype observed in vitro is normalized after grafting. Burns 32(2):135–138
Snyder D, Sullivan N, Margolis D, Schoelles K, Rockville SM (2020) Skin substitutes for treating chronic wounds. Technology Assessment Program - Technical Brief, Agency for Healthcare Research and Quality, (US)
Solovieva EV, Fedotov AY, Mamonov VE, Komlev VS, Panteleyev AA (2018) Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering. Biomed Mater 13(2):025007
Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C, Vandeskelde Y, Van der Elst J, Liebaers I, Sermon K (2008) Recurrent chromosomal abnormalities in human embryonic stem cells. Nat Biotechnol 26(12):1361–1363
Swaney MH, Kalan LR (2021) Living in your skin: microbes, molecules, and mechanisms. Infect Immun 89(4):e00695-e1620
Taghiabadi E, Nasri S, Shafieyan S, Firoozinezhad SJ, Aghdami N (2015) Fabrication and characterization of spongy denuded amniotic membrane based scaffold for tissue engineering. Cell J (yakhteh) 16(4):476
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Tamay DG, Dursun Usal T, Alagoz AS, Yucel D, Hasirci N, Hasirci V (2019) 3D and 4D printing of polymers for tissue engineering applications. Front Bioeng Biotechnol 7:164
Taylor CJ, Bolton EM, Bradley JA (2011) Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc b: Biol Sci 366(1575):2312–2322
Theoret C (2009) Tissue engineering in wound repair: the three “R” s—repair, replace, regenerate. Vet Surg 38(8):905–913
Tomford WW (1995) Transmission of disease through transplantation of musculoskeletal allografts. JBJS 77(11):1742–1754
Tong Z, Solanki A, Hamilos A, Levy O, Wen K, Yin X, Karp JM (2015) Application of biomaterials to advance induced pluripotent stem cell research and therapy. EMBO J 34(8):987–1008
Tripathi S, Singh BN, Divakar S, Kumar G, Mallick SP, Srivastava P (2021a) Design and evaluation of ciprofloxacin loaded collagen chitosan oxygenating scaffold for skin tissue engineering. Biomed Mater 16(2):025021
Tripathi S, Singh BN, Singh D, Srivastava P (2021b) Optimization and evaluation of ciprofloxacin-loaded collagen/chitosan scaffolds for skin tissue engineering. 3 Biotech 11(4):1–17
Troy J, Karlnoski R, Downes K, Brown KS, Cruse CW, Smith DJ, Payne WG (2013) The use of EZ Derm® in partial-thickness burns: an institutional review of 157 patients. Eplasty 13:e14
Unger C, Gao S, Cohen M, Jaconi M, Bergstrom R, Holm F, Galan A, Sanchez E, Irion O, Dubuisson JB (2009) Immortalized human skin fibroblast feeder cells support growth and maintenance of both human embryonic and induced pluripotent stem cells. Hum Reprod 24(10):2567–2581
Vacanti JP, Langer R (1999) Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. The Lancet 354:S32–S34
Vatankhah E, Prabhakaran MP, Jin G, Mobarakeh LG, Ramakrishna S (2014) Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J Biomater Appl 28(6):909–921
Vijayavenkataraman S, Lu W, Fuh J (2016) 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication 8(3):032001
Wallace HA, Basehore BM, Zito PM (2022) Wound healing phases. StatPearls. StatPearls Publishing, Treasure Island (FL), PMID: 29262065
Wang F, Wang M, She Z, Fan K, Xu C, Chu B, Chen C, Shi S, Tan R (2015) Collagen/chitosan based two-compartment and bi-functional dermal scaffolds for skin regeneration. Mater Sci Eng, C 52:155–162
Wang L, Wu S, Cao G, Fan Y, Dunne N, Li X (2019) Biomechanical studies on biomaterial degradation and co-cultured cells: mechanisms, potential applications, challenges and prospects. J Mater Chem B 7(47):7439–7459
Wang W, Wat E, Hui PC, Chan B, Ng FS, Kan C-W, Wang X, Hu H, Wong EC, Lau C (2016) Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci Rep 6(1):1–10
Wang Z, Wang Z, Lu WW, Zhen W, Yang D, Peng S (2017) Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater 9(10):e435–e435
Watt FM (2014) Mammalian skin cell biology: at the interface between laboratory and clinic. Science 346(6212):937–940
Whittam AJ, Maan ZN, Duscher D, Wong VW, Barrera JA, Januszyk M, Gurtner GC (2016) Challenges and opportunities in drug delivery for wound healing. Adv Wound Care 5(2):79–88
Wilkinson HN, Hardman MJ (2020) Wound healing: cellular mechanisms and pathological outcomes. Open Biol 10(9):200223
Xu K, Chai B, Zhang K, Xiong J, Zhu Y, Xu J, An N, Xia W, Ji H, Wu Y (2020) Topical application of fibroblast growth factor 10-PLGA microsphere accelerates wound healing via inhibition of ER stress. Oxid Med Cell Longevity 2020:1–13
Yamanaka S (2020) Pluripotent stem cell-based cell therapy—promise and challenges. Cell Stem Cell 27(4):523–531
Yin H-M, Qian J, Zhang J, Lin Z-F, Li J-S, Xu J-Z, Li Z-M (2016) Engineering porous poly (lactic acid) scaffolds with high mechanical performance via a solid state extrusion/porogen leaching approach. Polymers 8(6):213
Yin J, Fang Y, Xu L, Ahmed A (2021) High-throughput fabrication of silk fibroin/hydroxypropyl methylcellulose (SF/HPMC) nanofibrous scaffolds for skin tissue engineering. Int J Biol Macromol 183:1210–1221
Yousef H, Alhajj M, Sharma S (2021) Anatomy, skin (Integument), epidermis. StatPearls. StatPearls Publishing, Treasure Island (FL), PMID: 29262154
Yousef H, Sharma S (2018) Anatomy, skin (Integument), epidermis. StatPearls Treasure Island (FL). StatPearls Publishing LLC, St Petersburg, FA, USA
Yu H, Chen X, Cai J, Ye D, Wu Y, Fan L, Liu P (2019) Novel porous three-dimensional nanofibrous scaffolds for accelerating wound healing. Chem Eng J 369:253–262
Yu J, Wang M-Y, Tai H-C, Cheng N-C (2018) Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater 77:191–200
Yuan L, Li X, Ge L, Jia X, Lei J, Mu C, Li D (2018) Emulsion template method for the fabrication of gelatin-based scaffold with a controllable pore structure. ACS Appl Mater Interfaces 11(1):269–277
Zehra M, Mehmood A, Yar M, Shahzadi L, Riazuddin S (2020) Development of NSAID-loaded nano-composite scaffolds for skin tissue engineering applications. J Biomed Mater Res B Appl Biomater 108(8):3064–3075
Zhang J, Chan Y-C, Ho JC-Y, Siu C-W, Lian Q, Tse H-F (2012) Regulation of cell proliferation of human induced pluripotent stem cell-derived mesenchymal stem cells via ether-a-go-go 1 (hEAG1) potassium channel. Am J Physiol Cell Physiol 303(2):C115–C125
Zhang J, Yang S, Yang X, Xi Z, Zhao L, Cen L, Lu E, Yang Y (2018) Novel fabricating process for porous polyglycolic acid scaffolds by melt-foaming using supercritical carbon dioxide. ACS Biomater Sci Eng 4(2):694–706
Zhang Q, Chen S, You R, Tariq Z, Huang J, Li M, Yan S (2017a) Silk fibroin/hyaluronic acid porous scaffold for dermal wound healing. Fibers Polym 18(6):1056–1063
Zhang X, Jia C, Qiao X, Liu T, Sun K (2017b) Silk fibroin microfibers and chitosan modified poly (glycerol sebacate) composite scaffolds for skin tissue engineering. Polym Testing 62:88–95
Zhu P, Zhang S, Kumar R, Zhang Z, Zhang Z, Wang Y, Jiang X, Lin K, Kaur G, Yung KKL (2022) Rhamnolipids from non-pathogenic Acinetobacter calcoaceticus: bioreactor-scale production, characterization and wound healing potency. New Biotechnol 67:23–31
Zomer HD, Trentin AG (2018) Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci 90(1):3–12
Acknowledgements
The authors are thankful to the School of Biochemical Engineering, IIT (BHU) Varanasi for providing technical support. This work was financially supported by the Council of Scientific and Industrial Research, India under CSIR-JRF Ph.D program, for providing fellowship to author Soumya Katiyar during the tenure of this study [CSIR File No.- 09/1217(0066)/2019EMR-I].
Funding
This work was financially supported by the Council of Scientific and Industrial Research (CSIR), India.
Author information
Authors and Affiliations
Contributions
SoK performed the writing, literature search, figure production, and data analysis, while ShK and DS performed the editing and revising. This review paper was designed, conceptualized, and submitted with the help of PS and AM.
Corresponding author
Ethics declarations
Ethical approval
Not applicable. This article does not involve any studies related with animal and human participants performed by any of the authors.
Consent to participate
All authors acknowledge their work and agree to participate in the publication of this paper.
Consent for publication
All authors agreed to publish this review paper in 3 Biotech.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katiyar, S., Singh, D., Kumari, S. et al. Novel strategies for designing regenerative skin products for accelerated wound healing. 3 Biotech 12, 316 (2022). https://doi.org/10.1007/s13205-022-03331-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03331-y