Abstract
Cellulase is the most important enzyme in the world enzyme market, which can be synthesized by growing fungi on lignocellulosic substrates. In this study, cellulase was produced by using Trichoderma species. Twenty-three Trichoderma species were isolated and screened out for their cellulase-producing ability. Selected species, Trichoderma reesei, was further optimized on Leptochloa fusca, a perennial grass. For the higher production of enzyme, different culture conditions were optimized in flask fermenters. Our study points out that overall cellulase production was highest as 1.165 IU/ml/min at 70% moisture level, 120-h incubation period, 30 °C incubation temperature, 6 initial pH, 20% inoculum size, 0.3% NH4NO3 concentration and 0.3% concentration of surfactant (Tween 80), respectively. Under solid-state fermentation, the recovery of the cellulase from fermented substrate was optimized which yielded 1.785 IU/ml/min. Among different solvents tested, optimal extraction was attained by using citrate buffer. The optimal conditions for extraction were 90 min soaking time, 1:5 solid-to-solvent ratio, 140 revolution per minute agitation. It was detected that two washes were enough for maximum leaching of the enzyme. Results specify the admirable scope of utilizing kallar grass for biosynthesis of cellulase in solid-state fermentation employing Trichoderma reesei commercially.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most abundant polysaccharide present on the earth is cellulose. It is the major part of plant cell wall along with lignin, hemicellulose and pectin. Nearly, 40% of plant part is made up of cellulose. Although it is the most abundant carbon source, a few organisms have the ability to utilize it in efficient manners. Left over of the material from agro-wastes is one of the causes of environmental pollution. Recycling of these agro-wastes not only minimizes the risk of pollution but can also be used as an energy source and food for the future. The use of cheaper carbon source will reduce the overall production cost of enzyme or biofuel and make the process environment friendly and economically viable (Fernandes et al. 2009; Brijwani et al. 2010).
These lignocellulosic substrates can only be utilized by those microorganisms which have the capability to produce cellulytic enzymes. These enzymes are also referred as cellulases (Chinedu and Okochi 2003). Cellulase is the 3rd most important enzyme in the global enzyme market (Ilyas et al. 2013). Basically, it is not a single enzyme; it is the complex of cellulose-degrading enzymes. It consists of endo 1, 4-β glucanases, exo 1, 4-β-D glucanases and β-D glucosidases (Pothiraj et al. 2006). These enzymes synergistically act on the cellulosic substrates and convert the long biopolymer of the cellulose into the reducing sugars by cleaving their β-1-4 glycosidic linkages (Ilyas et al. 2013).
There are many microorganisms which are able to produce the large amount of the cellulase enzymes. But only few organisms have the ability to produce the cell-free cellulase enzyme which acts on the substrate in vitro condition efficiently. Filamentous fungi are best in cellulase production process, but few bacterias and some actinomycetes are also used for this purpose. In cellulase-producing fungi, mostly species of Trichoderma and Aspergillus are used (Ilyas et al. 2013). Nearly 20% of enzyme is produced from these organisms. It is also reported that Trichoderma reesei is the most used commercial organism for cellulase production (Murphy and Horgan 2005; Pirzadah et al. 2014).
Solid substrates are reported best for providing nourishing environment for the filamentous fungi. These fungi can not only grow on their surface but also penetrate in the substrate by their hyphae (Bisen and Sharma 2012). Cheap and easily available substrates can be used for this purpose such as agro-waste, agro industrial wastes, different grasses, trimmings of lawns, cotton stalk, rice husk, coffee husk, nut cakes, bagasse, fruit pulp, residues of the crops and many more (Zhang et al. 2010).
In Pakistan large area of the land is barren due to salinity. Salt tolerant grasses such as kallar grass can be grown on this area. Kallar grass Leptochloa fusca L.Kunth is also known as Diplachne fusca. It belongs to the family Poaceae and a perennial summer-growing grass. Now a days this grass is cultivated in different areas of Pakistan and India. It has the c4 mechanism for the production of the cellulosic material, so it can survive in the alkaline conditions. It also helps the soil condition to be better as it also has the ability for nitrogen fixation. This grass can produce 50 tons biomass/hectare, so it can be used as the cheap carbon source for the production of the different enzymes or biofuel production. Grasses can be the best source of lignocellulosic substrate for enzyme and biofuel production as they have less ash content and high cellulosic content. But the cost of transportation and handling should be overcome (Kretschmer et al. 2012).
In solid-state fermentation, after production of enzyme extraction is a significant step. Extraction means to leech down the maximum amount of enzyme from the fermented bran. So it is very important to select a proper solvent for leaching out the product. According to the Pal and Khanum (2010), best solvent is that which extracts maximum amount of enzyme with minimum contact time at room temperature. Selection of solvent depends upon its cost, efficacy and availability. It should reduce the cost of further downstream processing.
Present study is based upon the hypothesis that by optimizing the conditions of fermentation and extraction, more quantity of cellulase can be obtained. So, in the year 2015–2016, an effort was made to utilize the kallar grass for the production of cellulase by locally isolated spp. of Trichoderma from Lahore and Multan (Pakistan). Evaluation of different physio-chemical and nutritive parameters was also done on their cellulase-producing ability. The current communication also deals with the efficiency of leaching of the cellulase produced by Trichoderma reesei by using kallar grass (Leptochloa fusca L.Kunth) as substrate.
Materials and methods
Collection and preparation of substrate
Three different types of agro-waste were used as the substrate which were collected from different places. Cotton residual plant was taken from a field near Manga Mandi Lahore. Rice husk was taken from a rice factory in Sialkot, and Leptochloa fusca commonly called as kallar grass was collected from bank of River Ravi Lahore. These agro-wastes were dried and then ground into powder form using 3-mm mesh. Then they were packed in polythene bags and stored at ambient temperature for subsequent studies.
Isolation and identification of fungi
Different species of Trichoderma were isolated from canal water, air, leaf litter and diseased samples of plants. From canal water and agricultural soil samples, fungal colonies were obtained by serial dilution method. While pieces of rotten and disease fruit, leaf and wood samples were cut into small pieces, surface-sterilized them with 1% sodium hypochlorite and then kept on the potato dextrose agar medium (PDA) for 5 days at 28 °C. By re-culturing, single colony pure cultures were obtained. Colonial morphological characteristic was observed for one week. Microscopic identification of pure cultures was carried out by using the identification key illustrated by Samuels and Hebbar (2015).
Plate screening assay of Trichoderma spp
Screening assay of isolated species of Trichoderma was done with the method of Ariffin et al. (2008). Briefly, 1 μl spores suspension of each fungus was inoculated in the well made on the screening plates. These plates were made by PDA supplemented with 2% (w/v) carboxymethylcellulose (CMC). Then they were incubated at 29 ± 1 °C for 48 h. Then they were first stained with 1% Congo red stain for 15 min. and then distained by washing them with 1 M NaCl. Formation of a clear zone around the colony represented the synthesis of extra cellular cellulase by the tested fungal isolate.
Selection of substrate
Three substrates such as Kallar grass, cotton stalk and rice husk were used for the fermentation process with the fungi selected from plate screen assay. First solid-state fermentation process was carried out under non-optimized conditions. For this purpose, 5gm crushed substrates was taken in the flask and moistened with the 5 ml of vogel’s media and distilled water. Fermentation mixture was autoclaved at 121 °C for 15 min at 80% initial moisture level. Flasks containing fermentation mixture were left for cooling down and inoculated with 1 ml of spore suspension of selected Trichoderma sp. Then they were placed in incubator for 7 days in static condition at 25 °C.
Optimization of factors affecting cellulase production
At selected substrate, various process parameters were optimized to obtain the maximum cellulase production. The plan adopted for this was to determine the effect of a specific parameter and integrate it at optimum level before standardizing the succeeding parameter. Moisture level is the key factor in SSF. So, different moisture levels such as 65, 70, 75, 80, 85 and 90% were used to optimize maximum production of cellulase. Only quantity of distilled water was changed but quantity of vogel’s media remains constant as 5 ml. Best fermentation period was investigated by withdrawing the fermented flasks after regular intervals of time from 72 to 216 h. Both organic (peptone, yeast extract, peptone, malt extract and urea) and inorganic (NH4Cl, (NH4)2SO4, NH4NO3, (NH4)3PO4, NaNO3) nitrogen sources were used to examine the suitable nitrogen source for enzyme production. All nitrogen sources were used at concentration of 1% w/w at pre-optimized conditions of incubation time and moisture level. Different concentrations of best selected nitrogen source were also optimized. The optimum pH was determined by adjusting the pH of the vogel’s media within the range of 3.5–7.5. For investigating the impact of inoculum size, different levels of inoculum 15, 20, 25, 30, 35 and 40% were used in duplicate. For exploration of the effect of surfactants (Tween 20 and Tween 80) on synthesis of cellulase enzyme, five different concentration levels as 0.1, 0.2, 0.3, 0.4, 0.5 of Tween 20 and Tween 80 were studied. They were used in duplicate in pre-optimized conditions.
Enzyme extraction and assay
This process was conducted by soaking 5 g of fermented substrate in 250-ml conical flask with a suitable solvent for 30–120 min. Then crude enzyme extract was squeezed out through a cotton cloth. The clear extract was obtained after centrifugation at 8,000 rpm for 20 min and then assayed for cellulolytic activity. In this study, different parameters of extraction were optimized such as selection of best solvent and its ratio to the substrate, incubation time for soaking, physical states of leaching and no. of washes. Three, three replicates were used for each parameter in order to support their validity.
Enzyme assay was performed according to Acharya et al. (2008). The reducing sugar released in this process was determined with DNS (3, 5-dinitrosalicylic acid) by spectrophotometer at 540 nm. One unit of enzyme activity can be define as amount of reducing sugar released by the action of enzyme per ml per minute in standard assay conditions.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) using Co-stat version 3.03 software. Differences among means were analyzed through Duncan’s multiple range test at significant level of p ≤ 0.05 (Steel et al. 1997).
Results and discussion
Selection of fungi and substrate
Twenty-three isolates of Trichoderma were obtained from different sources and were identified as shown in Table 1. Results of the screening plate assay reveal that Trichoderma reesei was most potential cellulase-producing organism. Out of three substrates used in the experiment, Kallar grass showed the highest enzyme activity 0.158 U/ml than cotton stalk 0.099 IU/ml and rice husk 0.0263 IU/ml. So Kallar grass was selected as substrate for further studies. Previously, Rajoka (2004) also reported kallar grass as the best substrate for the production of the cellulase by Cellulomonas flavigena.
Optimization of physical parameters
All organisms need appropriate amount of water for their survival and supreme performance. So the proper moisture level of the substrate is one of the key factors in the solid-state fermentation (SSF). It depends upon the requirements of the microorganisms for better production of enzymes. Experiment with different substrate moisture level was carried out in flasks which were incubated at room temperature for 7 days. Maximum cellulase production 0.1368 IU/ml/min was observed at the 70% moisture level as represented in Fig. 1a. Same observations for Trichoderma reesei were reported by Latifian et al. (2007) who reported that the moderate amount of water produces maximum cellulase, whereas low and high amounts of solvent decrease the cellulase production. These types of results were also detected by Toor and Ilyas (2014). Like availability of the moisture, the fermentation period also has a great effect on enzyme production. Proper incubation time was significant for enzyme production. Experiment was carried out for the determination of optimum incubation period for the Trichoderma reesei on kallar grass. It was observed that at 120 h, the enzyme activity reached the maximum (0.275 IU/ml/min) with 70% moisture level at room temperature. After 120 h, the enzyme activity began to decrease. Figure 1b describes that after 216 h enzyme activity became 0.0991 IU/ml/min. This decrease in the enzyme productivity may be due to the shortage of nutrients or space in the fermentation media which lead to the disturbance in cellulase-producing machinery of the fungi or it may be due to the toxic effect of the fungal byproducts or degradation of produced cellulase. Bilal et al. (2015) also conduct an experiment for the investigation of the incubation period required for Trichoderma reesei for cellulase production. He indicated the same time as 120 h best for cellulase production. Cellulolytic enzymes were produced by Aspergillus phoenix at 120 h. incubation by Dedavid e Silva et al. (2009).
All organisms and chemicals are temperature-sensitive. Fluctuations in temperature may lead to the death of fungi, disturbance in enzyme production system or the degradation of the produced enzyme. So it is necessary to define the optimal temperature of fugal environment to produce maximum cellulase. Figure 1c shows that optimum cellulase production (1.165 IU/ml) was found at 30 °C with 70% moisture level and 120 h of incubation time. It was apparent that when the incubation temperature increased up to 30 °C, the enzyme activity decreased. At 50 °C it became very less as 0.612 IU/ml/min. Bilal et al. (2015) also point toward the same conditions. He reported that cellulase and xylanase remain active in range of 28–32 °C. Liu and Yang (2007) also confirm the present study by observing the same temperature for Trichoderma koningii. Guowei et al. (2011) also reported the same incubation temperature for Trichoderma reesei for cellulase production. It was observed that fungus grows well and produces cellulase in slightly acidic conditions as that maximum enzyme production 1.165 IU/ml/min was observed at pH 6 with pre-optimized conditions of incubation period, moisture level and temperature. Further raise in pH resulted in a lessening trend in production of enzyme. At pH 8 enzyme production was 0.917 IU/ml/min. It was also described in Fig. 1d. It was noticeable Liu and Yang (2007) also reported that acidic conditions at pH 5 are best for CMCase production. Toor and Ilyas (2014) also observed that low pH level is best for the cellulase production by filamentous fungi. Bilal et al. (2015) also reported that pH 5.5 of media gives minimum cellulase and xylanase production. Fatokun et al. (2016) also reported pH 6 for cellulase production.
Optimization of chemical parameters
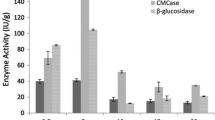
To sustain life, fungi need different nutrients. These compounds not only stimulate the fungal growth and reproduction but also have some effect on the production of the enzymes and metabolites. Different organic (malt extract, yeast extract, peptone, tryptone and urea) and inorganic (ammonium chloride, ammonium sulfate, ammonium nitrate, ammonium phosphate and sodium nitrate) compounds were added to the culture media to measure their effect on cellulase production at pre-optimized physical parameters. Figure 2a, b depicts that supplementation with different nitrogen sources stimulates the cellulase-producing ability in the fungi. All examined compounds except urea stimulated the growth and cellulase production. The inorganic compounds stimulated higher cellulase yields compared with organic compounds. It may be due to the rapid mode of action of inorganic compounds. Among the organic compounds, the highest cellulase production 0.275 IU/ml/min was obtained with tryptone and in inorganic sources ammonium nitrate gives the best cellulase production which is 0.673 IU/ml/min. The results are also in close conformity to Singhania et al. (2006) who reported maximum CMCase production at 7 pH, 35 °C of incubation temperature and NH4NO3 as nitrogen source. NaNO3, KNO3 and NH4NO3 were the best sources for cellulase production reported by Rajoka (2004).
Effect of chemical parameters on the cellulase production. a Organic nitrogen source, b inorganic nitrogen source, c concentration of selected inorganic nitrogen source, d inoculum size, e Tween 80, f Tween 20. The value with different letters shows the significant difference (P ≤ 0.05) determined by DMRT
Further Trichoderma reesei was grown in the presence of different NH4NO3 concentrations (0.1–0.6%; w/w). Higher cellulase production was obtained at all tested concentrations. But the superlative enzyme production was obtained with 0.3%. Further increase in concentration does not have any significant effect on cellulase production as shown in Fig. 2c. This may be due to saturation of the media at 0.3% with nitrogen source. Further increase leads to the decreased production of enzyme, as it becomes the toxic to fungi. The effect of spores per milliliter of suspension on the production of cellulase was also studied. The maximum production of cellulase 1.165 IU/ml/min was observed at 20% inoculums size. Further increase in the conc. of inoculum decreases the production of enzyme as shown in Fig. 2d. Enzyme production became 1.117 IU/ml/min at 40% inoculum size. Ilyas et al. (2012) also reported that 20% of inoculum and 0.2% concentration of inorganic nitrogen source are enough for cellulase production by using A. terreus. Alam et al. (2005) reported that the higher size of inoculums size is (20–25%).
The effect of surfactants was inspected by providing different concentrations of them to the growth medium in different concentrations. The results in Fig. 2e, f reveal that Tween 80 increases the cellulase production more than Tween 20. The highest cellulase yield 1.073 IU/ml/min was obtained in medium containing (0.4%) Tween 20 and 1.118 IU/ml/min in medium containing (0.3%) of Tween 80. These are in similarity to the results of the Pardo (1996) and Gashe (1992) who observed that 0.1% and 0.2% of Tween 80 concentration in medium is best for enhanced cellulase production, respectively. Basically Tween 80 reduces the pallet formation in the media. The factors which reduce the pallet formation ultimately increase the cellulase production as fungus becomes more active in degradation of cellulose.
Optimization of different extraction parameters for cellulase enzyme
The efficiency of extraction process is most critical thing in recovery of the enzyme from the fermented bran. Extraction is a process in which solute is recovered from the solid biomass by using suitable solvent. So, selection of an appropriate solvent, solid-to-solvent ratio, contact time, physical state of extraction and no. of washes used for enzyme extraction are all key factors. Among the different chosen solvents, 0.1 M citrate buffer (pH 4.6) served as the best leachate in extracting cellulase 1.474 IU/ml/min from the fermented Kallar grass as shown in Fig. 3a. Therefore, subsequent optimization experiments were carried out only with citrate buffer. As in SSF system, free flowing amount of solvent is limited. So for the extraction of enzyme sufficient amount of the solvent is required. If the amount of solvent is less, then recovery of enzyme becomes hard because less amount of solvent is availed to penetrate in the solid mass to leech out the enzyme. In other hand, if the large amount of the solvent is used, then the leachates become very dilute and extra efforts are required to make it concentrated for further processing. So it is necessary to maintain the product in a concentrated form to achieve the highest leaching efficiency. To optimize the best ratio between solid and solvent, four ratios were used 1:5, 1:10, 1:15 and 1:20. The results are represented in Fig. 3b. At the solid-to-solvent ratio of 1:5, 25 mL solvent was best for the extraction of enzyme from 5 g of fermented substrate. Enzyme activity was decreased when higher volume of the solvent used for extraction due to dilution factor. Similar results were depicted by Chandra et al. (2010) in extraction process of Fpase from fermented bran of Aspergillus niger. After selection of suitable substrates and its volume, contact time of solid to solvent should also be optimized. In this study, incubation time period was varied from 30 to 150 min. But 90 min incubation time of solvent and solid achieved maximum recovery of cellulase, and thereafter no valuable effect on the enzyme extraction was observed and described in Fig. 3c. Further increase in the contact time slightly decreased the leaching of the enzyme. This decrease is may be due to inactivation of the enzyme. In leaching of enzyme, conditions (stationary and shaking) are also very important.
Represents the factors affecting the extraction process of cellulase from the fermented bran of Kallar Grass a different solvent used, b ratio of selected solvent to the solid substrate, c time, d agitation speed, e no. of washes. The value with different letters shows the significant difference (P ≤ 0.05) determined by DMRT
The results in Fig. 3d also showed that all shaking conditions were more effective for the leaching practice then the stationary 1.54 IU/ml/min. Among the shaking condition, optimum enzyme was extracted at 140 rpm 1.776 IU/ml/min.
It can be easily defensible as on agitation, fermented substrate distributed evenly in the solvent, so reduction in concentration polarization resulted. Among the shaking condition optimum enzyme was extracted at 140 rpm. But there is not much difference between the 120 and 140 rpm. In earlier experiments, cellulase extraction process was carried out by a single wash with the solvent. For the determination, whether a single wash is enough for completely recover the enzyme or not, 2nd, 3rd and 4th no. of washes were done. It was done with the fermented substrate collected from the previous wash by adding fresh solvent. Results indicate that the enzyme recovery was maximum in the first two washings only as shown in Fig. 3e. First wash was more effective which gave 1.785 IU/ml/min, while the last wash only contained 0.61 IU/ml enzyme.
These studies are also supported by different workers. The use of citrate buffer for 1 h at room temperature is also reported for the leaching of enzyme cellulase by Fadel (2000). Chandra et al. (2010) also reported distal water as the best solvent for the extraction of β-endoglucanase when use for 30 min, at 1:4 ratio of solid to solvent. Pirota et al. (2013) have also shown that a single wash was enough to provide effective endoglucanase recovery.
Conclusion
This study revealed that environment is full of beneficial microorganisms. By plate screen method, we can guess their cellulolytic ability and then can further use them on different cheap and abundantly available substrates such as kallar grass. Kallar grass has great potential to be used as carbon source in the SSF. This also resolve the environmental problem by using grass as lignocellulosic substrate which otherwise becomes the agro-wastes. Production of cellulase by Trichoderma reesei can be significantly enhanced by optimizing different physio-chemical and nutritional conditions. Due to optimization, biosynthesis of cellulase becomes the cost-effective process and can further be used for producing the economical biofuel on commercial basis. After production, leaching of enzyme is also very critical step. Effective leaching makes the further studies more convenient and effective. Further investigations on large scale are required to make this study fruitful and beneficial.
References
Acharya PB, Acharya DK, Modi HA (2008) Optimization for cellulose production by Aspergillus niger using saw dust as substrate. Afr J Biotechnol 22:4147–4152
Alam Z, Muhammad N, Mahmat ME (2005) Production of cellulase from oil palm biomass as substrate by solid state bioconversion. J Appl Sci 2:569–572
Ariffin H, Abdullah N, Umikalsom MS, Shirai Y, Hassan MA (2008) Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by Bacillus pumilus EB3. J Biosci Bioeng 106:231–236
Bilal T, Malik B, Reiaz RU, Kumar M (2015) Influence of various parameters on cellulase and xylanase production by different strains of trichoderma species. Aust J Anal Pharm Chem 2(1):1034
Bisen PS, Sharma A (2012) Introduction to instrumentation in life sciences. CRC Press, Boca Raton
Brijwani KH, Oberoi S, Vadlani PV (2010) Production of a cellulolytic enzyme system in mixed-culture solid state fermentation of soybean hulls supplemented with wheat bran. Process Biochem 45:120–128
Chandra MA, Kalra PK, Sharma H, Kumar, Sangwan RS (2010) Optimization of cellulases production by Trichoderma citrinoviride on marc of Artemisia annua and its application for bioconversion process. Biomass Bioenerg Article in press
Chinedu SN, Okochi IV (2003) Cellulase production by wild-type Aspergillus niger, Penicillium chrysogenum and Trichoderma harzianum using waste cellulosic materials. Idi-Araba, Lagos, Nigeria
Dedavid e Silva LA, Lopes FC, Silveira ST, Brandelli A (2009) Production of cellulolytic enzymes by Aspergillus phoenicis in grape waste using response surface methodology. Appl Biochem Biotechnol 152:295–305
Fadel M (2000) Production physiology of cellulases and β-glucosidase enzymes of Aspergillus niger grown under solid state fermentation conditions. J Biol Sci 1:401–411
Fatokun EN, Nwodo UU, Okoh AI (2016) Classical optimization of cellulase and xylanase production by a marine streptomyces species. Appl Sci 6:286. https://doi.org/10.3390/app6100286
Fernandes P, Marques MP, Carvalho F, Cabral J (2009) A simple method for biocatalyst immobilization using PVA-based hydrogel particles. J Chem Technol Biotechnol 84:561–564
Gashe BA (1992) Cellulase production and activity by Trichoderma sp. A-001. J Appl Microbiol 73(1):79–82. https://doi.org/10.1111/j.1365-2672.1992.tb04973.x
Guoweia S, Man H, Shikai W, He C (2011) Effect of some factors on Production of cellulase by Trichoderma reesei HY07. Procedia Environ Sci 8:357–361
Ilyas U, Ahmed S, Majeed A, Nadeem M (2012) Biohydrolysis of Saccharum spontaneum for cellulase production by Aspergillus terreus. Afr J Biotechnol 11(21):4914–4920. https://doi.org/10.5897/AJB11.1194
Ilyas U, Gohar F, Saeed S, Bukhari Z, Ilyas H (2013) Screening of locally isolated Aspergillus species for their cellulolytic potential and their optimization on Vigna mungo in solid state fermentation. Br Biotechnol J 3(3):350–358
Kretschmer B, Allen B, Hart K (2012) Mobilising Cereal Straw in the EU to feed Advanced Biofuel Production. Report produced for Novozymes. Institute for European Environmental Policy (IEEP), London
Latifian M, Hamidi-Esfahani Z, Barzegar M (2007) Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermentation conditions. Bioresource Technol 98:3634–3637
Liu J, Yang J (2007) Cellulase Production by Trichoderma koningii AS3.4262 in Solid-State Fermentation Using Lignocellulosic Waste from the Vinegar Industry. Food Technol Biotechnol 45:420–425
Murphy RA, Horgan KA (2005) Antibiotics, enzymes and chemical commodities from Fungi. Biol Appl, Fungi, pp 113–143
Pal A, Khanum F (2010) Production and extraction optimization of xylanase from Aspergillus niger DFR-5 through solid-state-fermentation. Bioresource Technol 101:7563–7569
Pardo AG (1996) Effect of surfactants on cellulase production by Nectria catalinensis. Curr Microbiol 33:275. https://doi.org/10.1007/s002849900113
Pirota RDPB, Miotto LS, Delabona PS, Farinas CS (2013) Improving the extraction conditions of endoglucanase produced by Aspergillus niger under solid-state fermentation. Braz J Chem Eng 30:117
Pirzadah T, Garg S, Singh J, Vyas A, Kumar M, Gaur N (2014) Characterization of Actinomycetes and Trichoderma spp. for cellulase production utilizing crude substrates by response surface methodology. BMC Springer Plus 3: 622
Pothiraj C, Balaji P, Eyini M (2006) Enhanced production of Cellulases by various fungal cultures in solid state fermentation of cassava waste. Afr J Biotechnol 5:1882–1885
Rajoka MI (2004) Influence of various fermentation variables on exo-glucanase production in Cellulomonas flavigena. Electr J Biotechnol 7(3):0717–3458
Samuels JG, Hebbar KP (2015) Trichoderma identification and agricultural applications. The American phytopathological society St. Paul, Minnesota, USA
Singhania RR, Sukurmaran RK, Pillai A, Prema P, Szakacs G, Panday A (2006) Solid state fermentation of lignocellulosic substrates for cellulase production by Trichoderma reesei NRRL 11460. Indian J Biotechnol 5:332–336
Steel RGD, Torrie JH, Dickey DA (1997) Principles and procedures of statistics: A biometrical approach, 3rd edn. McGraw Hill Book Co. Inc, New York, pp 400–438
Toor Y, Ilyas U (2014) Optimization of Cellulase Production by Aspergillus ornatus by the Solid State Fermentation of Cicer arietinum. Am J Res Commun 2(1):125–141
Zhang Z, Lohr L, Escalante C, Wetzstein M (2010) Food versus fuel: what do prices tell us? Energy Policy 38:445–451
Acknowledgements
Authors want to thank Dr. Shahjahan Baig for providing the laboratory facilities for enzyme analysis at Pakistan Council of Scientific and Industrial Research (PCSIR) Lahore, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibiility: M. Abbaspour.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kalsoom, R., Ahmed, S., Nadeem, M. et al. Biosynthesis and extraction of cellulase produced by Trichoderma on agro-wastes. Int. J. Environ. Sci. Technol. 16, 921–928 (2019). https://doi.org/10.1007/s13762-018-1717-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1717-8