Abstract
Low-lying coastal ecosystems are rapidly salinizing due to sea level rise and associated saltwater intrusion (SWI). In agricultural soils, SWI can alter biogeochemical cycling of key nutrients such as nitrogen (N), phosphorus (P), and iron (Fe). The main objective of this study was to quantify the amount of nitrate–N (NO3–N), ammonium–N (NH4–N), soluble reactive P (SRP), and total dissolved iron (TDFe) released from agricultural soils undergoing SWI to determine their potential loss to downstream waterways. Agricultural soils were incubated for 0, 15, and 30 days (under oxic and anoxic conditions) with various salt solution combinations of sodium chloride (NaCl), sodium sulfate (Na2SO4), calcium sulfate (CaSO4), and Instant Ocean® to mimic (1) different ionic constituents of saltwater at different ionic strengths and (2) the presence or absence of gypsum, a soil amendment, through the addition of CaSO4. We also included a set of incubations treated with deionized water as a no ionic strength control (0.00 M). To increase statistical power, we grouped individual salt treatments based on our initial hypotheses at the end of the incubation period (day 30) to determine the effects of high (0.26–0.28 M) and low (0.03–0.04 M) ionic strength on inorganic N release and combinations of Ca and SO42− additions on SRP release to microcosm soil solution. Calcium additions decreased SRP release relative to saltwater that contained only NaCl additions under oxic and anoxic conditions. Additionally, high ionic strength treatments, which were about 7 times the ionic strength of low ionic strength treatments, released two times as much NH4–N to the soil solution, which suggests a non-linear relationship between ionic strength and NH4–N release. At day 30, anoxic microcosm soils treated with Instant Ocean® to simulate 15 parts per thousand seawater (ionic strength 0.26 M) released significantly more NH4–N (by 782 times), SRP (by 6 times), and TDFe (by 197 times) to the soil solution than oxic microcosm soils (P < 0.05). This treatment was designed to reflect a field inundated by brackish seawater for almost a month, which is representative of conditions on farm fields undergoing SWI. Under these anoxic conditions, as much as 22% of bioavailable soil P (as SRP) and 45% of total inorganic N (as NH4–N) could be released to overlying water when inundated with saltwater. Our work indicates that the influx of salts and inundation of SWI-affected farm fields could lead to a large export of N and P from agricultural soils and potentially affect downstream water quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Saltwater intrusion (SWI) into freshwater systems is a growing problem in coastal regions worldwide (White and Kaplan 2017; Tully et al. 2019a). Driven by sea level rise, the frequency and intensity of storms and droughts, water extraction for human use, and the connectivity of the landscape to sources of saltwater (e.g. canals, ditches, etc.), SWI can push sea salts inland far beyond the high tide line (Schoepfer et al. 2014; Bhattachan et al. 2018; Tully et al. 2019b). By 2050, global sea levels are expected to rise by 20–30 cm (Stocker et al. 2013). The effects of SWI include, but are not limited to, marsh migration, forest die-off, the spread of invasive species, crop yield declines, and nutrient pollution through changes in biogeochemical cycles in soils (Tully et al. 2019a). In this study, we focus on biogeochemical effects of SWI that alter nitrogen (N) and phosphorus (P) cycling in agricultural soils and can lead to pollution in coastal waterways downstream of SWI-affected farm fields (Ardón et al. 2013; Williams et al. 2014).
Many coastal farms are located on former wetlands that were drained for agricultural use (Moomaw et al. 2018). In areas very close to saline water bodies, such as tidal creeks and salt marshes, ditches can regularly flood with saltwater, even during baseflow conditions (Bhattachan et al. 2018). In coastal regions with low elevations, extensive ditch networks were historically carved into the landscape to allow excess water to flow off farm fields (Reddy and DeLaune 2008). However, as SWI moves inland, ditches serve the reverse purpose and frequently act as conduits that allow saltwater to concentrate on fields during high tides, storms, and droughts (Tully et al. 2019a). Saltwater can remain on these fields for days or even weeks and spur major changes in soil chemistry and nutrient release (Weissman and Tully 2020).
Though the theoretical understanding of the biogeochemical processes that lead to nutrient release in soils exposed to inundation and saltwater has been well established (Ardón et al. 2013; Hartzell et al. 2017), the rate and extent of their occurrence vary greatly from site to site depending on prevailing environmental conditions such as soil texture, organic matter content, pH, and temperature (Roman and Burdick 2012). Further, most studies on SWI have been conducted on freshwater wetlands that are becoming salinized (Neubauer et al. 2013; Helton et al. 2014; White and Kaplan 2017). Agricultural soil properties are distinct from freshwater wetland soils, which causes nutrient cycles to behave differently in each of these environments. For example, since wetlands are frequently inundated, nitrate (NO3−) is leached quickly from or denitrified in wetland soils, while sulfate (SO42−) is consumed by sulfate reduction (Megonigal and Neubauer 2009). In contrast, drier conditions in well-drained agricultural soils allow for accumulation of NO3− and SO42− (McLay et al. 1991). Because wetland soils usually contain a thicker horizon of carbon (C)-rich organic material than agricultural soils, biological activity is often more C-limited in agricultural soils (Roman and Burdick 2012). Finally, wetland soils tend to be more oxygen depleted and release soluble reactive P (SRP) into solution more readily than well-drained agricultural soils (Nair et al. 2015). Thus, in order to better understand the potential consequences of large-scale SWI in coastal farmlands, we conducted a detailed incubation study with agricultural soil to mimic different aspects of SWI (e.g. oxygen levels, ionic strength, SO42− addition, and a soil amendment containing calcium; Ca) and assess their impacts on N and P release to overlying waters.
In SWI-affected areas, N loss from soils and subsequent movement to downstream waterways increases with inundation and ionic strength (Tully et al. 2019a). Because of its negative charge, NO3− is repelled by negatively charged soil particles and hence tends to leach quickly from saturated soils (Goyne et al. 2008). On the other hand, ammonium (NH4+) ions tend to readily sorb to exchange sites on agricultural soil but are easily displaced by cations found in saltwater, such as Na+. Both of these processes can lead to a large export of N into local waterways (Ardón et al. 2013).
Interactions between SO42− and iron (Fe) can enhance P release from SWI-affected soils (Williams et al. 2014). In well-aerated soils, Fe is present in its oxidized form [Fe(III)] within poorly-crystalline oxide minerals that strongly bind phosphate (PO43−), the bioavailable form of P (McDowell and Sharpley 2001). However, Fe(III) is biotically and abiotically reduced to Fe(II) under low oxygen conditions, such as in a field that has been inundated. The Fe reduction process releases PO43− into the soil porewater (Nair et al. 2013). Unlike freshwater, saltwater is rich in SO42−, which provides another terminal electron acceptor in saturated soils. Microbial populations reduce SO42− to sulfide (a compound containing one or more S2− ions), which can bind to Fe(II), forming Fe sulfides (FeSx) in reducing environments (Schoepfer et al. 2014). This process immobilizes Fe and makes it unavailable to immediately rebind P (Caraco et al. 1990; Jordan et al. 2008). Since soils in SWI-affected areas tend to undergo wetting and drying cycles (Tully et al. 2019b), FeSx could be reoxidized upon soil drying and release hydrogen protons (acid), SO42−, and Fe(III), which can then reprecipitate as Fe oxides and rebind PO43− (Luther et al. 1992; Roden and Edmonds 1997). However in a SWI-affected system, soil drying and consequently Fe reoxidation reactions would occur relatively slowly compared to the rate at which mobilized PO43− tends to diffuse or move with tidal pulses out of porewater of inundated soil, into overlying water, then into downstream water bodies (Tully et al. 2019b).
Other transformations of N and P are also greatly affected by changing soil redox conditions that co-occur with SWI. Aerobic microbial respiration pathways (where oxygen is reduced) are the most thermodynamically favorable, and thus are the dominant form of respiration in well-aerated (oxic) soil. However, soils are depleted in oxygen as they become saturated, and other respiration pathways begin to dominate. The next most widespread and energetically favorable pathways are NO3− reduction, followed by Fe reduction, and SO42− reduction (Arndt et al. 2013). In saturated soils, much of the NO3− present is rapidly reduced to N2 gas through the process of denitrification. However, many studies have shown that a large portion of NO3− can also be reduced to NH4+ through dissimilatory nitrate reduction to ammonium (DNRA), particularly in saline soils (Tobias et al. 2001; Burgin and Hamilton 2007; Giblin et al. 2013).
Finally, soil amendments can also affect soluble reactive P (SRP; a proxy for phosphate) concentrations in soil porewater (Grubb et al. 2012). Calcium is often added to fields as gypsum (CaSO4∙2H2O) to remediate sodic soils (Fowler et al. 2014) and as agricultural lime [Ca(OH)2] to raise soil pH (Brady and Weil 2016). Because Ca also binds and sequesters SRP in soils, it has been studied for its potential to reduce P pollution in agricultural ditches (Moore and Miller 1994; Grubb et al. 2012). Thus, the addition of CaSO4 to soil as management response to SWI can also affect soil P bioavailability.
There is a growing body of literature using laboratory incubation (Weston et al. 2011; Williams et al. 2014; Steinmuller and Chambers 2018; Neubauer et al. 2019) studies to assess the effects of SWI on wetland soils. Additionally, in field studies, we have previously demonstrated how the synergistic effects of salinity additions and frequent inundation result in redox-mediated chemicals reactions that drive N and P loss from soils of coastal farmlands (Tully et al. 2019b; Weissman and Tully 2020). However, no prior laboratory incubation studies have examined how incoming saltwater affects nutrient release from agricultural soils undergoing SWI. Therefore, our incubation study represents a novel effort to quantify N and P release from coastal agricultural soils into downstream waterways under controlled conditions.
The main objective of this study was to quantify the effect of different ionic constituents of saltwater and CaSO4 on the release of dissolved inorganic N (DIN), SRP, and total dissolved Fe (TDFe) release from an agricultural soil under oxic and anoxic conditions. We also measured total Fe oxides to determine if the amount of Fe dissolved into soil solution caused a significant change in overall pools of soil Fe after 30 days. Because of DNRA and cation exchange on soil particle surfaces, we hypothesized that anoxic high ionic strength treatments would enhance NH4–N release to the soil solution while driving NO3–N concentrations to near-zero levels. Additionally, we expected to observe increased SRP release under anoxic conditions due to Fe and SO42− reduction. Finally, because Ca2+ readily binds to SRP, we expected that calcium sulfate (+ CaSO4) treatments would suppress SRP release from both oxic and anoxic soils. To achieve our study objective, we simulated four moderators of nutrient release: (1) change in ionic strength (Ardón et al. 2013); (2) enhanced SO42− reduction (Williams et al. 2014); (3) decreased oxygen levels (Nair et al. 2013); and (4) soil amendment with Ca (Grubb et al. 2012). Saltwater is comprised of a variety of ions that can affect biogeochemical cycling in soils such as base cations like sodium (Na+), calcium (Ca2+), magnesium, and potassium, and the anions SO42− and chloride (Cl−). Combined with lowered oxygen levels in soils that result from inundation, ions have synergistic effects on nutrient release. Our study results can be used to develop improved nutrient reduction targets for ecologically-sensitive coastal areas.
Materials and methods
Study site
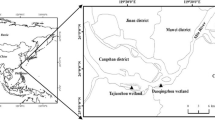
In Maryland, the Chesapeake Bay surrounds the western shores of Dorchester, Wicomico, and Somerset counties (Fig. 1a). We collected soils on a farm field near Princess Anne, in Somerset County, the southernmost county in Maryland (38.2° N, 75.7° W; Fig. 1b). The farm field has been planted in a corn/soy rotation for the past ten years. In 2018, the year prior to soil sample collection, the field was planted in corn. Urea ammonium nitrate fertilizer was applied to the field at a rate of 90 kg N/ha. The field had been limed three years prior with calcium carbonate (CaCO3). The soil was comprised of a mesic Aquic Hapludult loam mainly from the Manokin soil series (Soil Survey Staff 2018). Additional physical and chemical characteristics of the soil are described in Table 1. We collected soils from the top 10 cm of the agricultural field in April 2019 and stored the field-moist soils at 4 °C. The sampling point was 500 m from the closest salt marsh (Fig. 1b), and while neighboring fields had been affected, the focal field did not yet show signs of SWI. The nearby marsh soils were comprised of a combination of Transquaking and Mispillion soil series and had an average salinity of 22 parts per thousand (ppt) in the top 50 cm of the soil porewater (Soil Survey Staff 2018). By 2050, most of the county is expected to undergo regular tidal flooding (Maryland Department of Natural Resources and NOAA 2008). Based on estimates, sea levels are projected to rise 0.34–0.91 m near Cambridge, a nearby town in Dorchester County by 2050 (NOAA 2020). The area receives an average of 1040 mm of precipitation annually. The mean annual maximum temperature is 21 °C and the mean annual minimum temperature is 6 °C (Oceanic et al. 2018).
a Map of the Chesapeake Bay region, United States. Somerset County is outlined by the dotted rectangle. Blue dot is location of soil collection site b Satellite imagery of the soil collection site (Google Earth 2019). (Color figure online)
Soil solution preparation and analysis
In order to examine the effect of SWI on soil N, P, and Fe dynamics, we created a suite of salt solution treatments to determine the relative effect of different ionic constituents and ionic strength on N, P, and Fe release from soils (Table 2). Throughout this paper, we refer to “release” as the sum of biotic and abiotic processes that spur the movement of ions out of the soil and into the soil solution. Treatments containing calcium sulfate (+ CaSO4 treatments) were designed to simulate gypsum additions to SWI-affected fields. Treatments containing sodium sulfate (+ Na2SO4 treatments) were intended to simulate SO42− addition from saltwater without including the suppressive effects of Ca on SRP release. Treatments containing sodium chloride (+ NaCl treatments; 5 treatments total) were designed to determine the effects of high ionic strength (0.26–0.28 M) on nutrient release to soil porewater, and the Na2SO4 and CaSO4 treatments (2 treatments total) mimicked low ionic strength (0.03–0.04 M; Table 2). We also included deionized water (DIW) as a freshwater control and Instant Ocean® Sea Salt (Spectrum Brands, VA, USA) as a treatment to mimic natural seawater.
In total, we created eight salt treatment solutions with the ionic strength noted in parentheses: (1) DIW (0.00 M); (2) Na2SO4 (0.04 M); (3) CaSO4 (0.03 M); (4) NaCl + CaSO4 (0.26 M); (5) NaCl + Na2SO4 (0.27 M); (6) NaCl (0.26 M); (7) NaCl + Na2SO4 + CaSO4 (0.28 M); and (8) Instant Ocean® Sea Salt (0.26 M; Table 2). Instant Ocean® Sea Salt is comprised of the major cations Na+, K+, Mg2+, Ca2+, Sr+, the major anions Cl−, SO42−, and trace metals, all found in a similar ratio to that of natural seawater (Atkinson et al. 1997). The Na2SO4, CaSO4, NaCl + Na2SO4, and NaCl + CaSO4 treatments contained SO42− at the same concentration found in 15 ppt (brackish) saltwater (1.14 g SO42−/l) and the addition of NaCl brought those treatments up to 15 ppt total. The 15 ppt salt treatments ranged in ionic strength from 0.26 to 0.28 M. Exact stock solution mixtures are detailed in Table 2. We selected 15 ppt salinity to represent high salinity (and hence high ionic strength) salt treatments for this experiment based on the results of a previous field study we conducted on actively farmed fields undergoing SWI in Somerset County where soil porewater salinity in the top 25 cm of SWI-affected parts of these fields ranged from approximately 5–25 ppt throughout the data collection period (Tully et al. 2019b).
Each microcosm consisted of a slurry of 120 ml of treatment water added to 20 g well-mixed and homogenized field-moist soil and incubated at 25 °C. Microcosm slurries were made in duplicate and one was maintained aerated and the other anoxic to simulate high and low redox potential saturated environments. Though aerated microcosm slurries were also saturated with solution, we herein will refer to them as “oxic” for consistency. Oxic microcosm slurries were created in 250 ml beakers, sealed with gas-permeable membranes and stirred daily, to maintain aeration and prevent SRP from being released due to Fe reduction from FePO4 complexes under lower redox conditions, and also to prevent the reductive dissolution of Fe oxide minerals, a process that can release sorbed SRP (Musolff et al. 2017). Anoxic microcosm slurries were placed into 125 ml Erlenmeyer flasks and sealed with airtight rubber septa. These slurries were purged for ten minutes with purified N2 gas. Each oxygen level by salt solution combination was replicated four times for each microcosm soil solution sampling date (day 0, 15, and 30; ntotal = 192). Microcosms were destructively sampled to avoid changing the total soil and soil solution volume throughout the experiment. To limit the introduction of air to the water sample, anoxic microcosms were sampled via a syringe through the rubber septa. Oxic microcosms were sampled by syringes placed directly into the soil solution. Day 0 microcosms were sampled immediately after they were set up (< 30 min after salt treatment was applied).
All microcosm soil solution samples were immediately filtered through 1 μm glass fiber filters, and frozen until analysis. A subsample of each sample to be analyzed for dissolved inorganic phosphorus (measured as SRP) was acidified to pH ~ < 2 with hydrochloric acid to prevent PO43− coprecipitation with Fe upon sample exposure to oxygen. Samples were analyzed colorimetrically on a LACHAT QuikChem (LACHAT Instruments Loveland, CO) using the sulfanilamide method for NO3–N, the salicylate-nitroprusside method for NH4–N, and the molybdate-blue method for SRP.
All microcosm soil solutions were analyzed for total dissolved Fe (TDFe) using a modified method to reduce all dissolved Fe(III) to Fe(II) (Viollier et al. 2000). A reducing agent, 1.4 M hydroxylamine hydrochloride (H2NOH.HCl), was prepared in 2 M hydrochloric acid. A 10 M ammonium acetate (C2H7NO2) buffer was prepared and adjusted to pH 9.5 with a solution of ammonium hydroxide (NH4OH). Then, 2 ml of the reducing agent and 1.67 ml of the buffer was added to 10 ml of the water sample. All microcosm soil solutions were analyzed for Fe, which represents the total dissolved Fe(II) and Fe(III) oxyhydroxides present in the water samples, on an atomic adsorption spectrometer (PinAAcle 900; Perkin Elmer; CT, USA) using an air-acetylene flame.
Soil analyses
Soil samples from the agricultural field were analyzed at Brookside Laboratories in New Bremen, OH, USA to determine baseline soil nutrient levels. Where noted, the Mehlich III method was used to extract bioavailable (inorganic) nutrients (Mehlich 1984; Table 1). A potassium chloride method was used to extract NH4–N and NO3–N from the soils and extracts were run on a flow injection analyzer (FIAlyzer; FIAlab; WA, USA). Inductively coupled plasma optical emission spectroscopy (ICAP 7600; Thermo Fisher Scientific; MA, USA) was used to quantify concentrations of Ca, Mg, K, Na, B, Fe, Mn, Cu, Zn, and Al in the samples. Concentrations of Cl− and SO42− were water extracted from the soil and measured on an ion chromatograph (Mehtrom AG, Herisau, Switzerland). Soil pH and EC were measured in a 1:1 water extraction (wt:vol) on a SevenExcellence probe (Mettler Toledo; OH; USA) and soil organic matter percentage was estimated using the loss-on-ignition method (Heiri et al. 2001). Total soil C and N were analyzed at AgroLab in Harrington, DE, USA on a LECO CN-2000 dry combustion analyzer (MI, USA).
At each sampling point in the experiment (day 0, 15, and 30), we determined total Fe oxides in all of the microcosm soils in order to determine whether measurable iron transformations occurred in the overall pool of soil Fe over time. Soil samples from each microcosm were air-dried, ground, and passed through a 2 mm sieve. They were then extracted for total (crystalline + poorly-crystalline) Fe oxides via the dithionite citrate bicarbonate (DCB) extraction method, which uses a powerful reductant, dithionite, to reduce Fe(III) oxides to Fe(II). The supernatants from the extractions were decanted, filtered through Whatman 42 filter paper (2.5 µm) and analyzed for Fe on an atomic absorption spectrometer (PinAAcle 900; Perkin Elmer; CT, USA) using an air-acetylene flame.
Scaling nutrient release to the field level
In the final portion of this study, we calculated potential nutrient release from the soils used for this experiment under oxic and anoxic conditions (top 10 cm of topsoil from the field) in kg/ha as this is a unit that is useful to land managers when making decisions about fertilizer application (measured in kg/ha or lbs/acre). To area-scale concentrations, we used a soil bulk density of 1.55 g/cm3 from Web Soil Survey (Soil Survey Staff 2018) and results from analyte concentrations for different salt treatment and oxygen treatment combinations at day 30 of the experiment. The calculation used is as follows (Eq. 1):
where x is the particular salt and oxygen treatment, c is the analyte concentration (in mg/l), v is the volume of microcosm soil solution (in l), m is the mass of the dried microcosm soil sample (in g), dx is the depth to which the soil sample was taken in the field (10 cm) and the last two terms are conversion units for square cm to hectares and mg to kg.
To compare results to initial microcosm soil solution analyte concentrations, values in Table 1 were scaled from mg/kg to kg/ha using a similar equation (Eq. 2):
where ai is the initial analyte soil solution concentration (in mg/kg), di is the depth to which the original soil sample was taken in the field (10 cm) and the last three terms are conversion units.
Statistical approach
To examine differences in NO3–N, NH4–N, SRP, and TDFe in microcosm soil solutions and total Fe oxides in microcosm soil samples, we used a linear fixed-effects (LM) model lme4 package for R (Bates et al. 2018). Salt treatment, oxygen level, and day (0, 15, and 30) were included as fixed effects. We examined the main effect of salt treatment, oxygen level, and day and interactive effects for each variable. For each day sampled, we used Tukey post-hoc tests to examine pairwise differences in water chemistry among treatment combinations (multcomp package; Hothorn et al. 2017). A Welch’s two sample t-test (Welch 1947) was used to determine whether microcosm soil pH in anoxic versus oxic treatments was significantly different at day 30. We used the Box-Cox method (Box and Cox 1964) for log transformations prior to analysis to meet the assumptions of the statistical model when needed. All statistics were computed in R Studio (R Studio Team 2019).
Oxygen level and individual salt treatments were the initial variables in this study. However, when individual salt treatments were not significantly different, individual treatments were grouped to test the effects of ionic strength in the case of the different N species and combinations of Ca and SO42− additions in the case of SRP at the end of the incubation period (day 30). Because we hypothesized that higher ionic strength treatments would result in greater NH4–N release to microcosm soil solution, we grouped salt treatments into two categories: high ionic strength (0.26–0.28 M: NaCl, NaCl + Na2SO4, NaCl + CaSO4, NaCl + Na2SO4 + CaSO4, and Instant Ocean®) and low ionic strength (0.03–0.04 M: Na2SO4 and CaSO4) to better interpret our findings on inorganic N release. The DIW treatment (no ionic strength) was not included because it was the only treatment in its group and did not allow for adequate statistical power to detect significance between this treatment and the other treatment groups. Since PO43− sequestration in soils is enhanced by the presence of Ca (Penn et al. 2011) and SO42− often provides a control on PO43− release, to better interpret our findings on SRP microcosm soil solution concentrations, we grouped data from the salt treatments at day 30 based on whether the treatments contained added Na2SO4 (Na2SO4 and NaCl + Na2SO4 treatments), CaSO4 (CaSO4 and NaCl + CaSO4 treatments), neither constituent (NaCl and DIW treatments), or a combination of both (NaCl + Na2SO4 + CaSO4 treatments). These treatments are referred to as: (1) + Na2SO4 treatments; (2) + CaSO4 treatments; (3) −SO42− treatments; (4) or + Na2SO4 and CaSO4 treatments, respectively. We conducted a Pearson correlation to determine the linear relationship between TDFe and SRP concentrations but omitted data from day 0 as this was our baseline before there was adequate time for chemical transformations to take place in the microcosm soils.
Results
Overall, we found that oxygen levels and salt treatments had major effects on N, P, and TDFe release into the microcosm soil solution, with anoxic soils releasing more NH4–N, SRP, and TDFe to the overlying water than oxic soils after 15 and 30 days (P < 0.05). Values expressed in parentheses are a mean of all salt treatments with standard error of the mean, unless otherwise indicated. Concentrations of NH4–N, SRP, and TDFe were lowest in anoxic microcosm soil solutions at day 0 (0.23 ± 0.06 mg N/l, 0.30 ± 0.02 mg P/l, and 0.99 ± 0.23 mg Fe/l, respectively), higher at day 15 (3.93 ± 0.36 mg N/l, 5.67 ± 0.65 mg P/l, and 7.48 ± 1.14 mg Fe/l, respectively), and highest at day 30 (5.08 ± 0.41 mg N/l, 9.31 ± 0.67 mg P/l, and 16.64 ± 1.51 mg Fe/l, respectively), while NO3–N concentrations decreased from 7.65 ± 0.46 mg N/l at day 0 to near zero levels by day 15. Nitrate–N release from oxic soils was lowest at day 0 (7.68 ± 0.5 mg N/l), intermediate at day 15 (8.87 ± 0.88 mg N/l), and highest at day 30 (10.04 ± 1.19 mg N/l) while NH4–N and TDFe concentrations remained near zero throughout the incubation period. Though there was no discernable significant difference in DIN concentrations between anoxic and oxic treatments, DIN concentrations in oxic treatments were consistently higher than those of the anoxic treatments for each salt treatment at day 15 and day 30. In oxic microcosm soils, SRP release was lowest at day 0, but similar between day 15 and day 30 (0.29 ± 0.02 mg P/l, 2.19 ± 0.19 mg P/l, and 2.57 ± 0.20 mg P/l, respectively; Fig. 2). After exploring trends through time, we shifted our focus to the effects of the grouped salt treatments as described in the methods and oxygen level at day 30, as this was the end of the incubation period.
Microcosm soil solution nitrate-nitrogen (NO3–N) concentrations in mg NO3–N/l, ammonium-N (NH4–N) concentrations in mg NH4–N/l, dissolved inorganic N (DIN; NO3–N + NH4–N) concentrations in mg N/l, soluble reactive phosphorus (SRP) concentrations in mg P/l, and total dissolved iron (TDFe) concentrations in mg Fe/l at days 0, 15, and 30 of the experiment under oxic and anoxic conditions (oxygen level). Error bars represent standard error of the mean. Statistically significant differences within each day are indicated by different letters at P < 0.05. (Color figure online)
By day 30, significantly more NO3–N was released to the overlying microcosm soil solution under oxic conditions in both low and high ionic strength treatments (7.85 ± 2.47 and 12.14 ± 1.39 mg NO3–N/l, respectively) as compared to anoxic conditions (0.06 ± 0.05 to 0.08 ± 0.05 mg NO3–N/l, respectively; P < 0.05). However, there was no effect of salt treatment or ionic strength on NO3–N release regardless of oxygen level (Table 3 and Fig. 3a). Twice as much NH4–N was released to overlying waters in high ionic strength solution than in the low ionic strength solution, under both oxic and anoxic conditions (P < 0.05; Fig. 3b). Additionally, in both low and high ionic strength treatments, NH4–N concentrations were much higher under anoxic conditions (3.25 ± 0.49 and 6.09 ± 0.50 mg NH4–N/l; respectively) than under oxic conditions (0.04 ± 0.01 and 0.06 ± 0.04 mg NH4–N/l, respectively; P < 0.0001; Table 3 and Fig. 3b).
a Microcosm soil solution nitrate-nitrogen (NO3–N) concentrations in mg NO3–N/l at day 30 of the experiment grouped by low ionic strength (Na2SO4, CaSO4; 0.03 M and 0.04 M, respectively) and high ionic strength (NaCl, NaCl + Na2SO4, NaCl + CaSO4, NaCl + Na2SO4 + CaSO4, and Instant Ocean®; 0.26 M, 0.26 M, 0.27 M, 0.28 M, 0.26 M, respectively) treatments. Statistically significant differences in means are indicated by different letters at P < 0.05. b Microcosm soil solution ammonium nitrogen (NH4–N) concentrations in mg NH4–N/l at day 30 of the experiment grouped by low ionic strength (Na2SO4, CaSO4; 0.03 M and 0.04 M, respectively) and high ionic strength (NaCl, NaCl + Na2SO4, NaCl + CaSO4, NaCl + Na2SO4 + CaSO4, and Instant Ocean®; 0.26 M, 0.26 M, 0.27 M, 0.28 M, 0.26 M, respectively) treatments. Statistically significant differences in means are indicated by different letters at P < 0.05. c Microcosm soil solution DIN (dissolved inorganic N; NO3–N + NH4–N) concentrations in mg N/l at day 30 of the experiment grouped by low ionic strength (Na2SO4, CaSO4; 0.03 M and 0.04 M, respectively) and high ionic strength (NaCl, NaCl + Na2SO4, NaCl + CaSO4, NaCl + Na2SO4 + CaSO4, and Instant Ocean®; 0.26 M, 0.26 M, 0.27 M, 0.28 M, 0.26 M, respectively) treatments. Statistically significant differences in means are indicated by different letters at P < 0.05. (Color figure online)
At the end of the incubation period, concentrations of SRP were significantly lower in CaSO4-only (simulating gypsum soil amendment) treatments than in Na2SO4-only treatments with other treatments releasing intermediate amounts of SRP to microcosm soil solution (Fig. 4; P < 0.05). Additionally, SRP release was significantly lower under oxic conditions (1.46 ± 0.20–3.54 ± 0.31 mg SRP/l) than under anoxic conditions (6.22 ± 0.93–11.67 ± 1.78 mg SRP/l; P < 0.05; Fig. 4). There was a moderate and significant positive correlation between TDFe and SRP concentrations in microcosm soil solution (R2 = 0.49, P < 0.0001; Fig. 5). Concentrations of TDFe were significantly higher under anoxic conditions (16.64 ± 1.51 mg Fe/l) than under oxic conditions (0.43 ± 0.12 mg Fe/l; P < 0.05; Fig. 2). We observed no effect of salt treatment on the release of total dissolved Fe to solution (Table S1 and Figure S1). Total Fe oxides (6.48 ± 0.06 mg Fe/g dry soil) did not vary significantly by day, oxygen treatment, or salt treatment (Table 3). Additionally, at day 30, soil pH in anoxic treatments was significantly higher than that in the oxic treatments (6.2 ± 0.0 versus 5.7 ± 0.1, respectively; P = 0.00617).
Microcosm soil solution soluble reactive phosphorus (SRP) concentrations in mg P/l at day 30. Treatments are grouped by the type of sulfate (SO42−) salt they contained: sodium sulfate (Na2SO4), calcium sulfate (CaSO4), neither constituent (sodium chloride or deionized water), or a combination of both (Na2SO4 and CaSO4). Treatments containing CaSO4 are designed to simulate soil amendments used to reclaim sodic soils. Treatments containing Na2SO4 are designed to simulate the sulfate component of saltwater. Statistically significant differences in means are indicated by different letters at P < 0.05. (Color figure online)
When scaled to kg/ha, potential N release was similar between the Instant Ocean® and NaCl + Na2SO4 + CaSO4 (representing a field with added gypsum amendments) salt treatments. Under anoxic conditions, soils released a negligible amount (0.1 ± 0.0 kg of NO3–N/ha) to overlying solution in both Instant Ocean® and NaCl + Na2SO4 + CaSO4 treatments, whereas they released an average of 74 kg NH4–N/ha to solution in the two salt treatments. On the other hand, SRP and TDFe release was much lower in the soils treated with NaCl + Na2SO4 + CaSO4 than when exposed to Instant Ocean (90.4 ± 7.1 and 119.7 kg ± 6.4 P/ha, respectively), illustrating the suppressive effects of Ca on nutrient release. Under oxic conditions, NO3–N, NH4–N, SRP, and TDFe release was similar between the Instant Ocean® and NaCl + Na2SO4 + CaSO4 salt treatments. In the Instant Ocean® treatment, NO3–N release was almost 1450 times greater under oxic than anoxic conditions (143.6 ± 30.3 kg N/ha and 0.1 ± 0.0 kg N/ha, respectively) while NH4–N release was over 700 times greater under anoxic versus oxic conditions (78.2 ± 6.0 kg N/ha and 0.1 ± 0.0 kg N/ha, respectively). Finally, SRP was over 6 times greater under anoxic versus oxic conditions (119.8 ± 6.0 kg P/ha and 19.9 ± 0.9 kg P/ha, respectively). TDFe was almost 200 times greater under anoxic versus oxic conditions (295.3 ± 27.1 kg Fe/ha and kg 1.5 Fe/ha ± 0.4, respectively; Table 4). For the complete dataset of nutrient release scaled to kg/ha for each salt treatment, see Table S2.
Discussion
Overall, our results have major implications for how N, P, and Fe may be transformed and released from agricultural soils undergoing SWI. The soil used for this study was from a farm field that is at risk for SWI in the coming years as it is located only 500 m from a tidal salt marsh and hydrologically connected to the marsh through agricultural ditches. This farm soil shows a high potential for N and P release when exposed to saltwater. For example, when the soil becomes anoxic due to inundation, concentrations of NH4–N, SRP, and TDFe may increase in overlying waters. Although release of NH4–N, SRP, and TDFe was lower under oxic soil conditions, NO3–N release was greatly increased. Ionic strength was a strong driver of NH4–N release from anoxic soils while the addition of Ca suppressed SRP release from both oxic and anoxic soils.
Nitrogen
The main form of DIN release differed between oxic and anoxic microcosm soil solutions due to divergent redox pathways for N. By day 15, NO3–N concentrations were near zero under anoxic conditions (Fig. 3a), which indicates that NO3–N was either denitrified or converted to NH4–N via DNRA, as has been observed in previous studies of anoxic soils (Tobias et al. 2001; Koop-jakobsen and Giblin 2010; Giblin et al. 2013). Research has shown that DNRA rates tend to be higher in saline soils because bisulfide (HS−) produced from anaerobic SO42− reduction is utilized as a reactant and oxidized back to SO42− through one of the major DNRA pathways. This reaction occurs as:
\({\text{HS}}^{ - } + {\text{NO}}^{ - } {\text{ + H}}^{ + } {\text{ + H}}_{{2}} {\text{O}} \to {\text{SO}}_{{4}}^{2 - } {\text{ + NH}}_{{4}}^{ + }\) 2013)
High ionic strength treatments resulted in a greater NH4–N release to overlying water than low ionic strength treatments (Fig. 3b) due to cations in saltwater replacing NH4+ ions on soil exchange sites (Ardón et al. 2013). This effect was more pronounced under anoxic conditions where concentrations of NH4–N in solution were higher than under oxic conditions. Of note, high ionic strength treatments contained 10 times the salts and were about 7 times the ionic strength of low ionic strength treatments, but NH4–N release was only two times as much in the high ionic strength treatments, which suggests a non-linear relationship between ionic strength and NH4–N release (Fig. 3b). Similarly, other studies have demonstrated a non-linear increase in NH4–N released in soils exposed to incremental increases in ionic strength. As ionic strength increases, Na+ cations compete with NH4+ cations for exchange sites on soil colloidal surfaces (Seitzinger et al. 1991; Weston et al. 2010). The lyotropic series describes the order of cations according to their adsorptive power to soil colloidal particles, where ions with lower hydrated radii have higher adsorptive power, which is mechanistically defined by Coulomb’s Law (Huang 1980). Further, the non-linear nature of the replacement rate of NH4+ cations with Na+ cations has been modeled through the Langmuir adsorption isotherm (Abukhadra et al. 2020).
As expected, NO3–N was the dominant form of DIN in oxic microcosm soil solutions and NH4–N concentrations were close to zero. Additionally, salt treatments had no effect on NO3–N release. Nitrate–N concentrations remained relatively constant under oxic conditions throughout the incubation period as most of the NO3–N was immediately released into solution (Fig. 3a). Because NO3–N is prone to rapid leaching from soils (Jessen et al. 2017), SWI may spur a large amount of N loss to downstream waterways through inundation, before soils even become anoxic. Since there are no studies that have attempted to quantify NO3–N losses from an agricultural field initially undergoing SWI, this hypothesis should be tested through future research.
Consistently greater though not statistically significant DIN released to microcosm soil solution in the oxic versus anoxic microcosms suggests that other chemical pathways such as N mineralization and nitrification may also have played an important role in final N soil solution concentrations at the end of the incubation period. The significantly lower pH we observed in oxic soils versus anoxic soils could be attributed to greater rates of nitrification from aerobic microbial metabolic pathways. This difference in nitrification rates has been observed in other studies because N mineralization rates are greater in the presence of oxygen (Robertson and Vitousek 1981; Fellman and D’Amore 2007). Additionally, N mineralization and nitrification are tightly coupled. When NH4–N is produced from the decomposition of organic compounds under oxic conditions, it is quickly converted to NO3–N through aerobic microbial respiration (Groffman and Rosi-Marshall 2013).
Phosphorus
Overall, the soil used in this study had a very high level of bioavailable P (PO4–P; 352.5 ± 7.2 mg P/kg dry soil), which was over three times higher than bioavailable N (NO3–N + NH4–N) concentrations (116.0 ± 6.7 mg N/kg dry soil; Table 1). Due to the historic over-application of poultry manure, a fertilizer with a low N to P ratio (Waldrip et al. 2015), these P concentrations are more than four times what is needed for optimal crop growth in the study region (Sharpley et al. 2003). Remarkably, no poultry manure or P fertilizer was applied to the field the year that the soils were collected. Thus, the high bioavailable P levels reflect a legacy of nutrient buildup in the soil from past years of fertilizer application. Therefore, with exposure to SWI, agricultural soils with a history of P applications may become a source of nutrient pollution to downstream water bodies.
Calcium played an important role in P dynamics under both oxic and anoxic conditions. By day 30, we observed a significant suppression of SRP release in + CaSO4 treatments versus + Na2SO4 treatments under both oxygen levels (Fig. 4) because Ca ions tend to bind to SRP and form insoluble Ca-phosphate precipitate minerals (Ca10(PO4)6(OH,F,Cl)2; Zak et al. 2009), thus removing this form of P from the soil solution (Grubb et al. 2011). Additionally, by day 30, + CaSO4 oxic treatments released significantly less SRP to solution than the oxic −SO4 treatments (Fig. 4). However, this was not the case for the corresponding anoxic treatments, potentially due to differences in redox pathways under oxic and anoxic conditions. Consequently, as an agricultural field is initially undergoing SWI, Ca amendments such as gypsum may provide a moderate ability to prevent SRP loss downstream. However, the fact that we observed greater SRP release from anoxic treatments than oxic treatments suggest that as the field becomes anoxic, this ability is reduced.
Many incubation studies in wetland soils have demonstrated increases in SRP release with SO42− addition under anoxic conditions because of interacting relationships between Fe, sulfur (S), and P cycling (Weston et al. 2006; Williams et al. 2014; Steinmuller and Chambers 2018). Field observations show that sulfide (reduced S) tends to bind reduced Fe in solution in anoxic sediments and form insoluble FeSx complexes that prevent Fe from sequestering SRP (Jordan et al. 2008). In contrast, we did not clearly observe this effect from the agricultural soil used for our study, possibly because the background levels of SO42− in this soil were several orders of magnitude higher than those of freshwater wetland soils (compare to Alphin et al. 2007; Williams et al. 2014) due to the application of SO42−-rich poultry manure on the farm field from which the soil was collected. In our study, the soil used for our incubations already contained a high background level of SO4-S (23.3 ± 1.3 mg SO4–S/kg dry soil; Table 1; Flynn 2015) and salt treatments with SO42− increased SO42− in the incubations by only ~ 11% based on scaling salt treatment solution concentrations of SO4–S to mg/g dry soil (Calculation 1). Therefore, any effects of the added SO42− from these treatments may have been muted by high background levels of SO42− in the incubation soils.
Iron
Over the course of our incubations, we observed significantly greater TDFe concentrations in solution in the anoxic microcosms than in the oxic microcosms (Fig. 2). Therefore, it is clear that anoxic conditions spurred the reductive dissolution of Fe (Hartzell et al. 2017). Under oxic conditions, Fe oxyhydroxides tend to coprecipitate with SRP, thus removing it from solution (Weston et al. 2006), which is why we observed near zero concentrations of TDFe in the oxic microcosms (Fig. 2). In contrast to TDFe concentrations, total Fe oxide pools in microcosm soils remained constant throughout the entire incubation period (Figure S1). Crystalline and poorly-crystalline Fe(III) oxides have low solubility in water with circumneutral pH, and it can take years for a significant proportion of the Fe oxides to undergo dissolution to dissolved forms as a soil undergoes SWI (Weston et al. 2006). The 30 day length of our incubations appears to have been an insufficient amount of time for dissolution to significantly shift the overall pool of total Fe oxides even though it significantly increased TDFe concentrations. In the anoxic treatments, TDFe concentrations were less than 3% of total Fe oxide concentrations (Figs. 2 and S1), which illustrates that TDFe constitutes a small but chemically important pool of Fe in SWI-affected systems. The fact that we observed a moderate and significant positive correlation between TDFe and SRP concentrations (Fig. 5) suggests that TDFe plays an important role in sequestering and releasing SRP from inundated soils, as is supported by studies conducted in wetland ecosystems (Chambers and Odum 1990; Hartzell and Jordan 2012).
Additionally, S plays an important role in preventing Fe from re-binding SRP under changing oxygen conditions. As previously mentioned, SO42− in soil and soil amendments is reduced to sulfide under anoxic conditions and tends to bind Fe in FeSx complexes (Schoepfer et al. 2014). The fact that we still observed high levels of TDFe in solution under anoxic conditions suggests that the rate of Fe dissolution is faster than its precipitation with S. Therefore, Fe reduction is likely the driver of SRP release from inundated soils while FeSx formation prevents Fe from sequestering SRP if soil conditions become oxic again. Fields affected by SWI are subject to these interacting processes because they undergo wetting and drying cycles, which cause them to fluctuate between oxic and anoxic redox states (Tully et al. 2019b).
Potential soil nutrient release
Because we generated constant oxygen levels and salinity exposure in our incubations, it is important to note that our estimates reflect a potential for nutrient release from soils over time. Thus, this portion of our study represents a thought experiment representing the effects of an extreme case of SWI on nutrient release on the agricultural field. Unlike in a controlled laboratory setting, soil conditions in agricultural fields are dynamic. For example, the level of salinity exposure in an SWI-affected can change spatially and temporally, depending on factors such as tidal inundation, and precipitation and evapotranspiration rates (Weissman and Tully 2020). Consequently, the quantity and form of nutrients released from SWI-affected soils is highly dependent on the timing and magnitude of environmental variables. For example, it is possible that much of the NO3–N in the soil would rapidly be lost to leaching with saltwater inundation, before the soils could become oxygen-depleted. In turn, less NO3–N would be present in the soil to undergo DNRA, thus releasing less NH4–N to porewater when soils finally became anoxic. Therefore, the total proportion of inorganic N released as NO3–N versus NH4–N over time would vary depending on field conditions.
The pattern of enhanced NH4–N, SRP, and TDFe release and reduction of NO3–N concentrations in saline inundated soils has also been observed in other incubation studies. For example, in one study, a marsh soil that had been formerly diked and drained was subjected to full strength (~ 32 ppt) seawater over a period of 20 months. The soil showed a roughly sevenfold increase in PO4-P and a 28-fold increase in NH4–N (Portnoy and Giblin 1997). However, in contrast to the increase in soil solution TDFe after 30 days of anoxic conditions observed in our study, after the 20 month incubation in this study, TDFe concentrations decreased by 42% which was attributed to Fe sequestration in FeSx compounds (Portnoy and Giblin 1997). Another study focused solely on NH4–N exchange from wetland soils showed that at 0 ppt salinity, about 69% of NH4–N was bound to soil particles, 14% as salinity increased to 3 ppt, 6% at 10 ppt salinity, and less than 1% at 34 ppt (Weston et al. 2010). This decrease in soil bound NH4–N is comparable to the sevenfold increase in soil solution NH4–N concentrations we observed in our study between 1.5 and 15 ppt. In another study, where salinity was experimentally raised to 0.9, 2.25, 4.5, and 9 PSU with seawater in wetland soils over 2.5 years, researchers found lower porewater P concentrations as salinity increased. This was attributed to an increase in Ca concentrations due to seawater addition as well as enhanced mobilization of Ca through mechanisms of cation exchange on soil particles (van Dijk et al. 2019). We also observed a suppressive effect of Ca on P release to soil solution in our study. Finally, a 2.5 year study on wetland soils where salinity was increased from 0.0 to 0.1 ppt to a range of 1.7 to 3.8 ppt, showed a marked increase in the abundance of the microbial community responsible for DNRA (Neubauer et al. 2019). Taken together with our results, these studies suggest that processes associated with SWI mechanistically cause similar patterns of nutrient release in wetland soils as in agricultural soils. However, because of the legacy of intensive soil inputs, agricultural soils have a capacity to release nutrients at a greater magnitude than wetland soils.
Overall, our results show that large quantities of N and P are poised for loss from agricultural fields undergoing SWI. Soils were collected in April 2019, when precipitation rates in our study region are high, spring tides frequently flood coastal areas, and about a month before farmers tend to plant cash crops (Tully et al. 2019b). Consequently, nutrient export is likely highest during the spring, when there is less plant nutrient uptake because fields are planted in cover crops, conditions are prime for saltwater to move onto fields, and soils tend to remain saturated, sometimes for weeks. After 30 days of inundation with saltwater (e.g. Instant Ocean® treatment), as much as 45% of the total inorganic N could be released (as NH4–N) if soils were under anoxic conditions. As much as 22% of the bioavailable soil P could be released as SRP to overlying water after a month of inundation with saltwater (when initial soil N and P concentrations in Table 1 are scaled to kg/ha and compared to Table 4).
As mentioned previously, bioavailable P levels in the soil were over four times the maximum recommended level to optimize crop yield while minimizing P runoff losses (Sharpley et al. 2003). Thus, SRP release from SWI-affected fields could be a major source of nutrient pollution to nearby waterways. In order to better understand rates of nutrient loss, future research could focus on determining how conditions such as soil porewater salinity and redox potential change with nutrient concentrations in SWI-affected fields over time.
Conclusions
As sea levels rise, SWI will cause extended periods of soil inundation in coastal agroecosystems. Here we simulated this effect on an agricultural soil collected from a farm field vulnerable to SWI. Overall, we found that oxygen depletion in inundated soils contributes to SRP release from soils and shifts N cycling so that the DIN pool is dominated by NH4–N. Higher ionic strength, in turn, enhances NH4–N release from both oxic and anoxic soils. Additionally, even without the addition of added salts, high levels of Fe in agricultural soils can cause a large release of SRP from anoxic soils as a result of reductive dissolution. However, Ca addition from soil amendments such as agricultural lime or gypsum can bind to P and suppress its release into water overlying inundated soil. These findings imply that SWI-affected fields may be hotspots of nutrient pollution, particularly during the spring, when they have the potential to release a large proportion of inorganic soil N and P to downstream waterways. Therefore, defining mechanisms through which ions interact in solution is critical for quantifying N and P export in coastal agricultural soils affected by SWI.
Abbreviations
- NH4 + :
-
Ammonium
- NH4–N:
-
Ammonium nitrogen
- Ca:
-
Calcium
- CaCO3 :
-
Calcium carbonate
- CaSO4 :
-
Calcium sulfate
- C:
-
Carbon
- Cl- :
-
Chloride
- DNRA:
-
Dissimilatory nitrate reduction to ammonium
- Fe:
-
Iron
- FeSx :
-
Iron sulfide
- NO3 − :
-
Nitrate
- NO3–N:
-
Nitrate nitrogen
- N:
-
Nitrogen
- ppt:
-
Parts per thousand
- PO4 3 − :
-
Phosphate
- P:
-
Phosphorus
- SWI:
-
Saltwater intrusion
- Na:
-
Sodium
- NaCl:
-
Sodium chloride
- Na2SO4 :
-
Sodium sulfate
- SRP:
-
Soluble reactive phosphorus
- SO4 2 − :
-
Sulfate
- SO4–S:
-
Sulfate sulfur
- TDFe:
-
Total dissolved iron
References
Abukhadra MR, Abukhadra MR, Ali SM et al (2020) Effective sequestration of phosphate and ammonium ions by the bentonite/zeolite Na-P composite as a simple technique to control the eutrophication phenomenon: realistic studies. ACS Omega 5:14656–14668. https://doi.org/10.1021/acsomega.0c01399
Alphin T, Anderson CJ, Avery GB et al (2007) Ecology of tidal freshwater forested wetlands of the Southeastern United States. Springer, Dordrecht
Ardón M, Morse JL, Colman BP, Bernhardt ES (2013) Drought-induced saltwater incursion leads to increased wetland nitrogen export. Glob Change Biol 19:2976–2985. https://doi.org/10.1111/gcb.12287
Arndt S, Jørgensen BB, LaRowe DE et al (2013) Quantifying the degradation of organic matter in marine sediments: a review and synthesis. Earth-Sci Rev 123:53–86. https://doi.org/10.1016/j.earscirev.2013.02.008
Atkinson MJ, Bingman C, Box PO et al (1997) Elemental composition of commercial seasalts. J Aquaric Aquat Sci 8(2):39–43
Bates D, Maechler M, Bolker B et al (2018) lme4 linear mixed-effects models using “Eigen” and S4. https://cran.r-project.org/web/packages/lme4/lme4.pdf. Accessed 15 Dec 2019
Bhattachan A, Emanuel RE, Ardon M et al (2018) Evaluating the effects of land-use change and future climate change on vulnerability of coastal landscapes to saltwater intrusion. Elem Sci Anthropocene 6:62. https://doi.org/10.1525/elementa.316
Box GEP, Cox DR (1964) An analysis of transformations. J R Stat Soc Ser B 26:190–197
Brady NC, Weil RR (2016) The nature and properties of soils, 15th edn. Pearson, Columbus
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96. https://doi.org/10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2
Caraco N, Cole J, Likens GE (1990) A comparison of phosphorus immobilization in sediments of freshwater and coastal marine systems. Biogeochemistry 9:277–290. https://doi.org/10.1007/BF00000602
Chambers RM, Odum WE (1990) Porewater oxidation, dissolved phosphate and the iron curtain—iron-phosphorus relations in tidal freshwater marshes. Biogeochemistry 10:37–52. https://doi.org/10.1007/BF00000891
Darke AK, Walbridge MR (1994) Estimating non-crystalline and crystalline aluminum and iron by selective dissolution in a riparian forest soil. Commun Soil Sci Plant Anal 25:2089–2101
Dehaan RL, Taylor GR (2002) Field-derived spectra of salinized soils and vegetation as indicators of irrigation-induced soil salinization. Remote Sens Environ 80:406–417. https://doi.org/10.1016/S0034-4257(01)00321-2
Google Earth (2019) Google Earth imagery. http://www.earth.google.com. Accessed 4 Dec 2018
Fellman JB, D’Amore DV (2007) Nitrogen and phosphorus mineralization in three wetland types in Southeast Alaska, USA. Wetlands 27:44–53. https://doi.org/10.1672/0277-5212(2007)27[44:NAPMIT]2.0.CO;2
Flynn R (2015) Interpreting soil tests: unlock the secrets of your soil. New Mexico State University Cooperative Extension Service Circular, Las Cruces
Fowler DN, King SL, Weindorf DC (2014) Evaluating abiotic influences on soil salinity of inland managed wetlands and agricultural croplands in a semi-arid environment. Wetlands 34:1229–1239. https://doi.org/10.1007/s13157-014-0585-3
Giblin AE, Tobias CR, Song B et al (2013) The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26:124–131. https://doi.org/10.5670/oceanog.2013.54
Goyne KW, Jun H-J, Anderson SH, Motavalli PP (2008) Phosphorus and nitrogen sorption to soils in the presence of poultry litter-derived dissolved organic matter. J Environ Qual 37:154–163. https://doi.org/10.2134/jeq2007.0141
Groffman PM, Rosi-Marshall EJ (2013) Chapter 7—the nitrogen cycle. In: Weathers KC, Strayer DL, Likens GE (eds) Fundamentals of ecosystem science. Academic Press, Cambridge, pp 137–158
Grubb KL, McGrath JM, Penn CJ, Bryant RB (2011) Land application of spent gypsum from ditch filters: phosphorus source or sink? Agric Sci 02:364–374. https://doi.org/10.4236/as.2011.23048
Grubb KL, McGrath JM, Penn CJ, Bryant RB (2012) Effect of land application of phosphorus-saturated gypsum on soil phosphorus in a laboratory incubation. Appl Environ Soil Sci 2012:9–12. https://doi.org/10.1155/2012/506951
Hartzell JL, Jordan TE (2012) Shifts in the relative availability of phosphorus and nitrogen along estuarine salinity gradients. Biogeochemistry 107:489–500. https://doi.org/10.1007/s10533-010-9548-9
Hartzell JL, Jordan TE, Cornwell JC (2017) Phosphorus sequestration in sediments along the salinity gradients of Chesapeake Bay subestuaries. Estuar Coasts 40:1607–1625. https://doi.org/10.1007/s12237-017-0233-2
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110. https://doi.org/10.1023/A:1008119611481
Helton AM, Bernhardt ES, Fedders A (2014) Biogeochemical regime shifts in coastal landscapes: the contrasting effects of saltwater incursion and agricultural pollution on greenhouse gas emissions from a freshwater wetland. Biogeochemistry 120:133–147. https://doi.org/10.1007/s10533-014-9986-x
Herbert ER, Schubauer-Berigan J, Craft CB (2018) Differential effects of chronic and acute simulated seawater intrusion on tidal freshwater marsh carbon cycling. Biogeochemistry 138:137–154. https://doi.org/10.1007/s10533-018-0436-z
Hothorn T, Bretz F, Westfall P et al (2017) Simultaneous inference in general parametric models. https://cran.r-project.org/web/packages/multcomp/. Accessed 1 Dec 2019
Huang PM (1980) Adsorption processes in soil. Reactions and processes. Springer, Heidelberg, pp 47–59
Jessen S, Postma D, Thorling L et al (2017) Decadal variations in groundwater quality: a legacy from nitrate leaching and denitrification by pyrite in a sandy aquifer. Water Resour Res 53:184–198. https://doi.org/10.1002/2016WR018995
Jordan TE, Cornwell JC, Boynton WR et al (2008) Changes in phosphorus biogeochemistry along an estuarine salinity gradient: the iron conveyer belt. Limnol Oceanogr 53:172–184
Koop-jakobsen K, Giblin AE (2010) The effect of increased nitrate loading on nitrate reduction via denitrification and DNRA in salt marsh sediments. Limnol Oceanogr 55:789–802
Luther GW, Kostka JE, Church TM et al (1992) Seasonal iron cycling in the salt-marsh sedimentary environment: the importance of ligand complexes with Fe(II) and Fe(III) in the dissolution of Fe(III) minerals and pyrite, respectively. Mar Chem 40:81–103. https://doi.org/10.1016/0304-4203(92)90049-G
Machado RMA, Serralheiro RP (2017) Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae. https://doi.org/10.3390/horticulturae3020030
Maryland Department of Natural Resources, NOAA [National Oceanic and Atmospheric Administration] (2008) Somerset County, Maryland: Rising Sea Level Guidance. https://dnr.maryland.gov/ccs/Publication/SeaLevel_Somerset.pdf. Accessed 1 May 2019
McDowell RW, Sharpley AN (2001) Soil phosphorus fractions in solution: influence of fertiliser and manure, filtration and method of determination. Chemosphere 45:737–748. https://doi.org/10.1016/S0045-6535(01)00117-5
McLay CDA, Cameron KC, McLaren RG (1991) Effect of time of application and continuity of rainfall on leaching of surface-applied nutrients. Soil Res 29:1–9. https://doi.org/10.1071/SR9910001
Megonigal JP, Neubauer SC (2009) Biogeochemistry of tidal freshwater wetlands. Coast Wetl An Integr Ecosyst Approach. https://doi.org/10.1016/B978-0-444-53103-2.00019-3
Mehlich A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416. https://doi.org/10.1080/00103628409367568
Moomaw WR, Chmura GL, Davies GT et al (2018) Wetlands in a changing climate: science, policy and management. Wetlands 38:183–205. https://doi.org/10.1007/s13157-018-1023-8
Moore PA, Miller DM (1994) Decreasing phosphorus solubility in poultry litter with aluminum, calcium, and iron amendments. J Environ Qual 23:325. https://doi.org/10.2134/jeq1994.00472425002300020016x
Musolff A, Selle B, Büttner O et al (2017) Unexpected release of phosphate and organic carbon to streams linked to declining nitrogen depositions. Glob Change Biol 23:1891–1901. https://doi.org/10.1111/gcb.13498
Nair VD, Clark MW, Reddy KR (2015) Evaluation of legacy phosphorus storage and release from wetland soils. J Environ Qual 44:1956. https://doi.org/10.2134/jeq2015.03.0154
Nair VD, Reddy KR, DeLaune RD et al (2013) Phosphorus sorption and desorption in wetland soils. In: DeLaune RD, Reddy KR, Richardson CJ, Megonigal JP (eds) Methods in biogeochemistry of wetlands. American Society of Agronomy and Soil Science Society of America, Madison, pp 667–681
National Oceanic and Atmospheric Administration [NOAA] (2020) NOAA sea level rise viewer. https://coast.noaa.gov/digitalcoast/tools/slr.html. Accessed 1 Dec 2020
National Oceanic and Atmospheric Administration - National Centers for Environmental Information [NOAA-NCEI] (2018) Climate Data Online. In: Natl. Ocean. Atmos. Adm. - Natl. Centers Environ. Inf. https://www.ncdc.noaa.gov/cdo-web/. Accessed 20 Nov 2018
Neubauer SC, Franklin RB, Berrier DJ (2013) Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 10:8171–8183. https://doi.org/10.5194/bg-10-8171-2013
Neubauer SC, Piehler MF, Smyth AR, Franklin RB (2019) Saltwater intrusion modifies microbial community structure and decreases denitrification in tidal freshwater marshes. Ecosystems 22:912–928. https://doi.org/10.1007/s10021-018-0312-7
Penn CJ, Bryant RB, Callahan MP, Mcgrath JM (2011) Use of industrial by-products to sorb and retain phosphorus. Commun Soil Sci Plant Anal 42:633–644. https://doi.org/10.1080/00103624.2011.550374
Portnoy JW, Giblin AE (1997) Biogeochemical effects of seawater restoration to diked salt marshes. Ecol Appl 7:1054–1063. https://doi.org/10.1890/1051-0761(1997)007[1054:BEOSRT]2.0.CO;2
R Studio Team (2019) R Studio: Integrated Development Environment for R (1.1.447). R Studio PBC. http://www.rstudio.com
Reddy KR, DeLaune RD (2008) Biogeochemistry of Wetlands. CRC Press, Boca Raton
Robertson GP, Vitousek P (1981) Nitrification potentials in primary and secondary succession. Ecology 62:376. https://doi.org/10.2307/1936712
Roden EE, Edmonds JW (1997) Phosphate mobilization in iron-rich anaerobic sediments: microbial Fe(III) oxide reduction versus iron-sulfide formation. Arch Hydrobiol 139:347–378
Roman CT, Burdick DM (2012) Tidal marsh restoration. Island Press/Center for Resource Economics, Washington
Schoepfer VA, Bernhardt ES, Burgin AJ (2014) Iron clad wetlands: soil iron-sulfur buffering determines coastal wetland response to salt water incursion. J Geophys Res Biogeosci Res 119:2209–2219. https://doi.org/10.1002/2014JG002739
Seitzinger SP, Gardner WS, Spratt AK (1991) The effect of salinity on ammonium sorption in aquatic sediments: implications for benthic nutrient recycling. Estuaries 14:167–174. https://doi.org/10.2307/1351690
Sharpley AN, Daniel T, Sims T et al (2003) Agricultural phosphorus and eutrophication. U. S. Dev Agric Res Serv 149:38
Soil Survey Staff (2018) Web soil survey. In: USDA-NRCS Web Soil Surv. Website. https://websoilsurvey.sc.egov.usda.gov/. Accessed 4 Dec 2018
Steinmuller HE, Chambers LG (2018) Can saltwater intrusion accelerate nutrient export from freshwater wetland soils? An experimental approach. Soil Sci Soc Am J 82:283. https://doi.org/10.2136/sssaj2017.05.0162
Stocker TF, Dahe Q, Plattner G-K et al (2013) Technical Summary. Clim Chang 2013 Phys Sci Basis Contrib Work Gr I to Fifth Assess Rep Intergov Panel Clim Chang 33–115. https://doi.org/10.1017/CBO9781107415324.005
Tobias CR, Macko SA, Anderson IC et al (2001) Tracking the fate of a high concentration groundwater nitrate plume through a fringing marsh: a combined groundwater tracer and in situ isotope enrichment study. Limnol Oceanogr 46:1977–1989. https://doi.org/10.4319/lo.2001.46.8.1977
Tully KL, Gedan K, Epanchin-Niell R et al (2019a) The invisible flood: the chemistry, ecology, and social implications of coastal saltwater intrusion. Bioscience 69:368–378. https://doi.org/10.1093/biosci/biz027
Tully KL, Weissman D, Wyner WJ et al (2019b) Soils in transition: saltwater intrusion alters soil chemistry in agricultural fields. Biogeochemistry 142:339–356. https://doi.org/10.1007/s10533-019-00538-9
van Dijk G, Lamers LPM, Loeb R et al (2019) Salinization lowers nutrient availability in formerly brackish freshwater wetlands; unexpected results from a long-term field experiment. Biogeochemistry 143:67–83. https://doi.org/10.1007/s10533-019-00549-6
Viollier E, Inglett P, Hunter K et al (2000) The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl Geochem 15:785–790. https://doi.org/10.1016/S0883-2927(99)00097-9
Waldrip HM, Pagliari PH, He Z et al (2015) Legacy phosphorus in calcareous soils: effects of long-term poultry litter application. Soil Sci Soc Am J 79:1601. https://doi.org/10.2136/sssaj2015.03.0090
Weissman DS, Tully KL (2020) Saltwater intrusion affects nutrient concentrations in soil porewater and surface waters of coastal habitats. Ecosphere. https://doi.org/10.1002/ecs2.3041
Welch BL (1947) The generalization of ‘student’s’ problem when several different population variances are involved. Biometrika 34:28–35. https://doi.org/10.1093/biomet/34.1-2.28
Weston NB, Dixon RE, Joye SB (2006) Ramifications of increased salinity in tidal freshwater sediments: geochemistry and microbial pathways of organic matter mineralization. J Geophys Res Biogeosci 111:1–14. https://doi.org/10.1029/2005JG000071
Weston NB, Giblin AE, Banta GT et al (2010) The effects of varying salinity on ammonium exchange in estuarine sediments of the parker river, Massachusetts. Estuar Coasts 33:985–1003. https://doi.org/10.1007/s12237-010-9282-5
Weston NB, Neubauer SC, Velinsky DJ, Vile MA (2014) Net ecosystem carbon exchange and the greenhouse gas balance of tidal marshes along an estuarine salinity gradient. Biogeochemistry 120:163–189. https://doi.org/10.1007/s10533-014-9989-7
Weston NB, Vile MA, Neubauer SC, Velinsky DJ (2011) Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102:135–151. https://doi.org/10.1007/s10533-010-9427-4
White E, Kaplan D (2017) Restore or retreat? Saltwater intrusion and water management in coastal wetlands. Ecosyst Health Sustain 3:e01258. https://doi.org/10.1002/ehs2.1258
Williams AA, Lauer NT, Hackney CT (2014) Soil phosphorus dynamics and saltwater intrusion in a florida estuary. Wetlands 34:535–544. https://doi.org/10.1007/s13157-014-0520-7
Zak D, Rossoll T, Exner H-J et al (2009) Mitigation of sulfate pollution by rewetting of fens—a conflict with restoring their phosphorus sink function? Wetlands 29:1093–1103. https://doi.org/10.1672/09-102D.1
Acknowledgements
This work was supported by several funding sources: a Seed Grant through the University of Maryland National Science Foundation-ADVANCE Institutional Transformation grant (grant no. HRD-1008117); a USDA National Institute for Food and Agriculture (NIFA) Integrated Agriculture and Natural Resources Extension and Research Program Grant administered through the University of Maryland (project no. MD-PSLA-18496/project accession no. 1016911); a grant from the USDA NIFA Resilient Agroecosystems in a Changing Climate Challenge Area Grant No. 12451226/project accession no. 1015143); a Maryland Sea Grant Coastal Resilience and Sustainability Fellowship (Grant No. 20171-002); and the National Socio-Environmental Synthesis Center. The National Socio-Environmental Synthesis Center is supported by an award from the US National Science Foundation (Grant #s DBI-1052875 and DBI-1639145) to the University of Maryland, with additional support from University of Maryland, University of Maryland Center for Environmental Science, and Resources for the Future. The authors would like to thank Quentin Read for his help with the statistical analysis for this study. We would also like to thank Keryn Gedan, Kristal Jones, Margaret Palmer, Stephanie Yarwood, and the anonymous reviewers for their helpful insight and suggestions to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Kate Lajtha
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Weissman, D., Ouyang, T. & Tully, K.L. Saltwater intrusion affects nitrogen, phosphorus and iron transformations under oxic and anoxic conditions: an incubation experiment. Biogeochemistry 154, 451–469 (2021). https://doi.org/10.1007/s10533-021-00796-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-021-00796-6