Abstract
Purpose of Review
Phosphorus (P) loss from agricultural land to surface water is a leading cause of water quality deterioration. We reviewed the climate change impacts on sources and transport of P and how they can exacerbate P loss from agricultural soils to waterways.
Recent Findings
The effects of climate change include extreme precipitation events, increased temperature, elevated atmospheric carbon dioxide (eCO2), and saltwater intrusion induced by sea level rise. Extreme precipitation (EP) events cause accelerated transport of dissolved and particulate P from soils, exacerbated after the application of fertilizers and manures or drought. The unpredictability of EP leads to greater incidental P losses as appropriately timing nutrient applications is more challenging. Increased soil and air temperatures influence soil microbial communities and P-solubilizing microbes, but their effects on P losses are uncertain. Likewise, eCO2 may increase plant growth, P demand, and soil P cycling, but its impact on P losses is unclear. Saltwater intrusion caused by sea level rise can further mobilize P in high (legacy) P soils and enhance P loss from land to water.
Summary
Climate change is likely to increase P losses due primarily to increases in extreme precipitation and saltwater intrusion in coastal areas. These impacts will be geographically variable. Current P loss models could be improved by including climate change effects on P sources and transport, the inclusion of legacy P soil data, and accounting for P losses from legacy P soils.
Graphical Abstract
Climate change effects on phosphorus loss

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Overview
Eutrophication caused by excess phosphorus (P) is a major contributor to fresh and estuarine water quality degradation worldwide [1]. Excess P in water bodies stimulate algae, which in turn causes undesirable effects, including hypoxic or anoxic conditions, reduced water clarity, and onset of algal blooms [2]. In the USA, a majority of fresh waters contain elevated nutrient levels, resulting in an estimated economic loss of $2.2 billion annually [2].
This review focuses specifically on agricultural soils of the world, which are a major contributor of P loss to surface water. There is an emphasis on legacy P soils that reflect a long-term history of P applications, as these soils contribute a disproportionate amount of P loss and are more vulnerable to P loss due to climate change impacts. The Intergovernmental Panel on Climate Change (IPCC) has concluded that extreme precipitation (EP), increased temperature, elevated atmospheric carbon dioxide (eCO2), and saltwater intrusion (SWI) induced by sea level rise (SLR) are expected in future [3]. A body of soil P research has investigated how these effects may impact P source and transport mechanisms; however, to our knowledge, a detailed review of the above four key climate change variables on P Loss has not yet been compiled.
Forms of P in Soils
Natural soils typically contain small amounts of P as a product of weathering from the parent material. Certain regions of the world have large deposits of igneous or sedimentary phosphate rock that are mined for fertilizer use [4, 5], typically in the form of apatite [6]. Agricultural soils are often supplemented with additional P, either as inorganic fertilizer or as organic P from manure, biosolids, compost, crop residues, or other recycled organic sources [7,8,9,10]. In agricultural soils, P is most commonly found in inorganic forms consisting mainly of phosphate (PO4) bound to metals such as calcium (Ca), iron (Fe), and aluminum (Al) depending on the soil type, pH, type of P fertilizer or manure used, and soil formation factors [11, 12]. Phosphate also binds with other elements such as magnesium (Mg), manganese (Mn), copper (Cu), lead (Pb), and others to form an array of minerals in the soil [13]. Organic P is a significant pool in agricultural soils, comprising about 30–65% of the total P (TP) [14]. In regions with livestock production, organic P is often found in the soils as phytic acid, as it is present in livestock feed and excreted in manure [12, 15,16,17]. Figure 1 overviews various P pools and mineral groups found in soils.
While P is a limiting nutrient in many of the world’s soils and impacts crop production [18], soil P has accumulated to excessive amounts in some regions with intensive agricultural production. The P accumulation often occurs as a result of concentrated animal agriculture because it is not economical and feasible to transport excess manure long distances [19, 20]. Additionally, commercial P fertilizers are sometimes added to soils above crop P requirements in many developed regions (Fig. 2). This occurs because soil fertility recommendations have been based on the “build and maintain” philosophy of P fertilization, a long-standing yet debated philosophy that calls for adding P above crop requirements to increase soil test P to levels correlated to crop yield maximum and then maintaining P level in that category [21, 22]. The applications of P, from manure or commercial fertilizers, above crop applications lead to elevated soil test P that can persist for decades, defined here as legacy P.
World map showing P deficits and surpluses in agricultural soils (used with permission from MacDonald et al. [19]). The deep red color indicates the top quartile of P surpluses and is the most pronounced in eastern China, North Korea, South Korea, Taiwan, Japan, Vietnam, much of Europe, Chile, and the east coast and central west coast of the USA. These regions are also primarily coastal, with access to ports and water resources for humans and animals
The use of manure and fertilizer has significantly increased labile soil P forms in agricultural soils compared to native soils [23]. In addition to increased labile pools, legacy P soils contain a large reserve of inorganic P as insoluble or less soluble “stable” minerals, such as fluorapatite that may become available once soluble pools of P are exhausted [24]. For example, PO4 sorbed to Al or Fe contributes to the labile pool as soluble P is exhausted, particularly in highly weathered soils, as P cycles through sorption-desorption reactions between the solid phase and soil solution. Soils with significant Al and Fe can sorb significant amounts of P; however, when the Al and Fe binding sites in soils become saturated, the soils cannot hold more P, resulting in P loss to waterways [20, 25,26,27].
Forms of P in Water
Once lost from soils, P present in various pools in water or extracted solutions can be categorized as reactive, unreactive, dissolved, and particulate (Fig. 3). This is based on both the (1) size: whether the P is present in the dissolved or particulate fraction, with the distinction made by filtration through a filter paper (usually 0.45 or 0.2 µm), and (2) digestibility: whether the P is present in a reactive or unreactive fraction, with the distinction made by digesting the water with some form of acid or oxidizing agent [28]. This results in four categories of P forms with two main groups: reactive and unreactive. Reactive P is thought to mainly consist of orthophosphate (PO43−) in dissolved fraction and P in particulate pool, whereas unreactive dissolved P contains labile organic P compounds and unreactive particulate P contains recalcitrant organic P compounds.
Illustrations showing A various P forms present in water, B calculations of four P fractions in water samples, and C resulting P forms commonly referenced in literature with descriptions of the chemical nature of P. TRP, total reactive P; TP, total P; DRP, dissolved reactive P; TDP, total dissolved P; PRP, particulate reactive P; DUP, dissolved unreactive P; TPP, total particulate P; PUP, particulate unreactive P. Redrawn from Toor et al. [28] and Yang et al. [29]
Differentiation of P into these forms is useful to predict the bioavailability of P in water bodies. For example, dissolved reactive P (DRP) is considered readily available to aquatic biota, whereas dissolved unreactive P (DUP) may become available in water due to enzymatic hydrolysis and other P transformations [30]. Of particular concern are dissolved and long-term algal-available P forms [31] as dissolved P contributes to the growth of harmful algae, especially when P is a limiting nutrient and is readily available for uptake by autotrophs [32]. The growth of algae, and subsequent hypoxic or anoxic conditions that are harmful to aquatic life, is caused by eutrophication [2]. Particulate reactive P (PRP) and particulate unreactive P (PUP) forms in the environment are more recalcitrant and have lower bioavailability; therefore, they pose a less immediate threat to water quality; however, they can become available over the long term as more labile P forms are depleted.
Overview of P Loss to Water
Non-point agricultural pollution occurs where a source of P, namely soil P or P containing amendments (manure, biosolids, other waste products), interacts with the hydrology, such as overland flow or subsurface flow. There are three main pathways for P transport: (1) movement of dissolved P in surface runoff, (2) erosion of P bound to soil particles in surface runoff (i.e., particulate P), and (3) subsurface flow of dissolved P, as depicted in Fig. 4 [33,34,35]. A “critical source area” [36] indicates a “hotspot” for P loss where the P source meets hydrology, while other areas in the field are susceptible to some degree of P loss. This concept of where P sources interact with transport pathways is the premise of the P indices advocated by regulatory agencies to manage the P applications on high P loss risk fields [37]. Prioritizing field management using critical source areas is a useful strategy to minimize P losses to surface waters [38].
Climate Change Impacts on P Loss
An overview of the P transfer continuum related to climate change has been outlined by Forber et al. [39]; we expand and explore this continuum by discussing specific impacts that should be considered in future predictions of P loss. While climate change effects are myriad, complex, and vary worldwide, we focused this review on four main impacts that may affect P cycling and transformations in soil and flux from land to water. These include (i) EP events, (ii) increased soil temperature, (iii) elevated CO2 (eCO2) in the atmosphere, and (iv) SWI induced by SLR.
The objectives of this review are to explore how these four aspects of climate change may impact P source and transfer pathways, discuss management solutions, and outline suggestions for future research to understand the impacts on P. Although relevant to most soils worldwide, this review focuses on agricultural regions with legacy P soils that reflect a long-term history and mismanagement of P applications. These legacy P soils contribute a disproportionate amount of P loss and might be more vulnerable to P loss due to climate change impacts.
Effect of Extreme Climatic Events on P Losses
In this review, extreme climatic events are predominately defined as EP, with some discussion on droughts. Occurrence of EP is defined as the amount (volume) of precipitation exceeding the volume of the top 1% of all daily precipitation events from a reference period (e.g., 1901–1960), which may vary in different studies [40]. Global increases in EP have been measured and modeled based on wet and dry regions since the 1950s [41], and the average annual maximum daily precipitation has shown an increase of 5.73 mm day−1 (8.53%) from 1901 to 2010 [42]. In the USA, EP has significantly increased since the 1960s [43]; however, a few regions, such as the Southwest USA, have experienced decreased precipitation [44]. The increase in EP is correlated with a warming atmosphere, as total precipitation from extreme events almost doubles per degree of warming [45]. Along with increased rainfall amount, the increased intensity has been documented and correlated with increased dissolved P (DP), particulate P (PP), and TP loss from soils that received mineral fertilizer and manure [46]. An increase in EP events has resulted in greater P loss in watersheds worldwide, including Lake Mendota watershed, WI, USA [47], lower Great Lakes Basin, USA [48], Sichuan Basin, China [49], Eden River catchment, Cumbria, UK [50], and others.
Myhre et al. [45] depicted changes in precipitation for Europe (Fig. 5), with increases in both frequency and intensity of precipitation in recent years (1986–2015) than earlier years (1906–1935). Their research highlighted that larger amounts of daily precipitation events have mildly increased intensity and significantly increased frequency per unit Kelvin (K) increase in surface temperature [45]. These data have meaningful implications for increased potential for P transport because of the greater frequency of high-intensity, high-volume precipitation events.
A schematic illustration of the probability density function (PDF) of daily precipitation frequency and intensity. The purple line shows a reference PDF for earlier years (1906–1935), and the orange line shows how it changes with higher temperatures in later years (1986–2015). Once EP reaches a certain threshold, the increase in frequency and intensity leads to a change compared to the 1906–1935 reference timeframe, indicated by the “total change” filled area on the plot above. The data are the mean of 15 rain gauge stations in the Netherlands. Redrawn from Myhre et al. [45]
Dissolved P Losses
An increase in DP loss from soils with an increase in rainfall can be expected as water added with the rain will displace the soil solution P, which is in equilibrium with P in the solid phase. This addition of new water will affect the P equilibria, as P from the solid phase will be displaced to the soil solution pool, depending upon the kinetics of P release in soils with inherent differences in soil properties and other management and environmental conditions [51]. The forms of P present in the soil also impact the risk of DP losses. Through a combination of agronomic and environmental soil tests, molecular techniques, field lysimeter measurements, rainfall simulation, and field measurements on soils in Sweden, it was observed that soils with more Al-P compounds limited DP release, whereas soils with more Fe-P compounds led to greater DP release in rainfall [52]. The organic fractions in this study did not lead to significant differences in DP, but low sorption capacity and high agronomic and environmental soil P were also linked to the elevated risk of DP loss. New field interventions and the development of conservation practices at the edge-of-the-field (EOF) [53] could be useful for intercepting and reducing the transport of DP in soils with a high risk of P loss.
Depending on the hydrology of the site, there will be either direct P loss to surface water, groundwater, or a combination of surface and ground water. Sharpley et al. [54] compared base and storm flow P loss in a 39.5 ha primarily agricultural sub-watershed in PA, USA (with non-glaciated ultisols and inceptisols), considering storm size by return period (< 1, 1–3, 3–5, 5–10, and > 10 years). They found that over 10 years, 65% of DP and 76% of TP were exported during storm flow, with the remainder exported during base flow. Overall, DP concentration increased with storm size based on the return period, which was categorized using the peak and total flow. Ross et al. [48] found that DP was greater during EP events in the Great Lakes Basin, USA, where runoff is also the main transport pathway. The study also revealed that TDP to TP ratio was highest on the falling limb of the hydrograph, indicating that DRP (main component of TDP) continued to be transported once PP loss decreased. Increased DP during and after EP events is also observed in tile-drained systems. For example, storm intensity and event discharge were linked to increased DRP loading in tile-drained sites in North Central OH, USA [55]. Climate change has led to more frequent and intense precipitation; therefore, one would expect an increase in DP loading from watersheds. In one sub-watershed in IA, USA, storm events contributed 74.9 to 93.8% of annual DRP export over a 2-year study, and in one storm event, DRP was 84% of the annual DRP [56]. However, predicting quantitative DP losses based on storm size has proven challenging, with large variability between storm events and a hysteresis effect, which means that mobilization of DP occurs as a function of flow type (surface or subsurface) and source impacts (e.g., recent nutrient applications, tillage, crop stage, and type) [48, 57,58,59].
Erosion and Particulate P Losses
Studies around the world have been conducted to predict how rainfall intensity, storm pattern, vegetative cover, slope, soil type, and soil disturbance from management practices influence erosion [60,61,62,63]. While recent studies have become increasingly complex compared to earlier erosion studies, a commonality among them is that erosion increases with increases in storm intensity. Ezzati et al. [64] found that the combination of water discharge and suspended solids were the best predictors of TP measured in the runoff. Therefore, we expect increased contributions of PP with climate change, but to quantitatively predict the increased PP loading, models would need to estimate the existing P in the soil. Furthermore, estimating the fraction that may exist as PP would depend on management history and soil conditions. This estimate of PP could be paired with erosion estimates considering how the aforementioned variables influence erosion.
Most of the P loss in both base flow and storm flow measured by Sharpley et al. [54] in a PA, USA, catchment over a 10-year period was in the form of PP, and as storm size increased, PP constituted a larger percentage of TP loss. The annual PP loss was ~ 476 g ha−1 year−1 in the PA catchment, with 407 g ha−1 year−1 occurring during storm flow. High-intensity rainfall of > 9 mm h−1 led to average PP losses of 319 g ha−1 h−1 in tilled fields in South West England [65]. A similar effect was noted in a southern Brazilian agricultural catchment, where ~ 88% of transported P was PP, and the highest fraction of PP in the flow occurred closest to the maximum discharge of the storm events [57]. On a seasonal scale, New Zealand grassland soils receiving dairy manure and fertilizer P lost more PP in leachate during the irrigated growing season (November–April) but more DP in the non-irrigated season (May–October) with less rainfall and no irrigation water [66]. Seasonality of EP varies in different regions around the world, impacting plant growth [67], so continued studies of P forms and leachate across seasons are critical to understanding how EP may differently impact P losses at various temporal scales.
The impact of EP events is compounded if they occur after drought conditions. The reasons behind this are a combination of factors, such as worsening soil structure due to the inability of organic material to hold soil into aggregates, physical surface crusting, and poor crop growth, which can expose soil to erosion. Khan et al. [68] found that flooding soils in drought conditions resulted in significantly more P loss and more DP forms compared to flooding the same soils at field moisture capacity. The authors speculated this could be attributed to the breakdown of organic matter (OM) and biomass due to soil drying, reduced P sorption capacity due to increased crystallization of soil minerals, redox changes, or a combination of these factors. Similarly, increased TP was measured in Swedish catchments that experienced drought in the previous season, which flushed TP with heavy rainfall [64]. Moderate Resolution Imaging Spectroradiometer (MODIS) data and the Revised Universal Soil Loss Equation (RUSLE) model have demonstrated a significant correlation between calculated drought indices and soil loss [69]. The initial rainfall after drought increased measured P losses to surface water from agricultural soils in the Finger Lakes of NY, USA, compared to other land uses in the watershed [70]. While lower DP loss is expected during periods of drought due to the absence of rainfall, increased PP loss due to EP after drought will likely increase overall P loading, as predicted by the Soil and Water Assessment Tool (SWAT) in China [71]. Similarly, in areas around the world, such as Africa and the Middle East, where soils are prone to higher erosion losses [72], PP can be expected to be a main P form lost after EP events, further declining soil fertility.
Incidental P Losses Following Recent P Applications
When EP occurs shortly after manure or fertilizer P application, the risk of P loss is greater, even when application rates are based on crop P removal. In manured fields, the type and rate of manure have significantly different contributions of DRP and TP, with dairy manure contributing less DRP than poultry or swine manure [73]. It is also well-established that increased time between manure application and rainfall results in less P loss to surface water, allowing P to be sorbed in the soil and used by plants before a runoff event [74, 75]. The forms of P in dairy manure (predominately DRP), for example, react with the soil after application. The leachate from perennial pasture after dairy manure application showed a higher percentage of PUP likely consisting of orthophosphate monoesters and diesters and only a small fraction of DRP (1–7%) compared to that of the dairy manure effluent (38–76%) [15]. Therefore, the time and quantity of manure applied before EP events are critical. Researchers have developed various watershed models to estimate the impact of precipitation events on manured fields. At a basic level, models predict EP will cause increased DP and TP loss when manure is applied [76].
Fertilizer P has a greater risk of loss when EP occurs shortly after application. In one study, losses of 13–19% of common P fertilizers (e.g., monoammonium phosphate, diammonium phosphate, triple superphosphate, and single superphosphate) occurred after one simulated rainfall event when the fertilizers were surface applied [77]. Smith et al. [78] found that manure may pose greater water quality concerns if the rain event occurs in the first several days after application; however, the commercial fertilizer source (triple superphosphate) presents an elevated risk for a longer period until the rainfall event occurs. The incidental losses of recently applied P inputs are a major contributor to P losses, and with farmers facing less predictable weather, it may be more challenging to plan fertilizer and manure applications. Integration of incidental P losses into models may require assessments of the frequency that EP occurs unexpectedly after nutrient applications to fields, which may only be achievable through collecting detailed farm management and weather data.
A limitation in assessing the impact of EP on eutrophication is the varied and delayed response of cyanobacterial and algal blooms after precipitation and P loss. In 2019, heavy precipitation limited the planting of crops in the Maumee River watershed, USA, which flows into Lake Erie, USA. Thus, P applications were reduced by an estimated 62%, with measured DRP 29% less than expected and PP loads as predicted, leading to less severe harmful algal blooms in Lake Erie than were estimated based on rainfall and discharge [79]. This research highlights the importance of incidental P losses and proper management of P applications; however, the authors also indicate that other factors (e.g., no-tillage, fallow ground cover, and potentially reduced intensity of the rainfall) could have contributed to the measured P loss reduction. In an exploration of EP, discharge, and P load to Lake Mendota, WI, USA, there was a 1 to 60 days lag time between extreme P loading and the next significant cyanobacterial bloom [80]. This suggests the importance of long-term memory of P-loading events, though the impact may not be immediately apparent, large P reservoirs are deposited in surface water during EP events, which may become available and cause cyanobacterial growth when conditions are ideal.
Impact of Increased Temperature on Soil Processes
Global surface air temperatures are projected to increase by 1.5 to 2 °C through the mid-century and further, unless serious interventions to reduce greenhouse gas emissions are achieved [3]. An increase in air temperature will lead to higher soil temperature, which can lead to higher microbial respiration, particularly in cold regions [81]. However, microbial taxa respond variably to temperature increases, which leads to uncertainty in changes in nutrient cycling [81]. For example, measured activities of organic P-solubilizing enzyme, phosphatases, increase with increased temperature but do not always result in increased available P in soils [82]. Furthermore, an increase in P availability does not always translate to increased P loss [82, 83]. For instance, in soils with high P-fixing capacity, P loss in leachate can decrease with increasing temperature due to the increased net microbial P immobilization and reaction rate between P in solution and soil [84]. To our knowledge, the microbial impacts on P availability due to temperature rise in most soils, specifically legacy P soils, are unknown.
The inconclusive effects of temperature on P loss are also apparent in climate models. Most models include temperature as a variable when predicting the impacts of climate change on P loss; however, most simulated results suggest that temperature is not an important driver for changes in P loading compared to precipitation [85•]. In fact, model simulations suggest the impact of temperature on P loading is inconclusive, with literature indicating that increased temperatures will lead to increases and decreases in P loading; these inconsistencies stem from differences in geography and model parameters [86,87,88,89]. Furthermore, most models and studies do not investigate temperature impacts in isolation, as is practical, since global climate change will incur many changing effects in conjunction with temperature. Without this isolation, concluding the direct effects of temperature on P loading is difficult to predict. Nevertheless, present models do not fully consider the direct or indirect effects of increased temperature on P loading. These effects may include changes in the microbial population, land use (i.e., different crop types and changes in cropping zones), and biomass production. More research is needed to elucidate and disentangle the direct and indirect effects of temperature on P loss so that they can be included in future models.
Changes in Evapotranspiration and Soil Moisture
An increase in temperature is likely to increase evapotranspiration (ET) [90] and decrease soil moisture, which will have different effects on crop yield depending on the region due to specific crop tolerance [91]. The increase in ET is projected to offset some P loading caused by increased precipitation and runoff during the summer months [87]. Furthermore, potential ET is projected to increase at a higher rate than precipitation [92,93,94], which could result in soil moisture deficits leading to more droughts. Current projections of global soil drought associated with climate change may be simplistic. For instance, looking at the paleo-evidence of the mid-Pliocene Warm Period, where temperatures reflect projected climate changes, there was no expansion of arid ecosystems, and widespread vegetation flourished [95]. This inconsistency with climate model projections, referred to as the aridity paradox, along with recent literature attempting to reconcile these inconsistencies, creates hesitation to incorporate simplistic soil drought approaches in climate models when predicting P loss [96]. Regardless, ET and further soil drying can lead to disaggregation of soil particles making PP transport in runoff more likely when significant storm events occur [97]. While the effect of temperature on ET, soil moisture, and crop yield is often considered in climate change models, other effects, such as impacts on freeze-thaw cycles and P-solubilizing microbes, are not considered.
Temperature Effects on Freeze-Thaw Cycles and Associated P Loss
Freeze–thaw cycles (FTCs) commonly occur in the Northern Hemisphere and can significantly alter soil structure, water and heat movement, and organic matter [98, 99]. The FTCs can drive P into the solution phase by physically disturbing soil aggregates, plant biomass, and microorganisms, causing increased loss of DP in runoff and downward migration of P during snowmelt [100, 101]. The FTCs also threaten the efficacy of cover crops as a best management practice, as they have been shown to increase the TP, mainly DP, in leachate from cover-cropped soils versus a control soil without cover crops [102]. The FTCs can also increase soil erodibility [103], which can positively affect the frequency and magnitude of future FTCs [104]. As temperatures increase and reduce the insulating effects of snow cover, FTCs are projected to increase in intensity, frequency, and duration [89, 105,106,107]. Research has shown that simulated increases in FTCs cause both increases and decreases in soil water content (SWC) and available soil P, such as Mehlich 3–extractable P (M3-P) [100, 108], where discrepancies are attributed to soil properties, namely P-fixing sites on soil surfaces and SWC, which result in P (and metal ions) release due to the breakdown of organic and mineral complexes and exposing of additional P sorption sites [109].
Cheng et al. [110] found that FTCs increased P loss in runoff when SWC was low (10–15%); however, when SWC was high (20–30%), FTC-treated soils had less P loss than non-FTC-treated soils likely due to the exclusion effects of ice on ions. For example, as more soil water freezes and expands, P migrates downwards in the soil where it can sorb to vacant P binding sites. However, exploration of this mechanism in legacy P soils, where soil P-fixing sites are saturated even in lower soil depths, should be investigated as there is a risk for increased P loss even at high SWC, primarily due to macropore/preferential flow [111]. The decrease in P loading at high SWC is also attributed to disaggregation into smaller soil particles, which increases the specific surface area and the P sorption capacity. Therefore, FTCs can result in a balance between releasing P from lysed cells and physical occlusion and then resorption of P on newly vacated or exposed soil P binding sites. Clearly, the soil’s physical and chemical properties will be increasingly important as increased temperature and ET may decrease soil moisture and exacerbate the effects of increased FTCs.
Impacts of Temperature on P Pools and Phosphate-Solubilizing Microbes
Phosphate-solubilizing microbes (PSM) are critical for nutrient cycling and overall soil and plant health, especially in low P soils, where various microbes help plants access P. Networks of fungal hyphae can form “highways for PSM” to access both organic and inorganic P forms in the soil [112]. Several known strategies include enzyme production (e.g., phosphatase), exudate effects on pH, NH4+-driven proton release, exudation of organic acid anions, and siderophore production, yet not all have been thoroughly explored in situ [113]. Other mechanisms are still being discovered, including the recent discovery of antibiotics produced by some microbes that enhance P release from Fe-phosphate minerals [114]. In low P soils, microbial populations contribute to P availability and may reduce the required P inputs. Some microbes, such as mycorrhizae in legacy P soils, could help reduce P losses by immobilizing P being leached [115, 116]. Siebers et al. [117] suspected that the shift from labile P forms to more stable and residual P forms under increasing temperature conditions was due to increased biological processing; however, no direct measures of microbial populations were taken in this study. Most PSM exhibit a parabolic response to a wide range of temperatures, where maximum efficiency at specific temperatures is species dependent [118]. Thermotolerant species of PSM exist; however, it is unclear whether they will adapt at the current rate of climate change and how this will translate to the risk of P loading.
Arbuscular mycorrhizal (AM) fungi are a group of PSM important in agricultural systems [119, 120]. Staddon et al. [121] found that two repercussions of increased temperature (i.e., winter warming and summer drought) increased the percent root length colonized by the AM fungi but decreased the AM fungi extraradical hyphal length density, suggesting a trade-off between symbiosis and soil exploration. Reduced soil exploration by microbes can affect P loss in low and high P environments. In low P soils, this may induce the need for extra fertilizer P application, while in high P soils, microbial immobilization of P could be reduced, leaving more P in the soluble form.
As with many field studies, other factors such as changes in plant diversity and biomass across treatments complicate the interpretations of the direct effects of increased temperature on microbes and related P cycling. A recent study investigated the effects of a 4 °C increase on AM fungi and P leaching from two pasture plant species, tall fescue and lucerne [122], under P-limiting conditions, consistent with scenarios where AM fungi provide the most benefit to their host plants. For tall fescue, the decrease in P leachate caused by AM fungi disappeared under increased temperature, mainly due to a 67% decrease in AM colonization, whereas the increased temperature did not increase P leaching or decrease AM fungi colonization for lucerne. Increased temperatures are known to enhance the carbon (C) demand of AM fungi on their hosts [123], and for plants with a high P demand, like lucerne, the C cost from plants may be necessary to receive P benefits from microbes. These results suggest that the effects of temperature on AM fungi presence and efficacy at mitigating P loss are plant species dependent and may not be as strong in high P soils; however, global conclusions are far from warranted with the limited available literature.
While increased temperature is the most certain outcome of future climate change, there is still considerable uncertainty concerning how it will impact P loss. Increases in air and soil temperature will not occur alone but will come along with a variety of other environmental changes, so understanding the suite of indirect effects of temperature on SWC, FTCs, and P-solubilizing microbes is important to predict and mitigate the effects of climate change on P loss.
Elevated Atmospheric Carbon Dioxide
Carbon dioxide (CO2) concentrations in the atmosphere have increased from ~ 278 ppm in 1750 to ~ 410 ppm in 2019 and are expected to continue to increase in all likely future climate change scenarios [3]. Because soil CO2 concentrations are generally one to two orders of magnitude higher than the atmosphere, eCO2 is not likely to affect soil processes directly [124]. However, it may affect soil P indirectly by stimulating plant growth and P demand and by increasing soil P cycling [124, 125].
A review by Jin et al. [125] found that eCO2 increases the rate of photosynthesis and net primary productivity, which in turn increases plant P demand. They also summarized that plants show the potential to adapt to this higher P demand through changes in root morphology, root exudates, and root-microbial associations. To understand how these changes affect soil P pools and therefore P loss potential, we reviewed 17 journal articles that reported the effects of eCO2 on soil available P. A few of these papers also reported changes in other soil P pools.
Forber et al. [39] suggest using the four-step P transfer continuum to study the complicated dynamics of P loss under climate change. Using this framework as a guideline, we locate the effects of eCO2 primarily in the first two stages: sources and mobilization (solubilization). Sources refer to the significant changes in yields, crops, and rotations that will result from climate change, which in turn will change P fertilizer application needs and flows. Mobilization determines how much DP and PP is available for the third stage of the continuum, delivery/transport. As we were not able to find any studies that directly measured P loss in eCO2 environment, we use measurements of soil P availability as a proxy for P loss.
eCO2 and P Sources
The eCO2 is likely to stimulate greater demand for P, as plants are fertilized with C from the atmosphere and become limited by other nutrients [125, 126]. In two partly overlapping reviews, Jin et al. [125] and Guo et al. [127] found that eCO2 increased plant P uptake in all or most studies. Although a part of the increased P demand may be met by plant adaptations to forage for P in soil, Jin et al. [125] still concluded that increased P fertilization will be required to improve the adaptability of cropping systems to eCO2. Plants may also have a higher demand for P in response to specific stresses from climate change. Edwards et al. [128] found that eCO2 could stimulate increased N fixation in clover, but only with high P supply. In another study, plants needed P fertilizer for maximum drought resistance under eCO2 conditions [125].
eCO2 and P Mobilization
As eCO2 stimulates plant productivity, it will likely induce changes in soil P pools and cycling. Several studies have shown that plants use the extra C to increase foraging abilities through adaptations, such as root morphology, root exudates, and microbial associations [125, 129,130,131], where the net influx of C to the soil stimulates soil microbial growth.
Guo et al. [127] reviewed eight studies reporting the effects of eCO2 and temperature on soil P availability in arable crops. We added nine papers on eCO2 to this review, including arable crops and grassland. Of the 17 papers [129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145], the majority (9) reported no change to soil available P, three reported a decrease, and five reported an increase. Although the experiments ranged from 4 weeks to 22 years, the time had no clear influence on the results. This lack of a clear trend contrasts the findings of the meta-analysis by Yue et al. [146] which found that eCO2 caused a net decline in soil available P in seven studies; however, these mainly were in forest and natural ecosystems, not agricultural environments.
As soil (plant) available P is typically a small fraction of total soil P and is governed by many complex interactions in the soil, it may therefore be more useful to look at changes in the other soil P pools. Eight of the studies in our review also included measurements of soil organic P; of these, seven showed an increase and one study showed a decrease. Jin et al. [125] attributed this increase to the increased C allocation belowground and subsequent stimulation of microbial biomass and activity, which immobilized more P.
In a review of eCO2 effects on soil pools and fluxes of N, Kuzyakov et al. [124] found that there were few changes to N pools but large increases in fluxes and, therefore, biogeochemical cycling. Although their review focused on N, the findings should be applicable to other nutrients, including P. They also highlight another mechanism by which eCO2 may change P mobility in soil by increasing mycorrhization of roots, which increases the exudation of glycoproteins (e.g., glomalin) that serve as the glue for soil aggregates. The result is an increase in the formation and stability of soil aggregates, which can increase infiltration and reduce runoff loss of PP [124].
eCO2 Implications for P Losses
In most agricultural fields, P is primarily lost as PP in erosion and surface runoff, while DP can also be lost via surface runoff in addition to subsurface flow in some settings, such as tile-drained fields and fields with shallow groundwater, where DP may account for most of the P loss. The changes in P pools due to eCO2 are not likely to have a major impact on P loss pathways; however, it is possible that increased belowground C and improved soil aggregation may increase water infiltration, which could reduce P loss in the runoff, though it could simultaneously increase P leaching loss. These changes may be compounded by differences in farm management in response to climate change, such as soil health and soil C sequestration practices, which often reduce runoff and increase infiltration [147].
Interactions with Other Climate Change Effects
It is important to highlight that any potential eCO2 effects on soil P losses will be affected by other climate change effects (i.e., soil moisture, temperature, and N availability); each of which will also affect plant growth and microbial activity [135]. For example, increases in plant growth due to eCO2 may be offset by decreases in growth due to temperature or drought [127]. In turn, plant response to eCO2 may affect other climate effects such as increased soil moisture from reduced leaf stomatal conductance [148]. Increased soil moisture, in turn, could increase P losses to leaching and erosion/runoff pathways since less precipitation is needed to reach saturation point and initiate leaching or runoff [124].
Impact of Sea Level Rise on P Loss
Global sea levels are predicted to rise between 0.3 and 2.5 m by 2100 as a result of climate change [149]. Expected SLR will place large areas of coastal land below sea level, thereby impacting coastal human population and land use, necessitating the urgency for climate change adaptation strategies [150,151,152,153]. Brown et al. [154] projected that by 2100, the 1- to 100-year flood plain area will increase from 540 × 103 km2 (in the year 2000) to 620 × 103 km2 under the climate change mitigation scenario and 740 × 103 km2 under the no-mitigation scenario. Agriculture in coastal areas will be affected by SLR, which will decrease the amount of agricultural land available and change the water and soil chemistry of nearshore land [152, 155,156,157]. Many coastal areas of the world likely to be affected by SLR already have the highest agricultural P surpluses [19, 158] and are likely to have legacy P soils, which implies that these areas will disproportionately lose more P under climate change scenarios.
MacDonald et al. [19] used available local, regional, and global data on fertilizer, livestock, and crop harvest worldwide to estimate global and regional agricultural P imbalances and found the greatest P surpluses in coastal areas (indicated by deep red colors in Fig. 2). If greenhouse gas emissions remain high (IPCC scenario SSP3-7.0), projections of SLR by 2150 are 1–2 m for many coastal regions with high P surpluses [159,160,161]. Using the Climate Central Coastal Risk Screening Tool [158] to visualize where land would be submerged by 1.5-m SLR, there is a significant overlap between P-saturated areas and SLR in many parts of the world, such as the Eastern Shore of the Chesapeake Bay in MD, USA, the Yellow Sea coast of China, and the North Sea coast of the Netherlands. More detailed research overlaying these data, in addition to soil P testing for validation, is critical to identify high-risk areas that are both high in P and at risk for submersion in the future.
Influence of Saltwater Intrusion and Changed Hydrology on P Release
Saltwater intrusion, often a precursor to tidal inundation, occurs when salty water containing elevated concentrations of cations (Na+, Ca2+, Mg2+, K+) and anions ( Cl−, SO42−, NO3−, PO43−) moves inland, impacting estuaries and/or the freshwater-saltwater interface in coastal aquifers [162,163,164,165]. Multiple drivers of SWI include SLR, storm and tidal size and frequency, drought duration and frequency, water use, and hydrologic connectivity [156, 166]. SWI increases ionic strength, changes pH, and causes sulfidation of coastal surface and groundwater in addition to changes in coastal watersheds, including forest loss, species invasion, yield declines, eutrophication, and marsh migration [156].
Agricultural P losses are affected by SWI in multiple ways (Fig. 6). Saltwater increases the ionic strength of groundwater beneath farm fields and in nearby surface waters, which may mobilize P as a part of the soil P (PO43−) is exchanged with saltwater anions [166,167,168]. Additionally, the elevated concentrations of base cations in groundwater from SWI increase alkalization (and potentially pH). Saltwater generally has a higher pH than freshwater and high Ca carbonates, which neutralize some soil acidity [169]. Increases in pH can solubilize OM-Al-inorganic P complexes in acidic soils [170]. However, some studies have shown that SWI can decrease soil pH due to cation exchange between Na+ and H+ [171], so changes in pH would be variable depending on the soil and cations present. Finally, chronic SWI can cause sulfidation (i.e., an increase in sulfide) in soil and groundwater, which can lead to soil P release [172]. In agricultural soils, PO43− is often bound to Fe, but sulfide from chronic SWI can dissociate Fe from PO43−, releasing PO43− into the solution [156, 173]. Ditches and soils with groundwater fluctuations may have fewer tightly bound P forms due to changing redox, and recent research has demonstrated that SO42− competes to displace PO43− in these soils [174].
A simplified visualization of impacts of saltwater intrusion (SWI), induced by sea level rise (SLR), on P in agricultural soils. Ions in saltwater, particularly sulfate (SO42−), can displace phosphate (PO43−), bound with Al, Fe, and Mn, increasing PO43− in the solution. Other impacts of SWI include changes in pH and dispersion of aggregates
Soil P mobilization may also be indirectly affected by SWI. As more agricultural land is affected by SWI, crop yield could be reduced in some fields, and some farmland could be abandoned. As an alternative to field abandonment, there may be a need to grow more salt-tolerant crops (e.g., soybeans or Sorghum) or potentially high biomass crops (e.g., switchgrass or Miscanthus) [175]. Field abandonment would decrease new P input, but P removal by using other crops will be lower, resulting in more legacy P soils and more P losses.
Evidence of the effects of SWI on soil nutrient release has been documented in multiple studies [156, 167, 172, 176, 177]. Tully et al. [156] reported soil P gradients from multiple SWI-impacted fields in MD, USA, by sampling soil transects from the center of fields to ditches in three farms with legacy P soils. Increases in soil P, electrical conductivity, and concentrations of associated saltwater ions were observed in all three fields in the direction of the ditch banks, and soil P concentrations were two to three times higher in SWI-impacted abandoned field edges compared to fields where crops were actively growing. Observations of soil chemistry suggested that soil P was mobilized and likely lost to receiving waters due to SWI and that agricultural ditches served as conduits for saltwater. Furthermore, SWI increased soluble P concentrations in soil porewater from actively farmed intruded fields and surface waters from adjacent agricultural ditches [172]. They attributed elevated P in porewater and near-field surface water to total dissolved Fe being too low to adequately buffer against P loss to receiving waters.
Hydrologic changes in the soil driven by SWI may lead to P release due to the flowing water and excessive saturation of soils, leading to a reduced environment, where the dispersion of aggregates due to the reduction of Fe and Mn oxides could release P [68, 170]. In the UK, flooding of two distinct soils (pH: 5.13 and 5.41, clay: 19 and 11%, organic C: 3.65 and 1.08%, total P: 1283 and 883 mg kg−1, respectively) under both drought and field-moist conditions increased TDP loss, which coincided with a reduction in redox potential and was attributed to dissolution of Fe and Mn minerals [68].
Future research is needed to investigate the effects of SWI on agricultural P mobilization in coastal environments and determine the mechanisms responsible for altering the fate and transport of P. Additionally, research is needed on the effectiveness of potential interventions or strategies to manage the effects of SWI on agricultural sustainability and on incorporating SWI effects in watershed nutrient loading models.
Modeling to Assess the Impact of Climate Change Effects on P Loss
Models are a valuable tool to predict P losses (e.g., Table 1); these models could be adapted to project future P loss under climate change scenarios. A major question about using models is the scale because many models are field-based, even finer scale, or watershed scale. There would be trade-offs in the scale and inputs required to accurately predict how climate change effects may impact P loss.
Researchers have predicted climate change impacts on nutrient transport to surface water using modeling at regional scales, with mixed results on nutrient loading [186,187,188,189]. In addition to the process-based SWAT model [178], the SPAtially Referenced Regression of contaminant transport On Watershed attributes (SPARROW) model is used to predict nutrient fluxes to surface water by layering precipitation, land use, soil, and water flow data [179, 190]. The framework of SPARROW allows users to adjust variables for regional conditions to improve the model to predict contaminant transport under regional conditions, such as was done in the Lake Michigan watershed, USA [88]. The model developed by Ockenden et al. [85•] used the Hydrological Predictions for the Environment (HYPE) [180] and Data-Based Mechanistic (DBM) [181] models to predict P losses. A spreadsheet-based model, Annual P Loss Estimator (APLE), has been used to predict field-scale soil P losses using inputs of runoff, erosion, and management conditions [182]. Lychuk et al. [191] simulated labile P losses under climate change using the Environmental Policy Integrated Climate (EPIC) model to find that reduced inputs and increased crop diversity may mitigate some P loss. Other commonly used models include the INtegrated CAtchment model of phosphorus dynamics (INCA-P) [184] and AGricultural Non-Point Source Pollution Model (AGNPS) [185]. Wellen et al. [192] reviewed these most commonly used watershed-scale water quality models and suggested a number of ideas to improve uncertainties in the current framework.
A challenge in modeling the climate change effects is that many of the variables included in the models have sources of error that are difficult to reduce. Many contradictory studies exist, and further research is needed to improve the accuracy of models of P loss, which must factor in the precipitation [193]. The impacts of aerosols on cloud formation patterns, and therefore precipitation, are still poorly understood. Some of the models consider fertilizer use patterns and animal production to estimate P loading in a region and, thus, assume a reduction in P application will reduce P loss to water. Changes in land use are also included in some models, which can greatly change the outcome of the P loss prediction [194, 195], but the impact of legacy P soil and the potential for continued P losses must be considered in the land use or field management change impact. Impacts of legacy P, dependent on land-use changes in the future, require additional research to develop accurate models [196]. Recent research has indicated that legacy P soils have the capacity to continue contributing substantial amounts of soluble P, even without further inputs of P [12, 20, 24, 197]. A major limitation to the inclusion of legacy P into models is the lack of soil P data for these soils. Recently, McDowell et al. [198] published a framework for mapping P in soils on a global scale. They found ~ 33,000 suitable soil analyses, cleaned from a set of ~ 575,000 samples from topsoil around the globe, which were then converted to Olsen P and used to predict the P content of soils. While this is a good effort to compile soil P data, many more soil samples need to be included to improve the accuracy of the predictions, and scientists and landowners from around the world should continue contributing data to this database or one similar. Models must also be of increasingly high resolution, as noted by researchers in Ireland and Sweden who noticed significant differences between catchments in relatively close proximity [64, 199].
Furthermore, we suggest enhancing future models by highlighting additional P source and transport mechanisms, including potential legacy P inputs and impacts of other climatic effects, such as the increased temperature, eCO2, and SLR. Ockenden et al. [85•] developed a model using indices to describe EP events and climate models with a focus on Europe to predict management changes required to mitigate the expected increased P loading. While this model serves as a well-validated framework for catchments in the UK, we suggest additional considerations may be incorporated in climate change modeling of P loss to make it adaptable to other regions or even a global scale.
Conservation Practices to Reduce P Losses in the Context of Climate Change Effects
To mitigate the climate change effects on P mobilization in soils and loss to waterways, there is a need to refine, develop, and customize a suite of conservation practices. This may involve identifying how the sources and transport pathways of P loss will be altered under climate change scenarios, which may allow the implementation of conservation practices to be cost-effective and successful at reducing P loss from land to water. Conservation practices used to prevent P loss to surface waters can be grouped into two broad categories: practices to control P in soils and practices to trap P at the EOF [200•]. For example, control practices may include reduced or no-till management and cover cropping, while trap practices may include building or maintaining wetlands or using vegetative buffers. In another way, the conservation practices can be grouped into infield, EOF, and beyond-field categories depending on the scale of implementation.
Infield practices such as nutrient management planning, which provides guidance to farmers on correctly applying P to meet crop needs and reduce losses, can help reduce both P source and transport. Similarly, concepts such as the 4R principles, which encourage strategies of proper rate, timing, placement, and source, have generally improved fertilizer application practices [201]. Maintaining soil P levels in the optimum range for crop growth is the single most important practice that can help farmers maximize crop yields while minimizing P loss to waterways. The climate change effects, such as increased air and soil temperature, have the potential to affect soil P availability, so it is imperative to conduct routine soil testing to ensure soil P levels remain in the optimum threshold levels for crop growth. Complications can arise in legacy P soils, which continue to lose P to waterways, even with proper P fertilizer management, leading to persistent dead zones in vulnerable water bodies like the Chesapeake Bay [202, 203]. Climate change will also impact the effectiveness of the 4R practices. For example, the timing of application could be more difficult to determine due to unpredictable extreme weather, and rates could be challenging to determine due to unpredictable yield impacts. Another infield practice, cover cropping, is effective at reducing erosion, and thus PP loss, but can result in high DP loss. Conservation tillage can be useful in mixing freshly applied fertilizer and manures with the plow layer, so EP does not lead to as much incidental P loss. Innovative conservation practices are needed to address legacy P soils impacted by SWI. More research is needed to identify salt-tolerant and flood-tolerant crop species that may be able to use more labile P in these conditions in addition to P removal in the biomass.
In the absence of a regulatory framework, farmers have little, if any, incentive to use their resources to implement conservation practices beyond infield. Therefore, some cost-share assistance from state and federal agencies and non-profits will be critical to ensure that any P that has been transported from the field and is on the way to downstream water bodies can be trapped. Several EOF practices exist, such as wetlands, bioretention ponds, buffer zones, and grass waterways. These practices aim to temporarily store runoff water or slow runoff water to increase the residence time of flowing waters, providing more opportunities for DP and PP to percolate through the soil, where P may be captured rather than running directly into the surface waters [204]. It is important to consider that some conservation practices used to reduce erosion and PP may have trade-offs of increasing soluble P or N losses [205, 206]. Subsurface flow is of particular concern in areas where the water table is shallow and may be exacerbated by artificial drainage. Various in-stream and end-of-tile treatments, such as flow-through cartridges made with natural materials (e.g., gypsum) or industrial by-products (e.g., blast furnace slag), have been tested to remove P from drainage water before it reaches surface water [207]. Depending on the filter material, cartridges eventually become saturated with P and must be disposed of through land application or other means. Some research is being done on biofiltration using various substrates and even periphytic biofilms, which use a synergistic relationship between algae and bacteria to filter nutrients at the field scale [208]. The benefit of these systems is that the algae and bacteria could be harvested to be used as an organic fertilizer or in the biofuel industry if sufficient processing facilities and markets are available.
One of the major drawbacks of many EOF practices is EP, which overwhelms the system with water, leading to bypassing water from the conservation practices, as these high-flow events are the greatest contributors to P losses to surface waters. Thus, under future climate change–induced EP scenarios, conservation practices aimed at reducing P losses during high-flow events should be explored, such as containment basins for runoff and high-volume filtration adjustments. The last opportunity to mitigate P that has escaped infield and EOF practices is implementing practices downstream of several fields, such as riparian forest buffers, which can slow down the running waters and trap P. As many of these downstream areas may be impacted by SWI in coastal areas, other interventions need to be explored.
In summary, a holistic approach involving a suite or stacking of conservation practices is required to reduce P loss to waterways, which takes into account management decisions along the flow pathways from infield, EOF, and beyond the field. Site-specific conservation practices need to be researched and implemented based on the most impactful drivers of P loss. Also, new practices need to be developed that can withstand the impacts of climate change where traditional practices have decreased in effectiveness of capturing lost P. By this, we mean that climate change effects may lead to a variable risk to P sources and transport pathways depending on the location. Hence, mitigation efforts should focus on the most relevant impacts for a particular area. Multiple stages of treatment may be necessary to trap PP but also remove DP in a way that does not become a future source for P loss once saturated with P. Importantly, the varied success and failure of these practices due to extreme weather and other climate change impacts highlight that P transport pathways can be challenging to control, predict, and manage to reduce P loss in agricultural watersheds [200•].
Knowledge Gaps, Future Directions, and Conclusions
This review examined individual effects of climate change to understand the breadth of impacts to be included in future predictions of P loss. More research on the combined climate change effects on P loss is necessary because the effects of climate change will occur simultaneously. Currently, literature is limited to many of the effects individually. For example, the effects on the microbial community and resulting P loss due to combined climate change effects are minimally studied at this time. In the future, researchers should document how the combination of effects associated with climate change (i.e., EP, increasing temperature, eCO2, and SWI) may impact P mobility and loss in soils. This review is informed by the fact that the increased availability of the P source combined with increased transport potential lead to greater P losses in many scenarios. This assumption has been historically validated and is used to manage P from agricultural fields [38]. However, the complexities of climate change may warrant more specific research at the soil-water interface to predict the magnitude of P loss with varying source, mobilization, and transport mechanisms, as also suggested by Forber et al. [39]. The give-and-take of outcomes from the effects in this review calls for targeted research in both P-deficient and P-sufficient agricultural regions. For example, eCO2 and temperature may lead to increased plant biomass, which will require more P uptake, yet EP and periods of drought could lead to diminished crop production and leave soil vulnerable to P losses. Improved modeling of these complex situations or scenarios is a challenge but may become more attainable with advancements in technology. The contribution of legacy P soils to P loss is underrepresented in models and must be included to effectively assess and target high-risk areas prone to P loss. This data should be combined with models that predict transport potential to identify critical source areas. Identification of these critical source areas will improve the prediction of climate change impacts on P loss and remediation strategies to be developed and implemented more effectively.
Site-specific research on developing and refining conservation practices to reduce P losses is needed to address the climate change effects on P losses. Development of drought and flood resistant crops that will successfully grow in marginal land to mitigate P loss is crucial in watersheds with legacy P reservoirs. Researchers should use new innovations in technology and in-situ sensors to understand the mechanistic drivers of P loss and develop solutions to curb P and other contaminants transported from land to water. Current limitations of P removal materials (e.g., water treatment residuals, slag, or gypsum) that have P binding sites (such as Fe and Al) include P saturation over time and becoming a P source if not well-maintained. In many cases, it is not cost-effective to place P removal filters at the EOF, which means good stewardship and front-end infield nutrient management practices should be targeted. Also, P removal filters to treat subsurface flow or concentrated flow pathways, such as end-of-tile or ditch filters, may have diminished effectiveness in the future, as EP may lead to increased P transport in overland flow than subsurface pathways [209]. Researchers should identify, develop, and use easily accessible and affordable soil amendments that allow P to be released slowly in legacy P soils without introducing problematic amounts of metals or harming crop growth. Much research has been done using water treatment residuals, biochar, magnesium sulfate, and others, but well-calibrated rates to reduce P runoff do not exist at the time, and considerations of P availability to crops are required when using these P-immobilizing amendments. More work is needed to understand the microbial drivers that impact P availability, how that translates to P transport, and how this may be impacted by climate change. Documenting changes in P-solubilizing microbes in soil and how they impact P availability and potential transport under varying climate change conditions is warranted. Microbes could also be used as a biofilter, which is already being researched; however, the exploration of additional microbial processes and chemical methods that immobilize P could lead to new discoveries and solutions to manage P in soils.
We conclude that P in soils will be impacted by climate change effects and P losses from the soils will likely increase globally; however, some regions may experience the opposite effect. A clear understanding of the contribution of agricultural soil P to surface water pollution in a changing climate will require experts from varying fields, encompassing soil, water, climate, agronomy, microbiology, GIS, data science, and more. While research is needed to find detailed outcomes of P loss as the climate changes, improvements can be made to existing models using the ideas outlined in this review. With EP, we conclude that more intense and higher volume storms will increase both DP and PP losses. The unpredictability of EP will lead to more incidental P losses after manure or fertilizer applications, and the impacts of EP after drought or fires need to be considered. The impacts of EP will be compounded in legacy P soils. When considering increased temperature, changes in above and belowground biomass production, increased ET, and changes in microbial communities and their P scavenging or cycling behavior need to be explored and included in projections. The implications of eCO2 are similar regarding biomass production and microbial impacts. Finally, SLR must be considered not only due to chemical impacts as ions in seawater interact with soil P and release P to the solution but also due to the changing hydrology of the landscape. Regions with legacy P soils that will collide with a rising sea are of particular importance. We call on the research community to improve the predictions of agricultural P losses and develop the best mitigation strategies to reduce P loss from land to water to keep P in soils and reduce impairment of downstream waters.
Data Availability
All the data used is available in the article.
Abbreviations
- AGNPS:
-
AGricultural Non-Point Source pollution model
- AM:
-
Arbuscular mycorrhizal fungi
- APLE:
-
Annual Phosphorus Loss Estimator
- DBM:
-
Data-Based Mechanistic model
- DP:
-
Dissolved phosphorus
- DRP:
-
Dissolved reactive phosphorus
- DUP:
-
Dissolved unreactive phosphorus
- eCO2 :
-
Elevated atmospheric carbon dioxide
- EOF:
-
Edge-of-the-field
- EP:
-
Extreme precipitation
- EPIC:
-
Environmental Policy Integrated Climate model
- ET:
-
Evapotranspiration
- FTCs:
-
Freeze-thaw cycles
- HYPE:
-
Hydrological Predictors for the Environment model
- INCA-P:
-
INtegrated CAtchment model of Phosphorus dynamics
- IPCC:
-
Intergovernmental Panel on Climate Change
- K:
-
Kelvin
- M3P:
-
Mehlich 3–extractable phosphorus
- MODIS:
-
Moderate Resolution Imaging Spectroradiometer
- OM:
-
Organic matter
- Ortho-P:
-
Orthophosphate
- PP:
-
Particulate phosphorus
- PRP:
-
Particulate reactive phosphorus
- PUP:
-
Particulate unreactive phosphorus
- PSM:
-
Phosphate solubilizing microbes
- RUSLE:
-
Revised Universal Soil Loss Equation
- SLR:
-
Sea level rise
- SPARROW:
-
SPAtially Referenced Regressions of contaminant transport On Watershed attributes model
- SWAT:
-
Soil and Water Assessment Tool
- SWC:
-
Soil water content
- SWI:
-
Saltwater intrusion
- TDP:
-
Total dissolved phosphorus
- TP:
-
Total phosphorus
- TPP:
-
Total particulate phosphorus
- TRP:
-
Total reactive phosphorus.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Le Moal M, Gascuel-Odoux C, Ménesguen A, Souchon Y, Étrillard C, Levain A, et al. Eutrophication: a new wine in an old bottle? Sci Total Environ. 2019;651:1–11. https://doi.org/10.1016/j.scitotenv.2018.09.139.
Dodds WK, Bouska WW, Eitzmann JL, Pilger TJ, Pitts KL, Riley AJ, et al. Eutrophication of U.S. freshwaters: analysis of potential economic damages. Environ Sci Technol. 2009;43(1):12–9. https://doi.org/10.1021/es801217q.
IPCC. Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge, United Kingdom and New York, NY, USA; 2021.
Cooper J, Lombardi R, Boardman D, Carliell-Marquet C. The future distribution and production of global phosphate rock reserves. Resour Conserv Recycl. 2011;57:78–86. https://doi.org/10.1016/j.resconrec.2011.09.009.
Stewart WM, Hammond LL, Van Kauwenbergh SJ. Phosphorus as a natural resource. Phosphorus Agric Environ. 2005;46:1–22. https://doi.org/10.2134/agronmonogr46.c1.
Smil V. Phosphorus in the environment: natural flows and human interferences. Annu Rev Energy Env. 2000;25:53–88. https://doi.org/10.1146/annurev.energy.25.1.53.
Sharpley AN, McDowell RW, Kleinman PJA. Amounts, forms, and solubility of phosphorus in soils receiving manure. Soil Sci Soc Am J. 2004;68(6):2048–57. https://doi.org/10.2136/sssaj2004.2048.
Stutter MI, Shand CA, George TS, Blackwell MSA, Dixon L, Bol R, et al. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma. 2015;257–258:29–39. https://doi.org/10.1016/j.geoderma.2015.03.020.
Liu J, Yang J, Cade-Menun BJ, Hu Y, Li J, Peng C, et al. Molecular speciation and transformation of soil legacy phosphorus with and without long-term phosphorus fertilization: Insights from bulk and microprobe spectroscopy. Sci Rep. 2017;7(1). https://doi.org/10.1038/s41598-017-13498-7.
Ringeval B, Nowak B, Nesme T, Delmas M, Pellerin S. Contribution of anthropogenic phosphorus to agricultural soil fertility and food production. Glob Biogeochem Cycles. 2014;28(7):743–56. https://doi.org/10.1002/2014GB004842.
Pierzynski GM, McDowell RW, Thomas Sims J. Chemistry, cycling, and potential movement of inorganic phosphorus in soils. Phosphorus Agric Environ. 2005;46:51–86. https://doi.org/10.2134/agronmonogr46.c3.
Lucas E, Mosesso L, Roswall T, Yang YY, Scheckel K, Shober A, et al. X-ray absorption near edge structure spectroscopy reveals phosphate minerals at surface and agronomic sampling depths in agricultural Ultisols saturated with legacy phosphorus. Chemosphere. 2022;308:136288. https://doi.org/10.1016/j.chemosphere.2022.136288.
Lindsay WL, Vlek PL, Chien SH. Phosphate minerals. Minerals Soil Environ. 1989;1:1089–130.
Harrison AF. Soil organic phosphorus: a review of world literature. Wallingford, U.K.: CAB International; 1987.
Toor GS, Cade-Menun BJ, Sims JT. Establishing a linkage between phosphorus forms in dairy diets, feces, and manures. J Environ Qual. 2005;34(4):1380–91. https://doi.org/10.2134/jeq2004.0232.
Giles CD, Cade-Menun BJ. Phytate in animal manure and soils: abundance, cycling and bioavailability. In: Applied manure and nutrient chemistry for sustainable agriculture and environment. Springer; 2014. p. 163–90.
Toor GS, Peak JD, Sims JT. Phosphorus speciation in broiler litter and turkey manure produced from modified diets. J Environ Qual. 2005;34(2):687–97. https://doi.org/10.2134/jeq2005.0687.
Toor GS. Enhancing phosphorus availability in low-phosphorus soils by using poultry manure and commercial fertilizer. Soil Sci. 2009;174(6):358–64. https://doi.org/10.1097/SS.0b013e3181a7e716.
MacDonald GK, Bennett EM, Potter PA, Ramankutty N. Agronomic phosphorus imbalances across the world’s croplands. Proc Natl Acad Sci. 2011;108(7):3086–91. https://doi.org/10.1073/pnas.1010808108.
Lucas ER, Toor GS, McGrath JM. Agronomic and environmental phosphorus decline in coastal plain soils after cessation of manure application. Agric Ecosyst Environ. 2021;311. https://doi.org/10.1016/j.agee.2021.107337.
Fulford AM, Culman SW. Over-fertilization does not build soil test phosphorus and potassium in Ohio. Agron J. 2018;110(1):56–65. https://doi.org/10.2134/agronj2016.12.0701.
Olson RA, Anderson FN, Frank KD, Grabouski PH, Rehm GW, Shapiro CA. Soil testing: sampling, correlation, calibration, and interpretation. SSSA Special Publications; 1987. p. 41–52.
Zhang TQ, Zheng ZM, Drury CF, Hu QC, Tan CS. Legacy phosphorus after 45 years with consistent cropping systems and fertilization compared to native soils. Front Earth Sci. 2020;8(183). https://doi.org/10.3389/feart.2020.00183.
Roswall T, Lucas E, Yang YY, Burgis C, Scott IS, Toor GS. Hotspots of legacy phosphorus in agricultural landscapes: revisiting water-extractable phosphorus pools in soils. Water. 2021;13(8):1006. https://doi.org/10.3390/w13081006.
Blombäck K, Bolster CH, Lindsjö A, Hesse K, Linefur H, Parvage MM. Comparing measures for determination of phosphorus saturation as a method to estimate dissolved P in soil solution. Geoderma. 2021;383. https://doi.org/10.1016/j.geoderma.2020.114708.
Maguire RO, Sims JT. Measuring agronomic and environmental soil phosphorus saturation and predicting phosphorus leaching with Mehlich 3. Soil Sci Soc Am J. 2002;66(6):2033–9. https://doi.org/10.2136/sssaj2002.2033.
Nair VD, Harris WG, Lennartz B. Soil phosphorus storage capacity for environmental risk assessment. Adv Agric. 2014;2014(2014):1–9. https://doi.org/10.1155/2014/723064.
Toor GS, Hunger S, Peak JD, Sims JT, Sparks DL. Advances in the characterization of phosphorus in organic wastes: environmental and agronomic applications. Advances in Agronomy. Academic Press; 2006. p. 1–72.
Yang YY, Asal S, Toor GS. Residential catchments to coastal waters: forms, fluxes, and mechanisms of phosphorus transport. Sci Total Environ. 2021;765:142767. https://doi.org/10.1016/j.scitotenv.2020.142767.
Darch T, Blackwell MSA, Hawkins JMB, Haygarth PM, Chadwick D. A meta-analysis of organic and inorganic phosphorus in organic fertilizers, soils, and water: implications for water quality. Crit Rev Environ Sci Technol. 2014;44(19):2172–202. https://doi.org/10.1080/10643389.2013.790752.
Haygarth PM, Sharpley AN. Terminology for phosphorus transfer. J Environ Qual. 2000;29(1):10–5. https://doi.org/10.2134/jeq2000.00472425002900010002x.
Correll DL. The role of phosphorus in the eutrophication of receiving waters: a review. J Environ Qual. 1998;27(2):261–6. https://doi.org/10.2134/jeq1998.00472425002700020004x.
Haygarth PM, Condron LM, Heathwaite AL, Turner BL, Harris GP. The phosphorus transfer continuum: linking source to impact with an interdisciplinary and multi-scaled approach. Sci Total Environ. 2005;344(1):5–14. https://doi.org/10.1016/j.scitotenv.2005.02.001.
McDowell R, Biggs B, Sharpley A, Nguyen L. Connecting phosphorus loss from agricultural landscapes to surface water quality. Chem Ecol. 2004;20(1):1–40. https://doi.org/10.1080/02757540310001626092.
McDowell RW, Sharpley AN. Phosphorus losses in subsurface flow before and after manure application to intensively farmed land. Sci Total Environ. 2001;278(1):113–25. https://doi.org/10.1016/S0048-9697(00)00891-3.
Sharpley AN, Daniel TC, Edwards DR. Phosphorus movement in the landscape. J Prod Agric. 1993;6(4):492–500. https://doi.org/10.2134/jpa1993.0492.
Sharpley AN, Weld JL, Beegle DB, Kleinman PJA, Gburek WJ, Moore PA, et al. Development of phosphorus indices for nutrient management planning strategies in the United States. J Soil Water Conserv. 2003;58(3):137.
Sharpley AN, Kleinman PJA, Flaten DN, Buda AR. Critical source area management of agricultural phosphorus: experiences, challenges and opportunities. Water Sci Technol. 2011;64(4):945–52. https://doi.org/10.2166/wst.2011.712.
Forber KJ, Withers PJA, Ockenden M, Haygarth PM. The phosphorus transfer continuum: a framework for exploring effects of climate change. Agric Environ Lett. 2018;3(1):180036. https://doi.org/10.2134/ael2018.06.0036.
USGCRP: heavy precipitation. 2023. https://www.globalchange.gov/browse/indicators/heavy-precipitation. Accessed 23 Apr 2023.
Donat MG, Lowry AL, Alexander LV, O’Gorman PA, Maher N. More extreme precipitation in the world’s dry and wet regions. Nat Clim Chang. 2016;6:508–13. https://doi.org/10.1038/nclimate2941.
Asadieh B, Krakauer NY. Global trends in extreme precipitation: climate models versus observations. Hydrol Earth Syst Sci. 2015;19(2):877–91. https://doi.org/10.5194/hess-19-877-2015.
Kunkel KE, Karl TR, Brooks H, Kossin J, Lawrimore JH, Arndt D, et al. Monitoring and understanding trends in extreme storms: state of knowledge. Bull Am Meteor Soc. 2013;94(4):499–514. https://doi.org/10.1175/bams-d-11-00262.1.
Walsh J, Wuebbles D, Hayhoe K, Kossin J, Kunkel K, Stephens G, et al. Ch. 2: Our changing climate. Climate change impacts in the United States: the third national climate assessment. In: Melillo JMT, Richmond GWY, editors. U.S. Global Change Research Program; 2014. p. 19–67. http://nca2014.globalchange.gov/report/our-changing-climate/introduction.
Myhre G, Alterskjær K, Stjern CW, Hodnebrog Ø, Marelle L, Samset BH, et al. Frequency of extreme precipitation increases extensively with event rareness under global warming. Sci Rep. 2019;9(1):16063. https://doi.org/10.1038/s41598-019-52277-4.
Shigaki F, Sharpley A, Prochnow LI. Rainfall intensity and phosphorus source effects on phosphorus transport in surface runoff from soil trays. Sci Total Environ. 2007;373(1):334–43. https://doi.org/10.1016/j.scitotenv.2006.10.048.
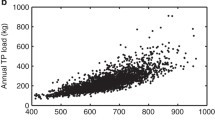
Carpenter SR, Booth EG, Kucharik CJ. Extreme precipitation and phosphorus loads from two agricultural watersheds. Limnol Oceanogr. 2018;63(3):1221–33. https://doi.org/10.1002/lno.10767.
Ross CA, Moslenko LL, Biagi KM, Oswald CJ, Wellen CC, Thomas JL, et al. Total and dissolved phosphorus losses from agricultural headwater streams during extreme runoff events. Sci Total Environ. 2022;848:157736. https://doi.org/10.1016/j.scitotenv.2022.157736.
Ding X, Xue Y, Lin M, Liu Y. Effects of precipitation and topography on total phosphorus loss from purple soil. Water. 2017;9(5):315. https://doi.org/10.3390/w9050315.
Ockenden MC, Deasy CE, Benskin CMH, Beven KJ, Burke S, Collins AL, et al. Changing climate and nutrient transfers: evidence from high temporal resolution concentration-flow dynamics in headwater catchments. Sci Total Environ. 2016;548–549:325–39. https://doi.org/10.1016/j.scitotenv.2015.12.086.
Menezes-Blackburn D, Giles C, Darch T, George TS, Blackwell M, Stutter M, et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: a review. Plant Soil. 2018;427(1):5–16. https://doi.org/10.1007/s11104-017-3362-2.
Djodjic F, Bergström L, Schmieder F, Sandström C, Agback P, Hu Y. Soils potentially vulnerable to phosphorus losses: speciation of inorganic and organic phosphorus and estimation of leaching losses. Nutr Cycl Agroecosyst. 2023. https://doi.org/10.1007/s10705-023-10298-6.
Penn CJ, Bryant RB, Kleinman PJ, Allen AL. Removing dissolved phosphorus from drainage ditch water with phosphorus sorbing materials. J Soil Water Conserv. 2007;62(4):269–76.
Sharpley AN, Kleinman PJ, Heathwaite AL, Gburek WJ, Folmar GJ, Schmidt JP. Phosphorus loss from an agricultural watershed as a function of storm size. J Environ Qual. 2008;37(2):362–8. https://doi.org/10.2134/jeq2007.0366.
Hanrahan BR, King KW, Williams MR. Controls on subsurface nitrate and dissolved reactive phosphorus losses from agricultural fields during precipitation-driven events. Sci Total Environ. 2021;754:142047. https://doi.org/10.1016/j.scitotenv.2020.142047.
Brendel CE, Soupir ML, Long LAM, Helmers MJ, Ikenberry CD, Kaleita AL. Catchment-scale phosphorus export through surface and drainage pathways. J Environ Qual. 2019;48(1):117–26. https://doi.org/10.2134/jeq2018.07.0265.
Bender MA, dos Santos DR, Tiecher T, Minella JPG, de Barros CuAP, Ramon R. Phosphorus dynamics during storm events in a subtropical rural catchment in southern Brazil. Agric Ecosyst Environ. 2018;261:93–102. https://doi.org/10.1016/j.agee.2018.04.004.
Bowes MJ, House WA, Hodgkinson RA, Leach DV. Phosphorus–discharge hysteresis during storm events along a river catchment: the River Swale, UK. Water Res. 2005;39(5):751–62. https://doi.org/10.1016/j.watres.2004.11.027.
Kelly PT, Renwick WH, Knoll L, Vanni MJ. Stream nitrogen and phosphorus loads are differentially affected by storm events and the difference may be exacerbated by conservation tillage. Environ Sci Technol. 2019;53(10):5613–21. https://doi.org/10.1021/acs.est.8b05152.
Gholami L, Khaledi Darvishan A, Spalevic V, Cerdà A, Kavian A. Effect of storm pattern on soil erosion in damaged rangeland; field rainfall simulation approach. J Mt Sci. 2021;18(3):706–15. https://doi.org/10.1007/s11629-019-5633-2.
Huang J, Wu P, Zhao X. Effects of rainfall intensity, underlying surface and slope gradient on soil infiltration under simulated rainfall experiments. CATENA. 2013;104:93–102. https://doi.org/10.1016/j.catena.2012.10.013.
Khan MN, Gong Y, Hu T, Lal R, Zheng J, Justine MF, et al. Effect of slope, rainfall intensity and mulch on erosion and infiltration under simulated rain on purple soil of south-western Sichuan Province, China. Water. 2016;8(11):528. https://doi.org/10.3390/w8110528.
Nearing MA, Pruski FF, O’Neal MR. Expected climate change impacts on soil erosion rates: a review. J Soil Water Conserv. 2004;59(1):43–50.
Ezzati G, Kyllmar K, Barron J. Long-term water quality monitoring in agricultural catchments in Sweden: impact of climatic drivers on diffuse nutrient loads. Sci Total Environ. 2023;864:160978. https://doi.org/10.1016/j.scitotenv.2022.160978.
Fraser A, Harrod T, Haygarth P. The effect of rainfall intensity on soil erosion and particulate phosphorus transfer from arable soils. Water Sci Technol. 1999;39(12):41–5. https://doi.org/10.1016/S0273-1223(99)00316-9.
Toor GS, Condron LM, Di HJ, Cameron KC. Seasonal fluctuations in phosphorus loss by leaching from a grassland soil. Soil Sci Soc Am J. 2004;68(4):1429–36. https://doi.org/10.2136/sssaj2004.1429.
Zeppel MJB, Wilks JV, Lewis JD. Impacts of extreme precipitation and seasonal changes in precipitation on plants. Biogeosciences. 2014;11(11):3083–93. https://doi.org/10.5194/bg-11-3083-2014.
Khan SU, Hooda PS, Blackwell MSA, Busquets R. Effects of drying and simulated flooding on soil phosphorus dynamics from two contrasting UK grassland soils. Eur J Soil Sci. 2022;73(1):e13196. https://doi.org/10.1111/ejss.13196.
Masroor M, Sajjad H, Rehman S, Singh R, Hibjur Rahaman M, Sahana M, et al. Analysing the relationship between drought and soil erosion using vegetation health index and RUSLE models in Godavari middle sub-basin, India. Geosci Front. 2022;13(2):101312. https://doi.org/10.1016/j.gsf.2021.101312.
Lisboa MS, Schneider RL, Sullivan PJ, Walter MT. Drought and post-drought rain effect on stream phosphorus and other nutrient losses in the Northeastern USA. J Hydrol Reg Stud. 2020;28:100672. https://doi.org/10.1016/j.ejrh.2020.100672.
Qiu J, Shen Z, Xie H. Drought impacts on hydrology and water quality under climate change. Sci Total Environ. 2023;858:159854. https://doi.org/10.1016/j.scitotenv.2022.159854.
Thapa GB, Weber KE. Soil erosion in developing countries: a politicoeconomic explanation. Environ Manage. 1991;15(4):461–73. https://doi.org/10.1007/BF02394737.
Kleinman PJA, Sharpley AN. Effect of broadcast manure on runoff phosphorus concentrations over successive rainfall events. J Environ Qual. 2003;32(3):1072–81. https://doi.org/10.2134/jeq2003.1072.
Sharpley AN. Rainfall frequency and nitrogen and phosphorus runoff from soil amended with poultry litter. J Environ Qual. 1997;26(4):1127–32. https://doi.org/10.2134/jeq1997.00472425002600040026x.
Schroeder PD, Radcliffe DE, Cabrera ML. Rainfall timing and poultry litter application rate effects on phosphorus loss in surface runoff. J Environ Qual. 2004;33(6):2201–9. https://doi.org/10.2134/jeq2004.2201.
Motew M, Booth EG, Carpenter SR, Chen X, Kucharik CJ. The synergistic effect of manure supply and extreme precipitation on surface water quality. Environ Res Lett. 2018;13(4):044016. https://doi.org/10.1088/1748-9326/aaade6.
Smith DR, Harmel RD, Williams M, Haney R, King KW. Managing acute phosphorus loss with fertilizer source and placement: proof of concept. Agric Environ Lett. 2016;1(1):150015. https://doi.org/10.2134/ael2015.12.0015.
Smith DR, Owens PR, Leytem AB, Warnemuende EA. Nutrient losses from manure and fertilizer applications as impacted by time to first runoff event. Environ Pollut. 2007;147(1):131–7. https://doi.org/10.1016/j.envpol.2006.08.021.
Guo T, Johnson LT, LaBarge GA, Penn CJ, Stumpf RP, Baker DB, et al. Less agricultural phosphorus applied in 2019 led to less dissolved phosphorus transported to Lake Erie. Environ Sci Technol. 2021;55(1):283–91. https://doi.org/10.1021/acs.est.0c03495.
Carpenter SR, Gahler MR, Kucharik CJ, Stanley EH. Long-range dependence and extreme values of precipitation, phosphorus load, and Cyanobacteria. Proc Nat Acad Sci. 2022;119(48):e2214343119. https://doi.org/10.1073/pnas.2214343119.
Wang C, Morrissey EM, Mau RL, Hayer M, Piñeiro J, Mack MC, et al. The temperature sensitivity of soil: microbial biodiversity, growth, and carbon mineralization. ISME J. 2021;15(9):2738–47. https://doi.org/10.1038/s41396-021-00959-1.
Shaw AN, Cleveland CC. The effects of temperature on soil phosphorus availability and phosphatase enzyme activities: a cross-ecosystem study from the tropics to the Arctic. Biogeochemistry. 2020;151(2):113–25. https://doi.org/10.1007/s10533-020-00710-6.
Eghball B, Wienhold BJ, Woodbury BL, Eigenberg RA. Plant availability of phosphorus in swine slurry and cattle feedlot manure. Agron J. 2005;97(2):542–8. https://doi.org/10.2134/agronj2005.0542.
Silveira ML, O'Connor GA. Temperature effects on phosphorus release from a biosolids-amended soil. Appl Environ Soil Sci. 2013;2013:981715. https://doi.org/10.1155/2013/981715.
• Ockenden MC, Hollaway MJ, Beven KJ, Collins AL, Evans R, Falloon PD, et al. Major agricultural changes required to mitigate phosphorus losses under climate change. Nat Commun. 2017;8(1):161. https://doi.org/10.1038/s41467-017-00232-0. This paper describes one of the most comprehensive examples of a P loss model for climate change, which could be expanded using some additional variables described in this article.
Johnson T, Butcher J, Deb D, Faizullabhoy M, Hummel P, Kittle J, et al. Modeling streamflow and water quality sensitivity to climate change and urban development in 20 U.S. watersheds. J Am Water Resour Assoc. 2015;51(5):1321–41. https://doi.org/10.1111/1752-1688.12308.
Kalcic MM, Muenich RL, Basile S, Steiner AL, Kirchhoff C, Scavia D. Climate change and nutrient loading in the western Lake Erie Basin: warming can counteract a wetter future. Environ Sci Technol. 2019;53(13):7543–50. https://doi.org/10.1021/acs.est.9b01274.
Robertson DM, Saad DA, Christiansen DE, Lorenz DJ. Simulated impacts of climate change on phosphorus loading to Lake Michigan. J Great Lakes Res. 2016;42(3):536–48. https://doi.org/10.1016/j.jglr.2016.03.009.
Wang L, Flanagan DC, Wang Z, Cherkauer KA. Climate change impacts on nutrient losses of two watersheds in the Great Lakes region. Water. 2018;10(4):442. https://doi.org/10.3390/w10040442.
Reshmidevi TV, Nagesh Kumar D, Mehrotra R, Sharma A. Estimation of the climate change impact on a catchment water balance using an ensemble of GCMs. J Hydrol. 2018;556:1192–204. https://doi.org/10.1016/j.jhydrol.2017.02.016.
Lobell DB, Gourdji SM. The influence of climate change on global crop productivity. Plant Physiol. 2012;160(4):1686–97. https://doi.org/10.1104/pp.112.208298.
Fu Q, Feng S. Responses of terrestrial aridity to global warming. J Geophys Res Atmos. 2014;119(13):7863–75. https://doi.org/10.1002/2014JD021608.
Scheff J, Frierson DM. Scaling potential evapotranspiration with greenhouse warming. J Clim. 2014;27(4):1539–58. https://doi.org/10.1175/JCLI-D-13-00233.1.
Scheff J, Frierson DMW. Terrestrial aridity and its response to greenhouse warming across CMIP5 climate models. J Clim. 2015;28(14):5583–600. https://doi.org/10.1175/JCLI-D-14-00480.1.
Salzmann U, Williams M, Haywood AM, Johnson ALA, Kender S, Zalasiewicz J. Climate and environment of a Pliocene warm world. Palaeogeogr Palaeoclimatol Palaeoecol. 2011;309(1):1–8. https://doi.org/10.1016/j.palaeo.2011.05.044.
Berg A, Sheffield J. Climate change and drought: the soil moisture perspective. Curr Clim Change Rep. 2018;4:180–91. https://doi.org/10.1007/s40641-018-0095-0.
Macleod CJA, Falloon PD, Evans R, Haygarth PM. Chapter two - the effects of climate change on the mobilization of diffuse substances from agricultural systems. In: Sparks DL, editor. Advances in agronomy. Academic Press; 2012. p. 41–77.
Fu Q, Hou R, Li T, Wang M, Yan J. The functions of soil water and heat transfer to the environment and associated response mechanisms under different snow cover conditions. Geoderma. 2018;325:9–17. https://doi.org/10.1016/j.geoderma.2018.03.022.
Zhao Y, Li Y, Yang F. Critical review on soil phosphorus migration and transformation under freezing-thawing cycles and typical regulatory measurements. Sci Total Environ. 2021;751:141614. https://doi.org/10.1016/j.scitotenv.2020.141614.
Messiga AJ, Ziadi N, Morel C, Parent L-E. Soil phosphorus availability in no-till versus conventional tillage following freezing and thawing cycles. Can J Soil Sci. 2010;90(3):419–28. https://doi.org/10.4141/CJSS09029.
Oztas T, Fayetorbay F. Effect of freezing and thawing processes on soil aggregate stability. CATENA. 2003;52(1):1–8. https://doi.org/10.1016/S0341-8162(02)00177-7.
Liu J, Ulén B, Bergkvist G, Aronsson H. Freezing–thawing effects on phosphorus leaching from catch crops. Nutr Cycl Agroecosyst. 2014;99:17–30. https://doi.org/10.1007/s10705-014-9615-z.
Wang L, Shi Z, Wu G, Fang N. Freeze/thaw and soil moisture effects on wind erosion. Geomorphology. 2014;207:141–8. https://doi.org/10.1016/j.geomorph.2013.10.032.
Zhang K, Liu H. Research progresses and prospects on freeze-thaw erosion in the black soil region of Northeast China. Sci Soil Water Conserv. 2018;16(1):17–24.
Gao D, Bai E, Yang Y, Zong S, Hagedorn F. A global meta-analysis on freeze-thaw effects on soil carbon and phosphorus cycling. Soil Biol Biochem. 2021;159:108283. https://doi.org/10.1016/j.soilbio.2021.108283.
Henry HAL. Climate change and soil freezing dynamics: historical trends and projected changes. Clim Change. 2008;87(3):421–34. https://doi.org/10.1007/s10584-007-9322-8.
Sinha T, Cherkauer KA. Impacts of future climate change on soil frost in the midwestern United States. J Geophys Res Atmos. 2010;115(D8). https://doi.org/10.1029/2009JD012188.
Qian D, Fan H, Zhou L, Wu M, Guo P. Effects of freeze–thaw cycles on Brown Forest soil available phosphorus in northeastern China. Commun Soil Sci Plant Anal. 2013;44(16):2361–70. https://doi.org/10.1080/00103624.2013.803567.
Peltovuori T, Soinne H. Phosphorus solubility and sorption in frozen, air-dried and field-moist soil. Eur J Soil Sci. 2005;56(6):821–6. https://doi.org/10.1111/j.1365-2389.2005.00726.x.
Cheng Y, Li P, Xu G, Li Z, Wang T, Cheng S, et al. The effect of soil water content and erodibility on losses of available nitrogen and phosphorus in simulated freeze-thaw conditions. CATENA. 2018;166:21–33. https://doi.org/10.1016/j.catena.2018.03.015.
Svanbäck A, Ulén B, Etana A. Mitigation of phosphorus leaching losses via subsurface drains from a cracking marine clay soil. Agr Ecosyst Environ. 2014;184:124–34. https://doi.org/10.1016/j.agee.2013.11.017.
Jiang F, Zhang L, Zhou J, George TS, Feng G. Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol. 2021;230(1):304–15. https://doi.org/10.1111/nph.17081.
Jones DL, Oburger E. Solubilization of phosphorus by soil microorganisms. In: Bünemann E, Oberson A, Frossard E, editors. Phosphorus in action: biological processes in soil phosphorus cycling. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. p. 169–98.
McRose DL, Newman DK. Redox-active antibiotics enhance phosphorus bioavailability. Science. 2021;371(6533):1033–7. https://doi.org/10.1126/science.abd1515.
Bender SF, Conen F, Van der Heijden MGA. Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biol Biochem. 2015;80:283–92. https://doi.org/10.1016/j.soilbio.2014.10.016.
Corkidi L, Merhaut DJ, Allen EB, Downer J, Bohn J, Evans M. Effects of mycorrhizal colonization on nitrogen and phosphorus leaching from nursery containers. HortScience. 2011;46(11):1472–79. https://doi.org/10.21273/HORTSCI.46.11.1472.
Siebers N, Sumann M, Amelung W. Climatic effects on phosphorus fractions of native and cultivated North American grassland soils. Soil Sci Soc Am J. 2017;81(2):299–309. https://doi.org/10.2136/sssaj2016.06.0181.
Musarrat J, Khan MS. Factors affecting phosphate-solubilizing activity of microbes: current status. Phosphate Solubilizing Microorganisms Princ Appl Microphos Technol. 2014:63–85. https://doi.org/10.1007/978-3-319-08216-5_3.
Powell JR, Rillig MC. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018;220(4):1059–75. https://doi.org/10.1111/nph.15119.
Rillig MC, Aguilar-Trigueros CA, Camenzind T, Cavagnaro TR, Degrune F, Hohmann P, et al. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019;222(3):1171–5. https://doi.org/10.1111/nph.15602.
Staddon PL, Thompson K, Jakobsen I, Grime JP, Askew AP, Fitter AH. Mycorrhizal fungal abundance is affected by long-term climatic manipulations in the field. Glob Change Biol. 2003;9(2):186–94. https://doi.org/10.1046/j.1365-2486.2003.00593.x.
Zhang H, Powell JR, Plett JM, Churchill AC, Power SA, Macdonald CA, et al. Climate warming negates arbuscular mycorrhizal fungal reductions in soil phosphorus leaching with tall fescue but not lucerne. Soil Biol Biochem. 2021;152:108075. https://doi.org/10.1016/j.soilbio.2020.108075.
Hawkes CV, Hartley IP, Ineson P, Fitter AH. Soil temperature affects carbon allocation within arbuscular mycorrhizal networks and carbon transport from plant to fungus. Glob Change Biol. 2008;14(5):1181–90. https://doi.org/10.1111/j.1365-2486.2007.01535.x.
Kuzyakov Y, Horwath WR, Dorodnikov M, Blagodatskaya E. Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: no changes in pools, but increased fluxes and accelerated cycles. Soil Biol Biochem. 2019;128:66–78. https://doi.org/10.1016/j.soilbio.2018.10.005.
Jin J, Tang C, Sale P. The impact of elevated carbon dioxide on the phosphorus nutrition of plants: a review. Ann Bot. 2015;116(6):987–99. https://doi.org/10.1093/aob/mcv088.
Gentile R, Dodd M, Lieffering M, Brock SC, Theobald PW, Newton PCD. Effects of long-term exposure to enriched CO 2 on the nutrient-supplying capacity of a grassland soil. Biol Fertil Soils. 2012;48(3):357–62. https://doi.org/10.1007/s00374-011-0616-7.
Guo L, Li Y, Yu Z, Wu J, Jin J, Liu X. Interactive influences of elevated atmospheric CO2 and temperature on phosphorus acquisition of crops and its availability in soil: a review. Int J Plant Prod. 2021;15(2):173–82. https://doi.org/10.1007/s42106-021-00138-4.
Edwards EJ, McCaffery S, Evans JR. Phosphorus availability and elevated CO2 affect biological nitrogen fixation and nutrient fluxes in a clover-dominated sward. New Phytol. 2006;169(1):157–67. https://doi.org/10.1111/j.1469-8137.2005.01568.x.
Jin J, Tang C, Hogarth TW, Armstrong R, Sale P. Nitrogen form but not elevated CO2 alters plant phosphorus acquisition from sparingly soluble phosphorus sources. Plant Soil. 2014;374(1–2):109–19. https://doi.org/10.1007/s11104-013-1870-2.
Jin J, Tang C, Robertson A, Franks AE, Armstrong R, Sale P. Increased microbial activity contributes to phosphorus immobilization in the rhizosphere of wheat under elevated CO2. Soil Biol Biochem. 2014;75:292–9. https://doi.org/10.1016/j.soilbio.2014.04.019.
Bhattacharyya P, Roy KS, Dash PK, Neogi S, Shahid M, Nayak AK, et al. Effect of elevated carbon dioxide and temperature on phosphorus uptake in tropical flooded rice (Oryza sativa L.). Eur J Agron. 2014;53:28–37. https://doi.org/10.1016/j.eja.2013.10.008.
An S, Niu X, Chen W, Sheng H, Lai S, Yang Z, et al. Mechanism of matrix-bound phosphine production in response to atmospheric elevated CO2 in paddy soils. Environ Pollut. 2018;239:253–60. https://doi.org/10.1016/j.envpol.2018.03.082.
Dey SK, Chakrabarti B, Purakayastha TJ, Prasanna R, Mittal R, Singh SD, et al. Interplay of phosphorus doses, cyanobacterial inoculation, and elevated carbon dioxide on yield and phosphorus dynamics in cowpea. Environ Monit Assess. 2019;191:223. https://doi.org/10.1007/s10661-019-7378-3.
Dijkstra FA, Pendall E, Morgan JA, Blumenthal DM, Carrillo Y, Lecain DR, et al. Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol. 2012;196(3):807–15. https://doi.org/10.1111/j.1469-8137.2012.04349.x.
Guenet B, Lenhart K, Leloup J, Giusti-Miller S, Pouteau V, Mora P, et al. The impact of long-term CO2 enrichment and moisture levels on soil microbial community structure and enzyme activities. Geoderma. 2012;170:331–6. https://doi.org/10.1016/j.geoderma.2011.12.002.
Jin J, Armstrong R, Tang C. Long-term impact of elevated CO2 on phosphorus fractions varies in three contrasting cropping soils. Plant Soil. 2017;419(1–2):257–67. https://doi.org/10.1007/s11104-017-3344-4.
Jin J, Tang C, Armstrong R, Butterly C, Sale P. Elevated CO2 temporally enhances phosphorus immobilization in the rhizosphere of wheat and chickpea. Plant Soil. 2013;368(1–2):315–28. https://doi.org/10.1007/s11104-012-1516-9.
Jin J, Tang C, Armstrong R, Sale P. Phosphorus supply enhances the response of legumes to elevated CO 2 (FACE) in a phosphorus-deficient vertisol. Plant Soil. 2012;358(1–2):91–104. https://doi.org/10.1007/s11104-012-1270-z.
Ma HL, Zhu JG, Liu G, Xie ZB, Wang YL, Yang LX, et al. Availability of soil nitrogen and phosphorus in a typical rice-wheat rotation system under elevated atmospheric [CO2]. Field Crop Res. 2007;100(1):44–51. https://doi.org/10.1016/j.fcr.2006.05.005.
Satapathy SS, Swain DK, Pasupalak S, Bhadoria PBS. Effect of elevated [CO2] and nutrient management on wet and dry season rice production in subtropical India. Crop J. 2015;3(6):468–80. https://doi.org/10.1016/j.cj.2015.08.002.
Shi S, Luo X, Dong X, Qiu Y, Xu C, He X. Arbuscular mycorrhization enhances nitrogen, phosphorus and potassium accumulation in vicia faba by modulating soil nutrient balance under elevated co2. J Fungi. 2021;7(5):361. https://doi.org/10.3390/jof7050361.
Touhami D, Condron LM, McDowell RW. Plant species rather than elevated atmospheric CO2 impact rhizosphere properties and phosphorus fractions in a phosphorus-deficient soil. J Soil Sci Plant Nutr. 2021;21(1):622–36. https://doi.org/10.1007/s42729-020-00388-7.
Touhami D, McDowell RW, Condron LM, Lieffering M, Newton PCD. Long-term atmospheric carbon dioxide enrichment decreases soil phosphorus availability in a grazed temperate pasture. Geoderma. 2020;378(July):114621–114621. https://doi.org/10.1016/j.geoderma.2020.114621.
Yu K, Chen X, Pan G, Zhang X, Chen C. Dynamics of soil available phosphorus and its impact factors under simulated climate change in typical farmland of Taihu Lake region, China. Environ Monit Assess. 2016;188(2):1–8. https://doi.org/10.1007/s10661-015-5087-0.
Zhang Y, Chen X, Zhang C, Pan G, Zhang X. Availability of soil nitrogen and phosphorus under elevated [CO2] and temperature in the Taihu Lake region, China. J Plant Nutr Soil Sci. 2014;177(3):343–8. https://doi.org/10.1002/jpln.201200526.
Yue K, Yang W, Peng Y, Peng C, Tan B, Xu Z, et al. Individual and combined effects of multiple global change drivers on terrestrial phosphorus pools: a meta-analysis. Sci Total Environ. 2018;630:189–202. https://doi.org/10.1016/j.scitotenv.2018.02.213.
Duncan EW, Osmond DL, Shober AL, Starr L, Tomlinson P, Kovar JL, et al. Phosphorus and soil health management practices. Agric Environ Lett. 2019;4(1):190014–190014. https://doi.org/10.2134/ael2019.04.0014.
Morgan JA, LeCain DR, Pendall E, Blumenthal DM, Kimball BA, Carrillo Y, et al. C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature. 2011;476(7359):202–5. https://doi.org/10.1038/nature10274.
Sweet W, Kopp RE, Weaver CP, Obeysekera JTB, Horton RM, Thieler ER, et al. Global and regional sea level rise scenarios for the United States. NOAA Tech Rep NOS CO-OPS. 2017;83. https://doi.org/10.7289/V5/TR-NOS-COOPS-083.
Hauer ME, Fussell E, Mueller V, Burkett M, Call M, Abel K, et al. Sea-level rise and human migration. Nat Rev Earth Environ. 2020;1(1):28–39. https://doi.org/10.1038/s43017-019-0002-9.
Hinkel J, Aerts JCJH, Brown S, Jiménez JA, Lincke D, Nicholls RJ, et al. The ability of societies to adapt to twenty-first-century sea-level rise. Nat Clim Change. 2018;8(7):570–8. https://doi.org/10.1038/s41558-018-0176-z.
Kirwan ML, Gedan KB. Sea-level driven land conversion and the formation of ghost forests. Nat Clim Change. 2019;9(6):450–7. https://doi.org/10.1038/s41558-019-0488-7.
Kulp SA, Strauss BH. New elevation data triple estimates of global vulnerability to sea-level rise and coastal flooding. Nat Commun. 2019;10(1):4844–4844. https://doi.org/10.1038/s41467-019-12808-z.
Brown S, Nicholls RJ, Goodwin P, Haigh ID, Lincke D, Vafeidis AT, et al. Quantifying land and people exposed to sea-level rise with no mitigation and 1.5 °C and 2.0 °C rise in global temperatures to year 2300. Earth's Future. 2018;6(3):583–600. https://doi.org/10.1002/2017EF000738.
Kheir AMS, El Baroudy A, Aiad MA, Zoghdan MG, Abd El-Aziz MA, Ali MGM, et al. Impacts of rising temperature, carbon dioxide concentration and sea level on wheat production in north Nile Delta. Sci Total Environ. 2019;651:3161–73. https://doi.org/10.1016/J.SCITOTENV.2018.10.209.
Tully KL, Weissman D, Wyner WJ, Miller J, Jordan T. Soils in transition: saltwater intrusion alters soil chemistry in agricultural fields. Biogeochemistry. 2019;142(3):339–56. https://doi.org/10.1007/s10533-019-00538-9.
Vu DT, Yamada T, Ishidaira H. Assessing the impact of sea level rise due to climate change on seawater intrusion in Mekong Delta, Vietnam. Water Sci Technol. 2018;77(6):1632–9. https://doi.org/10.2166/wst.2018.038.
ClimateCentral: Coastal Risk Screening Tool. 2000. https://coastal.climatecentral.org/. Accessed 7 Dec 2022.
Fox-Kemper B, Hewitt HT, Xiao C, Aðalgeirsdóttir G, Drijfhout SS, Edwards TL, et al. Ocean, cryosphere and sea level change. In: Masson-Delmotte VP, Zhai A, Pirani SL, Connors C, Péan S, Berger N, Caud Y, Chen L, Goldfarb MI, Gomis M, Huang K, Leitzell E, Lonnoy JB, Matthews R, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B, editors. Climate change 2021: the physical science basis contribution of working group I to the sixth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2021.
Garner GG, Kopp RE, Hermans T, Slangen ABA, Koubbe G, Turilli M, et al. Framework for Assessing Changes To Sea-level (FACTS). 2022. https://sealevel.nasa.gov/data/tools/ipcc-ar6-projections-licensing-and-acknowledgements. Accessed 7 Dec 2022.
PO.DAAC, CA, USA. 2021. https://podaac.jpl.nasa.gov/announcements/2021-08-09-Sea-level-projections-from-the-IPCC-6th-Assessment-Report. Accessed 7 Dec 2022.
Barlow PM. Ground water in freshwater-saltwater environments of the Atlantic Coast Circular 1262. Reston: US Department of the Interior, US Geological Survey; 2003.https://pubs.usgs.gov/circ/2003/circ1262/.
Knighton AD, Mills K, Woodroffe CD. Tidal-creek extension and saltwater intrusion in Northern Australia. Geology. 1991;19(8):831–831. https://doi.org/10.1130/0091-7613(1991)019%3c0831:TCEASI%3e2.3.CO;2.
Servais S, Kominoski JS, Charles SP, Gaiser EE, Mazzei V, Troxler TG, et al. Saltwater intrusion and soil carbon loss: testing effects of salinity and phosphorus loading on microbial functions in experimental freshwater wetlands. Geoderma. 2019;337:1291–300. https://doi.org/10.1016/J.GEODERMA.2018.11.013.
White E, Kaplan D. Restore or retreat? Saltwater intrusion and water management in coastal wetlands. Ecosyst Health Sust. 2017;3(1):e01258–e01258. https://doi.org/10.1002/ehs2.1258.
Tully K, Gedan K, Epanchin-Niell R, Strong A, Bernhardt ES, BenDor T, et al. The invisible flood: the chemistry, ecology, and social implications of coastal saltwater intrusion. Bioscience. 2019;69(5):368–78. https://doi.org/10.1093/biosci/biz027.
Weissman D, Ouyang T, Tully KL. Saltwater intrusion affects nitrogen, phosphorus and iron transformations under oxic and anoxic conditions: an incubation experiment. Biogeochemistry. 2021;154(3):451–69. https://doi.org/10.1007/s10533-021-00796-6.
Weston NB, Dixon RE, Joye SB. Ramifications of increased salinity in tidal freshwater sediments: geochemistry and microbial pathways of organic matter mineralization. J Geophys Res. 2006;111(G1):G01009–G01009. https://doi.org/10.1029/2005JG000071.
Arslan H, Demir Y. Impacts of seawater intrusion on soil salinity and alkalinity in Bafra Plain, Turkey. Environ Monit Assess. 2013;185(2):1027–40. https://doi.org/10.1007/s10661-012-2611-3.
Darke AK, Walbridge MR. Al and Fe biogeochemistry in a floodplain forest: implications for P retention. Biogeochemistry. 2000;51(1):1–32. https://doi.org/10.1023/A:1006302600347.
Tan LV, Thanh T. The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam. Open Chem. 2021;19(1):471–80. https://doi.org/10.1515/chem-2021-0037.
Weissman DS, Tully KL. Saltwater intrusion affects nutrient concentrations in soil porewater and surface waters of coastal habitats. Ecosphere. 2020;11(2):e03041–e03041. https://doi.org/10.1002/ecs2.3041.
Hartzell JL, Jordan TE, Cornwell JC. Phosphorus sequestration in sediments along the salinity gradients of Chesapeake Bay subestuaries. Estuaries Coasts. 2017;40(6):1607–25. https://doi.org/10.1007/s12237-017-0233-2.
Szerlag KD, Elavarthi M, Siebecker MG, Gu C, McCrone C, Sparks DL. Systematic study of legacy phosphorus (P) desorption mechanisms in high-P agricultural soils. Minerals. 2022;12(4):458. https://doi.org/10.3390/min12040458.
Zheng C, Iqbal Y, Labonte N, Sun G, Feng H, Yi Z, et al. Performance of switchgrass and Miscanthus genotypes on marginal land in the Yellow River Delta. Ind Crops Prod. 2019;141:111773. https://doi.org/10.1016/j.indcrop.2019.111773.
Kristensen E, Quintana CO, Valdemarsen T, Flindt MR. Nitrogen and phosphorus export after flooding of agricultural land by coastal managed realignment. Estuaries Coasts. 2021;44(3):657–71. https://doi.org/10.1007/s12237-020-00785-2.
Steinmuller HE, Chambers LG. Can saltwater intrusion accelerate nutrient export from freshwater wetland soils? An experimental approach. Soil Sci Soc Am J. 2018;82(1):283–92. https://doi.org/10.2136/sssaj2017.05.0162.
Arnold JG, Srinivasan R, Muttiah RS, Williams JR. Large area hydrologic modeling and assessment part I: model development 1. J Am Water Resour Assoc. 1998;34(1):73–89.
Schwarz GE, Hoos AB, Alexander R, Smith R. The SPARROW surface water-quality model: theory, application and user documentation. 2006;6–B3. https://doi.org/10.3133/tm6B3.
Lindström G, Pers C, Rosberg J, Strömqvist J, Arheimer B. Development and testing of the HYPE (Hydrological Predictions for the Environment) water quality model for different spatial scales. Hydrol Res. 2010;41(3–4):295–319. https://doi.org/10.2166/nh.2010.007.
Young P. Top-down and data-based mechanistic modelling of rainfall–flow dynamics at the catchment scale. Hydrol Process. 2003;17(11):2195–217. https://doi.org/10.1002/hyp.1328.
Vadas PA, Joern BC, Moore PA. Simulating soil phosphorus dynamics for a phosphorus loss quantification tool. J Environ Qual. 2012;41(6):1750–7. https://doi.org/10.2134/jeq2012.0003.
Williams JR, Dagitz S, Margre M, Meinardus A, Steglich E, Taylor R. EPIC User’s Manual v. 0810. Temple, TX: Texas A&M AgriLife Blackland Research and Extension Center; 2015.https://epicapex.tamu.edu/manuals-and-publications/.
Jackson-Blake LA, Wade AJ, Futter MN, Butterfield D, Couture RM, Cox BA, et al. The INtegrated CAtchment model of phosphorus dynamics (INCA-P): description and demonstration of new model structure and equations. Environ Model Softw. 2016;83:356–86. https://doi.org/10.1016/j.envsoft.2016.05.022.
Bingner R, Theurer F, Yuan Y. AnnAGNPS technical processes: documentation version 2. USDA-ARS National Sedimentation Laboratory, Oxford, Miss: Unpublished Report; 2001.
Crossman J, Futter MN, Elliott JA, Whitehead PG, Jin L, Dillon PJ. Optimizing land management strategies for maximum improvements in lake dissolved oxygen concentrations. Sci Total Environ. 2019;652:382–97. https://doi.org/10.1016/j.scitotenv.2018.10.160.
Dunn SM, Brown I, Sample J, Post H. Relationships between climate, water resources, land use and diffuse pollution and the significance of uncertainty in climate change. J Hydrol. 2012;434–435:19–35. https://doi.org/10.1016/j.jhydrol.2012.02.039.
Hallin-Pihlatie L, Rintala J, Hansen H. Integration of climate change and land-use scenarios in nutrient leaching assessment. Int J Climate Chang Strateg Manag. 2013;5(3):285–303. https://doi.org/10.1108/IJCCSM-04-2011-0016.
El-Khoury A, Seidou O, Lapen DR, Que Z, Mohammadian M, Sunohara M, et al. Combined impacts of future climate and land use changes on discharge, nitrogen and phosphorus loads for a Canadian river basin. J Environ Manage. 2015;151:76–86. https://doi.org/10.1016/j.jenvman.2014.12.012.
Smith RA, Schwarz GE, Alexander RB. Regional interpretation of water-quality monitoring data. Water Resour Res. 1997;33(12):2781–98. https://doi.org/10.1029/97WR02171.
Lychuk TE, Moulin AP, Lemke RL, Izaurralde RC, Johnson EN, Olfert OO, et al. Modelling the effects of climate change, agricultural inputs, cropping diversity, and environment on soil nitrogen and phosphorus: a case study in Saskatchewan, Canada. Agric Water Manag. 2021;252:106850. https://doi.org/10.1016/j.agwat.2021.106850.
Wellen C, Kamran-Disfani A-R, Arhonditsis GB. Evaluation of the current state of distributed watershed nutrient water quality modeling. Environ Sci Technol. 2015;49(6):3278–90. https://doi.org/10.1021/es5049557.
Tao WK, Chen JP, Li Z, Wang C, Zhang C. Impact of aerosols on convective clouds and precipitation. Rev Geophys. 2012;50(2). https://doi.org/10.1029/2011RG000369.
Liu LH, Ouyang W, Lin CY. Future warming-induced phosphorus loss mitigated by land conversion and degradation. Soil Tillage Res. 2022;224. https://doi.org/10.1016/j.still.2022.105526.
Ekstrand Sam S, Ekstrand S, Wallenberg P. Climate change impact on nutrient loss in regions with pronounced winter seasons. J Water Clim Chang. 2010;1(3):181–92. https://doi.org/10.2166/wcc.2010.019.
MacDonald GK, Bennett EM, Taranu ZE. The influence of time, soil characteristics, and land-use history on soil phosphorus legacies: a global meta-analysis. Glob Change Biol. 2012;18(6):1904–17. https://doi.org/10.1111/j.1365-2486.2012.02653.x.
McDowell R, Dodd R, Pletnyakov P, Noble A. The ability to reduce soil legacy phosphorus at a country scale. Front Environ Sci. 2020;8(6). https://doi.org/10.3389/fenvs.2020.00006.
McDowell RW, Noble A, Pletnyakov P, Haygarth PM. A global database of soil plant available phosphorus. Sci Data. 2023;10(1):125. https://doi.org/10.1038/s41597-023-02022-4.
Mellander P-E, Jordan P. Charting a perfect storm of water quality pressures. Sci Total Environ. 2021;787:147576. https://doi.org/10.1016/j.scitotenv.2021.147576.
• Kleinman PJA, Osmond DL, Christianson LE, Flaten DN, Ippolito JA, Jarvie HP, et al. Addressing conservation practice limitations and tradeoffs for reducing phosphorus loss from agricultural fields. Agric Environ Lett. 2022;7(2). https://doi.org/10.1002/ael2.20084. This paper describes how conservation practices to reduce P loss have tradeoffs and unintended consequences, and that climate change is reducing their effectiveness.
Johnston AM, Bruulsema TW. 4R nutrient stewardship for improved nutrient use efficiency. Procedia Eng. 2014;83:365–70. https://doi.org/10.1016/j.proeng.2014.09.029.
Sharpley A, Jarvie HP, Buda A, May L, Spears B, Kleinman P. Phosphorus legacy: overcoming the effects of past management practices to mitigate future water quality impairment. J Environ Qual. 2013;42(5):1308–26. https://doi.org/10.2134/jeq2013.03.0098.
Kleinman PJA, Fanelli RM, Hirsch RM, Buda AR, Easton ZM, Wainger LA, et al. Phosphorus and the Chesapeake Bay: lingering issues and emerging concerns for agriculture. J Environ Qual. 2019;48(5):1191–203. https://doi.org/10.2134/jeq2019.03.0112.
Dorioz JM, Wang D, Poulenard J, Trévisan D. The effect of grass buffer strips on phosphorus dynamics—a critical review and synthesis as a basis for application in agricultural landscapes in France. Agr Ecosyst Environ. 2006;117(1):4–21. https://doi.org/10.1016/j.agee.2006.03.029.
Heathwaite L, Sharpley A, Gburek W. A conceptual approach for integrating phosphorus and nitrogen management at watershed scales. J Environ Qual. 2000;29(1):158–66. https://doi.org/10.2134/jeq2000.00472425002900010020x.
Jarvie HP, Johnson LT, Sharpley AN, Smith DR, Baker DB, Bruulsema TW, et al. Increased soluble phosphorus loads to Lake Erie: unintended consequences of conservation practices? J Environ Qual. 2017;46(1):123–32. https://doi.org/10.2134/jeq2016.07.0248.
King KW, Williams MR, Macrae ML, Fausey NR, Frankenberger J, Smith DR, et al. Phosphorus transport in agricultural subsurface drainage: a review. J Environ Qual. 2015;44(2):467–85. https://doi.org/10.2134/jeq2014.04.0163.
Marella TK, Saxena A, Tiwari A, Datta A, Dixit S. Treating agricultural non-point source pollutants using periphyton biofilms and biomass volarization. J Environ Manage. 2022;301:113869. https://doi.org/10.1016/j.jenvman.2021.113869.
Mehan S, Aggarwal R, Frankenberger JR. Assessment of hydrology and nutrient losses in a changing climate in a subsurface-drained watershed. Sci Total Environ. 2019;688:1236–51. https://doi.org/10.1016/j.scitotenv.2019.06.314.
Funding
Gurpal S. Toor received funding from USDA-NIFA Hatch project 1014496, USDA-AFRI competitive grants 2019-68008-29911 and 2019-68012-29904, Harry R. Hughes Center for Agroecology, and the Maryland Department of Agriculture. This funding was used to support EL, BK, TR, and CB.
Author information
Authors and Affiliations
Contributions
EL, in conjection with BK, TR, CB, and GT wrote the first draft of the manuscript. EL prepared all the figures. All authors reviewed the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lucas, E., Kennedy, B., Roswall, T. et al. Climate Change Effects on Phosphorus Loss from Agricultural Land to Water: A Review. Curr Pollution Rep 9, 623–645 (2023). https://doi.org/10.1007/s40726-023-00282-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-023-00282-7