Abstract
Rising salinity in agricultural land is a major barrier limiting yields of crops like rice, which has been reported as a salt-sensitive plant. An improvement in salt tolerant ability of rice has been achieved by obstructing the sodium (Na) transpiration flow via apoplastic route. Here we assess the potential of an antitranspirant (AT) in modulating Na enrichment and yield in rice under salt stress. Sodium concentration in flag leaf was enriched in relation to the salt exposure time and significantly decreased in plants grown under 0.2% AT foliar application. Free proline accumulation in salt-stressed plants was increased by 15.3 folds over the control, whereas it was stable in plants grown under 0.2% AT foliar application. Chlorophyll a, chlorophyll b, total chlorophyll, total carotenoids, photon yield of photosystem II (PSII), net photosynthetic rate, transpiration rate, and stomatal conductance in salt-stressed plants were lower than the control with the exogenous foliar spray of 0.2% AT. Positive relationships between total chlorophyll and photon yield of PSII, photon yield of PSII and net photosynthetic rate, and net photosynthetic rate and fertile seed were also evident. Number of seeds per panicle in salt-stressed plants was significantly enhanced by 0.2% AT foliar application, whereas other yield attributes declined. Antitranspirants could be a promising option to improve the growth and yield of rice cultivated on salt-affected soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil salinity is a major abiotic constraint regulated by global climate change (Corwin 2021), which negatively affects plant growth and development and reduces crop yield (Shahid et al. 2018; Sahab et al. 2021). Salt-affected area in the arid and semi-arid regions is gradually and steadily increasing (Kılıc et al. 2022; Singh 2022). A soil is saline due to the presence of high-concentrated salts, such as sodium chloride (NaCl), sodium sulfate (Na2SO4) and magnesium sulfate (MgSO4) (Kumar and Sharma 2020; Hopmans et al. 2021) In halophytic species, adaptive mechanisms, i.e., Na+ homeostasis, compartmentalization, secretion, osmoregulation, antioxidant production, and hormonal regulation provide tolerance to high salt concentration (Loconsole et al. 2019; Rahman et al. 2021; Islam et al. 2022). In contrast, glycophytic plants, including many cultivated crop species, are very sensitive to high soil salinity, due to the lack of key salt-defense mechanisms (Hasegawa et al. 2000; Hasegawa 2013).
Rice (Oryza sativa L.) is a staple food crop for more than half of the world’s population, especially for 3.5 billion people in Asia (Devkota et al. 2019). It is a major source of carbohydrate, antioxidants, oils, fiber, minerals, and vitamins, which also help against metabolic disorders and cardiovascular diseases (Khush 2005; Sen et al. 2020; Behl et al. 2021). Rice has been reported as a salt-susceptible crop and salt stress leads to the inhibition of plant growth, development, and crop yield (Fahad et al. 2019; Schneider and Asch 2020). In general, sodium (Na) is a dominant cation, which is accumulated in large amounts in the root tissues (restriction) of rice (Plett and Møller 2010; Chakraborty et al. 2019) and bypass flow or apoplastic route to translocate Na from root to other organs (Faiyue et al. 2010, 2012) via transpiration stream (Hasanuzzaman et al. 2018), thereby reducing photosynthetic efficiency (Gadelha et al. 2021). Moreover, the application of abscisic acid (ABA), a plant hormone, which controls the stomatal closure, has been reported to limit the Na movement via transpiration flow (Parveen et al. 2021; Sharmin et al. 2021).
Antitranspirants have been widely applied for enhancing abiotic defense mechanisms in various crops grown under drought (AbdAlla et al. 2019), salt stress (Boari et al. 2016), high temperature stress (Cirillo et al. 2021), and ozone pollution (Francini et al. 2011), and are constituted by three classes, such as metabolic (s-ABA, chitosan, fulvic acid), reflective (kaolin, CaCO3, CaO), and film-forming (di-1-p-menthene, poly-1-p-menthene, acrylic polymers) (Mphande et al. 2020). The performance of the application of antitranspirants is highly dependent on plant species, developmental stage, and plant density (Morsy and Mehanna 2022; Mphande et al. 2022). Antitranspirant (AT)-induced negative effects have also been reported, including stomatal closure to limit CO2 assimilation, leaf temperature increase, and sunburn (Rodriguez et al. 2019). Pathumthani 1 rice genotype has been reported as a salt-susceptible species, exhibiting reduced plant growth and development when subjected to high salt treatment (Cha-um et al. 2009). It was hypothesized that the foliar spray of AT on the leaf tissues of Pathumthani 1 might limit transpiration, which in turn could reduce Na translocation from root to shoot and alleviate Na toxicity in terms of osmotic adjustment, photosynthetic pigment stabilization, photosynthetic capacity, and yield stability. The objective of this study was to evaluate the potential of an AT in modulating crop growth and yield in rice under salt stress.
2 Material and methods
2.1 Plant material, antitranspirant, and salt treatments
Seeds of indica rice (Oryza sativa L. ssp. indica cv. Pathumthani 1) were collected from the Pathum Thani Rice Research Center, Rice Department, Ministry of Agriculture and Cooperatives, Thailand. The seeds were surfaced sterilized by 20% Clorox® [8.25% ai sodium hypochlorite (w/v), Clorox, CA, USA] for 15 min, primed in the water for 12 h, and sowed in the water-saturated soil (EC = 2.69 dS m−1, pH = 5.7, organic matter = 12.26%, total nitrogen = 0.30%, total phosphorus = 578 mg kg−1, total potassium = 3073 mg kg−1, calcium = 7020 mg kg−1, and magnesium = 1034 mg kg−1). Four weeks after seed germination, individual rice seedling was directly transplanted into clay pots (ϕ 15 cm and 30 cm in height) containing soil. Fertilizer (16-16-16: N-P-K) was applied three times after 15 days (initial establishment stage), 45 days (vegetative stage), and 90 days (booting stage) of transplanting, based on the recommendation of Rice Department, Ministry of Agriculture and Cooperatives, Thailand. At booting stage, 50 mL per plant of 0.0% and 0.2% AT [Gustec-S®, 25.2% ai (w/v) sucrose esters of fatty acids, BIOSAFFER Co., Ltd., Thailand] were applied as a foliar spray using a hand-held sprayer. Then, 0 and 200 mM NaCl solution were applied to the pots, representing the control and salt stress treatments, respectively. Concentrations of Na+, K+, and Ca2+ were determined in the flag leaf collected at 1, 3, 5, 7, and 14 days after NaCl treatments. Biochemical and physiological data were also collected 14 days after NaCl treatments. Yield attributes were recorded at harvesting stage in each treatment.
2.2 Na+, K+, and Ca2+ assay
Na+, K+, and Ca2+ concentrations [mg g−1 dry mass (DM)] were assayed following the modified method of Tanaka et al. (1999) and Hossain et al. (2006). Flag leaf tissue of rice was washed by deionized water to remove excess ions on the surface of each leaf. Plant samples were dried at 80 °C for 3 days, ground into powder using mortar with liquid nitrogen, and then dissolved by deionized water. The ions in plant tissues were extracted after boiling in water for 15 min and centrifuged at 10,000 × g for 10 min. Supernatant was then diluted into 100 times and filtered through a 0.45 µm-filter membrane (VertiPure™, Vertical®, Thailand). Fifty µL of solution was applied into WATER IC-PACKTM ion exclusion column. The column was equipped with high-performance liquid chromatography (HPLC) system (e595 Alliance, Waters Associates, Milford, MA, USA) and 432-Conductivity Detector (Waters Associates, Milford, MA, USA). A mobile phase of 3 mM nitric acid and 0.1 mM EDTA was supplied with a flow rate at 1 mL min−1. Concentrations of Na+, K+, and Ca2+ were determined, based on standard curve of each ion (Sigma®, MA, USA).
2.3 Biochemical and physiological traits
Free proline concentration [μmol g−1 fresh mass (FM)] in the flag leaf was estimated according to the method of Bates et al. (1973). In brief, 50 mg of fresh samples were ground in a mortar with liquid nitrogen. One mL of aqueous sulfosalicylic acid (3%, w/v) was added into the homogenate powder and then filtered through a filter paper (Whatman™ #1). The supernatant was reacted with an equal volume of glacial acetic acid and ninhydrin reagent (1.25 mg ninhydrin in 30 mL glacial acetic acid and 20 mL 6 M H3PO4) and boiled at 95 °C in water bath for 1 h. Then, the reaction was stopped by incubating the reaction mixture in an ice bath. The reaction mixture was mixed vigorously with 2 mL of toluene. After cooling at 25 °C, the chromophore (pink color) was collected and measured at 520 nm using UV–VIS spectrophotometer (HACH DR/4000; HACH Company, Loveland, CO, USA). L-proline (0 − 20 μmol mL−1) standard was validated as the calibration standard.
Chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (TChl), and total carotenoid (Cx+c) concentrations in the flag leaf were measured according to the method of Shabala et al. (1998). In brief, 100 mg of leaf tissues were chopped and transferred to glass vials containing 10 mL of 99.5% acetone and blended using a homogenizer. The glass vials were sealed with Parafilm® (Sigma-Aldrich, USA) to prevent acetone evaporation, and kept at 4 °C in the darkness for 48 h. Chla, Chlb, and Cx+c concentrations [μg g−1 fresh mass (FM)] were measured at 662 nm, 644 nm, and 470 nm, respectively, using UV–VIS spectrophotometer against acetone (99.5%) as a blank.
Chlorophyll a fluorescence in the flag leaf was measured using a fluorescence monitoring system (FMS 2; Hansatech Instruments Ltd., Norfolk, UK) with the pulse amplitude modulation mode (Loggini et al. 1999). In brief, a dark-adapted leaf kept for 30 min was subsequently exposed to the modulated measuring beam of far-red light (LED source) with a typical peak at wavelength 735 nm. Initial fluorescence (F0) and maximum fluorescence (Fm) yields were measured under weakly-modulated red light (< 85 μmol m‒2 s‒1) with 1.6 s pulse of saturating light (> 1,500 μmol m−2 s−1photosynthetic photon flux density) and calculated using FMS software for Windows®. The variable fluorescence yield (Fv) was calculated by the equation: Fv = Fm – F0. The ratio of variable to maximum fluorescence (Fv/Fm) was calculated as the maximum quantum yield of PSII photochemistry. The photon yield of PSII (ΦPSII) in the light was calculated as: ΦPSII = (Fm′ − F)/Fm′ after 45 s of illumination when steady state was achieved (Maxwell and Johnson 2000), where F is a steady-state yield and Fm′ is a maximum fluorescence yield.
Net photosynthetic rate (Pn; μmol CO2 m‒2 s‒1), stomatal conductance (gs; mmol H2O m‒2 s‒1), and transpiration rate (E; mmol H2O m‒2 s‒1) in the flag leaf were recorded using a portable photosynthesis system fitted with an infra-red gas analyzer (LI 6400XT, LI-COR, Lincoln, NE, USA), following the method of Cha-um et al. (2007). The gs and E were auto-calculated by monitoring the H2O of air entering and exiting the infra-red gas analyzer head space chamber. The flow rate of air in sample line and micro-chamber temperature was set at 500 μmol m‒2 s‒1 and 27 ± 1 °C block temperature, respectively. The light intensity was set at 1,000 μmol m‒2 s‒1 photosynthetic photon flux density by 6400-02B red-blue light emitting diode (LED) light source.
2.4 Yield attributes
Panicle mass, panicle length, number of panicles per plant, number of seeds per panicle, fertile seed percentage, and one-hundred grain mass in each treatment were recorded (IRRI 2002).
2.5 Experimental layout and data analysis
The experiment was arranged in 2 × 2 factorials in completely randomized design with four replications. The data were subjected to a two-way analysis of variance (ANOVA) and were analyzed using Statistical Package for the Social Sciences (SPSS) software (version 11.5 for Window®). The mean values obtained from four treatment combinations, from either antitranspirant or NaCl, were compared using Tukey’s honest significant difference test at p ≤ 0.05.
3 Results
3.1 Morphology and biochemical changes
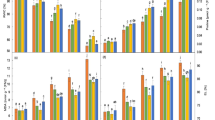
Leaf burning, leaf rolling, and leaf chlorosis in salt-stressed plants without the application of AT (0.0%) were observed, whereas the toxic damages were reduced by 0.2% AT foliar application (Fig. 1). Under 200 mM NaCl, Na+ concentration in older leaf (third from the top) without AT was the maximal at 31.1 mg g−1 DM, causing leaf chlorosis. A reduction of Na+ in the leaf tissues was observed (19.6 mg g−1 DM) when sprayed with 0.2% AT (Fig. S1). In flag leaf, Na+ was significantly increased in relation to NaCl exposure period, whereas the increment was comparatively slower under 0.2% AT application (Fig. 2a). In contrast, K+ concentration in plants under salt stress was significantly declined irrespective of AT applications when compared with the control (Fig. 2b). Na:K ratio was also the maximal in plants without foliar application of AT when exposed to 200 mM NaCl for 14 days (Fig. 2c). Likewise, Ca2+ concentration was also increased in salt-stressed plants and it was the highest in 0.2% AT-treated plants under salt stress. Free proline accumulation in flag leaf tissues of salt-stressed plants without AT was the maximal at 3.98 μmol g−1 FM and it was reduced to 1.08 μmol g−1 FM in 0.2% AT-treated plants (Table 1).
Sodium (Na+; a), potassium (K+; b), sodium:potassium ratio (Na+:K+; c), and calcium (Ca.2+; d) concentrations in flag leaf tissues of Pathumthani 1 rice genotype treated with or without antitranspirant (AT) and subsequently exposed to 0 and 200 mM NaCl for 1, 3, 5, 7, and 14 days. Data are presented as means of four replications ± standard errors. Different letters in a bar represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05
3.2 Physiological changes
Chla, Chlb, and Cx+c concentrations in the salt-stressed plants without AT were significantly decreased by 50.43%, 49.13%, and 80.16% over the control, respectively. Interestingly, in the salt-stressed plants with 0.2% AT, these parameters were reduced by 33.21%, 47.29%, and 68.61%, respectively, compared with the control (Table 1). Total chlorophyll (TChl) concentration in the leaf tissues of salt-stressed plants without AT was significantly declined by 49.83% over the control and TChl of salt-stressed plants with 0.2% AT was also degraded by 39.65% over the control without NaCl (Fig. 3a). A negative relationship between Na+ enrichment and TChl concentration was demonstrated (Fig. 3b; R2 = 0.87). Fv/Fm did not vary significantly among the treatments (Fig. S2a), whereas ΦPSII in the salt-stressed plants without AT and 0.2% AT was significantly diminished by 17.15% and 9.53% over the control, respectively (Fig. 3c). A positive relationship between TChl concentration and ΦPSII was evident (Fig. 3d; R2 = 0.57). Consequently, Pn in the salt-stressed plants without AT and 0.2% AT was sharply dropped by 93.94% and 75.14% over the control, respectively (Fig. 4a). Moreover, gs and E in the salt-stressed plants were also decreased (Fig. S2b; R2 = 0.80) in plants without AT (50.0% and 85.71% reduction over the control, respectively) (Table 1). A positive relationship between ΦPSII and Pn (Fig. 4b; R2 = 0.65) and Pn and fertile seed percentage of rice grain was demonstrated (Fig. 4d; R2 = 0.74).
Total chlorophyll concentration (a), relationship between sodium concentration and total chlorophyll concentration (b), photon yield of PSII (c), and relationship between total chlorophyll concentration and photon yield of PSII (d) in flag leaf tissues of Pathumthani 1 rice genotype treated with or without antitranspirant (AT) and subsequently exposed to 0 and 200 mM NaCl for 14 days. Data are presented as means of four replications ± standard errors. Different letters in a bar represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05
Net photosynthetic rate - Pn (a), relationship between photon yield of PSII and net photosynthetic rate - Pn (b), fertile seed (c), and relationship between net photosynthetic rate - Pn and fertile seed (d) in flag leaf tissues of Pathumthani 1 rice genotype treated with or without antitranspirant (AT) and subsequently exposed to 0 and 200 mM NaCl for 14 days. Data are presented as means of four replications ± standard errors. Different letters in a bar represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05
3.3 Yield attributes
Fertile seed percentage in salt-stressed plants with and without AT was significantly decreased by 75.14% and 93.94% over the control, respectively (Fig. 4c). Under 200 mM NaCl, panicle mass, panicle length, number of panicles per plant, number of seeds per panicle, and one-hundred grain mass were sharply declined by 81.70%, 39.92%, 91.67%, 71.15%, and 43.50% in plants without AT over the control, respectively. On the other hand, panicle mass, number of panicles per plant, and number of seeds per panicle in AT-treated plants were significantly dropped by 48.08%, 25.0%, and 31.23% over the control, respectively. Number of seeds per panicle of AT-treated plants under salt stress was retained. Moreover, yield attributes under salt stress were increased with the application of 0.2% AT (Table 2).
4 Discussion
Pathumthani 1 rice genotype exhibited leaf rolling, chlorosis, and leaf burn when plants were exposed to 200 mM NaCl. Previously, growth performance, including number of leaves, plant fresh mass, plant dry mass, and root length, of seedlings of Pathumthani 1 rice genotype under 342 mM NaCl has been reported to significantly decline (Cha-um et al. 2009). Similarly, tip burn and leaf chlorosis in 21-day-old seedlings of Pathumthani 1 genotype under hydroponic culture with 10 dS m−1 or 68 mM NaCl have been reported (Pongprayoon et al. 2019). The overall performance of rice crop under salt stress was markedly improved by 0.2% AT foliar application. Plant canopy and height of 6% kaolin (a member of antitranspirants) pretreated sesame (Sesamum indicum L.) under 2.3 dS m−1 NaCl salt stress have been reported to significantly improve over the control without AT (Gaballah et al. 2007). Likewise, the aboveground traits of tomato (Solanum lycopersicum L.) under salt stress have been observed to be slightly improved by kaolin application (Boari et al. 2016). The concentration of Na in the older leaves and flag leaf at the booting stage of rice under salt stress was the maximal. In IR2153-26–3-5–2 rice genotype, the maximal Na concentration (0.513 mmol g−1 DM) has been reported in old leaf (first from the base), whereas it has been observed as the lowest (0.170 mmol g−1 DM) in young leaf (fifth from the base) (Yeo et al. 1985). At the reproductive stage, Na concentration has been recorded in the order root > stem > middle leaf > flag leaf of Pokkali and Chettiviruppu rice genotypes under 8 dS m−1 NaCl for 4 weeks (Chakraborty et al. 2019).
An enrichment of Na and a decrement of K in antagonism have been well established in several rice genotypes grown under salt stress, leading to increased Na:K ratio (Sriskantharajah et al. 2020; Yong et al. 2020). However, the increase depends on the degree of salt concentration (Sriskantharajah et al. 2022), salt exposure period (Chakraborty et al. 2020; Gadelha et al. 2021), and environmental variations (Yong et al. 2020). Potassium has been reported as a macronutrient that controls the movement of Na in relation to transpiration rate, especially in K-starved plants (Quintero et al. 2007). In general, Na is directly absorbed by root tissues and translocated to other organs by apoplastic route (Yeo et al. 1987; Ochiai and Matoh 2002; Foster and Miklavcic 2017) and is closely related with transpiration stream (Naito et al. 1994; Quintero et al. 2008; Nomiyama et al. 2013; Hasanuzzaman et al. 2018). Abscisic acid (ABA) is one of signaling hormones induced by NaCl-mediated salt stress, which controls stomatal closure to limit the Na transpiration flow (Park et al. 2020; Parveen et al. 2021; Sharmin et al. 2021; Xue et al. 2021). Suppression of transpiration rate using polyethylene glycol, exogenous glycinebetaine, and proline has been reported as an effective way to limit transpiration rate, Na uptake, and apoplastic flow in rice crops (Ochiai and Matoh 2004; Sobahan et al. 2009).
Enrichment of Ca in epidermal cells near stomata plays a key role in stomatal closure, especially under environmental stress conditions (Fricke 2004). Alternatively, free proline accumulation is an important indicator of salt toxicity as it is a major osmolyte (Chakraborty et al. 2020; Sriskantharajah et al. 2022) due to increased Δ1-pyrroline-5-carboxylate synthetase and ornithine-δ-aminotransferase activities (Irakoze et al. 2022). An exogenous foliar spray of AT (chitosan) has been reported to strongly restrict Na concentration in the leaf tissues, stabilize photosynthetic pigments, and regulate enzymatic antioxidants (Attia et al. 2021; Zhang et al. 2021).
Chlorophyll degradation in salt-stressed rice without AT was observed, leading to diminished ΦPSII, reduced Pn, and impaired stomatal functions (declined gs and E). The photosynthetic abilities of 0.2% AT-treated plants grown under 200 mM NaCl were improved. In African rice (Oryza glaberima L.), Chla, Chlb, and Cx+c in the leaves of seedlings under 50 mM NaCl for 15 days have been observed to be significantly degraded, causing diminished ΦPSII and decreased Pn (Prodjinoto et al. 2021). In addition, chlorophyll degradation and ΦPSII diminution in indica rice genotypes have been reported as sensitive parameters under salt stress (Tsai et al. 2019; Chakraborty et al. 2020; Gadelha et al. 2021). Stomatal closure in salt-stressed rice is a primary adaptation response of plants, leading to decreased Pn, gs, and E (Fu et al. 2018; Prodjinoto et al. 2021), which is regulated by large amount of endogenous ABA synthesis (Park et al. 2020; Xue et al. 2021). A positive relationship between TChl concentration and ΦPSII (R2 = 0.72), ΦPSII and Pn (R2 = 0.84), and Pn and plant dry mass (R2 = 0.61) in Pathumthani 1 rice seedlings under salt stress has been reported in a previous study (Cha-um et al. 2009). A key function of antitranspirants in salt-stressed plant is to limit transpiration rate and Na translocation from root to shoot (Malash and Flowers 1984; Oddo et al. 2019).
Yield attributes of a crop species are very important criteria while considering salt tolerance improvement using antitranspirants. Increased percent sterility and reduced grain yield in rice crop grown under saline soil have been previously reported (Chakraborty et al. 2019). In the present study, the overall yield traits of salt-stressed Pathumthani 1 rice genotype were sustained by 0.2% AT foliar application. The harvest index and biological yield of wheat (Triticum aestivum L.) plants under salt stress have been reported to be retained by AT foliar spray (Gaballah and Moursy 2004). In tomato, the fruit yield traits have been reported to be strongly improved due to the application of AT even when exposed to salt stress (Boari et al. 2016; Ullah et al. 2020). Moreover, seed yield and oil percentage in sunflower (Helianthus annuus L.) grown under salt stress have been significantly improved by foliar application of AT (Bakhoum et al. 2020).
In conclusion, Na translocation from root to shoot via bypass flow (apoplastic route) in salt-stressed plants at the booting stage of Pathumthani 1 rice genotype was reduced by foliar application of antitranspirant, depending on stomatal closure. Photosynthetic pigments, photon yield of PSII, and net photosynthetic rate in salt-stressed plants with AT application were better retained than the control, thus maintaining grain yield attributes. Based on the present study, it can be concluded that the foliar application of antitranspirant in rice crop grown in saline soils is beneficial. However, studies are required to validate these findings under field conditions.
Data availability
All data are available under request.
References
AbdAllah AM, Mashaheet AM, Zobel R, Burkey KO (2019) Physiological basis for controlling water consumption by two snap beans genotypes using different anti-transpirants. Agric Water Manage 214:17–27. https://doi.org/10.1016/j.agwat.2018.12.029
Attia MS, Osman MS, Mohamed AS, Mahgoub HA, Garada MO, Abdelmouty ES, Abdel Latef AAH (2021) Impact of foliar application of chitosan dissolved in different organic acids on isozymes, protein patterns and physio-biochemical characteristics of tomato grown under salinity stress. Plants 10:388. https://doi.org/10.3390/plants10020388
Bakhoum GS, Sadak MS, Badr EAEM (2020) Mitigation of adverse effects of salinity stress on sunflower plant (Helianthus annuus L.) by exogenous application of chitosan. Bull Nat Res Centre 44:1–11. https://doi.org/10.1186/s42269-020-00343-7
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Behl T, Kumar S, Sehgal A, Singh S, Kumari S, Brisc MC, Munteanu MA, Brisc C, Buhas CL, Judea-Pusta C, Nistor-Cseppento C, Bungau S (2021) Rice bran, an off-shoot to newer therapeutics in neurological disorders. Biomed Pharmacother 140:111796. https://doi.org/10.1016/j.biopha.2021.111796
Boari F, Donadio A, Pace B, Schiattone MI, Cantore V (2016) Kaolin improves salinity tolerance, water use efficiency and quality of tomato. Agric Water Manage 167:29–37. https://doi.org/10.1016/j.agwat.2015.12.021
Chakraborty K, Chattaopadhyay K, Nayak L, Ray S, Yeasmin L, Jena P, Gupta S, Mohanty SK, Swain P, Sarkar RK (2019) Ionic selectivity and coordinated transport of Na+ and K+ in flag leaves render differential salt tolerance in rice at the reproductive stage. Planta 250:1637–1653. https://doi.org/10.1007/s00425-019-03253-9
Chakraborty K, Mondal S, Ray S, Samal P, Pradhan B, Chattopadhyay K, Kar MK, Swain P, Sarkar RK (2020) Tissue tolerance coupled with ionic discrimination can potentially minimize the energy cost of salinity tolerance in rice. Front Plant Sci 11:265. https://doi.org/10.3389/fpls.2020.00265
Cha-um S, Supaibulwatana K, Kirdmanee C (2007) Glycinebetaine accumulation, physiological characterizations and growth efficiency in salt-tolerant and salt-sensitive lines of indica rice (Oryza sativa L. ssp. indica) in response to salt stress. J Agron Crop Sci 193:157–166. https://doi.org/10.1111/j.1439-037X.2007.00251.x
Cha-um S, Charoenpanich A, Roytrakul S, Kirdmanee C (2009) Sugar accumulation, photosynthesis and growth of two indica rice varieties in response to salt stress. Acta Physiol Plant 31:477–486. https://doi.org/10.1007/s11738-008-0256-1
Cirillo A, Conti S, Graziani G, El-Nakhel C, Rouphael Y, Ritieni A, di Vaio C (2021) Mitigation of high-temperature damage by application of kaolin and pinolene on young olive trees (Olea europaea L.): a preliminary experiment to assess biometric, eco-physiological and nutraceutical parameters. Agronomy 11:1884. https://doi.org/10.3390/agronomy11091884
Corwin DL (2021) Climate change impacts on soil salinity in agricultural areas. Eur J Soil Sci 72:842–862. https://doi.org/10.1111/ejss.13010
Devkota KP, Pasuquin E, Elmido-Mabilangan A, Dikitanan R, Singleton GR, Stuart AM, Vithoonjit D, Vidiyangkura L, Pustika AB, Afriani R, Listyowati CL, Keerthisena RSK, Kieu NT, Malabayabas AJ, Hu R, Pan J, Beebout SE (2019) Economic and environmental indicators of sustainable rice cultivation: a comparison across intensive irrigated rice cropping systems in six Asian countries. Ecol Indic 105:199–214. https://doi.org/10.1016/j.ecolind.2019.05.029
Fahad S, Adnan M, Noor M, Arif M, Alam M, Khan IA, Ullah H, Wahid F, Mian IA, Jamal Y, Basir A, Hassan S, Saud S, Amanullah RM, Wu C, Khan MA, Wang D (2019) Major constraints for global rice production. In: Hasanuzzaman M, Fujita M, Nahar K, Biswas JK (eds) Advances in rice research for abiotic stress tolerance. Woodhead Publishing, Cambridge, pp 1–22
Faiyue B, Al-Azzawi MJ, Flowers TJ (2010) The role of lateral roots in bypass flow in rice (Oryza sativa L.). Plant Cell Environ 33:702–716. https://doi.org/10.1111/j.1365-3040.2009.02078.x
Faiyue B, Al-Azzawi MJ, Flowers TJ (2012) A new screening technique for salinity resistance in rice (Oryza sativa L.) seedlings using bypass flow. Plant Cell Environ 35:1099–1108. https://doi.org/10.1111/j.1365-3040.2011.02475.x
Foster KJ, Miklavcic SJ (2017) A comprehensive biophysical model of ion and water transport in plant roots. I. Clarifying the roles of endodermal barriers in the salt stress response. Front Plant Sci 8:1326. https://doi.org/10.3389/fpls.2017.01326
Francini A, Lorenzini G, Nali C (2011) The antitranspirant di-1-p-menthene, a potential chemical protectant of ozone damage to plants. Water Air Soil Pollut 219:459–472. https://doi.org/10.1007/s11270-010-0720-6
Fricke W (2004) Solute sorting in grass leaves: the transpiration stream. Planta 219:507–514. https://doi.org/10.1007/s00425-004-1262-1
Fu L, Shen Q, Kuang L, Yu J, Wu D, Zhang G (2018) Metabolite profiling and gene expression of Na/K transporter analyses reveal mechanisms of the difference in salt tolerance between barley and rice. Plant Physiol Biochem 130:248–257. https://doi.org/10.1016/j.plaphy.2018.07.013
Gaballah MS, Moursy M (2004) Reflectants application for increasing wheat plant tolerance against salt stress. Pak J Biol Sci 7:956–962
Gaballah MS, Abou B, Leila H, El-Zeiny A, Khalil S (2007) Estimating the performance of salt-stressed sesame plant treated with antitranspirants. J Appl Sci Res 3:811–817
Gadelha CG, Coutinho ÍAC, de Paiva Pinheiro SK, de Castro ME, de Carvalho HH, de Sousa LL, Gomes-Filho E (2021) Sodium uptake and transport regulation, and photosynthetic efficiency maintenance as the basis of differential salt tolerance in rice cultivars. Environ Exp Bot 192:104654. https://doi.org/10.1016/j.envexpbot.2021.104654
Hasanuzzaman MD, Shabala L, Zhou M, Brodribb TJ, Corkrey R, Shabala S (2018) Factors determining stomatal and non-stomatal (residual) transpiration and their contribution towards salinity tolerance in contrasting barley genotypes. Environ Exp Bot 153:10–20. https://doi.org/10.1016/j.envexpbot.2018.05.002
Hasegawa PM (2013) Sodium (Na+) homeostasis and salt tolerance of plants. Environ Exp Bot 92:19–31. https://doi.org/10.1016/j.envexpbot.2013.03.001
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Ann Rev Plant Biol 51:463–499. https://doi.org/10.1146/annurev.arplant.51.1.463
Hopmans JW, Qureshi AS, Kisekka I, Munns R, Grattan SR, Rengasamy P, Ben-Gal A, Assuline S, Javaux M, Minhas PS, Raats PAC, Skaggs TH, Wang G, van Lier QDJ, Jiao H, Lavado RS, Lazarovitch N, Li B, Taleisnik E (2021) Critical knowledge gaps and research priorities in global soil salinity. Adv Agron 169:1–191. https://doi.org/10.1016/bs.agron.2021.03.001
Hossain GS, Waditee R, Hibino T, Tanaka Y, Takabe T (2006) Root specific expression of Na+/H+ antiporter gene from Synechocystis sp. PCC6803 confers salt tolerance of tobacco plant. Plant Biotechnol 23:275–281. https://doi.org/10.5511/plantbiotechnology.23.275
Irakoze W, Quinet M, Prodjinoto H, Rufyikiri G, Nijimbere S, Lutts S (2022) Differential effects of sulfate and chloride salinities on rice (Oryza sativa L.) gene expression patterns: a comparative transcriptomic and physiological approach. Curr Plant Biol 29:100237. https://doi.org/10.1016/j.cpb.2022.100237
IRRI (2002) Standard evaluation system for rice. International Rice Research Institute, Los Baños, Laguna, Philippines
Islam MS, Haque KA, Jahan N, Atikullah M, Uddin MN, Naser AM, Faruk-E-Azam AKM, Islam MS (2022) Soil salinity mitigation by naturally grown halophytes in seawater affected coastal Bangladesh. Int J Environ Sci Technol 19:11013–11022. https://doi.org/10.1007/s13762-022-03912-7
Khush GS (2005) What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol 59:1–6. https://doi.org/10.1007/s11103-005-2159-5
Kılıc OM, Budak M, Gunal E, Acır N, Halbac-Cotoara-Zamfir R, Alfarraj S, Ansari MJ (2022) Soil salinity assessment of a natural pasture using remote sensing techniques in central Anatolia Turkey. PLoS ONE 17:e0266915. https://doi.org/10.1371/journal.pone.0266915
Kumar P, Sharma PK (2020) Soil salinity and food security in India. Front Sust Food Syst 4:533781. https://doi.org/10.3389/fsufs.2020.533781
Loconsole D, Murillo-Amador B, Cristiano G, de Lucia B (2019) Halophyte common ice plants: a future solution to arable land salinization. Sustainability 11:6076. https://doi.org/10.3390/su11216076
Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F (1999) Antioxidant defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol 119:1091–1100. https://doi.org/10.1104/pp.119.3.1091
Malash NMAR, Flowers TJ (1984) The effect of phenylmercuric acetate on salt tolerance in wheat. Plant Soil 81:269–279. https://doi.org/10.1007/BF02197160
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668. https://doi.org/10.1093/jexbot/51.345.659
Morsy ASM, Mehanna HM (2022) Beneficial effects of antitranspirants on water stress tolerance in maize under different plant densities in newly reclaimed land. Bull Nat Res Centre 46:248. https://doi.org/10.1186/s42269-022-00934-6
Mphande W, Kettlewell PS, Grove IG, Farrell AD (2020) The potential of antitranspirants in drought management of arable crops: a review. Agric Water Manag 236:106143. https://doi.org/10.1016/j.agwat.2020.106143
Mphande W, Farrell AD, Grove IG, Vickers LH, Kettlewell PS (2022) Metabolic and film antitranspirants both reduce drought damage to wheat yield despite having contrasting effects on leaf ABA. J Agron Crop Sci 208:143–157. https://doi.org/10.1111/jac.12567
Naito H, Tsuchiya M, Kumano S (1994) Physiological response to salinity in rice plant: II. Relationship of sodium exclusion to transpiration and root-respiration rates in NaCl-treated rice plant. Jpn J Crop Sci 63:320–325. https://doi.org/10.1626/jcs.63.320
Nomiyama R, Yasutake D, Sago Y, Kitano M (2013) Transpiration integrated model for root ion absorption under salinized condition. Biologia 68:1113–1117. https://doi.org/10.2478/s11756-013-0255-6
Ochiai K, Matoh T (2002) Characterization of the Na+ delivery from roots to shoots in rice under saline stress: excessive salt enhances apoplastic transport in rice plants. Soil Sci Plant Nutr 48:371–378. https://doi.org/10.1080/00380768.2002.10409214
Ochiai K, Matoh T (2004) Alleviation of salinity damage to rice plants by the use of polyethylene glycols (PEGs) through reduction of Na+ transport to shoots. Soil Sci Plant Nutr 50:129–133. https://doi.org/10.1080/00380768.2004.10408460
Oddo E, Russo G, Grisafi F (2019) Effects of foliar application of glycine betaine and chitosan on Puccinellia distans (Jacq.) Parl. subjected to salt stress. Biol Futura 70:47–55. https://doi.org/10.1556/019.70.2019.06
Park SI, Kim JJ, Shin SY, Kim YS, Yoon HS (2020) ASR enhances environmental stress tolerance and improves grain yield by modulating stomatal closure in rice. Front Plant Sci 10:1752. https://doi.org/10.3389/fpls.2019.01752
Parveen A, Ahmar S, Kamran M, Malik Z, Ali A, Riaz M, Abbasi GH, Khan M, Sohail AB, Rizwan M, Afzal S, Ali S (2021) Abscisic acid signaling reduced transpiration flow, regulated Na+ ion homeostasis and antioxidant enzyme activities to induce salinity tolerance in wheat (Triticum aestivum L.) seedlings. Environ Technol Innov 24:101808. https://doi.org/10.1016/j.eti.2021.101808
Plett DC, Møller IS (2010) Na+ transport in glycophytic plants: what we know and would like to know. Plant Cell Environ 33:612–626. https://doi.org/10.1111/j.1365-3040.2009.02086.x
Pongprayoon W, Tisarum R, Theerawitaya C, Cha-um S (2019) Evaluation and clustering on salt-tolerant ability in rice genotypes (Oryza sativa L. subsp. indica) using multivariate physiological indices. Physiol Mol Biol Plant 25:473–483. https://doi.org/10.1007/s12298-018-00636-2
Prodjinoto H, Irakoze W, Gandonou C, Lepoint G, Lutts S (2021) Discriminating the impact of Na+ and Cl− in the deleterious effects of salt stress on the African rice species (Oryza glaberrima Steud.). Plant Growth Regul 94:201–219. https://doi.org/10.1007/s10725-021-00709-5
Quintero JM, Fournier JM, Benlloch M (2007) Na+ accumulation in shoot is related to water transport in K+-starved sunflower plants but not in plants with a normal K+ status. J Plant Physiol 164:60–67. https://doi.org/10.1016/j.jplph.2005.10.010
Quintero JM, Fournier JM, Benlloch M, Rodríguez-Navarro A (2008) Na+ accumulation in root symplast of sunflower plants exposed to moderate salinity is transpiration-dependent. J Plant Physiol 165:1248–1254. https://doi.org/10.1016/j.jplph.2007.08.011
Rahman MM, Mostofa MG, Keya SS, Siddiqui MN, Ansary MMU, Das AK, Rahman MA, Tran LSP (2021) Adaptive mechanisms of halophytes and their potential in improving salinity tolerance in plants. Int J Mol Sci 22:10733. https://doi.org/10.3390/ijms221910733
Rodriguez J, Anoruo A, Jifon J, Simpson C (2019) Physiological effects of exogenously applied reflectants and anti-transpirants on leaf temperature and fruit sunburn in citrus. Plants 8:549. https://doi.org/10.3390/plants8120549
Sahab S, Suhani I, Srivastava V, Chauhan PS, Singh RP, Prasad V (2021) Potential risk assessment of soil salinity to agroecosystem sustainability: current status and management strategies. Sci Total Environ 764:144164. https://doi.org/10.1016/j.scitotenv.2020.144164
Schneider P, Asch F (2020) Rice production and food security in Asian Mega deltas—A review on characteristics, vulnerabilities and agricultural adaptation options to cope with climate change. J Agron Crop Sci 206:491–503. https://doi.org/10.1111/jac.12415
Sen S, Chakraborty R, Kalita P (2020) Rice-not just a staple food: a comprehensive review on its phytochemicals and therapeutic potential. Trend Food Sci Technol 97:265–285. https://doi.org/10.1016/j.tifs.2020.01.022
Shabala SN, Shabala SI, Martynenko AI, Babourina O, Newman IA (1998) Salinity effect on bioelectric activity growth, Na+ accumulation and chlorophyll fluorescence of maize leaves: a comparative survey and prospects for screening. Aust J Plant Physiol 25:609–616. https://doi.org/10.1071/PP97146
Shahid SA, Zaman M, Heng L (2018) Soil salinity: historical perspectives and a world overview of the problem. In: Zaman M, Shahid SA, Heng L (eds) Guideline for salinity assessment mitigation and adaptation using nuclear and related techniques. Springer, Cham, pp 43–53. https://doi.org/10.1007/978-3-319-96190-3_2
Sharmin S, Lipka U, Polle A, Eckert C (2021) The influence of transpiration on foliar accumulation of salt and nutrients under salinity in poplar (Populus x canescens). PLoS ONE 16:e0253228. https://doi.org/10.1371/journal.pone.0253228
Singh A (2022) Soil salinity: a global threat to sustainable development. Soil Use Manage 38:39–67. https://doi.org/10.1111/sum.12772
Sobahan MA, Arias CR, Okuma E, Shimoishi Y, Nakamura Y, Hirai Y, Mori IC, Murata Y (2009) Exogenous proline and glycinebetaine suppress apoplastic flow to reduce Na+ uptake in rice seedlings. Biosci Biotechnol Biochem 73:2037–2042. https://doi.org/10.1271/bbb.90244
Sriskantharajah K, Osumi S, Chuamnakthong S, Nampei M, Amas JC, Gregorio GB, Ueda A (2020) Contribution of two different Na+ transport systems to acquired salinity tolerance in rice. Plant Sci 297:110517. https://doi.org/10.1016/j.plantsci.2020.110517
Sriskantharajah K, Chuamnakthong S, Osumi S, Nampei M, Ueda A (2022) Varietal differences in salt acclimation ability of rice. Cereal Res Comm 50:419–427. https://doi.org/10.1007/s42976-021-00205-6
Tanaka K, Ohta K, Haddad PR, Fritz JS, Lee KP, Hasebe K, Ieuji A, Miyanaga A (1999) Acid-rain monitoring in East Asia with a portable-type ion-exclusion-cation-exchange chromatographic analyzer. J Chromatog 850:311–317. https://doi.org/10.1016/S0021-9673(99)00286-1
Tsai YC, Chen KC, Cheng TS, Lee C, Lin SH, Tung CW (2019) Chlorophyll fluorescence analysis in diverse rice varieties reveals the positive correlation between the seedlings salt tolerance and photosynthetic efficiency. BMC Plant Biol 19:403. https://doi.org/10.1186/s12870-019-1983-8
Ullah N, Basit A, Ahmad I, Ullah I, Shah ST, Mohamed HI, Javed S (2020) Mitigation the adverse effect of salinity stress on the performance of the tomato crop by exogenous application of chitosan. Bull Nat Res Centre 44:181. https://doi.org/10.1186/s42269-020-00435-4
Xue F, Liu W, Cao H, Song L, Ji S, Tong L, Ding R (2021) Stomatal conductance of tomato leaves is regulated by both abscisic acid and leaf water potential under combined water and salt stress. Physiol Plant 172:2070–2078. https://doi.org/10.1111/ppl.13441
Yeo AR, Caporn SJM, Flowers TJ (1985) The effect of salinity upon photosynthesis in rice (Oryza sativa L.): gas exchange by individual leaves in relation to their salt content. J Exp Bot 36:1240–1248. https://doi.org/10.1093/jxb/36.8.1240
Yeo AR, Yeo ME, Flowers TJ (1987) The contribution of an apoplastic pathway to sodium uptake by rice roots in saline conditions. J Exp Bot 38:1141–1153. https://doi.org/10.1093/jxb/38.7.1141
Yong MT, Solis CA, Rabbi B, Huda S, Liu R, Zhou M, Zhou M, Shabala L, Venkataraman G, Shabala S, Chen ZH (2020) Leaf mesophyll K+ and Cl− fluxes and reactive oxygen species production predict rice salt tolerance at reproductive stage in greenhouse and field conditions. Plant Growth Regul 92:53–64. https://doi.org/10.1007/s10725-020-00619-y
Zhang G, Wang Y, Wu K, Zhang Q, Feng Y, Miao Y, Yan Z (2021) Exogenous application of chitosan alleviate salinity stress in lettuce (Lactuca sativa L.). Horticulturae 7:342. https://doi.org/10.3390/horticulturae7100342
Acknowledgements
We thank the National Science and Technology Development Agency (Grant Number P-18-51456) for granting support and Professor Dr. Avishek Datta for grammatical proof reading.
Author information
Authors and Affiliations
Contributions
Conceptualization, SC. and CT.; methodology, RT. and TS.; formal analysis, RT. and CT.; data curation, DC.; writing—original draft preparation, CT. and SC.; and writing—review and editing, HPS. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Theerawitaya, C., Tisarum, R., Samphumphuang, T. et al. Antitranspirant modulates Na+ enrichment and yield in indica rice under salt stress. Theor. Exp. Plant Physiol. 35, 99–110 (2023). https://doi.org/10.1007/s40626-023-00272-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-023-00272-6