Abstract
Background & aims
Understanding the mechanism of how phosphorus (P) regulates the response of legumes to elevated CO2 (eCO2) is important for developing P management strategies to cope with increasing atmospheric CO2 concentration. This study aimed to explore this mechanism by investigating interactive effects of CO2 and P supply on root morphology, nodulation and soil P fractions in the rhizosphere.
Methods
A column experiment was conducted under ambient (350 ppm) (aCO2) and eCO2 (550 ppm) in a free air CO2 enrichment (FACE) system. Chickpea and field pea were grown in a P-deficient Vertisol with P addition of 0–16 mg P kg−1.
Results
Increasing P supply increased plant growth and total P uptake with the increase being greater under eCO2 than under aCO2. Elevated CO2 increased root biomass and length, on average, by 16 % and 14 %, respectively. Nodule biomass increased by 46 % in response to eCO2 at 16 mg P kg−1, but was not affected by eCO2 at no P supply. Total P uptake was correlated with root length while N uptake correlated with nodule number and biomass regardless of CO2 level. Elevated CO2 increased the NaOH-extractable organic P by 92 % when 16 mg P kg−1 was applied.
Conclusion
The increase in P and N uptake and nodule number under eCO2 resulted from the increased biomass production, rather than from changes in specific root-absorbing capability or specific nodule function. Elevated CO2 appears to enhance P immobilization in the rhizosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The concentration of global atmospheric CO2 has increased from around 270 μmol mol−1 prior to the Industrial Revolution to 384 μmol mol−1 in 2009 (Leakey et al. 2009). It is predicted that CO2 will reach 550 μmol mol−1 by the middle of this century and climb up to 700 μmol mol−1 by the end of the century (de Graaff et al. 2006; Ainsworth et al. 2008). Elevated CO2 (eCO2) has significant effects on plant growth and physiology (Stöcklin and Körner 1999; von Felten et al. 2007). However, the response of plants to eCO2 greatly depends on species and the availability of nutrients such as P (Conroy et al. 1992; Newbery et al. 1995). For example, the growth of legumes appears to be more responsive to eCO2 than non-legumes, especially under high-P conditions (Stöcklin et al. 1998; Stöcklin and Körner 1999).
Phosphorus (P) is involved in various metabolic processes such as conserving and transferring energy in cell metabolism (Raghothama 1999; Abel et al. 2002). It is expected that plants grown under eCO2 would require more P to maintain their physiological requirements due to increases in biomass normally associated with eCO2. Stöcklin and Körner (1999) reported a 166 % increase of biomass under eCO2 by Hippocrepis comosa when P was supplied but there was no response to eCO2 without P application. Furthermore, P plays a specific role in nodulation in legumes (Israel 1987; Bordeleau and Prevost 1994), as more P is required for nodule development and nodule function than for the host plant growth (Qiao et al. 2007). Under eCO2 environment, legumes exhibit stronger N2 fixation, resulting in positive photosynthetic and growth responses (Lee et al. 2003; Rogers et al. 2009). However, knowledge of the demand for P in N2-fixing legumes, and associated responses of N2 fixation and growth to P supply under eCO2 is limited.
Changes in root morphology and metabolism-driven rhizosphere processes occurring under eCO2 are believed to favour P acquisition (Barrett et al. 1998; Campbell and Sage 2002). For example, eCO2 has been shown to enhance root growth in Senecio vulgaris, Festuca ovina and Nardus stricta (Berntson and Woodward 1992; Fitter et al. 1996) and the formation of root hairs in Arabidopsis thaliana (Niu et al. 2011), which would, in turn, increase P uptake. In legumes, eCO2 may intensify rhizosphere acidification through differential cation/anion uptake during N2 fixation and hence benefit P mobilization (Tang et al. 2009). Also, the increased release of carbon-rich compounds under eCO2 including organic acid anions and phosphatases into the rhizosphere (Richardson 2001; de Graaff et al. 2006) might attract and stimulate soil microorganisms to mineralize or directly mobilize soil P (George et al. 2002). However, direct evidence is lacking as to whether rhizosphere processes under eCO2 facilitates P mobilization or immobilization.
Legume species differ markedly in their ability to take up P from soil. For example, chickpea has significantly higher root biomass and surface area than field pea (Srinivasarao et al. 2006; Erman et al. 2009). Chickpea also exudes large amounts of low-molecular weight carboxylates, which mobilize P by competing for the same adsorption sites in soil matrix (Gerke et al., 2000; Wouterlood et al. 2005; Veneklaas et al. 2003). In contrast, field pea, with a relatively small root system, secretes less carboxylates and phosphatases per root mass (Nuruzzaman et al. 2005), suggesting that field pea roots are less efficient in taking up P than chickpea roots.
In this study, a range of P application rates were added to two legume species grown in a P-deficient Vertisol within a free air CO2 enrichment facility to investigate the effect of eCO2 on P requirement, N uptake, and root and nodule characteristics. We hypothesized that eCO2 would increase the P demand, and this increased demand could be met by a greater capacity for P acquisition by the root system, and by increasing P-regulated nodulation and N2 fixation under eCO2, than under ambient CO2 (aCO2).
Materials and methods
Experimental design and plant growth
A column experiment was conducted at a free air CO2 enrichment (SoilFACE) facility at the Department of Primary Industries in Horsham, Victoria, Australia (36°42′S, 142°11′E) (Mollah et al. 2011). There were four FACE rings for elevated CO2 (550 ppm) and four ambient CO2 rings (350 ppm). The FACE array was engineered to achieve at least 80 % of the ring area with a CO2 concentration at or above 90 % of the target concentration at the ring-centre for 80 % of the time. The CO2 concentration in the elevated CO2 FACE rings was in a range of 512 to 580 ppm (Mollah et al. 2011). The experiment consisted of two levels of CO2, two leguminous species and five P levels in a split-plot design with CO2 as the main plot, and legume species and P application as sub-plot treatments. Each treatment had four replicates and each replicate was randomly allocated into one FACE-ring. Two grain legume species were chickpeas (Cicer arietinum L. cv. Genesis 836) and field pea (Pisum sativum L. cv. OzP0601) which differ in root morphology and physiology. Phosphorus was applied as KH2PO4 at five rates, i.e. 0, 2, 4, 8 and 16 mg P kg−1 soil. The soil was collected at a depth of approximately 10 to 30 cm from a virgin site under native vegetation that had not previously been used for farming near Horsham, Victoria, Australia. The soil type was a Vertosol (Isbell 1996) or a Vertisol (FAO - UNESCO 1976). It had organic C of 7.8 mg g−1 (Rayment and Higginson 1992), 2 M KCl-extractable NO3-N of 4.2 mg kg−1 and NH4-N of 1.0 mg kg−1, total P of 114 mg kg−1 (Guppy et al. 2000), Colwell P of 5 mg kg−1 (Colwell 1963) and a pH (1:5 in 0.01 M CaCl2) of 7.7. The experimental soil was air-dried and sieved through a 4-mm sieve, then mixed with siliceous sand (w:w=1:1) to aid root washing and collecting rhizosphere soil at harvest.
Each column used in this experiment comprised of two equal halves of a vertically-split PVC pipe (60 cm long, 10 cm in diameter). The two halves of pipe were taped together with plumbing tape with a PVC cap placed at the bottom of the column. Each column contained 8 kg of experimental soil mixed with the following basal nutrients (mg kg−1): K2SO4, 147; MgSO4.7H2O, 122; CaCl2, 186; CuSO4.5H2O, 6; ZnSO4.7H2O, 8; MnSO4.5H2O, 6; FeCl3, 0.6; CoCl2, 0.4; NaMoO4.2H2O, 0.4; and NaB4O7, 1.6 (Vu et al. 2010) and the required amount of P for each treatment.
Nine uniform germinated seeds of each species were hand-sown at a depth of 2 cm in each column and inoculated with rhizobium (Rhizobium ciceri for chickpea and Rhizobium leguminosarum for field pea) on the 19th September, 2010. The seedlings were thinned to 2 plants per column 3 weeks after sowing. The average temperatures during plant growth were 25.1 °C in the day and 10.1 °C at night. The total rainfall during the experiment was 116.8 mm. These meteorological observations were taken from Horsham Airport which is 6.6 km away from the SoilFACE site. The soil moisture in column was adjusted to 80 % of field capacity every 3 days by weighing and watering with reverse osmosis water adding up to 1,460 ml for each column during the experimental period.
Measurements
After 9 weeks of growth in the SoilFACE, plant shoots were cut off at ground level. To remove dust, shoots were washed with 0.1 M HCl and then rinsed twice in deionized water (Tang et al. 1990). Each column was opened and the soil was separated vertically into 4 layers, namely 0–10, 10–20, 20–40 and 40–60 cm. Roots in each layer were carefully removed by sliding out the entire root mass. The soil adhering to the roots was shaken off as rhizosphere soil (Marschner et al. 2004). The root system was washed with tap water until free of soil, and then soaked in 0.01 M CaCl2 solution for 5 min to desorb nutrients on root surface (Tang et al. 1990). Root nodules were counted and removed. The root morphology in terms of root length, surface area and diameter was determined by scanning roots on an EPSON EU-35 scanner (Seiko Epson Corp., Japan), and images were analysed using the Mac Rhizo Pro version 2003b programme (Régent Instruments Inc., Québec, CA).
All plant samples were dried at 70 °C for 72 h and then ground. Subsamples of ground shoots and roots were digested with a mixture of nitric and perchloric acid (4:1) (Yuen and Pollard 1954), and the concentrations of P in digests were colorimetrically measured using malachite green (Motomizu et al. 1980). The concentration of N in plant tissues was determined using an Elementar CNS analyser (Vario EL III, Elementar Analysensysteme GmbH, Germany).
Rhizosphere soil samples were mixed thoroughly, air-dried, and milled to <0.5 mm before further analysis. Phosphorus fractions were performed using the modified Hedley P fractionation scheme (Guppy et al. 2000). Total dissolved P including organic (Po) and inorganic P (Pi) in the bicarbonate (NaHCO3) and hydroxide (NaOH) extracts were determined after digesting in an autoclave at a pressure of 103 kPa at 121 °C for 1 h using acid ammonium persulphate (Butterly et al. 2009). The Po in these two fractions was determined by subtracting the Pi from total P. The Pi in extracts was determined using the malachite green method (Motomizu et al. 1980).
Statistical analysis
Statistical analyses were performed on parameters using SAS Release 6.12 for Windows (SAS Institute 1997). Protected ANOVA tests of LSD were used to assess the differences between treatment means (Steel and Torrie 1980). The data of plant biomass, root morphology, P and N parameters were statistically analyzed by factorial ANOVA to determine the effects of P, CO2, species and their interactions (Genstat, Version 13, VSN International software for bioscience).
Results
Shoot growth
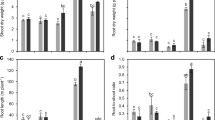
Shoot biomass of the legumes increased significantly with added P and with eCO2, and differed between the species with field pea producing a greater biomass than chickpea (Fig. 1a, Table 1). However, the relative shoot biomass response to eCO2 depended on the P treatment. The response of shoot biomass of both species to eCO2 was around 15 % with 0 mg P kg−1, and 32 % with 16 mg P kg−1, resulting in a significant P × CO2 interaction There was also a significant P × Species interaction, with chickpea having a 5.9-fold increase in shoot biomass when the P applied was increased from 0 to 16 mg kg−1 soil, compared to the 6.8-fold increase in field pea (Table 1). There was no significant CO2 × Species interaction, indicating that the species did not differ in their response to eCO2, nor was there any significant CO2 × P × Species interaction (Table 1).
The effects of CO2, P and species on shoot biomass (a), root biomass (b), root-to-shoot weight ratio (c) and root length (d) of chickpea (left) and field pea (right) after plants were exposed to CO2 treatments for 9 weeks in a P-deficient Vertisol supplied with 0 to 16 mg P kg-1 soil. The vertical bar in each panel indicates the LSD (P = 0.05) for the CO2 × P interaction
Root growth and biomass allocation
Unlike the effect on shoots, there was no significant CO2 × P interaction for roots (Table 1), but both of these main effect treatments increased root biomass (Fig. 1b). Between species, chickpea had significantly greater root biomass than field pea with the difference being greater at high P than at no or low P (Table 1; Fig. 1b). Chickpea responded more to eCO2 than field pea, increasing root biomass by 22 % when exposed to eCO2, whereas field peas had only 10 % increase (Fig. 1b).
The root-to-shoot ratio markedly declined as P supply increased, but was not affected by CO2 treatment. Irrespective of CO2 treatment, chickpea had higher root-to-shoot ratios than field pea (Table 1; Fig. 1c). A significant P × Species interaction was found, with the root-to-shoot ratio of chickpea decreasing more than field pea as P application rate increased. However, there were no significant CO2 × P, CO2 × Species or CO2 × P × Species interaction for the root-to-shoot ratio (Table 1).
Similar to the effects on root biomass, increasing P supply from 0 to 16 mg P kg−1 increased root length from 26.8 to 46.3 m plant−1 for chickpea and from 13.3 to 37.3 m plant−1 for field pea. Compared to aCO2, eCO2 increased average root length by 14 % for chickpea and by 12 % for field pea (Fig. 1d). However, there were no significant interactive effects on root length between any two treatments (Table 1).
Nodulation
Increasing P application increased nodule biomass, number and size but decreased N uptake per unit nodule biomass, while increasing CO2 concentration increased the total nodule biomass and nodule number but did not affect nodule size (single nodule mass) or N uptake per unit nodule biomass (Fig. 2, Table 1). Nodule density (nodule number per unit root length) also increased with P application, but was not affected by eCO2 across P treatments (Fig. 2d). Compared with field pea, chickpea on average produced a 6-fold greater nodule biomass and 49 % more nodules, and these nodules were 3 times larger. However, the plant N uptake per unit of nodule biomass was much lower in chickpea (Fig. 2e).
The effects of CO2, P and species on CO2 nodule biomass (a), nodule number (b), nodule size (c), nodule density (d) and N uptake per mg nodule (e) of chickpea (left) and field pea (right) after plants were exposed to CO2 treatments for 9 weeks in a P-deficient Vertisol supplied with 0 to 16 mg P kg-1 soil. The vertical bar in each panel indicates the LSD (P = 0.05) for the CO2 × P interaction
Although eCO2 did increase total nodule biomass, the response varied with P rate and between two species, resulting in significant CO2 × P, and CO2 × Species interactions (Table 1). The basis for the former interaction was 46 % greater nodule biomass under eCO2 with 16 mg P kg−1, compared to the lack of any difference in nodule biomass with nil applied P (Fig. 2a). Similarly, the response in nodule biomass to eCO2 by chickpea was 35 mg plant−1, compared with 5 mg plant−1 by field pea.
There were significant P × Species interactions on nodule biomass, number and size, and N uptake per unit nodule biomass (Table 1). Nodule biomass, number and size of chickpea increased more sharply than those of field pea as the rate of P application increased from 0 to 16 mg P kg−1 (Fig. 2a, b, and c). In contrast, with increasing P supply, N uptake per unit nodule biomass decreased more in field pea than in chickpea (Fig. 2e).
Irrespective of CO2 and P treatments, total N uptake was positively correlated (P < 0.001) with nodule number (R2 = 0.96–0.99), nodule biomass (R2 = 0.99) and total biomass production (R2 = 0.99) for both species.
Root and nodule distribution in soil profiles
Chickpea distributed 43–49 % of the root biomass and field pea distributed 30–48 % in 0–10 cm of soil profile. Applying P significantly decreased the distribution of root biomass in top 10 cm of the soil. Elevated CO2, however, did not affect the distribution of root biomass (Fig. 3a). There was no significant CO2 × P interaction on the distribution of root biomass for either species.
The distribution patterns at various soil depths of root biomass (a), root length (b) and nodule number (c) of chickpea (left) and field pea (right) grown for 9 weeks in a Vertisol supplied with 0–16 mg P kg−1 soil under ambient (350 ppm) (a) and elevated CO2 (550 ppm) (e). The vertical bars in each panel indicate the LSD (P = 0.05) for individual layers (0–10 cm, 10–20 cm, 20–40 cm and 40–60 cm) if the treatment effect or interaction is significant. n.s. not significant at P < 0.05
The relative proportion of root length located in the top 10 cm of soil tended to decrease as the rate of P application increased but was not affected by CO2. The distribution of root length throughout the soil profile varied with species, with chickpea having 9 % less root length in the topsoil than field pea (Fig. 3b). In general, chickpea had longer roots distributed deeper in the soil than field pea.
Increasing P application decreased nodule number in the 0–10 cm of soil depth (P < 0.05) (Fig. 3c). Elevated CO2 did not affect the nodule distribution. The two species differed in the distribution of nodule number (P < 0.01), with chickpea having 40 % of its nodules in the 10–20 cm soil layer while field pea had only 20 % of its nodules in the same soil layer.
Plant P concentration and uptake
Phosphorus application and eCO2 significantly affected the concentration of P in plants but this effect depended on the species (Table 2). Increasing P application generally increased P concentrations in shoots and roots of chickpea but not of field pea. On average, chickpea had higher tissue P concentrations than field pea. Elevated CO2 decreased the P concentration in shoots of chickpea by 12 %, but had no effect in field pea. There was no P × CO2 interaction on P concentration.
Total P uptake increased with increasing P application for both species but this increase was greater for field pea than chickpea. On average field pea had 29.5 % more total P than chickpea (Table 2). A significant CO2 × P interaction occurred on total P uptake, with total P uptake increasing more under eCO2 than aCO2 as P application increased. Total P uptake correlated positively with root length and root biomass of both species (data not shown).
Plant N concentration and uptake
Increasing P application generally decreased N concentration in shoots but not in roots (Table 2). Field pea had higher N concentrations in both shoots and roots than chickpea. However, CO2 treatment did not affect N concentration of either species (Table 2).
Total N uptake was affected by P and CO2 treatments (Table 2). Total N uptake increased as P application rate increased for both legume species with the increase being greater under eCO2 than under aCO2, resulting in a significant CO2 × P interaction. The basis for this was 9 % increase in N uptake with nil P, compared to the 27 % with 16 mg P kg−1 (Table 2). There was also a significant CO2 × Species interaction due to the greater N uptake response to eCO2 in chickpea than in field pea.
The N-to-P concentration ratio in the plant significantly decreased as the rate of P application increased, but it was not affected by eCO2 (Table 2). There was no P × CO2 interaction for the N-to-P ratio.
P fractionation in rhizosphere
Phosphorus supply and CO2 affected P pools in rhizosphere of the legumes. Increasing P application from 4 to 16 mg kg−1 significantly increased concentrations of both NaHCO3-Pi and NaOH-Po (Table 3). However, eCO2 only increased the NaOH-Po fraction, but this increase depended on P supply due to a significant CO2 × P interaction. This resulted from an 11 % increase in NaOH-Po with eCO2 at 4 mg P kg−1, compared to a 92 % of increase with eCO2 at 16 mg P kg−1, irrespective of species (Table 3). Species differences included higher concentrations of NaHCO3-Po and NaOH-Pi in the rhizosphere of field pea, compared with chickpea. Increased P application and CO2 concentration did not change the HCl-P or residual-P fractions in rhizosphere with averages of 16 and 83 mg P kg−1, respectively (data not shown).
Discussion
Plant growth
The two N2-fixing grain legume species grown in the P-deficient Vertisol soil required P addition to overcome the deficiency, before the shoot growth could respond to the eCO2. The maximum response to eCO2 occurred at the highest rate (16 mg P kg−1) while no response to eCO2 was observed when no P was added (Fig. 1), resulting in a highly significant P × CO2 interaction for shoot growth (Table 1). This finding is consistent with previous studies on pine seedlings (Pinus radiata D. Don) and strawberry (Fragaria virginiana R.), where responses to eCO2 were more pronounced under P adequate conditions than when P was deficient (Conroy et al. 1990; Whitehead et al. 1997). This result appears due to the effect of P deficiency on photosynthesis, a key physiological process underpinning plant responses to eCO2 (Conroy et al. 1992; Sinclair 1992; BassiriRad et al. 2001).
Root growth was enhanced by eCO2, but biomass allocation to the root was not affected by CO2 treatment in this study. The root biomass and total root length of both legume species increased significantly under eCO2, irrespective of P treatments (Fig. 1, Table 1). Thus, there was no P × CO2 interaction for root growth. Other work has reported similar root responses to eCO2. Fitter et al. (1996) found that Festuca ovina and Nardus stricta had increases of 41 % and 48 %, respectively, in root dry weight in response to elevated CO2. Rogers et al. (1992) demonstrated that CO2 enrichment significantly increased the root mass, length and diameter of soybean roots. Similarly, Berntson and Woodward (1992) showed that eCO2 resulted in longer roots and increased root branching in Senecio vulgaris. Thus, increased root growth is a widespread response to eCO2 resulting from increased photosynthate supply to the roots (Pritchard and Rogers 2000; Laby et al. 2000). Although there was increased root mass and length under eCO2, there was no effect on carbon partitioning between shoots and roots, as the root-to-shoot ratio did not change under eCO2 (Fig. 1). Furthermore, there was no effect of eCO2 on the distribution of roots in the soil profile (Fig. 4). Thus, the effect of eCO2 concentration in this study stimulated overall root growth without affecting the allocation of photosynthate between roots and shoots, or between shallower and deeper roots. Other studies that examined shoot and root growth under eCO2 reported different results. For example, root-to-shoot ratios increased under eCO2 in carrots and radish (Rogers et al. 1983, 1996), and corn (Idso et al. 1988). It is possible that species differences in the C-sink strength in the roots are responsible for these differences (Niu et al. 2011).
Nodulation and N uptake
The increase in total N uptake and total nodule biomass under eCO2 were the consequence of the increased biomass of the host plant, rather than specific effects on the components of symbiotic N2 fixation. This can be seen from the direct linear relationship between N uptake and total plant dry weight (data not shown), which was unaffected by eCO2. Similarly, eCO2 had no effect the linear relationship between total nodule number and N uptake (data not shown). Studies on Glycine max showed a similar result, in that CO2 enrichment did not influence specific nodule formation or nodule activity (Finn and Brun 1982). However, the N2-fixing activity in nodules significantly increased under eCO2 in other species such as alfalfa (Bertrand et al. 2007), mungbean (Srivastava et al. 2002), acacia (Schortemeyer et al. 2002) and Ormosia macrocalyx (Cernusak et al. 2011). These inconsistencies between studies may be attributed to (1) differences in the duration of eCO2 exposure that enable the N2-fixing capacity to be up-regulated (Srivastava et al. 2002); and/or (2) species variation in the nodule: root mass ratio determining the capacity to up-regulate N2 fixation under eCO2 (Cernusak et al. 2011); and/or (3) differences in rhizobial population affecting the N2 fixation to respond to extra photosynthate supply under eCO2 (West et al. 2005; Haase et al. 2007).
In contrast to eCO2, the addition of P to soil enhanced nodule formation and nodule development in the two legumes species. Similar results were also found in Stylosanthes humilis and Trifolium subterraneum (Robson et al. 1981; Gates 1974). Although increased P supply markedly increased total amount of N per plant, in parallel to increase in plant biomass, it decreased N concentration and N/P concentration ratio in the plant. Since the soil used in the experiment had an extremely low N concentration, the majority of N in the plant would have been derived from N2 fixation. Thus N2 fixation in the legumes was not inhibited by the P deficiency.
The importance of P supply for nodule formation and development has been highlighted in other studies. For example, nodule number and size in soybeans under P deficiency were only 9 % and 34 % of that under sufficient P addition (Israel 1987). This effect of P supply on nodule formation is probably because P supply affects the production of root-exudates including flavonoids that trigger nod-gene expression to form nodules, and also plays a role in nodule cell metabolism that affects nodule development (Raghothama et al. 1999; Abel et al. 2002).
Although P supply increased nodulation in the legumes, it did not affect the functioning or the N2-fixing capacity of the nodules. Reports in the literature on the effect of P on nodule function are inconsistent. Cassman et al. (1980) observed that increased P supply enhanced nodule function in Stylosanthes humilis, Glycine max and Medicago truncatula whereas Robson et al. (1981) found no effect of P supply on the N2-fixing capacity of nodules on the roots of Trifolium subterraneum. The discrepancy could be due to different P requirements for N2 fixation between species, as P supply in the nodule can regulate nitrogenase activity via ATP-dependent reactions (Sa and Israel 1991), and this regulation may differ between species.
P uptake by root system and its availability in rhizosphere
Elevated CO2 increased P uptake by both legumes when sufficient P was supplied (Fig. 1), indicating that the P demand under eCO2 increased significantly. This increase in total P uptake appeared to result from increased biomass production under eCO2, rather than from any enhanced ability of the roots to acquire soil P (Table 2). This can be seen by the fact that the linear relationships between total root length and total P uptake were not affected by eCO2 (data not shown). In addition, the P uptake per unit of root length or per unit of root surface area did not differ between eCO2 and aCO2 (data not shown), and the P concentration in the two legumes studied did not increase under eCO2 (Table 2). Similar findings have been reported in other studies where there was a decrease or no change of P concentration in wheat (Wolf 1996; Fangmeier et al. 1999), Eucalyptus grandis (Conroy et al. 1992), Calluna vulgaris (Whitehead et al. 1997), Lolium perenne (Gentile et al. 2011) or Agrostis capillaries (Newbery et al. 1995), although eCO2 did increase foliar P concentration of Bouteloua eriopoda (BassiriRad et al. 1997). Genetic differences in nutrient acquisition in response to eCO2 may explain the discrepancy, because the Bouteloua species was observed to have a stronger root absorption capacity for nutrient uptake than other species (BassiriRad et al. 1997). Although P demand increased with the biomass response to eCO2 in this study, we could not define the critical level of external and internal P concentrations, because maximum growth was not reached even at the highest P supply. Further research will be required to quantify the critical P concentrations in these species under eCO2.
Although eCO2 did not affect the P uptake capacity of the roots, it did alter P fractions in the rhizosphere of both legumes species. The effect was to increase the NaOH-extractable Po pool size in the rhizosphere (Table 3). This fraction contains a range of organic P compounds such as phosphate monoesters, phosphate diesters and phosphonate, which are derived from soil microbes and organic matter (Beck and Sanchez 1994; Turner et al. 2007). As these compounds can potentially be mineralized into labile Pi, they are considered to be the moderately labile P. On the other hand, eCO2 did not increase the NaHCO3-extractable Pi or Po pools, irrespective of P application (Table 3), suggesting that there was a net flux of Pi into the NaOH-extractable Po pool. The fact that the NaOH-extractable Po pool size was greater when 16 mg P kg−1 was applied compared with 4 mg P kg−1 supports this view. Immobilization of Pi by soil microbes in the rhizosphere and the formation of moderately stable Po compounds would explain this observation.
There are a number of possible mechanisms whereby eCO2 could increase the NaOH-extractable Po pool in the rhizosphere. The first is that root exudates could be increased under eCO2 and this would enhance the activity of microorganisms in the rhizosphere (Richardson 2001; de Graaff et al. 2006). Increased root exudation could have a priming effect on soil organic matter decomposition, and transfer more complex organic P to the NaOH-extractable Po pool (Fontaine et al. 2004). In addition, the increased microbial activity would also enable microbes to compete for labile Pi forms and increase the microbial P pool size that is extractable in NaOH (Binkley et al. 2000; Achat et al. 2010; Richardson and Simpson 2011).
Species differences on P and N uptake
There were marked differences between the two legumes in their ability to take up P from the Vertisol. It was proposed that chickpea would be more efficient in P uptake than field pea, because chickpea has a larger root system (Gerke et al. 2000) and releases more P-mobilizing root exudates than field pea (Nuruzzaman et al. 2005, 2006). However, in this study, field pea was able to accumulate more P in shoots and roots than chickpea. Despite the smaller root system of field pea (Fig. 1), P uptake per unit root length was greater than chickpea irrespective of CO2 treatments. Furthermore, P concentrations in the roots and shoots of field pea were higher than in chickpea, irrespective of CO2 or P supply (Table 2). The soil NaHCO3-extractable Po and NaOH extractable Pi concentrations in the rhizosphere were also higher with field pea than chickpea, indicating that the field pea roots could potentially mobilize more stable soil P pools into labile P. The explanation for the higher P acquisition efficiency of field pea may be due to its finer root system. Field pea roots had smaller diameters than chickpea roots (0.35 and 0.51 mm for field pea and chickpea, respectively). The field pea, therefore, produces more roots with lower tissue construction costs in energy and carbon, and this is likely to enable them to explore the soil with a lower metabolic investment, enabling the plant to take up P more efficiently (Lynch 2011).
The two legumes also differed in their ability to accumulate N in both shoots and roots. Nitrogen accumulation was greater for field pea, indicating a more efficient N2-fixing symbiosis. It had smaller roots, fewer nodules and smaller nodules than chickpea, resulting in lower nodule biomass (Fig. 2). However, N concentration in plants, total N uptake and the N uptake per unit nodule mass were greater in field pea than chickpea (Table 2). Rennie and Dubetz (1986) also confirmed that field pea nodules were more efficient in N2 fixation than chickpea when field pea was inoculated with Rhizobium leguminosarum strains of 175F1, 17SF2, 175F5 and 175F8, and chickpea with 27A2,27A7 and 27A9. The basis for this superior capacity of field pea requires further investigation.
Conclusion
Phosphorus addition is required for the two grain legume species studied to overcome P deficiency before the shoot growth could respond to the eCO2. Elevated CO2 increased P demand by both of these legumes and the resulting increase in P uptake under eCO2 resulted from increased biomass rather than any enhanced P acquisition capacity in the roots. The study could not establish critical concentrations of P for plant growth and nodulation under eCO2 because the maximum growth was not achieved at the highest level of P supply. When P is supplied under eCO2, the increase in the size of the root system would enhance exploration of the soil for P, and nodulation which also benefits N uptake and consequent plant growth. However, the specific uptake of P and N by roots and nodules was not influenced by eCO2. In the rhizosphere, eCO2 increased the moderately labile Po pool, indicating an increase of microbial P immobilization.
References
Abel S, Ticconi CA, Delatorre CA (2002) Phosphate sensing in higher plants. Physiol Plant 115:1–8
Achat DL, Morel C, Bakker MR, Augusto L, Pellerin S, Gallet-Budynek A, Gonzalez M (2010) Assessing turnover of microbial biomass phosphorus: combination of an isotopic dilution method with a mass balance model. Soil Biol Biochem 42:2231–2240
Ainsworth EA, Leakey ADB, Ort DR, Long SP (2008) FACE-ing the facts: inconsistencies and interdependence among field, chamber and modeling studies of elevated [CO2] impacts on crop yield and food supply. New Phytol 179:5–9
Barrett DJ, Richardson AE, Gifford RM (1998) Elevated atmospheric CO2 concentrations increase wheat root phosphatise activity when growth is limited by phosphorus. Aust J Plant Physiol 25:87–93
BassiriRad H, Reynolds JF, Virginia RA, Brunelle MH (1997) Growth and root NO -3 and PO 3-4 uptake capacity of three desert species in response to atmospheric CO2 enrichment. Aust J Plant Physiol 24:353–358
BassiriRad H, Gutschick VP, Lussenhop J (2001) Root system adjustments: regulation of plant nutrient uptake and growth responses to elevated CO2. Oecologia 126:305–320
Beck MA, Sanchez PA (1994) Soil-phosphorus fraction dynamics during 18 years of cultivation on a typic paleudult. Soil Sci Soc Am J 58:1424–1431
Berntson GM, Woodward FI (1992) The root-system architecture and development of Senecio vulgaris in elevated CO2 and drought. Funct Ecol 6:324–333
Bertrand A, Prevost D, Bigras FJ, Lalande R, Tremblay GF, Castonguay Y, Belanger G (2007) Alfalfa response to elevated atmospheric CO2 varies with the symbiotic rhizobial strain. Plant Soil 301:173–187
Binkley D, Giardina C, Bashkin MA (2000) Soil phosphorus pools and supply under the influence of Eucalyptus saligna and nitrogen-fixing Albizia facaltaria. For Ecol Manag 128:241–247
Bordeleau LM, Prevost D (1994) Nodulation and nitrogen-fixation in extreme environments. Plant Soil 161:115–125
Butterly CR, Buenemann EK, McNeill AM, Baldock JA, Marschner P (2009) Carbon pulses but not phosphorus pulses are related to decreases in microbial biomass during repeated drying and rewetting of soils. Soil Biol Biochem 41:1406–1416
Campbell CD, Sage RF (2002) Interactions between atmospheric CO2 concentration and phosphorus nutrition on the formation of proteoid roots in white lupin (Lupinus albus L.). Plant Cell Environ 25:1051–1059
Cassman KG, Whitney AS, Stockinger KR (1980) Root-growth and dry-matter distribution of soybean as affected by phosphorus stress, nodulation and nitrogen-source. Crop Sci 20:239–244
Cernusak LA, Winter K, Martinez C, Correa E, Aranda J, Garcia M, Jaramillo C, Turner BL (2011) Responses of legume versus non-legume tropical tree seedlings to elevated CO2 concentration. Plant Physiol 157:372–385
Colwell JD (1963) The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Aust J Exp Agric Anim Husb 3:190–198
Conroy JP, Milham PJ, Reed ML, Barlow EW (1990) Increases in phosphorus requirements for CO2-enriched pine species. Plant Physiol 92:977–982
Conroy JP, Milham PJ, Barlow EWR (1992) Effect of nitrogen and phosphorus availability on the growth-response of Eucalyptus grandis to high CO2. Plant Cell Environ 15:843–847
de Graaff MA, van Groenigen KJ, Six J, Hungate B, van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Chang Biol 12:2077–2091
Erman M, Yildirim B, Togay N, Cig F (2009) Effect of phosphorus application and rhizobium inoculation on the yield, nodulation and nutrient uptake in field pea (Pisum sativum sp arvense L.). J Anim Vet Adv 8:301–304
Fangmeier A, De Temmerman L, Mortensen L, Kemp K, Burke J, Mitchell R, van Oijen M, Weigel HJ (1999) Effects on nutrients and on grain quality in spring wheat crops grown under elevated CO2 concentrations and stress conditions in the European, multiple-site experiment ‘ESPACE-wheat’. Eur J Agron 10:215–229
FAO-UNESCO (1976) Soil map of the world, 1:5 000 000, vol. X, Australia. UNESCO, Paris
Finn GA, Brun WA (1982) Effect of atmospheric CO2 enrichment on growth, non-structural carbohydrate content, and root nodule activity in soybean. Plant Physiol 69:327–331
Fitter AH, Self GK, Wolfenden J, vanVuuren MMI, Brown TK, Williamson L, Graves JD, Robinson D (1996) Root production and mortality under elevated atmospheric carbon dioxide. Plant Soil 187:299–306
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Lett 7:314–320
Gates CT (1974) Nodule and plant development in Stylosanthes humilis H.B.K.: symbiotic response to phosphorus and sulphur. Aust J Bot 22:45–55
Gentile R, Dodd M, Lieffering M, Brock SC, Theobald PW, Newton PCD (2012) Effects of long-term exposure to enriched CO2 on the nutrient-supplying capacity of a grassland soil. Biol Fert Soil 48:357–362
George TS, Gregory PJ, Wood M, Read D, Buresh RJ (2002) Phosphatase activity and organic acids in the rhizosphere of potential agroforestry species and maize. Soil Biol Biochem 34:1487–1494
Gerke J, Beissner L, Romer W (2000) The quantitative effect of chemical phosphate mobilization by carboxylate anions on P uptake by a single root. I. The basic concept and determination of soil parameters. J Plant Nutr Soil Sci 163:207–212
Guppy CN, Menzies NW, Moody PW, Compton BL, Blamey FPC (2000) A simplified, sequential, phosphorus fractionation method. Commun Soil Sci Plant 31:1981–1991
Haase S, Neumann G, Kania A, Kuzyakov Y, Romheld V, Kandeler E (2007) Elevation of atmospheric CO2 and N-nutritional status modify nodulation, nodule-carbon supply, and root exudation of Phaseolus vulgaris L. Soil Biol Biochem 39:2208–2221
Idso SB, Kimball BA, Mauney JR (1988) Effects of atmospheric CO2 enrichment on root-shoot ratios of carrot, radish, cotton and soybean. Agric Ecosyst Environ 21:293–299
Isbell RF (1996) The Australian soil classification. CSIRO Publishing, Melbourne
Israel DW (1987) Investigation of the role of phosphorus in symbiotic dinitrogen fixation. Plant Physiol 84:835–840
Laby RJ, Kinkaid MS, Kim D, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23:587–596
Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60:2859–2876
Lee TD, Tjoelker MG, Reich PB, Russelle MP (2003) Contrasting growth response of an N2-fixing and non-fixing forb to elevated CO2: dependence on soil N supply. Plant Soil 255:475–486
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156:1041–1049
Marschner P, Crowley D, Yang CH (2004) Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 261:199–208
Mollah M, Partington D, Fitzgerald G (2011) Understand distribution of carbon dioxide to interpret crop growth data: Australian grains free-air carbon dioxide enrichment experiment. Crop Pasture Sci 62:883–891
Motomizu S, Wakimoto T, Toei K (1980) Spectrophotometric determination of phosphate in river waters with molybdite and malachite green. Analyst 108:361–367
Newbery RM, Wolfenden J, Mansfield TA, Harrison AF (1995) Nitrogen, phosphorus and potassium uptake and demand in Agrostis capillaris: the influence of elevated CO2 and nutrient supply. New Phytol 130:565–574
Niu Y, Jin C, Jin G, Zhou Q, Lin X, Tang C, Zhang Y (2011) Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. under elevated CO2. Plant Cell Environ 34:1304–1317
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2005) Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant Soil 271:175–187
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2006) Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 281:109–120
Pritchard SG, Rogers HH (2000) Spatial and temporal deployment of crop roots in CO2- enriched environments. New Phytol 147:55–71
Qiao YF, Tang CX, Han XZ, Miao SF (2007) Phosphorus deficiency delays the onset of nodule function in soybean. J Plant Nutr 30:1341–1353
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol 50:665–693
Raghothama KG, Muchhal US, Kim DH, Bucher M (1999) Molecular regulation of plant phosphate transporters. In: Lynch JP, Deikman J (eds) Phosphorus in plant biology: regulatory roles in molecular, cellular, organismic, and ecosystem processes, American society of plant physiologists, Maryland pp 271–280
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press, Melbourne
Rennie RJ, Dubetz S (1986) Nitrogen-15-determined nitrogen fixation in field-grown chickpea, lentil, fababean, and field pea. Agron J 78:654–660
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol 28:897–906
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability. Plant Physiol 156:989–996
Robson AD, O’Hara GW, Abbott LK (1981) Involvement of phosphorus in nitrogen-fixation by subterranean clover (Trifolium subterraneum L.). Aust J Plant Physiol 8:427–436
Rogers HH, Bingham GE, Cure JD, Smith JM, Surano KA (1983) Response of selected plant-species to elevated carbon-dioxide in the field. J Environ Qual 12:569–574
Rogers HH, Prior SA, Oneill EG (1992) Cotton root and rhizosphere responses to free-air CO2 enrichment. Crit Rev Plant Sci 11:251–263
Rogers HH, Prior SA, Runion GB, Mitchell RJ (1996) Root to shoot ratio of crops as influenced by CO2. Plant Soil 187:229–248
Rogers A, Ainsworth EA, Leakey ADB (2009) Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiol 151:1009–1016
Sa TM, Israel DW (1991) Energy status and functioning of phosphorus-deficient soybean nodules. Plant Physiol 97:928–935
SAS (1997) SAS user’s guide: statistics. Version 6.12. SAS Institute, Cary
Schortemeyer M, Atkin OK, McFarlane N, Evans JR (2002) N2 fixation by Acacia species increases under elevated atmospheric CO2. Plant Cell Environ 25:567–579
Sinclair TR (1992) Mineral nutrition and plant growth response to climate change. J Exp Bot 43:1141–1146
Srinivasarao C, Ganeshamurthy AN, Ali M, Venkateswarlu B (2006) Phosphorus and micronutrient nutrition of chickpea genotypes in a multi-nutrient-deficient typic ustochrept. J Plant Nutri 29:747–763
Srivastava AC, Pal M, Sengupta UK (2002) Changes in nitrogen metabolism of Vigna radiata in response to elevated CO2. Biol Plant 45:395–399
Steel RG, Torrie JH (1980) Principles and procedures of statistics: a biometrical approach, 2nd edn. McGraw-Hill, New York
Stöcklin J, Körner C (1999) Interactive effects of elevated CO2, P availability and legume presence on calcareous grassland: results of a glasshouse experiment. Funct Ecol 13:200–209
Stöcklin J, Schweizer K, Körner C (1998) Effects of elevated CO2 and phosphorus addition on productivity and community composition of intact monoliths from calcareous grassland. Oecologia 116:50–56
Tang C, Robson AD, Dilworth MJ (1990) A split-root experiment shows that iron is required for nodule initiation in Lupinus angustifolius L. New Phytol 115:61–67
Tang C, Han XZ, Qiao YF, Zheng SJ (2009) Phosphorus deficiency does not enhance proton release by roots of soybean [Glycine max (L.) Murr.]. Environ Exp Bot 67:228–234
Turner BL, Condron LM, Richardson SJ, Peltzer DA, Allison VJ (2007) Soil organic phosphorus transformations during pedogenesis. Ecosystems 10:1166–1181
Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197
von Felten S, Hättenschwiler S, Saurer M, Siegwolf R (2007) Carbon allocation in shoots of alpine treeline conifers in a CO2 enriched environment. Trees 21:283–294
Vu DT, Armstrong RD, Sale PWG, Tang C (2010) Phosphorus availability for three crop species as a function of soil type and fertilizer history. Plant Soil 337:497–510
West JB, HilleRisLambers J, Lee TD, Hobbie SE, Reich PB (2005) Legume species identity and soil nitrogen supply determine symbiotic nitrogen-fixation responses to elevated atmospheric CO2. New Phytol 167:523–530
Whitehead SJ, Caporn SJM, Press MC (1997) Effects of elevated CO2, nitrogen and phosphorus on the growth and photosynthesis of two upland perennials: Calluna vulgaris and Pteridium aquilinum. New Phytol 135:201–211
Wolf J (1996) Effects of nutrient supply (NPK) on spring wheat response to elevated atmospheric CO2. Plant Soil 185:113–123
Wouterlood M, Lambers H, Veneklaas EJ (2005) Plant phosphorus status has a limited influence on the concentration of phosphorus-mobilising carboxylates in the rhizosphere of chickpea. Funct Plant Biol 32:153–159
Yuen SH, Pollard AG (1954) Determination of nitrogen in agricultural materials by the Nessler Reagent. II. Micro-determination of plant tissue and soil extracts. J Sci Food Agric 5:364–369
Acknowledgment
We thank Dr. Clayton Butterly and anonymous reviewers for reviewing the manuscript. This research was supported by an Australian Research Council Linkage Project (LP100200757), and utilised the SOILFACE facilities at DPI Horsham which were developed with funding by the Victorian Department of Primary Industries (VDPI), the University of Melbourne (UM), the Grains Research and Development Corporation (GRDC), the Federal Department of Agriculture, Fisheries and Forestry (DAFF) and the Australian Greenhouse Office (AGO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Jin, J., Tang, C., Armstrong, R. et al. Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient vertisol. Plant Soil 358, 91–104 (2012). https://doi.org/10.1007/s11104-012-1270-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1270-z