Abstract
The present study deals with the cultivation of Trichoderma sp. for the production of cellulase in lignocellulosic materials. The highest cellulase activity was determined under optimal conditions of pH (5.0), incubation temperature (30 °C), inocula concentration (2 x 108 spores mL-1) and particle size (500 μm). Maximum activity for raw palm kernel cake (PKC), defatted PKC, and vegetable waste (VW) substrates were achieved as 6.9 FPU g−1, 16.1 FPU g−1 and 50.1 FPU g−1 correspondingly. It was observed that defatted PKC served as a better substrate than raw PKC for cellulase activity. A comparative study for the production of enzymes via solid state fermentation (SSF) indicated that cellulase activity produced by Trichoderma was about 1-fold higher in PKCs. However, Bacillus cereus scored 2-folds higher activity than VW substrates. On the basis of the significant yield of cellulase activity, palm oil industry wastes can be successfully used for the production of cellulase. Thus, environmental pollution can be controlled by utilizing palm oil industrial wastes to generate value-added product (cellulase).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The enzymatic degradation of plant polysaccharides has been widely used in industry-related purposes, including paper, food and feed industry, as well as providing sustainable production of fuels and chemicals (Abu-Sharar et al. 2012; Jain et al. 2016; Kuhad et al. 2011). The main components of plant cell wall are polysaccharides, cellulose, hemicelluloses and pectin (Caffall and Mohnen 2009; Kuhad et al. 2011). Cellulose is a β-(1, 4)-D-glucan that exists in the cell wall of plants and strongly coordinated with hemicelluloses and lignin (Medve 1997; Ramos 1992). Owing to these characteristics, lignocellulosic materials are generally used for the production of biomolecules, liquid fuels, and chemical supplies. Therefore, it is critical to develop eco-friendly methods that are able to weaken the polymeric associations through biological processes such as enzymatic hydrolysis and fermentation (Al-Gheethi and Ismail 2014; Medve 1997; Ramos 2003). Lignocellulose is one of the most abundant feed stock raw materials for the fermentation process. The main product of lignocellulose fermentation is fermentable sugars, which can be easily utilized as the source of carbon by several microorganisms (Jørgensen et al. 2007; Sola et al. 2013). Fermentable sugars can be produced via solid state fermentation process using fungi in its natural habitat (Ibrahim 2008). Thus, by applying the solid state fermentation method, the cellulosic industrial waste can be utilized for the production of cellulase. The ability of the fungi to provide a set of carbohydrate-active enzymes has made it an efficient potential biomass for degradation of polysaccharides (Srivastava and Sharma 2014; Van and De Vries 2011). Filamentous fungal strains, such as Trichoderma sp., are wood-degrading organisms that release a large quantity of cellulase and hemicellulase. There are three major components involved in the production of cellulase using fungi (endo glucanases) which have the ability to break down the internal glycosidic linkages; cellobio hydrolases that generate cellobiose from cellulose chain ends and β-glucosidases that produce glucose from the conversion of cellobiose. Other species, such as Trichoderma reesei, also produces cellulases and hemicellulases in a significant amount which can frequently be used for enzymatic saccharification of lignocellulosic materials. Among all wood-degrading fungi, T. reesei was studied as one of the most successful cellulases secreting species (Goyal et al. 1991; Shafawati and Siddiquee 2013; Teeri et al. 1998). Besides the degradation of polysaccharides, Trichoderma can also be used for the removal of organic and inorganic pollutants in industrial wastewaters (Al-Khashman 2009; Efaq et al. 2015; Keesari et al. 2015; Nik Norulaini Nik Ab Rahman et al. 2016; Nik Norulaini Nik Abd Rahman et al. 2014).
The extraction of oil from the palm oil industry generates a huge amount of palm kernel cake and vegetable wastes which have become a serious issue of environmental pollutions (Shafawati and Siddiquee 2013; Wong and Zahari 2011). The problem generated by these forms of waste disposal can be solved by transforming these wastes to value added products. Many studies related to the utilization of waste from the palm oil industry have been conducted worldwide (Ali and Sandi 2014; Sudiyani et al. 2013). However, the characteristics of the substrates used for the conversion of cellulose into fermentable sugars have remained unclear. Among the most influential characteristics are substrate accessibility, the degree of crystallinity, the degree of polymerisation as well as the distribution and composition of lignin (Palonen et al. 2004; Ramos et al. 1999; Teeri et al. 1998).
In this context, attention has been paid to utilize the palm oil industry waste for the production of cellulose via enzymatic fermentation. The fermentation process for the generation of animal feed was performed using PKC, which is a suitable source due to its high protein content (Mansour and Salem 2015; Wan et al. 2013; Wong and Wan Zahari 1992). A number of microbial isolates were applied during the conversion of PKC into the protein-rich feed. Some of the potential isolates included: T. koninggi, T. viride, A. niger and A. terreus. In spite of this, PKC can also be used as a substrate or a carbon source for the growth of fungal (Koyani et al. 2011; Moslim and Kamarudin 2014). The main aim of this study was to determine the cellulase activity of Trichoderma sp. using different particle sizes of PKC and vegetable wastes. In addition, other parameters such as the effect of pH, temperature, and inocula concentrations were examined for the optimal reaction of Trichoderma on the substrates. A comparison was also made by using Bacillus cereus as a cellulase producer.
2 Materials and Methods
2.1 Isolation and Identification of Trichoderma Sp.
The isolation of Trichoderma was carried out by using the same method described by Tengku Norsalwani (Norsalwani and Norulaini 2012; 2014). In this method, the sterilized chip of the oil palm wood was immersed into the commercially available agricultural fertilizer (Pro-Fil). It was cultured in a nutrient agar for seven days at room temperature (25 ± 2 °C), in the presence of white light. A portion of the seven-day cultured medium (wood chip) was transferred to a freshly prepared vegetable agar media (30 % w/w) and underwent an incubation period of five days at 30 ± 2 °C. 30 % vegetable agar (V8) was prepared by adding agar powder (Merck) with mixed vegetable juice (consisting of a vegetable juice blend with concentrated juices of tomatoes, carrots, celery, beets, parsley, lettuce, watercress and spinach). A small portion (2 mm3) of the culture was sub-cultured in a freshly prepared V8 media to maintain its viability. The identification of the cultured fungal medium was carried out on the basis of colonial morphology and Scanning Electron Microscopy (SEM) analysis (Bushra et al. 2015; Nabi et al. 2011; Shahadat et al. 2012).
2.2 Sample Preparation
A sample of PKC was obtained from the palm oil industry. One portion of the sample was immediately stored at 4 °C in a refrigerator for further use. Another portion of PKC (labeled as defatted) was prepared by applying soxhlet oil-extraction technique using hexane (as a solvent with hexane) for 8 h in order to remove the residual oil inside PKC (Khosrokhavar et al. 2014; Philippi et al. 2016). The solvent of the sample was evaporated using a rotary evaporator, and the defatted PKC was kept at 4 °C for further use. Meanwhile, fresh vegetable wastes were collected from a local market in Penang, Malaysia. The vegetables were dried at 60 °C in an oven for 24 h. The raw PKC, defatted PKC and dried vegetables were ground and screened in a sieve shaker to obtain mesh sizes of three ranges: (1) ≤ 250 μm, (2) > 250 μm to ≤500 μm and (3) > 500 μm to ≤1 mm, respectively.
2.3 Cultivation of Fungal Spore
The broth for the cultivation of fungi was prepared by mixing 163 mL of V8 solution and 380 mL sterile distilled water into 500 mL Erlenmeyer flask. The pH of the media was adjusted to pH 5.0 and autoclaved at 121 °C, 0.1 MPa for 15 min. About one block (1 cm × 1 cm) of Trichoderma sp. was cut from the periphery of the culture (the most activated growth part) that was inoculated into a sterile liquid of V8 media. The liquid was further incubated (5 days at 30 °C, 150 rpm) on a rotary shaker. The incubated broth and its biomass were collected by filtration. Collected biomass was rinsed with distilled water to remove the impurities. Finally, haemocytometer was employed to determine the conidia.
2.4 Fermentation and Extraction of Fungi
The fermentation of the fungus was performed by using Mandel’s medium. The Mandel’s medium was formulated with a fixed amount (g) of each salt in g L−1: (1) urea, 0.3; (2) peptone, 0.75; (3) yeast extract, 0.25; (4) (NH4)2SO4, 1.4; (5) KH2PO4, 2.0; (6) CaCl2, 0.3; (7) MgSO4.7H2O, 0.3; and trace elements (mg L−1): (1) FeSO4.7H2O, 5; (2) MnSO4. 4H2O, 1.6; (3) ZnSO4.7H2O, 1.4 and (4) CoCl2.6H2O, 20.0 (Ryu and Mandels 1980). The Mandel’s medium, the trace elements, and the PKC substrates were autoclaved separately at 1.03 × 10−5 Pa, 121 °C for 15 min. The flasks were cooled down at room temperature (25 ± 2 °C) and a fixed amount of Mandel’s medium (2 mL) and sterilized trace element (1 mL) were added to the substrate (5 g). After that, the PKC substrates were inoculated with different amounts of fungi filtrates: (1) 2x108, (2) 2x107, (3) 2x106 and (4) 2x105 spores mL−1 in the inocula filtrates while the control was prepared without fungi inoculation. Entire samples and controls were incubated for five days at an ambient temperature. The same procedure was also used for the vegetable waste.
For the extraction of the enzyme, a small portion of distilled water (20 mL) was added to each flask containing fermented PKCs. All the flasks were vigorously shaken for 30 min at 200 rpm. The biomass of the suspension was separated by using filter paper (Whatman No.1). The supernatant was employed as a source of crude enzyme. The procedure was repeated with a different treated raw and defatted PKC as well as vegetable waste.
2.5 Evaluation of Enzyme Activity of Different Assay at Different Conditions of pH and Temperature
The enzymatic activity of the fungi was determined using filter paper assay tests for the saccharifying cellulose (Ghose 1987; Ryu and Mandels 1980). In this experiment, 1 mL of 0.05 M citrate buffer of varying pH, 3.0, 4.0, 5.0, 5.5, 6.0 and 7.0 (warmed at 40 °C) were added to the test tube containing filter paper (1 cm × 6 cm) as a source of cellulose. A fixed volume (0.5 mL) of sample solution (supernatant) was added to each citrate buffer solution. The solution was mixed thoroughly and heated at 60 °C for 60 min (using a water-bath). A fixed volume of DNS solution (3 mL) was added to stop any enzymatic reaction. The test tubes were again boiled to develop color and cooled at 25 ± 2 °C. The absorbance of all the solutions was determined at 540 nm, and the activity of the enzyme was calculated in terms of FPU mL−1.
2.6 Statistical Analysis Using ANOVA
All the data were expressed as mean ± standard deviation (SD) for at least triplicate analyses on the same sample. The data were analyzed using one-way analysis of variance (ANOVA) with 95 % confidence intervals using Minitab 15 software.
3 Results and Discussion
The production of cellulase from the fungal spores (Fig. 1) is known as one of the most powerful enzymes for cellulose hydrolysis (Beldman et al. 1985). Therefore, attention has been paid to produce cellulose via enzymatic hydrolysis from the renewable source by recycling waste materials. The production of cellulose from the fungal enzymatic reaction depends on a number of factors such as the substrate, substrate concentration, temperature, pH, and incubation period (Nataraja et al. 2010). Generally, enzymes demonstrate maximum activity under the range of different pH (3–7) and temperature (40–60 °C) values.
3.1 The Effect of pH on Cellulase Activity
The pH of a medium plays an important role in the production of a cellulase that uses a natural catalyst (fungi). It is inferred in Fig. 2 that pH = 5 is found to be the optimal pH for cellulase assay using Trichoderma sp. as it provides a significant stability to the enzymes (Latifian et al. 2007; Nataraja et al. 2010). Owing to this noticeable activity, Trichoderma sp. has been successfully used for enzymatic saccharification of lignocellulosic materials (Fujii et al. 2009). Under optimal conditions of pH and temperature, these enzymes can easily break down cellulose to yield glucose.
3.2 The Effect of Temperature
Besides pH, the temperature of the medium also affects the activity of an enzyme. The best temperature for the enzyme activity was at 50 °C which is achieved at about 5 FPU g−1 efficiency of Trichoderma sp. (Fig. 3). Incubation at higher temperature reflected lower enzyme activity. An enzyme is a secondary metabolite produced during the exponential growth phase and the incubation at high temperature can lead to poor growth. This resulted in a reduction in enzyme yield (Sabu et al. 2002). The optimum growth temperature for Trichoderma sp. was considered at 30 °C (Norsalwani and Norulaini 2012), while the optimum temperature for maximum cellulase activity was found at 50 °C. Similar results were also observed during the evaluation of cellulase activity by Trichoderma reesei at 50 °C (Latifian et al. 2007). The effect of temperature on the cellulose activity of enzyme in the crude extract of fungi derived from solid waste showed that most of the cellulase enzymes demonstrated optimal activity at 55 °C (Bradley et al. 1982; Chandra et al. 2010; Lu et al. 2003). Previous studies on Aspergillus and Trichoderma sp. suggested that strain variations of the microorganisms affect the optimal temperature for cellulase production (Gautam et al. 2011).
3.3 Effect of Concentration
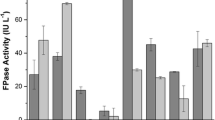
The effect of inoculum concentration is studied to examine the maximum activity of cellulose (Fig. 4a–c). It is anticipated that the inoculum in the form of spores will eventually germinate and proliferate into the mycelial mass of the fungus. The cellulase is produced by boosting the mycelial mass. Hence, a larger mass boosts up the cellulase excretion. By increasing the inoculum concentration from 2 x 105 to 2 x 107 spores mL−1, a small change in the cellulase activity was observed. The concentration was increased up to 2 x 108 spores mL−1 where the enzyme production became maximized. It was revealed that the increase in the inoculum concentration also increased the number of fungal spores as it enhanced the production of the enzyme as well as the growth of the fungal biomass (Zhang and Sang 2012). The enhancement of cellulase activity by Trichoderma sp. in both raw and defatted PKC was about 1-fold while the vegetable waste demonstrated the highest cellulose activity (2-folds). Thus, the increment in inoculum concentration results in a positive effect on cellular activity as shown in Fig. 4a–c. Lower inoculum concentration requires a longer time for the cell germination to proliferate into a vegetative biomass of fungi which induces the cellulase production and utilizes the substrate. Decrease in enzyme production is due to the concurrent depletion of nutrients which also recede the fungal mycelia growth and its metabolic activities (Kashyap et al. 2002). A synergic balance between the proliferating biomass and available nutrient yields an optimal activity of enzyme synthesis (Ramachandran et al. 2004).
3.4 Effect of Particle Size
The activity of cellulose mainly depends on the efficiency of exo and endo-splitting glucanases with regards to time. However, the effect of substrate particle size can be determined by examining their effects on the saccharification of cellulose. As shown in Figs. 4a–c, the highest enzyme activity (2-fold) of all three samples, i.e., (a) vegetable waste, (b) defatted PKC, and (c) raw PKC, are observed by using 500 μm inocula of vegetable waste followed by 250 μm and 1 mm, respectively (Goyal et al. 1991). Similar trends were also achieved using defatted PKC and raw PKC which resulted in lower enzyme activity (1-fold) compared to vegetable waste (Figs. 4b and c). Thus, the substrate particle size positively affects the cellulase activity. The reduction of substrate particle size makes it readily usable for the fungi because of decreasing crystallinity and degree of polymerisation, the increasing surface area as well as the bulk density of the raw materials. As a result, more cellulase is produced to break down the cellulosic compounds (Awafo et al. 2000).
It was also scrutinized that small size particles of palm oil empty fruit bunches (400 μm) produced high cellulase activity due to the larger specific surface area in fine particles but low porosity property (Tao et al. 1997). Lower porosity may cause less penetration of the fungal hypha into the pores of the substrate, so fungal growth can only be observed on the surface of the substrate. By using larger substrate particle size (> 400 μm) during fermentation, a network of aerial hypha grows into the inter-particle space for low fungal growth on the surface of substrate particle which resulted in decreasing enzyme activity (Krishna and Chandrasekaran 1996; Tao et al. 1997). Smaller particle size provides a larger surface area for the microbial attack; however, it faces difficulty in respirating due to the limited availability of inter-particle space. In contrast, larger particles provide better respiration opportunities with a lesser surface area (Pandey et al. 2000). During the bioprocess optimization, a varied range of particle size is applied to reduce the cost. The wheat bran, which is considered as the most commonly used substrate in SSF, is obtained in two forms: fine and coarse. The former contains a smaller particle size (mostly smaller than 500–600 μm) while the latter is larger.
3.5 Cellulase Activity of Different Wastes as Source of Carbon
The cellulose activity for vegetable waste is demonstrated at 50 FPU g−1 (Fig. 4a), while only16 FPU g−1 is observed using PKC and defatted PKC (Figs. 4a and b). Overall, the cellulase activity measured on the vegetable waste was higher than both types of PKCs with an increment of 2-fold of cellulase activity. Thus, different substrates showed significant effects on cellulase activity. The difference in the cellulase activity can also be used by distinctive substrate morphologies in their outward appearance. These materials comprised about 40–50 % cellulose, 20–30 % hemicellulose with lesser amounts of lignin in cereals and herbaceous plants with a higher amount in forestry residues (e.g., sawdust) (Wyman 2008). The PKC obtained from the kernels were similar to the hardwood which contained a higher lignin content compared to other substrates (which belong to the cereal family) (Milala et al. 2009). Lignin, a film formed around the cellulose could restrain or slow down cellulase from hydrolysingthe cellulose. Therefore, it may affect the secretion of cellulase by the microorganism as it can be observed within the PKC substrate. Low cellulase activity was also observed by using sawdust (from hardwood) as a substrate (Ojumu et al. 2003). Meanwhile, more fruit juice was extracted from the mango pulp using Trichoderma sp., despite the high amount of polysaccharides in the pulp (Buenrostro et al. 2010). This may be due to the fact that the polysaccharides in the pulp can be easily degraded to fermentable sugars using filamentous fungus. Enzyme activity is substrate-dependent which means that different types of polysaccharides structure of the substrate affect the enzyme activity differently. In addition, the cellulase activity in defatted PKC was measured as half when raw PKC was used as a substrate. After the expeller pressing oil extraction process, about 6–8 % w/w of the residual oil remained in the PKC. The high lipid content of PKC can affect the growth of microorganisms and enzyme productions as a result of its poor water absorption capacity which can prevent oxygen diffusion within the substrate or biomass (Pang et al. 2006). A comparison of three different substrates showed that vegetable waste demonstrated the highest cellulase activity (50 FPU g−1).
3.6 A Comparative Study of the Production of Cellulase Using Trichoderma and Bacillus Cereus
A comparative study of Trichoderma and Bacillus cereus in terms of pH, concentration and temperature was carried out to examine the production of cellulase activity. It is inferred from Table 1 that in comparison to Bacillus cereus, Trichoderma showed maximum pH and temperature for the production of cellulase activity. It might be due to the higher tolerance capacity of Trichoderma sp. in alkaline and warmer conditions. In terms of cellulase activity, Trichoderma demonstrated the maximum cellulase activity using 108 inocula while B. cereus covered the maximum activity by using inocula of the lower concentration (106 cells mL−1). Pang et al. (2006) examined the maximum level of F Pase by using a higher concentration of Trichoderma (1x108 spores mL−1) on sugarcane bagasse: palm kernel cake (Pang et al. 2006). Thus, the marginal differences in cellulase activity of the fungal and bacterial were of 1-fold and 2-fold increment by using Trichoderma and B. cereus, respectively, after 5 days of fermentation process on the vegetable substrate. Therefore, Trichoderma sp. and B. cereus have the potential to secrete more cellulase during the degradation of cellulose depending on the type of substrates used as the composition of the substrate differ from one to another.
4 Conclusions
Palm kernel cake and vegetable wastes have potential to produce cellulase using Trichoderma sp. via solid state fermentation. The optimal conditions for the maximum production of cellulase were achieved at pH 5.0 and 30 °C using 2 x 108 mL−1 spores of inocula. Among the three wastes, the maximum cellulose activity was obtained by Trichoderma on vegetable waste. On the basis of particle size, the inocula of 500 μm demonstrated the most significant cellulase activity in all waste samples. Trichoderma sp. can be effectively used for the production of cellulase enzyme by utilizing the lignocellulosic waste from the palm oil processing industry including palm kernel cake (raw PKC or defatted PKC) or domestic vegetable waste, either for commercial or industrial purposes. Thus, to create a pollution free environment, palm oil industrial wastes can be utilized for the production of the value-added product (cellulase).
PKC, Palm Kernel Cake; VW, Vegetable Waste; SSF, Solid State Fermentation; Sp., Species; T. reesei, Trichoderma reesei; T. koningii, Trichoderma koningii; T. viride, Trichoderma viride; A. terreus, Aspergillus terreus; V8, Vegetable juice; SEM, Scanning Electron Microscope; Rpm, Revolution per minute.
References
Abu-Sharar TM, Al-Karablieh EK, Haddadin MJ (2012) Role of virtual water in optimizing water resources management in Jordan. Water Resour Manag 26:3977–3993
Al-Gheethi AA, Ismail N (2014) Biodegradation of pharmaceutical wastes in treated sewage effluents by Bacillus subtilis 1556WTNC. Environl Process 1:459–481
Ali A, Sandi S (2014) Application of Ammoniation-fermentation technology based on palm plantation waste for increasing productivity of Pampangan buffalo. APCBEE Procedia 8:93–98
Al-Khashman OA (2009) Chemical evaluation of Ma’an sewage effluents and its reuse in irrigation purposes. Water Resour Manag 23:1041–1053
Awafo V, Chahal D, Simpson B (2000) Evaluation of combination treatments of sodium hydroxide and steam explosion for the production of cellulase-systems by two T. Reesei mutants under solid-state fermentation conditions. Bioresour Technol 73:235–245
Beldman G, Leeuwen MF, Rombouts FM, Voragen FG (1985) The cellulase of Trichoderma Viride. Eur J Biochem 146:301–308
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209
Buenrostro FJ, De La Garza-Toledo H, Ibarra-Junquera V, Aguilar CN (2010) Juice extraction from mango pulp using an enzymatic complex of Trichoderma sp. produced by solid-state fermentation. Food Sci Biotechnol 19:1387–1390
Bushra R, Naushad M, Adnan R, Md S, Ansari M, Ahmed A (2015) Electrical and Optical Properties of Synthesized Composite Material Polyaniline-Ti (IV) Arsenophosphate. Asian J Chem 27:1121
Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344:1879–1900
Chandra M, Kalra A, Sharma PK, Kumar H, Sangwan RS (2010) Optimization of cellulases production by Trichoderma citrinoviride on marc of Artemisia annua and its application for bioconversion process. Biomass Bioenergy 34:805–811
Efaq A, Rahman NNNA, Nagao H, Al-Gheethi A, Shahadat Md, Kadir, M. A. (2015). Supercritical carbon dioxide as non-thermal alternative technology for safe handling of clinical wastes. Environl Process 2:797–822.
Fujii T, Fang X, Inoue H, Murakami K, Sawayama S (2009) Enzymatic hydrolyzing performance of Acremonium cellulolyticus and Trichoderma Reesei against three lignocellulosic materials. Biotechnol Biofuels 2:24
Gautam S, Bundela P, Pandey A, Khan J, Awasthi M, Sarsaiya S (2011) Optimization for the production of cellulase enzyme from municipal solid waste residue by two novel cellulolytic fungi. Biotechnol Res Int 2011:1–8
Ghose T (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Goyal A, Ghosh B, Eveleigh D (1991) Characteristics of fungal cellulases. Bioresour Technol 36:37–50
Ibrahim C (2008) Development of applications of industrial enzymes from Malaysian indigenous microbial sources. Bioresour Technol 99:4572–4582
Jain CK, Malik DS, Yadav AK (2016) Applicability of plant based biosorbents in the removal of heavy metals: a review. Environl Process 3:495–523
Jørgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod Biorefin 1:119–134
Kashyap P, Sabu A, Pandey A, Szakacs G, Soccol CR (2002) Extra-cellular L-glutaminase production by Zygosaccharomyces rouxii under solid-state fermentation. Process Biochem 38:307–312
Keesari T, Ramakumar K, Prasad MBK, Chidambaram S, Perumal P, Prakash D, Nawani N (2015) Microbial evaluation of groundwater and its implications on Redox condition of a multi-layer sedimentary aquifer system. Environl Process 2:331–346
Khosrokhavar R, Griffiths S, Wolf KH (2014) Shale gas formations and their potential for carbon storage: opportunities and outlook. Environl Process 1:595–611
Koyani RD, Sanghvi GV, Rajput KS (2011) Comparative study on the delignification of Azadirachta indica (L) Del., wood by Chrysosporium asperatum and Trichoderma harzianum. Int Biodeter Biodegr 65:179–184
Krishna C, Chandrasekaran M (1996) Banana waste as substrate for α-amylase production by Bacillus Subtilis (CBTK 106) under solid-state fermentation. Appl Microbiol Biotechnol 46:106–111
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enz Res 2011
Latifian M, Hamidi-Esfahani Z, Barzegar M (2007) Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermentation conditions. Bioresour Technol 98:3634–3637
Lu W, Li D, Wu Y (2003) Influence of water activity and temperature on xylanase biosynthesis in pilot-scale solid-state fermentation by Aspergillus sulphureus. Enzym Microb Technol 32:305–311
Mansour MM, Salem MZ (2015) Evaluation of wood treated with some natural extracts and Paraloid B-72 against the fungus Trichoderma harzianum: wood elemental composition, in-vitro and application evidence. Int Biodeter Biodegr 100:62–69
Medve J (1997) Cellulose Hydrolysis by Trichoderma reesei cellulases: studies on adsorption, sugar production and synergism of cellobiohydrolase I. Lund University, II and endoglucanase II
Milala M, Shehu B, Zanna H, Omosioda V (2009) Degradation of agro-waste by cellulase from Aspergillus candidus. Asian J Biotechnol 1:51–56
Moslim R, Kamarudin N (2014) The use of palm kernel cake in the production of conidia and blastospores of Metarhizium anisopliae var. major for control of Oryctes Rhinoceros. J Oil Palm Res 26:133–139
Nabi S, Shahadat M, Bushra R, Shalla A, Azam A (2011) Synthesis and characterization of nano-composite ion-exchanger; its adsorption behavior. Colloids and Surfaces B 87:122–128
Nataraja S, Chetan D, Krishnappa M (2010) Effect of temperature on cellulose enzyme activity in crude extracts isolated from solid wastes microbes. Int J Microbiol Res 2:44
Norsalwani TT, Norulaini NN (2012) Utilization of lignocellulosic wastes as a carbon source for the production of bacterial cellulases under solid state fermentation. Inter J Environ Sci Develop 3:136–140
Ojumu TV, Solomon BO, Betiku E, Layokun SK, Amigun B (2003) Cellulase production by Aspergillus flavus Linn isolate NSPR 101 fermented in sawdust, bagasse and corncob. Afr J Biotechnol 2:150–152
Palonen H, Tjerneld F, Zacchi G, Tenkanen M (2004) Adsorption of Trichoderma Reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. J Biotechnol 107:65–72
Pandey A, Soccol CR, Mitchell D (2000) New developments in solid state fermentation: I-bioprocesses and products. Process Biochem 35:1153–1169
Pang P, Ibrahim D, Poppe L, Szakacs G, Che Omar I (2006) Production of cellulolytic enzymes by a newly isolated, Trichoderma sp. FETL c3-2 via solid state fermentation grown on sugar cane bagasse: palm kernel cake as substrates. Pak J Biol Sci 9:1430–1437
Philippi K, Tsamandouras N, Grigorakis S, Makris DP (2016) Ultrasound-assisted green extraction of eggplant peel (Solanum melongena) polyphenols using aqueous mixtures of glycerol and ethanol: optimisation and kinetics. Environl Process 3:369–386
Rahman NNNA, Md S, Won CA, Omar FM (2014) FTIR study and bioadsorption kinetics of bioadsorbent for the analysis of metal pollutants. RSC Adv 4:58156–58163
Rahman NNNA, Md S, Omar FM, Chew AW, Kadir MOA (2016) Dry Trichoderma biomass: biosorption behavior for the treatment of toxic heavy metal ions. Desalin Water Treat 57:13106–13112
Ramachandran S, Patel AK, Nampoothiri KM, Francis F, Nagy V, Szakacs G, Pandey A (2004) Coconut oil cake––a potential raw material for the production of α-amylase. Bioresour Technol 93:169–174
Ramos LP (1992) Steam pretreatment and enzymatic hydrolysis of Eucalyptus viminalis chips: Theses, University of Ottawa (Canada) 1910–2010.
Ramos LP (2003) The chemistry involved in the steam treatment of lignocellulosic materials. Quím Nov. 26:863–871
Ramos LP, Zandoná FA, Deschamps F, Saddler JN (1999) The effect of Trichoderma cellulases on the fine structure of a bleached softwood kraft pulp. Enzym Microb Technol 24:371–380
Ryu DD, Mandels M (1980) Cellulases: biosynthesis and applications. Enzym Microb Technol 2:91–102
Sabu A, Sarita S, Pandey A, Bogar B, Szakacs G, Soccol CR (2002) Solid-state fermentation for production of phytase by Rhizopus oligosporus. Appl Biochem Biotechnol 102:251–260
Shafawati SN, Siddiquee S (2013) Composting of oil palm fibres and Trichoderma spp. as the biological control agent: a review. Int Biodeter Biodegr 85:243–253
Shahadat M, Shalla A, Raeissi A (2012) Synthesis, characterization, and sorption behavior of a novel composite cation exchange adsorbent. Ind Eng Chem Res 51:15525–15529
Sola F, Vallejos A, López-Geta J, Pulido-Bosch A (2013) The role of aquifer media in improving the quality of seawater feed to desalination plants. Water Resour Manag 27:1377–1392
Srivastava S, Sharma YK (2014) Arsenic induced changes in growth and metabolism of black Gram seedlings (Vigna mungo L.) and the role of phosphate as an ameliorating agent. Environl Process 1:431–445
Sudiyani Y, Styarini D, Triwahyuni E, Sembiring KC, Aristiawan Y, Abimanyu H, Han MH (2013) Utilization of biomass waste empty fruit bunch fiber of palm oil for bioethanol production using pilot–scale unit. Energy Procedia 32:31–38
Tao S, Beihui L, Deming L, Zuohu L (1997) Effect of elevated temperature on Trichoderma Viride SL-1 in solid state fermentations. Biotechnol Lett 19:171–174
Teeri T, Koivula A, Linder M, Wohlfahrt G, Divne C, Jones T (1998) Trichoderma Reesei cellobiohydrolases: why so efficient on crystalline cellulose? Biochem Soc Trans 26:173–178
Van DBJ, De Vries RP (2011) Fungal enzyme sets for plant polysaccharide degradation. Appl Microbiol Biotechnol 91:1477–1492
Wan C, Zhao XQ, Guo SL, Alam MA, Bai FW (2013) Bioflocculant production from Solibacillus silvestris W01 and its application in cost-effective harvest of marine microalga Nannochloropsis oceanica by flocculation. Bioresour Technol 135:207–212
Wong H, Wan Zahari, M (1992) Characterization of oil-palm by-products as feed for ruminants. Proc. 15th. Annual Conference of the Malaysian Society of Animal Production.
Wong KK, Zahari MW (2011) Utilization of oil palm by-products as ruminant feed in Malaysia. J Oil Palm Res 23:1029–1035
Wyman CE (2008) Cellulosic ethanol: a unique sustainable liquid transportation fuel. MRS Bull 33:381–383
Zhang H, Sang Q (2012) Statistical optimization of cellulases production by Penicillium chrysogenum QML-2 under solid-state fermentation and primary application to chitosan hydrolysis. World J Microbiol Biotechnol 28:1163–1174
Acknowledgments
This work was supported by the research university grant of University Sains Malaysia (No.1001/PJJAUH/811198). Special thanks to United Fleet Palm Sdn. Bhd. and School of Industrial Technology for the materials and facilities provided for this research.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lah, T.N.T., Norulaini, N.A.N., Shahadat, M. et al. Utilization of Industrial Waste for the Production of Cellulase by the Cultivation of Trichoderma via Solid State Fermentation. Environ. Process. 3, 803–814 (2016). https://doi.org/10.1007/s40710-016-0185-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-016-0185-8