Abstract
This study was carried out to investigate possible seawater intrusion into groundwater along the coastal lines of the Bafra Plain and salinity–alkalinity problems over land areas irrigated with water exposed to seawater intrusion were evaluated. For this purpose, 32 groundwater wells were selected over the plain, water samples were taken from these wells between October 2007 and September 2008, and chemical analyses were performed over these samples. Soil samples were taken from the fields irrigated with this water at 32 different locations at the end of the irrigation season in September 2008 from 0–30, 30–60, 60–90, and 90–120 cm soil depths and textures. EC, pH, Na, Ca, Mg, and K analyses were performed over these samples. Excessive seawater intrusion was observed in some parts of the plain and impacts of seawater intrusion decreased with the distance from the coastal line. It was determined that groundwater quality was significantly affected from seawater intrusion. Salinity and especially alkalinity problems were observed in land areas irrigated with this water and alkalinity increased with the rate of intrusion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The expansion of irrigated agriculture and the intensive agricultural activities induce the risk of groundwater quality degradation through high groundwater pumping rates and overexploitation of the aquifers, leading to seawater intrusion in the coastal aquifers. The use of poor quality water for irrigation reduces crop productivity and can alter soil physicochemical properties causing soil degradation (Tanji 1990; Oster 1994).
Soil salinization is the accumulation of free salts in the soil to such an extent that it leads to degradation of soils and vegetation. The increase in the amounts of saline soils is due mainly to irrigation with salty water and a disregard for principles of soil drainage. Other contributors to the problem are intrusion of saline water (Van-Camp et al. 2004) and evapotranspiration of saline soil moisture (Hernandez et al. 2004).
Use of highly saline effluent water and improper drainage and soil management increase the risk of salinity in irrigated soils. In many irrigation regions, rising saline groundwater interacting with the soils in the root zone can compound the problem (Rengasamy 2006).
In coastal regions, the uncontrolled exploitation of groundwater resources, intended for domestic, industrial, and agricultural purposes, unbalances the dynamic equilibrium between seawater and the flowing groundwater, favoring saltwater intrusion. Under such conditions, saltwater penetration may cause serious repercussions on both the prevailing environmental and economic conditions (Moujabber et al. 2006).
The pumped water from the wells was observed and controlled, especially in the coastal areas during the dry seasons, in order to monitor their salinity and the water table drawdown (Khair et. al. 1994).
Cemek et al. (2007) carried out a research over the Bafra Plain and observed salinity and alkalinity problems over the plain and recommended continuous monitoring of groundwater table and quality. Demir et al. (2009) investigated groundwater level and quality by indicator kriging over the plain and determined drainage problems in eastern parts of the plain.
In this study, the groundwater of salinity conditions and source of the salt in coastal areas were investigated. In addition, the groundwater in areas of irrigated soils with known EC, pH, and ESP values determined and the relationships between groundwater quality and soil electrical conductivity (EC), exchangeable sodium percentage (ESP), and pH values were put forward.
Material and methods
Description of the study area
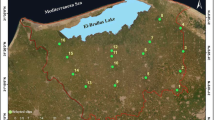
The study area lies on the Black Sea coastal region of Samsun (41º30′–41º 45′ latitude and 35 º 30′–36º 15′ longitude) in Northern Turkey (Fig. 1). The soils of the study area are composed of alluvial deposits at various elevations. The current climate in the region is semi-humid. Average temperature in July is 23.8°C and in January is 6.6°C. The annual average temperature is 14.4°C. The mean annual precipitation is 802.6 mm, most of which falls between September and April (Anonymous 2008).
The groundwater level and drainage greatly differ within the study area due to the differences in elevation, which slightly increases from the seaside and reaches to 10 m within approximately a 6-km distance. The study area has been under the conventional tillage system including the moldboard plough (about 20 cm depth) in fall, cultivator (about 15 cm depth) and disc harrow (about 10 cm depth), and under production of maize, peppers, watermelons, cucumbers, and tomatoes with sprinkler and furrow irrigations in the summer, and cabbage and leeks in the winter.
Groundwater–soil sampling and analyses
A network of 32 wells was installed in the Bafra Plain for groundwater quality. All samples were both filtered with a 0.45-μm filter as well as sealed in polyethylene bottles and stored at 4°C prior to analysis. Groundwater sampling was made from wells on a monthly basis between October 2007 and September 2008. Electrical conductivities (EC) of groundwater samples were measured in situ. The concentrations of major cations (Ca+2, Mg+2, K+, Na+) and anions (Cl−, SO −24 ) were determined by the ion chromatography technique (761 Compact IC Metrohm). The concentration of HCO3 was determined by titration.
Along with groundwater wells, 128 soil samples were collected at 32 different locations at the end of the irrigation season in September 2008. Four samples were taken from each sampling site from 0–30, 30–60, 60–90, and 90–120 cm depths of soil profile. The samples were air-dried, pass through a 2 mm sieve and analyzed for ESP, EC, and pH. Soluble salts were calculated from the measurement of EC in the soil extraction by the use of a conductivity meter (Rhoades 1982). Soil texture was determined according to the method Bouyoucos (1951) used. ESP was calculated from soluble salts. Soil pH was determined using a glass electrode pH meter (McLean 1982).
The percentages of seawater, soil salinity, exchangeable sodium percentage (ESP), and soil pH were used in ArcGIS 10.1 to visually analyze the spatial relationships among the variables. Groundwater level values were evaluated by making use of the analysis functions of GIS. Observation values were interpolated using the Inverse Distance Weight (IDW) method. IDW was conducted on the 32 points in the training set using the power of 2 and the neighborhood method. There are 15 neighbors included and have included at least 10 of them using the elliptical (quadrant) search window.
Results and discussion
Piezometric height
In Figure 2, the piezometric surfaces prevailing in the coastal alluvial aquifer for September 2008 is presented. Groundwater level in the research area was between 15 and 600 cm. Groundwater level in the northwest and southwest sections of study area was around 50 cm from sea level. Groundwater levels increased with increasing distances from the sea and decreasing distances to Kızılırmak River. Groundwater flow direction was along the northwest direction.
Groundwater quality and sea water intrusion
EC and Na+ play a vital role in suitability of water for irrigation. Higher salt content in irrigation water causes an increase in soil solution osmotic pressure (Thorne and Peterson 1954). The total concentrations of soluble salts in irrigation water can be classified as low (C1), medium (C2), high (C3) and very high (C4) salinity zones. These zones (C1 to C4) have EC less than 250 μS/cm, 250 to 750 μS/cm, 750 to 2,250 μS/cm, and more than 2,250 μS/cm, respectively (USSL 1954). Higher EC of water creates a saline soil. Another important chemical parameter for judging the degree of suitability of water for irrigation is sodium content or alkalinity hazard, which is expressed by the sodium adsorption ratio (SAR). The SAR is calculated by using the following equation (concentrations are expressed in meq/l);
The sodium hazard is expressed in terms of classification of irrigation water as low (S1: <10), medium (S2: 10 to 18), high (S3: 18 to 26), and very high (S4: > 26).
As it can be seen from Table 1, the annual average EC values vary between 1.86 and 10.47 dS/m, chlorine values between 9.65 and 90.26 meq/l, and sodium values between 10.52 and 107.22 meq/l. The SAR values are between 3.54 and 34.67. It was determined that excessive groundwater withdrawals caused an exposure of seawater intrusion and consequent groundwater salinization, and the EC–SAR values of groundwater wells were higher than the limit for irrigation water quality. Groundwater quality was seriously degraded in the Marathon-Nea Makri Plain from seawater intrusion due to over pumping and use of groundwater for irrigation, which in most cases could affect soil salinity and crop yields (Psychoyou et al. 2007).
When the relationships between the mixing percent of seawater and groundwater quality parameters (Table 2) were evaluated, correlations between the mixing percent of seawater and the EC, Na, HCO3, SO4, and SAR values were found to be significant at p < 0.01. Increasing seawater intrusion caused an increase in groundwater EC and SAR values.
There have been some methodologies to identify groundwater pollution or salinization by seawater intrusion like geophysical surveys, consecutive, or intermittent monitoring of groundwater chemistry (Jeen et al. 2001; Benkabbour et al. 2004; Sherif et al. 2006; Lee and Song 2007). Kim and Park (1998), Kurttaş (2002), Zakhem and Hafiz (2007), and Somay and Gemici (2009) determined freshwater–seawater mixing ratios using the value of Cl.
The freshwater–seawater mixing percentages can be determined mathematically by using freshwater and seawater concentrations as the end-members (Custodio 1987).
The lowest chloride content of water resources affecting groundwater within the study area was taken as the chloride content of freshwater. Therefore, chloride content of the Kızılırmak River effective in the Bafra Plain irrigations was taken into consideration regarding its calculations. Average chloride content of the river was 170 mg/l. The world average, 19,000 mg/l, was used as the chloride content of seawater.
Based on the conservative mass balance of the chloride concentration, theoretical mixing could be calculated by using the following equation:
where
- F :
-
mixing ratio expressed as the percentage of seawater (%)
- ClMIX :
-
chloride concentration in the mixed groundwater
- ClGW :
-
chloride concentration in fresh water (Kızılırmak River)
- ClSEA :
-
chloride concentration in seawater.
The range of variation in chemical parameters and ion concentrations of groundwater samples taken from October 2007 to September 2008 are given in Table 1.
It was determined that seawater mixture ratios in groundwater well samples varied between 0.45 % and 17.55 %. Mixing percentages of groundwater samples calculated based on chloride concentrations of samples taken from October 2007 to September 2008 are presented in Fig. 3. Maximum mixing percentage was found to be 15.07 %. These values indicate excessive seawater intrusion into the region. The sea water intrusion was presented in groundwater as far as 4 km inland. Significant variations were not observed in mixture rates during the year.
The level of ground water and the mixing percentage of seawater are seen to be a relationship between the opposites in Figs. 2 and 3. The mixing percentage of seawater was lower in areas close to the Kızılırmak River than the other areas and was higher along the coastal line where river effect diminished and decreased with distance from the coastal line. There is a recharge in these areas from the river as observed in Fig. 2. Therefore, there is a decrease in the mixing percentage of seawater in these areas.
A positive relationship was observed between seawater intrusion and the groundwater quality parameters. Increase in intrusion rates caused negative impacts on the groundwater quality parameters.
The effects of soil salinity and alkalinity of sea water intrusion
Statistical data about the research area are provided in Table 3. As it is seen from Table 3, sand contents of the soils vary between 3.85 % and 87.12 %, silt contents are between 3.38 % and 53.92 % and clay contents are between 5.13 % and 86.44 %. With regard to depths of soil layers, amount of sand increased with depth while amounts of clay decreased with depth.
Table 3 indicates that the EC values varied between 1.06 and 7.02 dSm−1. The average EC values were 2.41 dSm−1 for the 0–30 cm soil depth, 2.42 dSm−1 for 30–60 cm, 2.49 dSm−1 for 60–90 cm, and 2.43 dSm−1 for 90–120 cm. Salinity increased with the soil depth but the had little variation.
Salinity classes were determined in accordance with the Soil Survey Staff (1993), Janzen (1993), Smith and Doran (1996). Based on these classifications, soils with electrical conductivities between 0 and 0.98 dSm−1 were classified as non-saline, between 0.98 and 1.71 dSm−1 as very slightly saline, between 1.71 and 3.16 dSm−1 as slightly saline, between 3.16 and 6.08 dSm−1 as saline, and over 6.08 dSm−1 as very saline. Figure 4 shows that most of the soils at the 0–30 cm depth were classified as slightly saline, most of soils of 30–60 cm were classified as slightly saline, but some saline soils were also observed at the 30–60 cm soil layer. Similar classifications were also valid for 60–90 cm, mostly slightly saline and some saline soils were observed at this layer. The soil layer of 90–120 cm exhibited similar characteristics with its overlying layer.
Table 3 indicates that soil ESP values varied between 4.72 and 43.57. The average ESP values were 10.97 for the 0–30 cm soil depth, 14.26 for 30–60 cm, 15.14 for 60–90 cm, and 15.83 for 90–120 cm.
It is stated that ESP values over 6 create an alkalinity problem for soils. The soils with ESP values lower than 6 are classified as non-alkaline; the soils with ESP values between 6 and 10 are classified as alkaline, between 10 and 15 as very alkaline, between 15 and 25 as excessively alkaline, and over 25 as extremely alkaline (Isbell 1996).
Figure 5 shows that almost all soils of the research site have ESP values higher than 6. The ESP values were mostly between 10 and 15 for the 0–30 cm layer, between 15 and 25 for 30–60 cm, and an increased observation in areas with ESP values of 15–25 in the 30–60 cm layer. The number of soils with ESP of 15–25 increased also in the 60–90 cm layer, and soils with ESP values over 25 were also observed in this layer. The 90–120 cm layer had more areas having ESP values of 15–25 and over 25 than its overlying layer. It was determined that soil ESP values increased with increasing soil depth and were very alkaline; excessive alkalinity and even extreme alkalinity were observed over several parts of the research site. Alkalinity increased with increasing distances from the Kızılırmak River and decreasing distances from the coastal line. Mcmulen (2000) proposed the gypsum application rates according to the ESP values as 2–5 t/ha for 5 ≤ ESP ≤ 10 and 5 t/ha for ESP ≥ 10. The gypsum application should definitely be applied over the research site since the soil samples have very high ESP values.
As it can be seen from Table 3, pH values varied between 7.41–9.42. The average pH values were 7.86 for the 0–30 cm soil depth, 8.10 for 30–60 cm, 8.25 for 60–90 cm, and 8.29 for 90–120 cm. Soil pH values decreased with decreasing distances from the Kızılırmak River and increasing distances from the sea. An increase in pH values was observed with increasing soil depth.
When the pH map of the site was evaluated, it was observed that most of the site had pH values between 8.0–8.5 at the 0–30 and 30–60 cm depths (Fig 6). Increasing number of points with pH values between 8.50 and 9.0 were observed at the 60–90 cm soil depth and this increased continued throughout the layer of 90–120 cm. Most of the research site had pH values over the limits for plant growth; some points had pH values over 9.6. Values decreased with decreasing distances from the Kızılırmak River and increasing distance from the sea. An increase was also observed with increasing soil depths.
The Pearson correlation analysis was performed to determined the relationships among the electrical conductivity of the saturation extract (ECe), ESP and pH values of soils, which were irrigated by excessively saline groundwater due to sea water intrusion, with regard to soil layers and to determine the relationships of these values with groundwater quality parameters during the September 2008 period. Relationships between the groundwater quality parameters of September 2008 and soil characteristics are given in Table 4.
Significant correlations were observed between the groundwater SAR value and soil ESP value for 30–60 cm and 90–120 cm soil layers at p < 0.05 level and for 60–90 cm layer at p < 0.01 level. Also significant correlations were observed between the residual sodium carbonate (RSC) and ESP values for 0–30 cm and the 30–60 cm soil layers at the p < 0.05 level and for the 60–90 cm and 90–120 cm layers at the p < 0.01 level. The relationship between soil ESP and EC values was found to be significant for all soil depths at the p < 0.01 level. Significant correlations were found between soil ESP and pH values for the 0–60 cm depth at the p < 0.05 and for the 90–120 cm layer at the p < 0.01 level.
When the relationships between the soil pH value and quality parameters of groundwater and other soil characteristics were evaluated, significant relationships were observed between the groundwater RSC and SAR values for the 90–120 cm soil depth at the p < 0.05 level.
When the relationships between soil EC values and quality parameters of groundwater and other soil characteristics were evaluated, significant relationships were observed between the soil EC values and groundwater SAR values for the 60–90 and 90–120 cm soil depths at the p < 0.05 level. The relationship between soil EC and ESP values was found to be significant for all soil depths at the p < 0.01 level.
There is a significant relationship between the SAR values of irrigation water and sodium (Na+) absorption of soils. If water used for irrigation is high in sodium (Na+) and low in calcium, the cation change complex may become saturated with sodium (Na+). This can destroy the soil structure owing to dispersion of the clay particles (Todd 1980). Very high SAR values were observed in groundwater samples of the research site due to seawater intrusion and salinity, and especially very high ESP and pH values were observed over the lands irrigated with these waters.
Conclusions
In this study, possible seawater intrusion into groundwater along the coastal lines of the Bafra Plain was investigated; salinity–alkalinity problems regarding land areas irrigated with this water were evaluated and the relationships between the mixing percent of seawater and soil salinity–alkalinity were put forward.
Groundwater quality is seriously degradated in Bafra Plain from seawater intrusion due to overpumping. The use of groundwater for irrigation in most cases could affect soil salinity and crop yields.
Chloride values were used to determine mathematically the mixing percent of seawater into groundwater. Calculations revealed that the mixing percent of seawater varied between 3.82 % and 17.55 %. The mixing percent of seawater decreased with the decreasing distance to Kızılırmak River and increasing distances from the sea.
Excessive increases were observed in salinity and alkalinity of groundwater due to seawater intrusion. Close relationships were observed between seawater intrusion and the EC, Na, HCO3, SO4, and SAR values of groundwater, and quality of groundwater decreased with an increase of the mixing percent of seawater. The EC and Na values of groundwater wells with excessive seawater intrusion increased, and consequently, the SAR values increased accordingly.
It was observed that the entire research site had an ESP value over 6 and soil alkalinity existed throughout the research site. The ESP values increased along the soil depth; the values were between 15 and 25 at the 60–90 and 90–120 cm soil layers for most parts of the site. All these areas were classified as extremely alkaline.
When the pH values of the research site were evaluated, it was observed that values were way above the available levels for plants and increased throughout the soil depth. The soils of the research were generally slightly saline and some parts were saline and highly saline.
It was determined that seawater intrusion negatively affected the groundwater quality parameters, and the EC and SAR values of many wells were found to be beyond the allowable limits to be used in irrigation. It was observed that most parts of the soils had alkaline and pH values over the optimum values for plants.
A close relationship was observed between the SAR-RSC values of groundwater and the ESP values of soils irrigated with this water. Increasing SAR and RSC values of groundwater increased the ESP values of this water, and consequently, caused alkalinization of these soils. A close relationship was also determined between the ESP and pH values of soils and the pH values increased with increasing ESP values.
In this research, excessive alkalinity was seen among the soils of the research site. Reclamation of alkaline lands is a time-consuming, complex and costly process. Therefore, initially, a proper drainage system should be installed. Then, chemicals should be applied over alkaline lands for reclamation purposes. Alkalinity-resistant crops should be cultivated during the installation of the drainage systems and reclamation of alkaline lands. Another best proposal to prevent excessive alkalinization of lands is to immediately stop groundwater withdrawals.
References
Anonymous (2008). Samsun climatic data. State Meteorological Services (DMI): Ankara, Turkey.
Benkabbour, B., Toto, E. A., & Fakir, Y. (2004). Using DC resistivity method to characterize the geometry and the salinity of the Plioquaternary consolidated coastal aquifer of the Mamora plain, Morocco. Environmental Geology, 45, 518–526.
Bouyoucos, G. H. (1951). A recalibration of the hydrometer for making mechanical analysis of soils. Agronomy Journal, 43, 438.
Cemek, B., Güler, M., Kılıç, K., Demir, Y., & Arslan, H. (2007). Assessment of spatial variability in some soil properties as related to soil salinity and alkalinity in Bafra plain in northern Turkey. Environmental Monitoring and Assessment, 124, 223–234.
Custodio, E. (1987). Ground water problems in coastal areas. In: Studies and reports in hydrology (UNESCO).
Demir, Y., Erşahin, S., Güler, M., Cemek, B., Günal, H., & Arslan, H. (2009). Spatial variability of depth and salinity of groundwater under irrigated ustifluvents in the Middle Black Sea Region of Turkey. Environmental Monitoring and Assessment, 158, 279–294.
Hernandez, J. A., de Oro, N., & Ortiz, S. R. (2004). Electrolytic conductivity of semiarid soils (southeastern Spain) in relation to ion composition. Arid Land Research and Management, 18, 265–281.
Isbell, R. F. (1996). The Australian soil classification. Collingwood: CSIRO Australia.
Janzen, H. H. (1993). Soluble salts. In M. R. Carter (Ed.), Soil sampling and methods of analysis (pp. 161–166). Boca Raton: Canadian Society of Soil Science. Lewis Publ.
Jeen, S. W., Kim, J. M., Ko, K. S., Yum, B. W., & Chang, H. W. (2001). Hydrogeochemical characteristics of groundwater in a mid-western coastal aquifer system, Korea. Geosciences Journal, 5(4), 339–348.
Khair, K., Aker, N., Haddad, F., Jurdi, M., & Hachach, A. (1994). The environmental impacts of humans on groundwater in Lebanon. Water, Air, & Soil Pollution, 78(12), 37–49.
Kim, O., & Park, H. (1998). Variation of hydrochemical characteristic of major elements in groundwater by the seawater intrusion in the Byeonsan Peninsular, Korea. Geosystem Engineering, 1(2), 106–110.
Kurttaş, T. (2002). Karışım Sularında Kökensel Katkıların Belirlenmesi. Adana: Hidrolojide İzotop Tekniklerinin Kullanılması Sempozyumu, DSİ Genel Müdürlüğü.
Lee, J. Y., & Song, S. H. (2007). Evaluation of groundwater quality in coastal areas: implications for sustainable agriculture. Environmental Geology, 52, 1231–1242.
McLean, E.O. (1982). Soil pH and lime requirement. In Methods of soil analysis part II. Chemical and microbiological properties (pp. 199–224). Madison, WI, U.S.A.: ASA-SSSA.
McMulen, B. (2000). Soil pak for vegetable. Wagga Wagga: NSW Agriculture.
Moujabber, E.M., Samra, B., Darwısh, T., Atallah, T. (2006). Comparison of different indicators for groundwater contamination by seawater intrusion on the Lebanese coast. Water Resources Management, 20(2), 161–180.
Oster, J. D. (1994). Irrigation with poor quality water. Agricultural Water Management, 25(1994), 271–297.
Psychoyou, M., Mimides, T., Rizos, S., & Sgoubopoulou, A. (2007). Groundwater hydrochemistry at Balkan coastal plains—the case of Marathon of Attica, Greece. Desalination, 213(2007), 230–237.
Rengasamy, P. (2006). World salinization with emphasis on Australia. Journal of Experimental Botany, 57(5), 1017–1023.
Rhoades, J.D. (1982). Soluble salts. In Methods of soil analysis. Part II. Chemical and microbiological properties (pp. 167–179). Madison, WI, U.S.A.: ASA-SSSA.
US Salinity Laboratory Staff (1954). Diagnosis and improvement of saline and alkali soils. US Department of AgriculturalHand Book 60, Washington.
Sherif, M., El Mahmoudi, A., Garamoon, H., Kacimov, A., Akram, S., Ebraheem, A., & Shetty, A. (2006). Geoelectrical and hydrogeochemical studies for delineating seawater intrusion in the outlet of Wadi Ham, UAE. Environmental Geology, 49, 536–551.
Smith, J.L., Doran, J.W. (1996). Measurement and use of pH and electrical conductivity for soil quality analysis. p. 169–185. In: J.W. Doran and A.J. Jones (eds.) Methods for assessing soil quality. Soil Sci. Soc. Am. Spec. Publ. 49. SSSA, Madison, WI.
Soil Survey Staff (1993). Soil survey manual. United States Department of Agriculture. Hnbk no. 18. U.S. Gov. Printing Office, Washington, DC.
Somay, M. A., & Gemici, Ü. (2009). Assessment of the salinization process at the coastal area with hydrogeochemical tools and geographical ınformation systems (GIS): Selçuk Plain, Izmir, Turkey. Water, Air, and Soil Pollution, 201, 55–74.
Tanji, K. K. (1990). Agricultural salinity assessment and management, ASCE Manuals and Reports on Engineering Practice No. 71. New York: American Society of Civil Engineers.
Thorne, D. W., & Peterson, H. B. (1954). Irrigated soils. London: Constable and Company Limited.
Todd, D. K. (1980). Groundwater hydrology. Wiley International Edition. New York: Wiley.
Van-Camp, L., Bujarrabal, B., Gentile, A. R., Jones, R. J. A., Montanarella, L., Olazabal, C. (2004). Reports of the technical working groups established under the thematic strategy for soil protection. Volume II ERosion.EUR 21319 EN/2. Luxembourg; Office for Officical Publications of the European Communities.
Zakhem, B. A., & Hafez, R. (2007). Environmental isotope study of seawater intrusion in the coastal aquifer (Syria). Environmental Geology, 51, 1329–1339.
Acknowledgments
This study was supported by Ondokuz Mayıs University, Scientific Research Programs under the project no of PYO. ZRT. 1901.10.004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arslan, H., Demir, Y. Impacts of seawater intrusion on soil salinity and alkalinity in Bafra Plain, Turkey. Environ Monit Assess 185, 1027–1040 (2013). https://doi.org/10.1007/s10661-012-2611-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2611-3