Abstract
Arsenite oxidizing bacteria, isolated from industrial wastewater, showed high resistance against arsenite (40 mM) and other heavy metals (10 mM Pb; 8 mM Cd; 6 mM Cr; 10 mM Cu and 26.6 mM As5+). Bacterial isolates were characterized, on the basis of morphological, biochemical and 16S rRNA ribotyping, as Bacillus cereus (1.1S) and Acinetobacter junii (1.3S). The optimum temperature and pH for the growth of both strains were found to be 37 °C and 7. Both the strains showed maximum growth after 24 h of incubation. The predominant form of arsenite oxidase was extracellular in B. cereus while in A. junii both types of activities, intracellular and extracellular, were found. The extracellular aresenite oxidase activity was found to be 730 and 750 µM/m for B. cereus and A. junii, respectively. The arsenite oxidase from both bacterial strains showed maximum activity at 37 °C, pH 7 and enhanced in the presence of Zn2+. The presence of two protein bands with molecular weight of approximately 70 and 14 kDa in the presence of arsenic points out a possible role in arsenite oxidation. Arsenite oxidation potential of B. cereus and A. junii was determined up to 92 and 88 % in industrial wastewater after 6 days of incubation. The bacterial treated wastewater improved the growth of Vigna radiata as compared to the untreated wastewater. It indicates that these bacterial strains may find some potential applications in wastewater treatment systems to transform toxic arsenite into less toxic form, arsenate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a toxic heavy metal located in group V of the periodic table (Wackett et al. 2004). It’s a natural and ubiquitous element and released by different natural processes (e.g. weathering of rocks, land erosion, leaching, and volcanism). In 300 minerals, arsenic is present in association with sulphide ores and manganese or iron oxides (Brandstetter et al. 2000; Leist et al. 2000), like as arsenopyrite (FeAsS), realgar (AsS), enargite (Cu3AsS4) and orpiment (As2S3) (Donahoe et al. 2004). In anthropogenic processes, almost 90 % of all arsenic compounds come from wood preservatives [e.g. commonly chromated copper arsenate (CCA)] and remaining 10 % is coming from gold mining, smelting, glass manufacturing, pharmaceutical industries, electronics, chemical weapons, pigment production, rodent poisons, printing, tanning, metal alloys etc. and agricultural applications as pesticides, insecticides, herbicides, fungicides, soil sterilants, and silvicides (Campos et al. 2009).
Arsenic is 20th most abundant element in the earth’s crust that is released into the environment as toxic metalloid from natural and manmade activities as causes chronic diseases especially cancer in human beings (Bahara et al. 2012). Arsenic is mostly present in the form of arsenite and arsenate, former is more toxic and has high solubility than arsenate (Neff 1997). Both forms interfere with normal body function as arsenite has strong attraction for binding with sulfhydryl groups of cysteine and arsenate shows structural similarity with phosphate and disrupts the normal oxidative phosphorylation process (Ordonez et al. 2005).
Arsenic in As3+ oxidation state is more easily movable and more attractive for protein thiols or vicinal sulfhydryl groups of cysteine residues in proteins and causes endocrine disruption because of its property to bind to hormone receptors and resultant disruption of normal cell signaling that’s why it’s 100 times more toxic as compared to As5+ (Liu et al. 2001). The structures and chemical compositions of proteins, lipids, and DNA are changed by arsenite as it induces production of reactive oxygen species so generally it acts as carcinogenic (Liu et al. 2001).
Pakistan is under great threat of water pollution now a days due to lack of management to control industrial effluents, house hold sewage and saline drainage water fall into rivers and sea and this water contains large amount of heavy metals that are not environment friendly (Ghafoor et al. 1994; Ali et al. 2012).
There is a need to develop more advance methods that should be cheap, more effective and environmental friendly. The bioremediation approach which uses microorganisms because different microbes react to arsenic in different ways such as exclusion, chelation, immobilization, compartmentalization (Di Toppi and Gabbrielli 1999), methylation, reduction and oxidation to reduce, eliminate, or transform arsenic contaminants from soils, sediments, water and air. This process is also called microbial metabolism (Newman et al. 1998; Stools and Oremland 1999).
The present study aims at isolation and molecular characterization of arsenite resistant bacteria from industrial effluents and their potential use in wastewater treatment.
Materials and methods
Isolation and screening of arsenite resistant bacteria
Wastewater samples were taken in sterilized bottles from leather and pharmaceutical industries of Lahore, Pakistan. The physiochemical properties of samples were determined i.e. temperature, pH and color and arsenite concentration was also estimated from the wastewater samples. For bacterial isolation, 100 µL of each wastewater sample was spread on 100, 500, 1000, 1500, 2000, 2500, 3000, 3500 and 4000 µg/mL of arsenite supplemented in acetate minimal salt medium (MSM) (Pattanapipitpaisal et al. 2001).
Identification of arsenite resistant bacteria
For identification of arsenite resistant bacteria, morphological and biochemical tests, gram staining, spore staining, catalase, cytochrome oxidase, citrate utilization, motility, urease, triple sugar iron reaction, Voges–Proskauer test, indole test, methyl red, Eosin methylene blue agar, starch hydrolysis, MacConkey agar, mannitol salt agar and pigment production were performed according to Cappuccino and Sherman (2001). For molecular characterization selected bacterial isolate’s total DNA was extracted according to Sambrook et al. (2001). For 16S rRNA gene amplification universal primers i.e. RS1 (5-AAACTCAAATGAATTGACGG-3) and RS3 (5-ACGGGCGGTGTGTA-3) were used (Rehman et al. 2007). Amplification reactions were performed in 25 µL of distilled water containing 2.5 µL of each primer (20 pmol), 6 µL of genomic DNA (5 µg/mL) (Amersham Pharmacia, Piscataway, NJ, USA). PCR was performed by initial denaturation at 94 °C for 5 min, followed by 35 cycles at 94 °C for 1 min, annealing at 53 °C for 2 min and extension at 72 °C for 1 min, with a final extension at 72 °C for 10 min. Amplified products sequenced from Centre for Excellence in Molecular Biology (CEMB), results and their similarity analyzed by nucleotide BLAST database and submitted to GenBank to get accession numbers of the corresponding bacterial isolates.
Cross metal resistance
Arsenite resistant bacteria were also checked for their ability to resist other heavy metal ions. For this purpose various concentrations (100, 300, 500, 1000, 1500, 2000 and 2500 µg/mL) of different metal ions were used in minimal salt-broth medium i.e. arsenate (As5+), chromium (K2Cr2O7), lead [Pb(NO3)2] cadmium (CdCl2·H2O) and copper (CuSO4). The medium containing heavy metal ions was inoculated with 50 µL of bacterial cells growing in log phase and was incubated at 37 °C for 24–48 h.
Optimization of growth conditions
The most favorable temperature and pH for the growth of bacterial isolates were determined by growing 1 % of log phase growing bacterial cells at temperature i.e. 25, 30, 37 and 42 °C and pH i.e. 5, 6, 7, 8 and 9. Finally optical density was taken after 24 h at 600 nm.
Arsenite effect on the growth of bacterial isolates
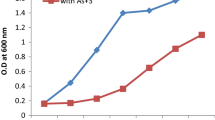
Arsenite effect on bacterial growth was determined by culturing the isolates in 1 mM (100 μg/mL) of NaH2AsO3, LB broth medium containing 1 % glucose. Growth was monitored from very start point of inoculation to after every 4–36 h by taking optical densities at 600 nm.
Determination of arsenite oxidase activity
Qualitative method (AgNO3 assay)
Acetate minimal agar supplemented with (1 mM) 100 µg/mL NaH2AsO3 after 48 h growth of bacterial isolates was flooded with 0.1 M silver nitrate solution and the result was noted according to Simeonova et al. (2004).
Quantitative methods
Safranine O spectrophotometric method
Acetate minimal medium supplemented with (1 mM) 100 µg/mL NaH2AsO3 was inoculated with bacterial isolates and without inoculation as a control for 24–96 h. Later on bacterial culture was centrifuged at 14,000 rpm for 10 min and supernatant used for extracellular enzyme activity. Bacterial cells were physically disrupted (sonicated 3 times for 15 s with an interval of 1 min); centrifuged at 14,000 rpm for 10 min and supernatant was used for intracellular enzyme activity.
According to Pasha and Narayana (2008) each sample was mixed with 1 mL of 2 % potassium iodate, 1 mL of 1 M hydrochloric acid, 0.5 mL of 0.02 % safranine O, volume made up to 100 mL with deionized water and gently shaked for 2 min. The pH was maintained at 4 by adding acetate buffer and absorbance was taken at 532 nm against reagent blank. The respective activities were compared with the culturing medium containing no arsenite in it. One unit of enzyme activity was defined as the amount of enzyme releasing arsenate equivalent to 1 μmol arsenite per minute under the assay condition.
Arsenate determination by molybdene blue
According to Lenoble et al. (2003) 4 mL of intracellular and extracellular enzyme was mixed separately with 200 µL of reagent A (dissolving 1390.5 g of (NH4)6Mo7O24·4H2O in 9 M H2SO4), 100 µL of reagent B (dissolving 109 0.5 g of ascorbic acid in 100 mL autoclaved distilled water) and 700 µL of deionized water. Optical density was measured at 870 nm after different time intervals (0, 5, 10, 15, 20, 25 and 30 min) and same procedure was followed in case of control.
SDS-polyacrylamide electrophoresis
According to Laemmli (1970) protocol SDS-PAGE was performed. Bacterial cells were grown in acetate minimal broth with NaH2AsO3 (1 mM) and without at 28 °C for 48 h. Medium was centrifuged at 14000 rpm for 10 min. The supernatant was mixed with 60 % of ammonium sulphate and was left at 4 °C for overnight, again centrifuged at 14,000 rpm for 10 min and lower bottom 3–4 mL residue was used as crude protein extract. A 40 µL of crude protein extract and 20 µL of loading dye were heat shocked at 100 °C for 5 min, then quickly transferred on ice for 5 min and centrifuged at 14000 rpm for 2 min. A 25 µL of supernatant was loaded in wells and electrophoresed for 2 h at 120 V. Later on gel was stained and destained according to Sambrook et al. (2001).
Amplification of arsenite oxidase gene
For amplification of arsenite oxidase gene degenerate primers aoxBM1-2F-(5-CCACTTCTGCATCGTGGGNTGYGGNTA-3) and aoxBM3-2R (5-TGTCGTTGCCCCAGATGADNCCYTTYTC-3) were used (Quéméneur et al. 2008). The reactions were performed in 50 µL of distilled water containing 5 µL of each primer (10 pmol), 6 µL of genomic DNA (5 µg/mL) (Amersham Pharmacia, Piscataway, NJ, USA). PCR was performed by initial denaturation at 94 °C for 5 min, followed by 34 cycles at 94 °C for 1 min (annealing temperature for 45 s, in first cycle decreases 1 °C on each cycle, from 55 °C until reaches to 46 °C and on 46 °C last 25 cycles were run) and extension was done on 72 °C for 1 min, with a final extension at 72 °C for 5 min (Jinbo et al. 2007).
Arsenite oxidation by bacterial isolates
Decrease in arsenite (As3+) concentration was determined in industrial wastewater by inoculating 3 % bacterial culture of 48 h grown in 1 L of industrial wastewater and two controls were used (autoclaved and un-autoclaved wastewater). Arsenite concentration was measured by calibration curve of standard arsenite according to Anderson et al. (1992).
Use of microbial treated wastewater for plant growth
Vigna radiata (mung beans) was cultivated in small pots using autoclaved soil. Three experimental pots were used; one was watered with wastewater treated with bacterial cells while two control pots were watered with tap water and untreated wastewater. The plants were grown for 10 days under 1:1 light and dark period and the growth was compared.
Statistical analysis
Observations were made and all the experiments run in triplicate. At least three separate flasks were usually maintained for one treatment. Each time three readings were taken, their mean, and standard error of the mean were calculated.
Results
Screening of arsenite resistant bacterial isolates
Wastewater sample’s physiochemical characteristics (Table 1) were present in ranged i.e. temperature (24–29 °C), pH (6–9) and color (grey to light blue). The arsenite concentration ranged between 1.23 ± 0.04 and 2.70 ± 0.03 µg/mL. Appearance of colonies number decreases as concentration of arsenite in MSM increases, only two colonies i.e. 1.1S and 1.3S appeared at 40 mM (3000 µg/ml) of arsenite and were selected for further research work. No growth was appeared at 45 mM (3500 µg/mL) and 50 mM (4000 µg/mL) of arsenite.
Morphological, biochemical and molecular characterization of bacterial strains
Morphological and biochemical characteristics of the bacterial isolates are given in Table 2. Both isolates (1.1S and 1.3S) observed as round shape cells. After blast (NCBI database), the nucleotide sequences coding for 16S rRNA gene were found homolog with Bacillus cereus (1.1S) and Acinetobacter junii (1.3S) and were submitted to NCBI database under the accession numbers of KF003021 and KF003019, respectively.
Metal resistance
Both strains have ability to tolerate other heavy metals i.e. Pb (10 mM), Cd (8 mM), Cr (6 mM), Cu (10 mM) and As5+ (26.6 mM).
Optimum growth conditions and arsenite effect on growth
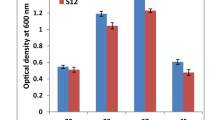
Both bacterial isolates showed optimal growth at 37 °C and pH of 7. The growth of both bacterial strains was increased with time and both showed maximum growth at 24 h (O.D. 1.121; B. cereus and O.D. 1.150; A. junii) in the presence of arsenite while in the absence of arsenite and in the presence of glucose both strains showed maximum growth at 24 h (O.D. 1.315; B. cereus and O.D. 1.321; A. junii) (Fig. 1).
Arsenite oxidase activity
AgNO3 assay
This assay results showed that both B. cereus and A. junii produced arsenite oxidase that converts highly toxic arsenite into less toxic arsenate as shown in Fig. 2.
By Safranine O method
Extracellular enzyme activity of B. cereus and A. junii was found to be 730 and 750 µM/m after 96 h growth which was more as compared to the intracellular enzyme activity 580 µM/m (B. cereus) and 630 µM/m (A. junii) to oxidize arsenite into arsenate.
By molybdenum blue method
Both B. cereus and A. junii were able to convert arsenite into arsenate i.e. 667 and 639 µg by extracellularly and 414 and 618 µg by intracellularly (Fig. 3).
SDS-PAGE analysis
SDS-PAGE was used to determine the molecular mass of extracellular arsenite oxidase which was induced by the presence of arsenite as compared to the control. The enzyme composed of two subunits protein i.e. 70 kDa as large subunit and 14 kDa as smaller subunit (Fig. 4).
Arsenite oxidase gene amplification
The arsenite oxidase gene was amplified by degenerate primers and a band of <450 bp was amplified in both bacterial strains indicating the presence of arsenite oxidase protein (Fig. 5).
Arsenite oxidation potential of bacterial isolates
The decrease in As3+ concentration in industrial wastewater by B. cerus and A. junii was measured after 3 days (82.8 and 79 %) and 6 days (92 and 88 %) of growth, respectively (Fig. 6).
Plant growth in microbial treated wastewater
There was a marked difference in the growth of plants watered with microbial treated wastewater and those plants which were watered with untreated wastewater (Fig. 7). All of the seeds sown in earlier case germinated well while in the latter case one or two seeds were germinated. The delayed growth in untreated wastewater is clearly shown in Fig. 5, indicating the potential role of bacterial strains in the conversion of toxic arsenite into less toxic arsenate.
Discussion
In biogeochemical cycling of arsenic, oxidation of arsenite by microbes is very beneficial. During arsenite oxidation in autotrophic microbes, arsenite donates electrons that act as the energy source for bacteria and carbon dioxide used in food synthesis process (Santini et al. 2000; Garcia-Dominguez et al. 2009) while heterotrophic bacteria transformed more toxic arsenite to less toxic arsenate by utilizing arsenite oxidase enzyme (Muller et al. 2003). In the present study, two bacteria resisting arsenite up to 3000 µg/mL or (40 mM) were isolated from tannery and pharmaceuticals industries of Lahore, Pakistan. Many researchers have already reported the isolation of arsenite resistant microorganisms from such environment (Duquesne et al. 2008; Rehman et al. 2010; Butt and Rehman 2011; Raja and Omine 2012).
Bacterial species (Acinetobacter, Aeromonas, Aureobacterium, Bacillus, Escherichia, Klebsiella, Micrococcus, Pseudomonas, Rhodococcus, Stenotrophomonas, Delftia, Arthrobacter, Microbacterium, Acidovorax, Ensifer, Janibacter, Janthinobacterium, Kocuria, Shewanella and Thauera) isolated from arsenic contaminated environment were highly resistant to arsenic (Anderson and Cook 2004; Jackson et al. 2005; Chang et al. 2007; Krumova et al. 2008). Arsenite oxidation character is present in both gram positive as well as gram negative bacteria but it is one of the established facts that gram positive bacteria are more tolerant than gram negative bacteria due to special metabolic characteristics and variations (Albarracin et al. 2008). Oremland and Stolz (2005) reported that arsenite oxidation ability is mostly present in gram negative bacteria while Mokashi and Paknikar (2002), Fisher and Hollibaugh (2008), Prasad et al. (2009), Cavalca et al. (2010) and Heinrich-Salmeron et al. (2011) reported only three genra from gram positive bacteria such as Bacillus, Arthrobacter and Microbacterium which have ability to transform arsenite into arsenate.
In the present study, heavy metals resistance by B. cereus and A. junii was shown in the following manner; As3+ > As5+ > Cu2+ > Pb2+ > Cd2+ > Cr6+. Raja and Omine (2012) reported the order of heavy metals resistance by Bacillus safensis in following order Zn2+ > Pb2+ > Cr6+ > Cu2+ > Cd2+. A large number of microorganisms are capable of growth in the presence of heavy metal ions and tolerate high concentrations as reported by Rehman et al. (2005). Bachate et al. (2013) reported the bacterial isolates that have ability to oxidize 50 mg/L of arsenite and reduce 15 mg/L of chromium.
The optimization experiments revealed that both bacterial strains showed maximum growth at 37 °C. The influence of temperature on arsenite oxidase production is related to the growth of the organism. Intracellular nature of the enzyme makes enzyme production dependent on the growth. Hence, the optimum temperature depends on whether the culture is mesophilic or thermophilic.
The pH of the medium is one of the important factors that determines the growth and morphology of microorganisms as they are sensitive to the concentration of hydrogen ions present in the medium. Both B. cereus and A. junii have shown maximum growth at pH 7 that is neutral pH. Arsenite oxidase producing bacterial strains exhibited maximum enzyme production at pH 7 (Butt and Rehman 2011; Raja and Omine 2012; Bachate et al. 2013). So the optimal pH of 6 and 7 for bacterial isolates is in good agreement with the reported literature.
It was observed that there was less growth (no. of cells) of all the bacterial isolates in MSM containing 100 µg/mL concentration of NaH2AsO3 as compared to the growth in salt medium containing 1 % glucose. Because all bacterial isolates showed maximum growth in the presence of glucose, which suggest that glucose serves as the more easily available energy source as it is easily metabolized and readily available to body cells for the growth of organisms (Butt and Rehman 2011). This indicates that both bacterial isolates prefer to utilize glucose as a simple carbon source as compared to the arsenite for growth. In the absence of glucose as a carbon source both bacterial isolates utilized arsenite for their growth (Fig. 1) and in literature it is reported that arsenite in the medium enhanced the production of arsenite oxidase (Butt and Rehman 2011).
In the present investigation, SDS-PAGE revealed two protein bands with molecular weight of approximately 70 and 14 kDa that may be involved in arsenite oxidation process. Same results were reported by Ellis et al. (2001), Conrads et al. (2002) and Stolz et al. (2006). Santini and Hoven (2004) reported that an enzyme called arsenite oxidase is induced in the presence of arsenite composed of two polypeptides, large catalytic subunit having molecular mass of 98 kDa and smaller subunit of 14 kDa. Arsenite oxidase is composed of two subunits of 88 and 14 kDa as reported by Anderson et al. (1992). Herminii monasarsenicoxydans contains protein of aoxB with molecular weight of 92 kDa (Koechler et al. 2010).
Many researchers have reported the presence of arsenite oxidase through amplification in different bacterial strains. Achour et al. (2007) reported a 530 bp band of aox fragment by using inverse PCR while Kashyap et al. (2006) obtained a product of 350 bp amplified arsenite oxidase in Agrobacterium tumefacien. Arsenite oxidase gene amplified product of 500 bp was reported by Wang et al. (2012) in Acinetobacter sp. and in the current study a 450 bp amplified product of arsenite oxidase gene was obtained. Similarly, a partial 435 bp arsenite oxidase gene aoxB was amplified from Agrobacterium sp. C13 (Jinbo et al. 2007).
The effect of bacterial treated industrial effluent on the growth of mung bean plants was monitored. Efficient plants growth was shown by bacterial treated wastewater as compared to the untreated wastewater that directly represents efficiency of bacterial arsenite oxidase. Firstly arsenite as start entry into the cell it is converted to arsenate by specific membrane bounded arsenite oxidase and if it gets the entry into the cell then again binding with specific periplasmic arsenite oxidase protein that transforms it into less toxic arsenate as reported by Mukhopadhyay et al. (2002), Silver and Phung (2005) and Mateos et al. (2006).
In the present study, both B. cereus and A. junii could tolerate As3+ up to 40 mM. The both arsenite oxidizing strains can transform more than 85 % of As3+ into As5+ from the industrial wastewater and bacterial treated wastewater can be at least used for irrigation purpose.
References
Achour AR, Bauda P, Billard P (2007) Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res Microbiol 158:128–137
Albarracin VH, Avila AL, Amoroso MJ, Abate CM (2008) Copper removal ability by Streptomyces strains with dissimilar growth patterns and endowed with cupric reductase activity. FEMS Microbiol Lett 288:141–148
Ali I, Hadi F, Bano A (2012) Microbial assisted phytoextraction of metals and growth of soybean (Glycine Max L. Merrill) on industrial waste water contaminated soil. Pak J Bot 44(5):1593–1599
Anderson CR, Cook GM (2004) Isolation and characterization of arsenate-reducing bacteria from arsenic contaminated sites in New Zealand. Curr Microbiol 48:341–347
Anderson GL, Williams J, Hille R (1992) The purification and characterization of arsenite oxidase from Alcaligenes faecalis, a molybdenum-containing hydroxylase. J Biol Chem 267:23674–23682
Bachate SP, Nandre VS, Ghatpande NS, Kodam KM (2013) Simultaneous reduction of Cr(VI) and oxidation of As(III) by Bacillus firmus TE7 isolated from tannery effluent. Chemosphere 90:2273–2278
Bahara MM, Megharaja M, Naidua R (2012) Kinetics of arsenite oxidation by Variovorax sp. MM-1 isolated from a soil and identification of arsenite oxidase gene. J Hazard Mater S0304-3894(12)01182-X
Brandstetter A, Lombi E, Wenzel WW, Adriano DC (2000) Arsenic-contaminated soils: I. Risk assessment. In: Wise DL, Trantolo DJ, Cichon EJ, Inyang HI, Stottmeister U (eds) Remediation engineering of contaminated soils. Dekker, New York, p 715
Butt AS, Rehman A (2011) Isolation of arsenite-oxidizing bacteria from industrial effluents and their potential use in wastewater treatment. World J Microbiol Biotechnol 27:2435–2441
Campos VL, Escalante G, Yañez J, Zaror CA, Mondaca MA (2009) Isolation of arsenite-oxidizing bacteria from a natural biofilm associated to volcanic rocks of Atacama Desert, Chile. J Basic Microbiol 49:S93–S97
Cappuccino JG, Sherman N (2001) Microbiology: a laboratory manual, 6th edn. Pearson Education, Benjamin Cummings, San Francisco
Cavalca L, Zanchi R, Corsini A, Colombo M, Romagnoli C, Canzi E, Andreoni V (2010) Arsenic-resistant bacteria associated with roots of the wild Cirsium arvense (L.) plant from an arsenic polluted soil, and screening of potential plant growth-promoting characteristics. Syst Appl Microbiol 33:154–164
Chang J-S, Yoon I-H, Kim K-W (2007) Isolation and ars detoxification of arsenite-oxidizing bacteria from abandoned arsenic-contaminated mines. J Microbiol Biotechnol 17:812–821
Conrads T, Hemann C, George GN, Pickering IJ, Prince RC, Hille R (2002) The active site of arsenite oxidase from Alcaligenes faecalis. J Am Chem Soc 124(38):11276–11277
Di Toppi LS, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Donahoe J, D’Imperio S, Jackson C, Inskeep W, McDermott T (2004) Arsenite-oxidizing Hydrogenobaculum strain isolated from an acid-sulfate-chloride geothermal spring in Yellowstone National Park. Appl Environ Microbiol 70:1865–1868
Duquesne K, Lieutaud A, Ratouchniak J, Muller D, Lett M-C, Bonnefoy V (2008) Arsenic oxidation by a chemoautotrophic moderately acidophilic Thiomonas sp.: from the strain isolation to the gene study. Environ Microbiol 10(1):228–237
Ellis PJ, Conrads R, Hille R, Kuhn P (2001) Crystal structure of the 100 kDa arsenite oxidase from Alcaligenes faecalis in two crystal forms at 1.64 Å and 2.03 Å. Structure 9:125–132
Fisher JC, Hollibaugh JT (2008) Selenate-dependent anaerobic arsenite oxidation by a bacterium from Mono Lake California. Appl Environ Microbiol 74:2588–2594
Garcia-Dominguez E, Mumford A, Rhine ED, Paschal A, Young LY (2009) Novel autotrophic arsenite-oxidizing bacteria isolated from soil and sediments. FEMS Microbiol Ecol 66:401–410
Ghafoor A, Rauf A, Arif M, Muzaffar W (1994) Chemical composition of effluents from different industries of the Faisalabad. Pak J Agric Sci 33:73–74
Heinrich-Salmeron A, Cordi A, Brochier-Armanet C, Halter D, Pagnout C, Abbaszadeh-fard E, Montaut D, Seby F, Bertin PN, Bauda P, Arsène-Ploetze F (2011) Unsuspected diversity of arsenite-oxidizing bacteria revealed by a widespread distribution of the aoxB gene in prokaryotes. Appl Environ Microbiol 77:4685–4692
Jackson CR, Harrison KG, Dugas SL (2005) Enumeration and characterization of culture able arsenate resistant bacteria in a large estuary. Syst Appl Microbiol 28(8):727–734
Jinbo X, Wenming W, Haoxin F, Gejiao W (2007) Arsenite-oxidizing Agrobacterium and arsenic resistant microorganisms of a Chinese coal mine ecosystem1. State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, http://www.paper.edu.cn
Kashyap DR, Botero LM, Lehr C, Hassett DJ, McDermott TR (2006) A Na+:H+ antiporter and a molybdate transporter are essential for arsenite oxidation in Agrobacterium tumefaciens. J Bacteriol 188:1577–1584
Koechler S, Cleiss-Arnold J, Proux C, Sismeiro O, Dillies M-A, Goulhen-Chollet F, Hommais F, Lièvremont D, Arsène-Ploetze F, Coppée J-Y, Bertin PN (2010) Multiple controls affect arsenite oxidase gene expression in Herminii monasarsenicoxydans. BMC Microbiol 10:53
Krumova K, Nikolovska M, Groudeva V (2008) Isolation and identification of arsenic-transforming bacteria from arsenic contaminated sites in Bulgaria. Biotechnol Biotechnol Equip 22:721–728
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriaphage T4. Nature 227:680–685
Leist M, Casey RJ, Caridi D (2000) The management of arsenic wastes: problems and prospects. J Hazard Mater 76:125–138
Lenoble V, Deluchat V, Serpaud B, Bollinger J-C (2003) Arsenite oxidation and arsenate determination by the molybdene blue method. Talanta 61:267–276
Liu SX, Athar M, Lippai I, Waldren C, Hei TK (2001) Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc Natl Acad Sci USA 98:1643–1648
Mateos LM, Ordonez E, Letek M, Gil JA (2006) Corynebacterium glutamicumas a model bacterium for the bioremediation of arsenic. Int Microbiol Off J Span Soc Microbiol 9:207–215
Mokashi SA, Paknikar KM (2002) Arsenic (III) oxidizing Microbacterium lacticum and its use in the treatment of arsenic contaminated groundwater. Lett Appl Microbiol 34:258–262
Mukhopadhyay R, Rosen BP, Phung LT, Silver S (2002) Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol Rev 26:311–325
Muller D, Lievremont D, Simeonova DD, Hubert JC, Lett MC (2003) Arsenite oxidase aox genes from a metal-resistant beta proteobacterium. J Bacteriol 185:135–141
Neff JM (1997) Ecotoxicology of arsenic in the marine environment. Environ Toxicol Chem 16:917–927
Newman DK, Ahmann D, Morel FM (1998) A brief review of microbial arsenate respiration. Geomicrobiology 15:255–268
Ordonez E, Letek M, Valbuena N, Gil JA, Mateos LM (2005) Analysis of genes involved in arsenic resistence in Corynobacterium glutamicumATCC1303. Appl Environ Microbiol 71:6206–6215
Oremland RS, Stolz JF (2005) Arsenic, microbes and contaminated aquifers. Trends Microbiol 13:45–49
Pasha C, Narayana B (2008) Determination of arsenic in environmental and biological samples using toluidine blue or safranine O by simple spectrophotometric method. Bull Environ Contam Toxicol 81:47–51
Pattanapipitpaisal P, Brown NL, Macaskie LE (2001) Chromate reduction and 16S rRNA identification of bacteria isolated from a Cr(VI)-contaminated site. Appl Microbiol Biotechnol 57:257–261
Prasad KS, Subramanian V, Paul J (2009) Purification and characterization of arsenite oxidase from Arthrobacter sp. Biometals 22:711–721
Quéméneur M, Heinrich-Salmeron A, Muller D, Lie`vremont D, Jauzein M, Bertin PN, Garrido F, Joulian C (2008) Diversity surveys and evolutionary relationships of aoxB genes in Aaerobic arsenite-oxidizing bacteria. Appl Environ Microbiol 74:4567–4573
Raja CE, Omine R (2012) Arsenic, boron and salt resistant Bacillus safensis MS11 isolated from Mongolia desert soil. Afri J Biotechnol 11(9):2267–2275
Rehman A, Ashraf S, Qazi JI, Shakoori AR (2005) Uptake of lead by a ciliate Stylonychia mytilus, isolated from industrial effluents: potential use in bioremediation of wastewater. Bull Environ Contam Toxicol 75:290–296
Rehman A, Ali A, Muneer B, Shakoori AR (2007) Resistance and biosorption of mercury by bacteria isolated from industrial effluents. Pak J Zool 39(3):137–146
Rehman A, Butt AS, Hasnain S (2010) Isolation and characterization of arsenite oxidizing Pseudomonas lubricansand its potential use in bioremediation of waste water. Afr J Biotechnol 9(10):1493–1498
Sambrook J, MacCallum P, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Santini JM, Hoven RNV (2004) Molybdenum-containing arsenite oxidase of the chemolithoautotrophic arsenite oxidizer NT-26. J Bacteriaology 186:1614–1619
Santini JM, Sly LI, Schnagl RD, Macy JM (2000) A new chemolithotrophic arsenite-oxidising bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl Environ Microbiol 66:92–97
Silver S, Phung LT (2005) A bacterial view of the periodic table: genes and proteins for toxic inorganic ions. J Ind Microbiol Biotechnol 32:587–605
Simeonova D, Lievremont D, Lagarde F, Muller D, Groudeva V, Lett M-C (2004) Microplate screening assay for detection of arsenite oxidizing and arsenate-reducing bacteria. FEMS Microbiol Lett 237:249–253
Stolz JF, Basu P, Santini JM, Oremland RS (2006) Arsenic and selenium in microbial metabolism. Annu Rev Microbiol 60:107–130
Stools JF, Oremland RS (1999) Bacterial respiration of arsenic and selenium. FEMS Microbiol Rev 23(5):615–627
Wackett LP, Dodge AG, Ellis LBM (2004) Microbial genomics and then periodic table. Appl Environ Microbiol 70:647–655
Wang X, Rathinasabapathi B, de Oliveira L, Guilherme L, Ma L (2012) Bacteria-mediated arsenic oxidation and reduction in the growth media of arsenic hyperaccumulator Pteris vittata. Environ Sci Technol 46:11259–11266
Acknowledgments
This work was supported by the research Grant No. 20/1373/R&D/10 from Higher Education Commission (HEC), Islamabad Pakistan which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naureen, A., Rehman, A. Arsenite oxidizing multiple metal resistant bacteria isolated from industrial effluent: their potential use in wastewater treatment. World J Microbiol Biotechnol 32, 133 (2016). https://doi.org/10.1007/s11274-016-2079-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-016-2079-3