Abstract
Enormous waste has been generated from the vegetable and fruit processing industries, which are a good source of carbohydrates. Such unused remnant imposes huge disposal and severe pollution problems. Due to the presence of cellulose, hemicellulose, pectin, minerals, and vitamins, these waste materials have a great prospective for its bioconversion into useful products, viz., acids, enzymes, fuels, and value-added products. To reveal their possible potential, separate sets of experiments have been conducted by using bottle gourd peel waste biomass as a carbon source for cellulase production. It was observed from experimental findings that 30 °C temperature and 0.56 g/l of inoculum dosages are the most promising situations for cellulase production by both the fungal strains. FPase and CMCase activity considerably increases by the inclusion of whey as well as starch hydrolysates in the media used in the production study. The present study portrays the utility of bottle gourd peel waste, whey, and starch-based hydrolysates in cellulase production by Trichoderma reesei and Neurospora crassa. The exploitation of cost-effective, cheap, bottle gourd vegetable peel waste for cellulase production could be an innovative, effective, sustainable, and green approach in cellulase production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The most abundant and renewable resources in the earth for the production of and value-added chemicals and biofuels are lignocellulosic biomass [39]. Agro-industrial waste materials are made up of complex polysaccharide that fortifies the growth and development of industrially important microbes. During the agriculture raw material processing for food, a bulk quantity of agro solid wastes was generated [7, 22, 37]. Economical and efficient depolymerization of lignocellulosic biomass is the basic necessity for large-scale production of biomass originated fuels and chemicals [34]. Due to awareness of the health welfares of fruit and vegetable, the demand for fruits and vegetables has increased considerably. Sound knowledge about the physicochemical properties of lignocellulosic derived biomass and further analytical characterization for those properties plays a vital role in the effective biomass conversion technology [7].
Bottle gourd is an annual climbing vine that belongs to cucurbitaceae family and also known by other names as calabash, lauki, trumpet gourd, calebassier, cojombro, guiro amargo, talayag, gucuzzi, and zucca melon [13]. This plant contains triterpenoid, Cucurbitacins, antioxidants, flavones, C-glycosides, ß-glycosides, vit C, thiamin, riboflavin, and niacin in fruits. Thus, it is a good source of vitamins, irons, and minerals as well as an excellent diet for people having digestive problems [23]. Bottle gourd fruits are conventionally used as a nutritive thing having cardioprotective, cardiotonic, purgative diuretic, and antidote to certain poisons [13]. It is also beneficial in insanity, epilepsy, and other nervous diseases.
These plants were found to retain anti-inflammatory activity, anti-diabetics, anti-hyperlipidemic, and anticancer activity [32, 35]. Bottle gourd peels are also utilized in the detection and quantification of lethal metals in industrial effluents and groundwater [1]. Bottle gourd peel based activated carbon acts as an adsorbent for the exclusion of leather dye (Direct Black 38) from aqueous solution [12]. It has also been used for the adsorption of hazardous Reactive red 195-A (RRD) and Reactive blue 222 (RBD) from aqueous solution [30]. Bottle gourd peel extract-based magnetic nanoparticles are also used for organic dyes degradation [33]. Behera and Gupta [6] reported the production of few edible mushroom cultures by the utilization of bottle gourd peel biomass. Waste produced after processing of fruit and vegetable is difficult to manage. Therefore, utilization of these wastes for the production of value-added chemicals not only valorizes waste biomass but also helpful in the reduction of environmental pollution [48]. Due to the importance of bottle gourd vegetables in various processes industries, effective and economical utilization of their peels is also important for complete exploitation. In the present investigation, a novel concept has been used to study the application of bottle gourd peel as a carbon and energy source for cellulase production.
Chemically and enzymatically processed starch are used in the food and pharmaceutical industry in different forms such as starch hydrolysates, glucose syrups, fructose, maltodextrin derivatives, or cyclodextrins as substitutes, mixtures, thickening agents, and fillers. In addition to that, the sugars produced can be fermented to produce value-added chemicals [4, 8, 15]. Starch is a glucose-based polymer mainly comprised of two main fractions: these are amylose and amylopectin, both having different structural properties. Amylose is highly hydrophilic, due to more number of hydroxyl groups. Amylose is a much smaller molecule than amylopectin, and it also contains alpha amylase linkage [2, 56]. Amylopectin entails of short α-1,4 linkage, linked to linear chains of glucose units and α-1,6 linkage, linked to side chains. Branched amylopectin contains both α-amylase and β-amylase linkages [3, 8]. Starch is plentiful renewable resources and used as an important feedstock for industrial applications [36]. Arrangement of starch molecules in the plant is in the form of semicrystalline granules with a unique granular size. Granular microstructure and the nanostructure of the growth rings collectively affect the enzymatic digestibility of granular starches [8, 50]. Granules of rice starch are relatively smaller (about 2 μm) than potato starch granules (up to 100 μm). Wheat starch grains are bimodal in size, smaller B-starch (15–20%) ,and the larger A-starch granules (80–85%) [25, 31]. Water-insoluble protein complex (wheat gluten) is present in the wheat endosperm. As literature suggested, the water-soluble starch hydrolysates also act as a better inducer for cellulase production. It stimulates the enzymatic system to the same extent as pure cellulose [10, 51].
Dairy industry waste (whey) is the byproduct of a cheese manufacturing process [38]. It is a severe pollutant that enforces excessive BOD of 30,000–50,000 mg/lit. Disposal of whey creates a substantial loss of possible nutrients in the form of lactose. It also promotes the process of eutrophication, causing excessive growth of microorganisms and aquatic plants [5, 27, 45]. Application of lactose (soluble carbon source) present in the whey for cellulase production consents much control on the environment, simplifies the fermentation operational process, and accelerates the cellulase production [46]. The present experimental work illustrates the effectiveness of innovative and cheap bottle gourd peel waste as an energy source for cellulase production, along with the utilization of dairy industry waste (whey) as well as starch hydrolysates on its production by Trichoderma reesei and Neurospora crassa.
2 Materials and methods
2.1 Materials
Chemicals, biochemicals, and reagents consumed to execute the present work were of Himedia, Sigma-Aldrich, and Merck make. Trichoderma reesei NCIM 1186 and Neurospora crassa NCIM 1021 were acquired from the National Chemical Laboratory (NCL) Pune, India. Whey was procured from the local dairy industry, whereas the local vegetable market was a center for the collection of bottle gourd.
2.2 Methods
2.2.1 Preparation of raw material
After peeling off the bottle gourd, the peel was dried, ground, and further sieved with a mesh screen. The ground raw material (850 μm) was used as a solid bed for cellulase production analysis.
2.2.2 Estimation of holocellulose and lignin content in bottle gourd Peel waste
Holocellulose and acid-insoluble lignin in raw materials were assessed by the TM1-A-9 and TM1-A-7 test method, respectively, as stated in the laboratory guide of Central Pulp and Paper Research Institute (CPPRI) Saharanpur, U.P., India [24].
2.2.3 Determination of ash and moisture content
Ash and moisture content were determined by the prescribed methods, as stated in the laboratory guide of Central Pulp and Paper Research Institute (CPPRI) Saharanpur, U.P., India [24].
2.2.4 FTIR/XRD/SEM analysis of bottle gourd Peel
Nicolet 6000 spectrophotometer was used to carry out Fourier transform infrared (FTIR) spectroscopic analysis. To perform this, oven-dried samples were mixed with KBr in the proportion of 1:200 mg (raw material: KBr) and further pressed under vacuum to form the pellets. Transmittance was quantifying over a scale from 4000 to 500 cm−1.
XRD (x-ray diffraction) analysis of Bottle gourd peels was estimated on a Bruker AXS D8 Advance diffractometer. The samples were imaged in the range from 0 to 70° angle.
Scanning electron microscopy (SEM) was used to determine the surface properties of treated and untreated raw materials. In this study, the samples were glazed with a gold film. The samples were then investigated using scanning electron microscope model LEO-435 VP.
2.2.5 Pretreatment of starch
Acid pretreatment of starch was performed by using a 2% HCl (v/v) solution. Ten grams of wheat, rice, and potato starch powdered biomass were taken separately after that 40 mL of diluted HCl solution were added separately in each starch sample, to maintain the slurry of about 25% consistency. Afterwards, starch hydrolysates were exposed to heat treatment in a pressure of 15 psi at 121 °C for 1 h time duration. The pretreated starch hydrolysates with different volumes were consumed in the production medium.
2.3 Inoculum development
Inoculum development experiments have been performed in respective culture media by the methods previously used by Verma et al. [47].
2.3.1 Dry weight determination
Cell dry weight of microbial suspensions was determined by the procedure used by Verma et al. [47]. The determination of fungal growth by cell dry weight was expressed as the mean of three independent readings.
2.3.2 Preparation of Production Media & Solid State Fermentation
Three types of production medium were used for production studies. (I) Normal basal salt media was used for production studies having the following constituents (g/L): urea, 0.3; (NH4)2SO4, 1.4; KH2PO4, 2.0; MgSO4.7H2O, 0.3; peptone, 1.0; Tween 80, 0.2; FeSO4.7H2O, 0.005; MnSO4.7H2O, 0.0016; ZnSO4.7H2O; 0.0014; CaCl2.2H2O, 0.4; CoCl2.6H2O, 0.02. (II) Whey containing production media: 15, 30, and 50% (v/v) whey was incorporated separately in the earlier described production media. (III) Whey + starch hydrolysates containing production media: 30% (v/v) whey along with 2 and 5% (v/v) of 2% HCl-treated starch hydrolysate (potato, wheat and rice starch) was incorporated in the earlier described production media.
Separate sets of batch experiment were performed in 250 mL Erlenmeyer flasks comprising sieved bottle gourd peels as the carbon source for the growth and production of organisms impregnated with the normal basic salt media. Bottle gourd peel waste bed soaked with basal salt media were autoclaved and then separately inoculated with 0.36, 0.46, 0.56, 0.66, and 0.76 (g/L) of potato dextrose (PD) broth culture solution of Trichoderma reesei and M2 broth culture solution of Neurospora crassa for 6 days. Further in another set of experiment, production medium containing flasks was put in an incubator at 25, 27, 30, 32, and 35 °C for 6 days. To investigate the influence of various initial pH (3 to 8) of the basal salt medium, separate sets of experiments were performed at 30 °C. Another set of experiments was carried out to study the effect of whey, wheat, potato, and rice starch hydrolysate. About this 15, 30, and 50% (v/v), whey was incorporated separately in the earlier described production media and now the whey containing supplementary production media was further expended for impregnation of bottle gourd peel based solid bed. Alternatively untreated, 2% and 5% (v/v) acid-treated wheat, potato and starch hydrolysate solution were incorporated separately in 30% (v/v) whey containing basal salt media which was further used for impregnation of Bottle gourd peel solid bed. All the bottle gourd peel bed containing production flasks inoculated with culture solution to study the effect of whey, wheat, potato, and rice starch hydrolysates was placed in an incubator at 30 °C for 6 days.
2.3.3 Extraction of enzyme
Extraction of enzyme was performed by the method previously used by Verma et al. [47]. The subsequent supernatant was collected and used as a crude enzyme source. All extractions were performed in duplicate.
2.3.4 Total Cellulase (filter paper activity) and CMCase activity
Filter paper (FPA) and carboxymethyl cellulase (CMCase) activity were analyzed by the method recommended by Ghose [16].
3 Results and discussions
3.1 Characterization of bottle gourd peel waste biomass
3.1.1 Evaluation of bottle gourd peel waste biomass
To establish the major constituent of bottle gourd peel waste biomass, proximate evaluation has been executed. To resolve the appropriateness and effectiveness of waste biomass for cellulase production, a distinct set of experiments and investigations have been performed.
It has been observed from the proximate analysis of dried bottle gourd peel waste that holocellulose (66.35 ± 4.65) component stated as major constituent followed by lignin (21.80 ± 3.38) on percentage (w/w%) basis.
3.1.2 XRD pattern of bottle gourd peel waste biomass
The crystallinity and surface area of peel based biomass are evaluated as the most important factors to interpret the structural evolution of biomass [20]. To determine the accessibility and nature of cellulose present in the bottle gourd peel waste biomass, XRD analysis has been executed.
XRD pattern of bottle gourd peel shows the lesser number of peaks with broader peak heights which proves its lower crystallinity as viewed from Fig. 1. Therefore, we can suggest that cellulose present in Bottle gourd peel is easily accessible for fungal attacks.
3.1.3 FTIR spectra of bottle gourd peel waste biomass
To identify the constituents of lignocellulosic waste materials, FTIR spectroscopy has been performed. This is a well-established analytical method for process monitoring and identifying the chemical species. It gives a total simultaneous chemical analysis of lignocellulosic waste material.
FTIR spectroscopy was used for recognizing the components of lignocellulosic biomass. The lignocellulosic constituents of bottle gourd peel could be examined from the peak existence in between 3448 cm−1 and 895 cm−1. Several peaks were observed by FTIR spectra of bottle gourd peel (3448 cm−1, 3343 cm−1, 3313 cm−1, 2925 cm−1, 2360 cm−1, 1641 cm−1, 1539 cm−1, 1426 cm−1, 1326 cm−1, 1247 cm−1, 1065 cm−1, and 895 cm−1) as shown in Fig. 2.
Strong bands have been observed in the FTIR spectra of bottle gourd peel at 3448 cm−1, 3343 cm−1, and 3313 cm−1with a higher percentage of absorbance. These bands are allied to the –OH distending vibration of hydroxyl groups present in the phenolics of lignin and aliphatic compounds [18].
FTIR spectra of bottle gourd peel at 2925 cm−1 shows extreme band at this region with a higher percentage of absorption, may be consigned to the (C–H) stretch band of methyl groups present in the lignin [19]. The bands in the region of 1326 cm−1 in bottle gourd peel spectra may be endorsed to phenolic syringyl ring C–O stretching of phenol, while bands at 1247 cm−1 in bottle gourd peel spectra may probably be due to C–O stretching in the acetyl and phenolic groups [44]. The peak in the spectrum near 1539 cm−1 in bottle gourd peel may be owing to the aromatic skeletal vibrations C=C present in the lignin.
The spectral band at 1641 cm−1 in Bottle gourd peel may be attributed primarily due to the C=O stretching vibration of alpha-keto carbonyl for cellulose [17, 19]. The presence of the vibrational peak at 1065 cm−1 in the spectra of bottle gourd peel may be due to the C–OH stretching vibration of the cellulose backbone [41]. The absorption band at 895 cm−1 in the spectra of bottle gourd peel may be assigned to the C–H distortion of cellulose as well as ß-glucosidic linkage between sugars [44, 54].
The FTIR spectral map of bottle gourd peel recommends that lignin and phenolic components are present in good amount in the bottle gourd peel which creates impediment to the uptake of cellulose by the fungal system even that microbes grow well under bottle gourd peel due to the presence of sizeable amount of cellulose which provides favorable conditions for fungal attack.
3.2 Bottle gourd peel used in cellulase production
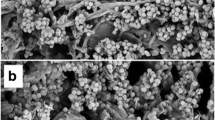
Separate sets of batch experiments were performed for the cellulase production studies by T. reesei and N.crassa for utilizing bottle gourd peel as raw material for solid support. Bottle gourd peels are soft and light green as observed from Fig. 3. It was observed that Trichoderma grew better than N.crassa under bottle gourd peel-based solid bed as shown by Fig. 3d, e.
This observation evidences that T. reesei can grow and produce cellulases under phenolic-based raw materials. On the other hand, N.crassa showed lesser tolerance under such harsh conditions. During heat treatment in autoclaving disruption of raw material cell membranes as well as cell walls are observed which hydrolyzes the bonds and making more accessible the cellulosic as well as other constituents such as antioxidants and phenolics. As literature reported, T. reesei has the forbearance to grow in the recalcitrant pollutants of a certain level [43]. Scanning electron microscopy has proved to be a precious and invaluable tool for analyzing the growth of the fungal system [47]. To study the amplified view of untreated and microbial treated bottle gourd peel, scanning electron microscopic analysis of the desired samples has been performed.
Scanning electron microscopic analysis also proves fruitful growth of T.reesei as compared with N.crassa under bottle gourd-based solid state fermentation as shown by Fig. 4.
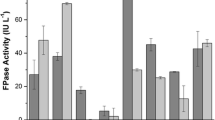
Fungal production of cellulases was compared under various operating parameters such as temperature, inoculum dosages, and pH. As shown in Table 1, a temperature higher or lower than 30 °C was somewhat less favorable for the production of cellulase by T. reesei and N. crassa. Under high temperature, decrement in the enzyme activity has been perceived, which might be because at higher temperature, thermal deactivation enzymes as well as microbes have been occurred [52]. At higher temperature, the hyphae appears with warped, reduced branches and thereby concentrates the cell nucleus which affect and diminish the microbial production [55], while at lower temperature, the substrates affinity for cells in microbial system is lowered, because of the thickening of lipids of the membrane and due to this the microbial production of enzyme is decreased [29]. Good cellulase activities were observed in terms of FPase and CMCase by T.reesei (3.38 ± 0.09 IU/mL, 3.45 ± 0.11 IU/mL) N.crassa (1.52 ± 0.06 IU/mL, 4.42 ± 0.13 IU/mL) under bottle gourd-based solid bed at 30 °C.
Alternatively, pH 5.0 was found to be the most suitable for cellulase production by T.reesei while N.crassa performs better at pH 6.0. pH regulates the speciation and concentrations of electron donors, acceptors, and reaction products, which in turn establish the energy yields of redox reactions [21]. Operational pH stimulates a stress response and eliciting a pH signal pathway to regulate the expression [49]. In higher or lower pH-based production medium, cellulase activity was substantially decreased. This indicates that a highly acidic or alkaline condition becomes unsuitable for fungal growth and production system.
If the pH is not suitable, microbial metabolism would be disturbed and ultimately affects fungal production system or in another word if there is no proper balance of ions, and the shape of the active site of enzyme would be distracted so that the substrate could not be bounded into the enzyme. Such conditions would favor decrement in enzyme activity [52]. Lower pH conditions endorses the dissemination of formic acid, acetic acid, and other short-chain fatty acids across the membrane, which disperses proton drive force across the membrane and deters the microbial growth [21]. An acidic pH favored cellulase production, while this was significantly decreased towards much acidic as well as neutral and slight alkaline pH, as observed from Table 1.
The size of inoculum seems to have a profound effect on microbial growth and enzyme production. Inoculum dosages of 0.56 g/l were found most appropriate dosages for cellulase production by both of the fungal strains. Inoculum size influences the utilization of carbon and nitrogen from the medium by microbial sources. Dhillon et al. [11] stated that maximum enzyme activity was analyzed using 5% inoculum. An increment in inoculum size from 5% showed a progressive decrease in enzyme activity reaching the lowest at 20% inoculums. Smaller inoculum sizes produced a transient mycelial stage, whereas a higher inoculum concentration becomes favorable, probably because of the reduction in the lag phase caused by highly concentrated inocula. Reduction in cellulase production on increasing the inoculum size could be due to competition between microorganism colonies for nutrients and probably the non-availability of nutrients for the large population limits the fungal growth [26]. Therefore, a suitable and appropriate inoculum size or dosages required for healthier fungal propagation and their enzyme production.
When compared the cellulase activities produced by T.reesei and N. crassa under bottle gourd peel-based solid state fermentation, it was observed that cellulases are produced from T.reesei having higher FPase activity as compared with N.crassa. On the other hand, cellulases produced from N.crassa showed higher CMCase activity as observed from Table 1.
To evaluate the effects of dairy industry waste (whey) and starch hydrolysates, separate set of experiments has been conducted by utilizing modified basal salt medium.
It was observed from Table 2 that FPase activities were enhanced by the incorporation of 30% whey in BSM. The highest increment in FPase was observed by T. reesei (3.80 ± 0.13 IU/mL) strain, and the least increment was observed with N.crassa (1.66 ± 0.08 IU/mL) on bottle gourd peel-based solid state fermentation, which suggests that T. reesei quite effectively utilized whey as inducer and carbon source. Morikawa et al. [28] reported that lactose may function as an inducer for cellulase formation if it is taken up in the mycelium of T. reesei PC-3-7. Induction capacity of whey was very low or negligible by N.crassa system. It has been observed from Table 2 that CMCase activities (3.58 ± 0.06; 4.58 ± 0.09) were not much enhanced by incorporation of whey in BSM. These finding insinuate that whey are not much effective inducers for CMCase in comparison to FPase activity produced by microbial system.
FPase activities were not improved by most of the untreated starch-based production systems. They imply that untreated starch having very little or nearly zero cellulase induction capability. FPase of T.reesei was further improved by inclusion of (2% v/v) wheat starch hydrolysate (2% HCl treated with 1 h pretreatment time) in whey based basal salt medium.
By increasing the wheat starch hydrolysate concentration (5% v/v), additional improvement in the FPase (4.45 ± 0.11 IU/mL) was observed, which suggests that wheat starch hydrolysates contain few sugars which induce the Trichoderma reesei cellulase production system. Growth and cellulase stimulation both take place in the hydrolyzates containing medium, apparently due to the presence of few dimeric sugars in the hydrolyzates which ultimately induces cellulase production [9]. Gao et al. [14] reported that transglycosylation products have been successfully used as the cellulase inducer by Trichoderma reesei. Earlier studies showed that starch itself was poor inducer for the cellulase induction, but it was declared highly operative by acid hydrolysis. This was due to the formation of reversion products, such as sophorose (disaccharide) during acid hydrolysis [42].
Mandhania et al. [26] suggested that sophorose, cellobiose, or galactose may provoke a putative lactose permease in T. reesei PC-3-7 which may be helpful in the induction process. Reasonable improvement in FPase (4.37 ± 0.08 IU/mL) was also observed by T.reesei under (5% v/v) rice starch hydrolyzates medium containing bottle gourd peel-based solid state fermentation, while potato starch hydrolysates were found not much effective for FPase induction by T.reesei. No satisfactory enhancement in FPase (4.16 ± 0.13 IU/mL) was observed under potato starch hydrolysate-based production. In contrast, N.crassa system showed different behaviors for starch hydrolyzate-based cellulase induction. It has been observed from Table 2 that N.crassa showed satisfactorily improvement in the FPase activities (2.09 ± 0.16 IU/mL) (2.01 ± 0.09 IU/mL) under 2% v/v potato and rice starch hydrolyzates containing bottle gourd peel bed, respectively, comparison to FPase activity (1.79 ± 0.09 IU/mL) under wheat starch hydrolysate-based fermentation. Significant improvement in FPase activity (2.28 ± 0.07 IU/mL) was also observed by increasing potato starch hydrolysates dosages (5% v/v). These findings suggest that sugars (maltose, maltodextrins) present in the potato starch hydrolysates may induce transcriptional factors for improved cellulase activity for Neurospora system rather than its growth [40] [53].
It has also been observed from Table 2 that CMCase activities were satisfactorily enhanced by incorporation of acid hydrolyzed starch in BSM. Higher increment in CMCase activity (5.43 ± 0.13 IU/mL) was observed by N.crassa system under potato starch hydrolysatebased fermentation.
It has been observed from Table 2 that whey and acid hydrolyzed starches induce cellulase activities diversely under bottle gourd peel-based solid state fermentation. Higher cellulase activities produced by T. reesei in terms of FPase (4.45 ± 0.11 IU/mL) and CMCase (4.20 ± 0.08 IU/mL) were observed under whey and wheat starch hydrolysate containing bottle gourd solid bed-based fermentation.
On the other hand, higher cellulase activities produced by N.crassa in terms of FPase (2.28 ± 0.07 IU/mL) and CMCase (5.43 ± 0.13 IU/mL) were observed under whey and potato starch hydrolysates containing bottle gourd peel bed-based fermentation.
4 Conclusions
Vegetable waste biomass is an easily available, inexpensive, and renewable, natural resource for large-scale production of bio-energy. bottle gourd peel serves as a promising candidate for cellulase production under solid state cultivation. Satisfactory improvement in enzyme activities was observed by Trichoderma reesei under whey based solid support as compared with Neurospora crassa. Among starch hydrolysates, Neurospora performed better under medium supplemented with potato starch hydrolysate. Utilization of starch hydrolysates, as well as dairy industry waste in cellulase production under bottle gourd peel-based fermentation, provides a sustainable, recyclable, green, and eco-friendly approach for solid as well as liquid waste management; therefore, the generation of renewable energy by the exploitation of vegetable wastes is gaining importance in the present scenario.
References
Ahmed D, Abid H, Riaz A (2018) Lagenaria siceraria peel biomass as a potential biosorbent for the removal of toxic metals from industrial wastewaters. Int J Environ Stud 75(5):63–773. https://doi.org/10.1080/00207233.2018.1457285
Ahmed S, Ru W, Han H, Cheng L, Bian X, Li G, Jin L, Wu P, Bao J (2019) Fine molecular structure and its effects on physicochemical properties of starches in potatoes grown in two locations. Food Hydrocoll 97(29)
Alvani K, Qi X, Tester RF (2011) Use of carbohydrates, including dextrins, for oral delivery. Starch-Special issue. Clinl Nutr-Appl Alpha-Glucans 63(7):424–431. https://doi.org/10.1002/star.201000110
An C, Ma SJ, Chang F, Xue WJ (2017) Efficient production of pullulan by Aureobasidium pullulans grown on mixtures of potato starch hydrolysate and sucrose. Braz J Microbiol 48(1):80–185. https://doi.org/10.1016/j.bjm.2016.11.001
Anekar S, Rao CR (2009) Ultra filtration – tool to recover valuable constituent from dairy waste water. J Appl Sci Environ Sanit 4(2):125–132
Behera S, Gupta N (2015) Utilization of vegetable waste for biomass production of some wild edible mushroom cultures. Tropic Plant Res 2(1):05–09
Cai J, He Y, Yu X, Banks SW, Yang Y, Zhang X, Yu Y, Liu R, Bridgewater AV (2017) Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew Sust Energ Rev 76:309–322. https://doi.org/10.1016/j.rser.2017.03.072
Carioca JOB, Arora HL, Panir Selvam PV, Tavares FCA, Kennedy JF, Knill CJ (1996) Industrial utilisation of starch and its derived products in Brazil. Starch 48(9):322–326. https://doi.org/10.1002/star.19960480904
Chen S, Wayman M (1992) Novel inducers derived from starch for cellulase production by Trichoderma reesei. Process Biochem 27:327–334. https://doi.org/10.1016/0032-9592(92)87010-E
Chen S, Wayman M (1993) Use of sorbose to enhance cellobiose activity in Trichoderma reesei cellulase system produced on wheat hydrolysates. Biotechnol Tech 7:345–350. https://doi.org/10.3389/fmicb.2016.00620
Dhillon SS, Gill RK, Gill SS, Singh M (2004) Studies on the utilization of citrus peel for pectinase production using fungus Aspergillus niger. Intl J Env Studies 61(2):199–210. https://doi.org/10.1080/0020723032000143346
Foletto EL, Weber CT, Paz DS, Mazutti M, Meili L, Basacco MM, Colazzo GC (2012) Adsorption of leather dye onto activated carbon prepared from bottle gourd: equilibrium, kinetic and mechanism studies. Water Sci Technol 67(1):201–209. https://doi.org/10.2166/wst.2012.555
Gajera RR, Joshi DC, Ravani A (2017) Processing potential of bottle gourd (L. siceraria). Fruits 5(4):106–109
Gao J, Qian Y, Wang Y, Qu Y, Zhong Y (2017) Production of the versatile cellulase for cellulose bioconversion and cellulase inducer synthesis by genetic improvement of Trichoderma reesei. Biotechnol Biofuels 10:272. https://doi.org/10.1186/s13068-017-0963-1
Gerits LR, Jay BP, Delcour A (2015) Wheat starch swelling, gelatinization and pasting: effects of enzymatic modification of wheat endogenous lipids. LWT Food Sci Technol 63(1):361–366
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268. https://doi.org/10.1351/pac198759020257
Guo GL, Hsu DC, Chen WH, Chen WH, Hwong WS (2009) Charecterisation of enzymatic saccharification for acid pretreated lignocellulosic materials with different lignin composition. Enzym Microb Technol 45(2):80–87. https://doi.org/10.1155/2012/276278
Ibrahim MM, Dufresne A, El-Zawawy WK, Agblevor FA (2010a) Banana fibers and microfibrils as lignocellulosic reinforcements in polymer composites. Carbohydr Polym 81:811–819. https://doi.org/10.1016/j.carbpol.2010.03.057
Ibrahim MNM, Ahmed-Haras MR, Sipaut CS, Aboul-Encin HY, Mohamed AA (2010b) Preparation and characterization of a newly water soluble lignin graft copolymer from oil palm lignocellulosic waste. Carbohydr Polym 80(4):1102–1110
Jiang LQ, Fang Z, Li X, Luo J (2013) Production of 2,3-butanediol from cellulose and jatropha hulls after ionic liquid pretreatment and dilute acid hydrolysis. AMB Express,3(1):48,1–8. https://doi.org/10.1186/2191-0855-3-48
Jin Q, Kirk MF (2018) pH as a primary control in environmental microbiology: 1. Thermodynamic Perspective, Front. Environ. Sci. https://doi.org/10.3389/fenvs.2018.00021
Kodagoda KHGK, Marapana RAUJ (2017) Utilization of fruit processing by-products for industrial applications: A review. Int J Food Sci Nutr 2(6):24–30. https://doi.org/10.22271/food
Kubde MS, Khadabadi SS, Farooqui IA, Deore SL (2010) Lagenaria siceraria: Phytochemistry,phamacognosy and pharmacological studies. Report Opin 2(3):91–98
Laboratory Manual. 2001. Laboratory manual of central pulp and paper research institute, analysis of fibrous raw materials, proximate chemical analysis, Saharanpur, 247001(U.P.), India, “Estimation of acid insoluble lignin in wood/nonwood, TM1A-7; Estimation of acid soluble lignin in wood/nonwood, TM1A-8; Estimation of holocellulose in wood/nonwood; TM1-A9
Leeman AM, Karlsson ME, Eliasson AC, Björck IME (2006) Resistant starch formation in temperature treated potato starches varying in amylose/amylopectin ratio. Carbohydr Polym 65(3):306–313
Mandhania S, Jain V, Malhotra SP (2010) Culture optimization for enhanced production of microbial pectin methylesterase under submerged conditions. Asian J Biochem 5:12–22. https://doi.org/10.3923/ajb.2010.12.22
Marwaha SS, Kennedy JF (2007) Whey pollution problem and potential utilization. Int J Food Sci Technol 23:323–336. https://doi.org/10.1111/j.1365-2621.1988.tb00586.x
Morikawa Y, Ohashi T, Mantani O, Okada H (1995) Cellulase induction by lactose in Trichoderma reesei PC-3-7. Appl Microbiol Biotechnol 44(1–2):106–111. https://doi.org/10.1007/BF00164488
Nedwell DB (1999) Effect of low temperature on microbial growth: lowered affinity for substrates limits growth at low temperature. FEMS Microbiol Ecol 30(2):101–111. https://doi.org/10.1111/j.1574-6941.1999.tb00639.x
Palamthodi S, Lele SS (2016) Optimization and evaluation of reactive dye adsorption on bottle gourd peel. J Environ Chem Eng 4(4, Part A):4299–4309. https://doi.org/10.1016/j.jece.2016.09.032
Pérez S, Baldwin PM, Gallant DJ (2009) Structural features of starch granules I., Starch,3rd edition., Chemistry and Technology.,149–192
Prajapati RP, Kalariya M, Parmar SK, Sheth NR (2010) Phytochemical and pharmacological review of Lagenaria sicereria. J Ayurveda Integr Med 1(4):266–272. https://doi.org/10.4103/0975-9476.74431
Prasad C, Karlapudi S, Rao CN, Venkateswarlu P, Bahadur I (2017) A highly resourceful magnetically separable magnetic nanoparticles from aqueous peel extract of Bottle gourds for organic dyes degradation. J Mol Liq 243:611–615
Qi B, Vu A, Wickramasinghe SR, Qian X (2018) Glucose production from lignocellulosic biomass using a membrane-based polymeric solid acid catalyst. Biomass Bioenergy 117:137–145. https://doi.org/10.1016/j.biombioe.2018.07.017
Roopan SM, Rajeswari VD, Kalpana VN, Elango G (2016) Biotechnology and pharmacological evaluation of Indian vegetable crop Lagenaria siceraria: an overview. Appl Microbiol Biotechnol 100(3):1153–1162. https://doi.org/10.1007/s00253-015-7190-0
Rosicka-Kaczmarek J.,Kwaśniewska-Karolak I.,Nebesny E., Komisarczyk A., 2018. The Functionality of wheat starch.,S tarch in Food., 2nd Edition, Woodhead Publishng Series in Food Science Technology and Nutrition., 325–352
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresources Bioprocess 5:1. https://doi.org/10.1186/s40643-017-0187-z
Silviya R, Macwan Bhumika K, Dabhi SC, Parmarand KD, Aparnathi (2016) Whey and its utilization. Int J Curr Microbiol AppSci 5(8):134–155. https://doi.org/10.20546/ijcmas.2016.508.016
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass--an overview. Bioresour Technol 199:76–82. https://doi.org/10.1016/j.biotech.2015.08.030
Słomińska L, Zielonka R, Jarosławski L (2013) The unconventional single stage hydrolysis of potato starch. Pol J Chem Technol 15(3):7–14
Spiance MAS, Lambert CS, Fermoselli KKG, De Paoli MA (2009) Characterization of lignocellulosic curaua fibers. Carbohydr Polym 77(1):47–53. https://doi.org/10.1016/j.carbpol.2008.12.005
Sternberg D, Mandels GR (1979) Induction of cellulolytic enzymes in T.reesei by sophrose. J. Bacteriol 139:761–769
Tripathi P, Singh PC, Mishra A, Chauhan P, Dwivedi S, Bais RT, Tripathi RD (2013) Trichoderma: a potential bioremediator for environmental cleanup. Clean Techn Environ Policy 15(4). https://doi.org/10.1007/s10098-012-0553-7
Tserki V, Matzinos P, Kokkou S, Panayiotou C (2005) Novel biodegradable composites based on treated lignocellulosic waste flour as filler. Part I, Surface chemical modification and caracterization of waste flour. Compos A: Appl Sci Manufact 36(7):965–974. https://doi.org/10.1016/j.compositesa.2004.11.010
Venkatraman K, Achi M (2004) To eliminate the disposal of salty whey from a dairy industry into the sewer in an environmental, social and economical way – a case study from dairy farmers, Toowoomba, Queensland, Australia. Int J Environ Technol Manag 4:365–374. https://doi.org/10.1504/IJETM.2004.005722
Verma, N., Kumar, V., Bansal, M. C., 2009. Various inducers involved in environmental viable cellulase biosynthesis. Proceedings of International Conference on Emerging Technologies in Environmental Science and Engineering, at Civil Engineering Department of Aligarh Muslim University (AMU) India in collaboration with Toledo University, U.S.A., Oct 26–28, 1690–1695
Verma N, Bansal MC, Kumar V (2011) Scanning electron microscopic analysis of Aspergillus niger pellets and biofilms under various process conditions. Int J Microbiol Res 2(1):08–11 http://idosi.org/ijmr/ijmr2(1)11/2.pdf
Verma N, Bansal MC, Kumar V (2018) Utility of Luffa cylindrica and Litchi chinensis peel, an agricultural waste biomass in cellulase production by Trichoderma reesei under solid state cultivation. Biocatal Agricult Biotechnol 16:483–492. https://doi.org/10.1016/j.bcab.2018.09.021
Virgilio S, Cupertino FB, Bernardes NE, Freitas FZ, Takeda AAS, Fontes MRM (2016) Molecular components of the Neurospora crassa pH signaling pathway and their regulation by pH and the PAC-3 transcription factor. PLoS ONE 11(8). https://doi.org/10.1371/journal.pone.0161659
Vriesekoop F, Rathband A, MacKinlay J, Bryce JH (2010) The Evolution of dextrins during the mashing and fermentation of all-malt whisky production. J Inst Brew 116(3):230–238. https://doi.org/10.1002/j.2050-0416.2010.tb00425.x
Wang CH, Hseu TH, Huang CM (1988) Induction of cellulases by cellooligosaccharides in Trichoderma koninghii G-39. J Biotechnol 9:47–60
Widowati E, Utami R, Mahadjoeno E, Saputro GP (2017) Effect of temperature and pH onpolygalacturonase production by pectinolyticbacteria Bacillus licheniformis strain GD2a insubmerged medium from Raja Nangka (Musaparadisiaca var. formatypica) banana peel waste. IOP Conf. Ser.: Mater. Sci. Eng. https://doi.org/10.1088/1757-899X/193/1/012018
Xiong Y, Wu VW, Lubbe A, Qin L, Deng S, Kennedy M, Bauer D, Singan VR, Barry K, Northen TR, Grigoriev IV, Glass NL (2017) A fungal transcription factor essential for starch degradation affects integration of carbon and nitrogen metabolism. PLoS Genet 13(5). https://doi.org/10.1371/journal.pgen.1006737
Yang S, Li J, Zheng Z, Meng Z (2009) Lignocellulosic structural changes of Spartina alterniflora after anaerobic mono and co digestion. Int Biodeterior Biodegrad 63:569–575. https://doi.org/10.1016/j.ibiod.2009.02.007
Zhang X, Zhang B, Miao R, Zhou J, Ye L, Jia D, Peng W, Yan L, Tan W, Li X (2018) Influence of temperature on the bacterial community in substrate and extracellular enzyme activity of Auricularia corne. Mycobiology 46(3):224–235. https://doi.org/10.1080/12298093.2018.1497795
Zhu F (2018) Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci Technol 78:234–242. https://doi.org/10.1016/j.tifs.2018.05.024
Acknowledgments
Authors gratefully acknowledged the ministry of human resource and development, India, for providing fellowship to carry out present research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, N., Kumar, V. Utilization of bottle gourd vegetable peel waste biomass in cellulase production by Trichoderma reesei and Neurospora crassa. Biomass Conv. Bioref. 12, 1105–1114 (2022). https://doi.org/10.1007/s13399-020-00727-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00727-9