Abstract
Salinity is a major abiotic stress that affects plant growth and development, especially in rice crop as it is a salt susceptible crop. Therefore, a wide range of rice genetic resources are screened in the germplasm banks to identify salt tolerant cultivars. The objective of this investigation was to develop effective indices for the classification of salt tolerant rice genotypes among Pathumthani 1, Khao Dawk Mali 105 (KDML 105), RD31, RD41, Suphanburi 1, RD43, RD49 and Riceberry. Rice seedlings were hydroponically grown with 10 dS m−1 NaCl treatment or without NaCl treatment (to serve as control) (WP; 2 dS m−1). Standard evaluation system peaked at a score of 9 in Pathumthani 1 and KDML 105, after 21 days of salt treatment, leading to leaf chlorosis, leaf burns and plant death. Chlorophyll a, chlorophyll b and total carotenoids were maintained better in the salt-stressed leaves of rice cvs. Riceberry and RD43, as compared to other cultivars. Salt stress induced a remarkable increase in the free proline accumulation (by 8.38 folds) in cv. Riceberry. Overall growth performance in rice cv. Riceberry was retained, whereas it declined in other cultivars. After 21 days of NaCl treatment at a concentration of 10 dS m−1, eight rice cultivars were classified into 3 groups based on multivariate physio-morphological indices, Group I: salt-tolerant rice, including cv. Riceberry; Group II: moderately salt tolerant, consisting of RD31, RD41, Suphanburi 1, RD43 and RD49 cultivars; Group III: salt-sensitive cultivars, namely Pathumthani 1 and KDML 105.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
World food demand is a critical issue, in view of the increasing world population, which is estimated to reach a mark of 9 billion in 2050 (Godfray et al. 2010). High salt concentrations, affecting soil and crop productivity have often been reported as a challenging issue, causing heavy yield loss (> 40% of crop yield or 27.3 billion US$ in each year of economic loss) (Qadir et al. 2014). Around the globe, 397 million ha or 3.1% of total land area is defined as salt affected soil, identified by the value of electrical conductivity (ECe) in the soil solution, being more than 4 dS m−1 (40 mM NaCl) (Munns and Tester 2008). Salt affected soil contains a large amount of Na+, which is absorbed by the root tissues and translocated to other organs of plant species (Eynard et al. 2005). Plants respond in a variety of ways to the ionic and osmotic stresses caused by toxic accumulation of Na+ ions, especially in glycophytes (salt sensitive), i.e. chlorophyll degradation (leaf chlorosis and leaf burns), reduced photosynthetic abilities, growth retardation and crop yield reduction (Munns and Tester 2008). In case of extreme salt contamination (> 16 dS m−1 or 160 mM NaCl), severe toxicity in the form of leaf burns and shoot die back, prior to plant death has been well demonstrated in glycophytic plant species (Polle and Chen 2015).
Rice crop has been reported as a Na+ hypersensitive plant species, leading to approximately 45% yield loss each year and economic losses up to 398 US$ ha−1 year−1 (Qadir et al. 2014). Therefore, salt tolerance ability has been tested in a wide range of rice genotypes using several methods, i.e. in vitro seedlings (Cha-um et al. 2010), hydroponic screening (Gregorio et al. 1997; Zeng et al. 2003; Zeng 2005), pot culture screening (Chunthaburee et al. 2016) and field trial selection (Mohammadi et al. 2014). Pokkali rice and FL478 (Pokkali × IR29) have been identified as the best salt tolerant cultivars that play a role as positive check, whereas IR29 has been selected as a salt susceptible cultivar or negative check in salt-tolerant screening programs (Ravikiran et al. 2018). In general, there are several tools for categorizing salt tolerant category in rice genotypes. These include: salt toxicity score (Kranto et al. 2016; Kordrostami et al. 2017), Hierarchical cluster analysis (Cha-um et al. 2010), Ward’s minimum-variance cluster analysis (Zeng et al. 2002; Zeng 2005; Ravikiran et al. 2018), Unweighted pair group method with arithmetic mean (UPGMA; de Leon et al. 2015) and Simple sequence repeats marker (SSR; Ali et al. 2014; Sakina et al. 2015). Based on those cluster ranking methods, Ward’s minimum-variance cluster analysis is a most popular way to classify the group depending on simple, high sensitivity, repeatable and high accuracy, especially in rice crop (Zeng et al. 2002; Zeng 2005; Ravikiran et al. 2018). Of these, multivariate cluster analysis using physiological and morphological indices, has been generally implemented for categorizing the groups of salt tolerant and salt sensitive genotypes during germination, vegetative and reproductive stages (Zeng et al. 2003; Zeng 2005; Sakina et al. 2015). It is a simple, highly accurate, repeatable, and user-friendly evaluated method (Kanawapee et al. 2012; Chunthaburee et al. 2016). In Thailand, the germplasm of aromatic rice (230 genotypes) has been screened for the identification of salt tolerant traits, resulting in the selection of 11 candidate cultivars based on chlorophyll stability index (Wanichananan et al. 2003). However, there is only one report concerning the salt tolerance in economic rice cultivars in both local (RD; Thai improved cultivars) and international (IR; IRRI improved cultivars) genotypes (Kanawapee et al. 2012). Moreover, the pigmented pericarp genotypes in both glutinous and non-glutinous rice cultivars, have been considered as suitable candidates for salt-tolerant screening choices (Chunthaburee et al. 2016; Kanawapee et al. 2012). In addition, only one study has reported the mechanism of regulation of salt stress on cyanidin-3-glucoside in the leaf tissues of rice cv. Riceberry (black pericarp of rice grain), a hybrid of Jao Hom Nil and KDML105 rice varieties (Daiponmak et al. 2010). Therefore, there lies a wide gap in the existing records, relating to the physio-morphological and biochemical adaptations of rice genotypes in irrigated low land rice during the salt stress conditions. In Thailand, paddy filed divides into two types, (1) rainfed lowland in the north and northeast region, a major production area of KDML105 (9.4 million ha with 24.1 million ton of rice grain production in Year 2017) and (2) irrigated lowland in the central region, a major production area of RD members, RD31, RD41, RD43 and RD49, Suphanburi 1 and Pathumthani 1 (1.9 million ha with 7.9 million ton of rice grain production in Year 2017). In the present study, Riceberry (cultivar with enriched anthocyanin), RD43 (cultivar with low glycemic index), RD member (RD31, RD41, RD49) and Suphanburi 1 (high yielding white grain varieties), KDML105 (jasmine rice; high quality cultivar) and Pathumthani 1 (salt sensitive cultivar for negative check) were chosen as potential candidates for salt tolerant screening and clustering using multivariate physio-morphological indices. The aim of this study was to develop the effective indices for the grouping of salt tolerance rice genotypes, Pathumthani 1, KDML 105, RD31, RD41, Suphanburi 1, RD43, RD49 and Riceberry.
Materials and methods
Plant materials, growing conditions and salt stress treatment
Seeds of eight rice (Oryza sativa L. spp. indica) cultivars, including Pathumthani 1 (negative check; salt susceptible); Khao Dawk Mali 105 (KDML 105; jasmine rice); RD31, RD41, Suphanburi 1 and RD49 (high yielding varieties); RD43 (low glycemic index), and Riceberry (black pericarp rice) (Table 1) were procured from Suphan Buri Rice Research Center (Rice Research Institute, Department of Agriculture, Ministry of Agriculture and Cooperative, Thailand). Seedlings were hydroponically grown in a greenhouse under 400 ± 50 µmol m−2 s−1 photosynthetic photon flux density and temperature shift at 32 ± 2 °C/28 ± 2 °C in the day/night intervals. Rice seeds were soaked in distilled water for 48 h before germinating on the sterile sand for 2 weeks. Thereafter, the rice seedlings were transferred to modified WP nutrient solution (580 mg L−1 KNO3, 500 mg L−1 CaSO4, 450 mg L−1 MgSO4·7H2O, 250 mg L−1 Triple super phosphate, 100 mg L−1 (NH4)2SO4, 160 mg L−1 Na2EDTA, 120 mg L−1 FeSO4·7H2O, 15 mg L−1 MnSO4·H2O, 5 mg L−1 H3BO3, 1.5 mg L−1 ZnSO4·7H2O, 1 mg L−1 KI, 0.1 mg L−1 Na2MoO4·2H2O, 0.05 mg L−1 CuSO4·5H2O, and 0.05 mg L−1 CoCl2·6H2O) for 2 weeks (Vajrabhaya and Vajrabhaya 1991). Twenty eight-days-old seedlings were subjected to modified WP nutrient solution (20 L hydroponic culture) containing 68 mM sodium chloride (NaCl) to achieve EC value of 10 dS m−1 with a parallel control (WP without NaCl at EC of 2 dS m−1) for 21 days. The WP nutrient solution was refreshed at weekly intervals during the whole experiment.
Growth characteristics
Four seedlings of each rice cultivar were randomly harvested from salt treated (10 dS m−1 NaCl) and control stock (WP; 2 dS m−1) after 21 days. Aerial and underground parts of the seedlings were separated to assay the fresh weights (FW) and dry weights (DW). For the estimation of dry weight, shoots and roots of the seedlings were dried in a forced-air oven at 60 °C for 48 h. Shoot height was measured from the base of the stem to the tip of the highest leaf. Leaf width was measured in the topmost fully expanded leaf. Visual damage toxic score of plants was observed and recorded using Standard Evaluation System of rice (SES) (Barua et al. 2015; Chunthaburee et al. 2016).
Determination of the free proline content
Free proline content was determined according to the method of Bates et al. (1973). Five hundred milligrams of leaf tissue was grounded in a mortar with liquid nitrogen. The homogenate powder was mixed with 1 mL of 3% sulfosalicylic acid and centrifuged at 5000×g for 15 min. The supernatant was collected and allowed to react with an equal volume of reaction mixture containing 1.25 mg of ninhydrin in 30 mL of glacial acetic acid and 20 mL of 6 M H3PO4. The homogenate was incubated at 95 °C for 1 h and then, immediately placed on an ice bath to stop the reaction. The reaction mixture was extracted with 2 mL of toluene and free proline was quantified by measuring the absorbance at 520 nm using l-proline as standard. The content was expressed as per g fresh weight (FW).
Photosynthetic pigments assay
The contents of chlorophyll a (Chla), chlorophyll b (Chlb) and total carotenoids (Cx+c) in the leaf tissues were analyzed according to the method of Shabala et al. (1998) and Lichtenthaler (1987). Chlorophyll a (Chla) and chlorophyll b (Chlb) were determined at wavelengths 662 and 644 nm, respectively, whereas total carotenoid concentration (Cx+c) was measured at 470 nm using a UV–Vis spectrophotometer (LibraS11 Biochrom, England). A solution of 80% (v/v) acetone was used as blank.
Experiment design and statistical analysis
The experiment was performed as 8 × 2 factorials with a completely randomized design (CRD) with four biological replicates (n = 4). The mean values of SES score, free proline content, chlorophyll a and b, total carotenoids, shoot fresh weight (SFW), shoot dry weight (SDW), root fresh weight (RFW) and root dry weight (RDW) were subjected to t test for analysis. The comparison of means, number of leaves, shoot height, leaf length, leaf width and root length using Turkey’s HSD at p ≤ 0.05 was carried out with Minitab software (ver. Minitab 16, Minitab Inc., USA). Salt tolerance indices were calculated by dividing the values of physio-morphological parameters in salt-stressed seedlings by the control as per the following equation (Cha-um et al. 2010):
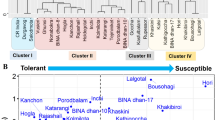
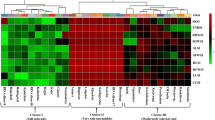
The cluster ranking groups were obtained based on Ward’s minimum variance cluster analysis (Zeng et al. 2002; Zeng 2005; Ravikiran et al. 2018) using the relative salt tolerance indices of 15 physio-morphological parameters i.e. SES toxic score and free proline accumulation, chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (TC), total carotenoids (Cx+c) degradation, as well as shoot fresh weight (SFW), shoot dry weight (SDW), root fresh weight (RFW) and root dry weight (RDW) reduction, number of leaves, shoot height, leaf length, leaf width and root length inhibition subjected to Hierarchical cluster analysis in SPSS software (version 11.5) (Cha-um et al. 2007; Pongprayoon et al. 2008).
Results
Growth characteristics in rice genotypes under salt stress
Morphological characters in all the rice cultivars under salt stress were demonstrated. Leaf chlorosis, leaf burns, delayed growth and expansion of leaves were evidently observed in the seedlings under salt stress (10 dS m−1 NaCl), especially in cvs. KDML 105 and Pathumthani 1, whereas healthy leaves and normal shoot and root developmental stages were seen in the control seedlings (WP; 2 dS m−1) (Fig. 1). In cvs. Pathumthani 1 and KDML 105, leaf burns and plant death was detected under10 dS m−1 NaCl (salt stress), due to which the availability of plant material was limited to be assayed for each parameter. Number of leaves in rice cultivars were significantly declined by 17–40% when subjected to salt stress, except for cv. RD43 (Table 2). Shoot height in cvs. RD43, RD49 and Riceberry under salt stress was retained, whereas it was sharply retarded by 31%, 27% and 37% in cvs. RD31, RD41 and Suphanburi 1, respectively. Leaf length in cvs. RD31, Suphanburi 1 and RD49 under salt stress was significantly decreased (28–30%) while it was maintained in cvs. RD41, RD43 and Riceberry. In Suphanburi 1, leaf width and root length were significantly declined by 25% and 24%, respectively, when exposed to salt stress for 21 days. Leaf width in cv. RD31 under salt stress was also decreased by 31%, whereas it was alleviated in other cultivars (Table 2).
SFW was maintained in cvs. RD49 (with only 32% reduction to that of control) and Riceberry (with only 28% reduction to that of control) under salt stress, although it was significantly declined by 68%, 55%, 72% and 46% in cvs. RD31, RD41, Suphanburi 1 and RD43, respectively (Fig. 2a). SDW in cv. Riceberry under salt stress (with only 21% reduction to that of control) was sustained, whereas it was sharply declined in other cultivars by 48–70% (Fig. 2b). RFW in rice cvs. RD31 and Suphanburi 1 under salt stress was inhibited by 66% and 76%, respectively, while it was maintained in cvs. RD41, RD43, RD49 and Riceberry (Fig. 2c). RDW in cvs. RD31, Suphanburi 1 and RD43 was dropped by 67%, 76% and 47%, respectively (Fig. 2d).
Shoot fresh weight, shoot dry weight, root fresh weight and root dry weight of eight rice cultivars grown under 2 dS m−1 (WP; control) and 10 dS m−1 (salt stress) for 21 days. Data presented as the mean of four replicates with standard error (± SE), and different letters in each cultivar represent significant difference at p ≤ 0.05, using t test
Salt evaluation score (SES), photosynthetic pigments and free proline osmolytes
Salt injury or salt evaluation score (SES) was determined following the standard score method of IRRI (Gregorio et al. 1997). It was clearly demonstrated that the toxicity score in cvs. Pathumthani 1 and KDML 105 was maximum (score = 9); moderate toxicity (score = 4–5) was noticed in cvs. RD31, RD41 and Suphanburi 1; and subsequently low toxicity (score = 1–2) was seen in cvs. RD43, RD49 and Riceberry (Fig. 3a).
Salt evaluation score (SES) (a), chlorophyll a (b), chlorophyll b (c) and total carotenoids (d) of eight rice cultivars grown under 2 dS m−1 (WP; control) and 10 dS m−1 (salt stress) for 21 days. Data presented as the mean of four replicates with standard error (± SE), and different letters in each cultivar represent significant difference at p ≤ 0.05, using t test
Chlorophyll a (Chla) and chlorophyll b (Chlb) in the leaf tissues of rice cvs. RD31, RD41 and Suphanburi 1 were significantly degraded (> 20% of control) when plants were subjected to salt stress. In contrast, these were maintained in cvs. RD43, RD49 and Riceberry (Fig. 3b, c). Total carotenoids (Cx+c) in the salt stressed seedlings of rice cvs. RD43 and Riceberry were maintained; however, these were sharply degraded in cvs. RD31, RD41, Suphanburi 1 and RD49 by 20%, 33%, 40% and 20%, respectively (Fig. 3d).
Free proline content in the salt stressed leaves was increased by 1.5 folds to that of control in cvs. RD31 (4.18 fold), RD41 (4.21 fold), Suphanburi 1 (2.05 fold), RD43 (1.62 fold), RD49 (4.94 folds), and Riceberry (8.38 fold) (Fig. 4a).
Free proline content (a) and relationships between salt injury score and chlorophyll a content (b), total carotenoids and free proline content (c), total chlorophyll and plant dry weight (d), of eight rice cultivars grown under 10 dS m−1 (salt stress) for 21 days. Data presented as the mean of four replicates with standard error (± SE)
Relationships between physiological data and salt tolerant classification using hierarchical cluster analysis
In the present study, a negative relationship was observed (r2 = 0.927) between SES and chlorophyll a in the leaf tissues of salt stressed seedlings (Fig. 4b). However, total carotenoids (NPQ function in photosystem II) and free proline (osmotic adjustment function) were found to be positively correlated (r2 = 0.724) (Fig. 4c). In addition, a positive relation between total chlorophyll (Chla + Chlb) and plant dry weight was demonstrated (Fig. 4d). The relative salt tolerance indices of 15 physio-morphological parameters (degradation and reduction % in each parameter) were subjected to the cluster ranking groups based on Ward’s minimum variance cluster analysis. Cluster ranking based on physiological and morphological changes in rice seedlings under salt stress, classified the tested cultivars in three groups: Group I: Riceberry was the only variety identified as salt tolerant, group II: RD31, RD41, RD43, RD49 and Suphanburi 1 were defined as moderate salt tolerant cultivars and group III: Pathumthani 1 and KDML 105 were classified as salt susceptible varieties (Table 2 and Fig. 5).
Discussion
Seedling stage of 8 rice cultivars was selected to evaluate the salt tolerant abilities. We found that Riceberry could be regarded as the only cultivar to effectively tolerate salt stress in comparison to other cultivars. Rapid screening of salt tolerant traits in rice germplasm has been studied in all the developmental stages, i.e. seed germination, vegetative and reproductive stages (Wankhade et al. 2013; Sakina et al. 2015). A high degree of growth retardation in rice seedlings grown under salt stress has been evidently observed, especially in the salt sensitive cultivars, which demonstrated the visual toxic symptoms, i.e. leaf chlorosis, leaf burns and plant death (cvs. Pathumthani 1 and KDML 105). Based on IRRI toxic score, leaf chlorosis and leaf burn in salt stressed seedlings were evidently observed. Chlorophyll degradation and chlorophyll a fluorescence diminution in the salt-stressed rice seedlings have been well documented, relating to a degree of salt concentration, salt exposure period and their interaction (Wankhade et al. 2013; Basu et al. 2017). Previously, rice cultivars, Pathumthani 1 and KDML105 have been identified as salt susceptible, due to their low survival rates and high levels of visual toxicity and growth inhibition (Kanawapee et al. 2012). Visual toxicity and plant death were also observed in rice cvs. Mekongga and Inpari-13 grown under 150 mM NaCl for 14 days (Hariadi et al. 2014). Our results demonstrated that the salt injury score (based on IRRI standard score 1–9) in cv. Riceberry and RD43 was very low, whereas free proline content was enriched, thereby, preventing the degradation of Chla, Chlb and Cx+c as well as maintaining overall growth performance under salt stress. An accumulation of free proline in the leaf tissues of Pokkali, FL496, and FL530 salt tolerant genotypes under salt stress is evidently observed, and attributed it to the cellular osmotic adjustment ability of proline to maintain the chlorophyll pigments and retain overall growth performance (Kanawapee et al. 2012; Chunthaburee et al. 2016). In contrast, IR29 (a salt sensitive check) was found to be highly sensitive to 200 mM NaCl provided for 14 days under photoautotrophic growth conditions, and resulted in chlorophyll degradation and reduction in shoot height by 24.44%, root length by 43.75%, plant dry weight by 60.19% and leaf area by 56.82% in comparison to control (Cha-um et al. 2010). Similarly, shoot height, plant fresh weight, plant dry weight and leaf area of salt-stressed seedlings in rice cv. Pathumthani 1 were reduced by 12.21%, 29.35%, 20.90% and 51.91% to that of the control seedlings (Cha-um et al. 2010). In the pigmented rice, the black color leaves of Riceberry may be enriched by anthocyanins [cyanidin-3-glucoside (C3G) and peonidin-3-glucoside (P3G)] that play a key role in salt antioxidant defense mechanism. C3G content in salt-stressed leaves (60 mM NaCl in Yoshida nutrient solution for 11 days) of pigmented rice cvs. Riceberry, Klum and Klum Doi Saket increased by 1.47, 1.71 and 5.95 folds over the control, respectively, to regulate the antioxidant salt-defense mechanism and thereby, leading to high survival rates and alleviated growth characters (Daiponmak et al. 2010). In the pigmented rice cv. Niewdam (GS number 00621; salt tolerant), the total anthocyanin content in the leaves of rice seedlings under 100 mM NaCl salt stress for 14 days was increased by 1.12 folds over the control, whereas SFW, SDW, RFW and RDW was declined by 38.17%, 41.18%, 8.33% and 46.67%, respectively (Chunthaburee et al. 2016). In the present study, shoot dry weight in Riceberry grown under salt stress was retained whereas it was significantly declined in other cultivars. The growth parameters i.e. shoot length (SL), root length (RL), SFW, SDW, RFW and RDW in the pigmented rice seedling cv. KKU-LLR-039 (salt susceptible) were significantly retarded when subjected to 150 mM NaCl for 10 days (Chunthaburee et al. 2014). Growth parameters in high yielding non-glutinous rice cvs. RD31, RD41 and RD49 (cultivars released by Rice Department of Thailand) and Suphanburi 1 as well as low yielding cv. RD43 with specific trait of low glycemic index, fluctuated depending on their salt tolerance abilities, when exposed to salt stress for 21 days. Similarly, 106 rice cultivars including Thai commercial cultivars (KDML105, Pathumthani 1, Pathumthani 60, RD6, RD15, Suphanburi 2, Suphanburi 90 and Surin 1) have been classified as salt tolerant on the basis of survival percentage, salt tolerance scores and Na+/K+ ratio (Kanawapee et al. 2012). In present study, seedlings of cvs. KDML105 and Pathumthani 1 was absolutely dried in the salt stress for 21 days, depending on the growth stages, screening systems and sensitive indices or selection criteria. The growth percentage or salt tolerance indices, measured for shoot length, root length, shoot dry weight and root dry weight during the vegetative growth stage of 33 rice genotypes have been used to classify the salt tolerant (i.e. Nana Bokra) and salt sensitive groups (i.e. Basmati-198) (Sakina et al. 2015). A decrease in plant growth parameters, root length and shoot length of the rice seedlings grown under 150 mM NaCl for 4 days, over the control, were applied to calculate the salt tolerance indices in different rice genotypes (Omisun et al. 2018). In addition, the relative salt tolerance based on shoot length, root length and plant biomass in the rice seedlings treated with different salt concentrations (EC 4–14 dS m−1) for 12 days was successfully elucidated as suitable morphological index for measuring the degree of salt tolerance using cluster analysis (Ali et al. 2014).
Salt injury score in cvs. RD43, RD49 and Riceberry was very low (1–2), whereas it was estimated to be 9 or symptoms of plant death were prevalent in cvs. Pathumthani 1 and KDML 105. In a previous study, the salt tolerance score was recorded to be very low (range 3.16–5.95; 4.34 score) in 13 genotypes of salt-tolerant rice cultivars grouped under cluster I (including cv. Pokkali-positive check), whereas it was very high (range 7.66–8.75; 8.00 score) in 7 genotypes of highly salt-sensitive rice cultivars grouped in cluster V (including cv. IR 29-negative check) (Kanawapee et al. 2012). Salt tolerance score in salt tolerant cultivar, FL478 was very low (score = 1) after 6 and 12 days of 12 dS m−1 salt treatment within the same group with cvs. Hassani, Ahlamitarom, and Binam, while it was very high (score = 9) in cvs. IR 29 and Sepidrood, grown under 12 dS m−1 salt treatment for 12 days (Kordrostami et al. 2017). In another study, the salt injury score was recorded to be maximum at 9.0 in salt sensitive cluster (15 genotypes), whereas it was minimum at 3.0 in salt tolerant cluster (12 genotypes) (Ravikiran et al. 2018). In summary, salt injury score is a simple tool to validate the overall toxic symptoms in rice crop under salt stress. Salinity stress impacted many aspects of a plant’s physiology such as photosynthetic pigment degradation and free proline osmolyte accumulation in rice seedlings under salt stress. In the present study, chlorophyll a, chlorophyll b and total carotenoids in rice cvs. Riceberry and RD43 under salt stress were maintained better than that in the other cultivars. The rate of chlorophyll degradation in rice cultivars has been validated as a parameter for classifying salt tolerance in various rice genotypes (Basu et al. 2017; Cha-um et al. 2010; Wanichananan et al. 2003). Correspondingly, there was a significant degradation of chlorophyll pigments during the vegetative stage of rice cvs. Bomba and Bahia, depending on the severity of salt stress (Wankhade et al. 2013). Free proline accumulation in the salt stressed leaves has been identified to play a key role in osmotic adjustment and thus, maintaining turgor pressure at the cellular level, which is an effective index for salt tolerance classification. In the present study, free proline in salt stressed rice cv. Riceberry was increased by 8.38 folds over the control. Previously, free proline in the leaf tissues of salt stressed seedlings of cv. Niewdam (GS number 00621) was enhanced by 8.74 folds over control (Chunthaburee et al. 2014).
In the present study, a negative relationship between chlorophyll a content and salt injury score was demonstrated with r2 value of 0.927. This suggests that the degradation rate of chlorophyll a in the leaf tissues may be related to high toxicity score. Chlorophyll degradation is a visual symptom in the leaves, turning the leaf color from dark green to yellow, when the plant is subjected to salt stress, especially in the salt sensitive species. Similarly, in another study, chlorophyll content in salt stressed seedlings of rice was negatively correlated with salt tolerance score (**: p ≤ 0.01) with r2 = − 0.103 (Kanawapee et al. 2012). However, total carotenoids in the salt stressed leaves exhibited a positive relationship with free proline accumulation. Maintenance of total carotenoids in the leaf tissues may function as non-enzymatic antioxidant system, when plants are exposed to salt stress. In 12 rice cultivars, the total anthocyanin and free proline content were found to be positively correlated (R2 = 0.649; *: p ≤ 0.05) (Chunthaburee et al. 2016). This can be attributed to the fact that the mechanism of proline accumulation plays a major role in maintaining the photosynthetic organelles including chlorophyll pigments (Wanichananan et al. 2003) as well as alternatively functioning as an antioxidant (Huque et al. 2007; Banu et al. 2009). Likewise, preliminary works have determined that the chlorophyll content is well maintained in salt tolerant plant genotypes under salt stress (Al-Aghabary et al. 2005). In addition, a positive relation between the total chlorophyll and plant dry weight was observed, which is in concordance with the results of previous studies (Chunthaburee et al. 2016). In general, free proline enrichment in salt tolerant cultivar may function as major osmolytes to maintain osmotic potential in the cellular levels, leading to prevent photosynthetic pigments as well as to retain overall growth performances.
In conclusion, the present study evaluated free proline enrichment, photosynthetic pigment degradation, salt injury score increment and overall growth retardation in salt stressed seedlings of 8 cultivars of rice crop and subsequently categorized these into three groups: (1) salt susceptible cultivars i.e. Pathumthani 1 and KDML105; (2) moderately salt tolerant cultivars including Suphanburi 1, RD31, RD41, RD43 and RD49, and (3) salt tolerant cultivars such as Riceberry, using multivariate cluster analysis.
References
Al-Aghabary K, Zhu Z, Shi Q (2005) Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative in tomato plants under salt stress. J Plant Nutr 27:2101–2115
Ali MN, Yeasmin L, Gantait S, Goswami R, Chakraborty S (2014) Screening of rice landraces for salinity tolerance at seedling stage through morphological and molecular markers. Physiol Mol Biol Plant 20:411–423
Banu MNA, Hoque MA, Watanabe-Sugimoto M, Matsuoka K, Nakamura Y, Shioishi Y, Murata Y (2009) Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J Plant Physiol 166:146–156
Barua R, de Ocampo M, Egdane J, Ismail AM, Mondal S (2015) Phenotyping rice (Oryza sativa L.) genotypes for physiological traits associated with tolerance of salinity at seedling stage. Sci Agric 12:156–162
Basu S, Giri RK, Kumar S, Rajwanshi R, Dwivedi SK, Kumar G (2017) Comprehensive physiological analyses and reactive oxygen species profiling in drought tolerant rice genotypes under salinity stress. Physiol Mol Biol Plant 23:837–850
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Cha-um S, Vejchasarn P, Kirdmanee C (2007) An effective defensive response in Thai aromatic rice varities (Oryza sativa L. spp. indica) to salinity. J Crop Sci Biotechnol 10:257–264
Cha-um S, Ashraf M, Kirdmanee C (2010) Screening upland rice (Oryza sativa L. ssp. indica) genotypes for salt-tolerance using multivariate cluster analysis. Afr J Biotechnol 9:4731–4740
Chunthaburee S, Sanitchon J, Pattanagul W, Theerakulpisut P (2014) Alleviation of salt stress in seedlings of black glutinous rice by seed priming with spermidine and gibberellic acid. Not Bot Horti Agrobot Cluj-Napoca 42:405–413
Chunthaburee S, Dongsansuk A, Sanitchon J, Pattanagul W, Theerakulpisut P (2016) Physiological and biochemical parameters for evaluation and clustering of rice cultivars differing in salt tolerance at seedling stage. Saudi J Biol Sci 23:467–477
Daiponmak W, Theerakulpisut P, Thanonkao P, Vanavichit A, Prathepha P (2010) Changes of anthocyanin cyaniding-3-glucoside content and antioxidant activity in Thai rice varieties under salinity stress. Sci Asia 36:286–291
de Leon TB, Lincombe S, Gregorio G, Subudhi K (2015) Genetic variation in Southern USA rice genotypes for seedling salinity tolerance. Front Plant Sci 6:374
Eynard A, Lal R, Wiebe K (2005) Crop response in salt-affected soils. J Sust Agric 27:5–50
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327:812–817
Gregorio GB, Senadhira D, Mendoza RD (1997) Screening rice for salinity tolerance. IRRI Discussion Series No. 22. International Rice Research Institute, Manila, Philippines
Hariadi YC, Nurhayati AY, Soeparjono S, Arif I (2014) Screening six varieties of rice (Oryza sativa) for salinity tolerance. Proc Environ Sci 28:78–87
Huque MA, Okuma E, Banu MNA, Nakamura Y, Shimoishi Y, Murata Y (2007) Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activity. J Plant Physiol 164:553–561
Kanawapee N, Sanitchon J, Lontom W, Threerakulpisut P (2012) Evaluation of salt tolerance at the seedlings stage in rice genotypes by growth performance, ion accumulation, proline and chlorophyll content. Plant Soil 358:235–249
Kordrostami M, Rabiei B, Kumleh HH (2017) Biochemical, physiological and molecular evaluation of rice cultivars differing in salt tolerance at seedling stage. Physiol Biol Plant 23:529–544
Kranto S, Chankaew S, Monkham T, Theerakulpisut P, Sanichon J (2016) Evaluation for salt tolerance in rice using multiple screening methods. J Agric Technol 18:1921–1931
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Mohammadi R, Mendioro MS, Diaz GQ, Gregorio GB, Singh RK (2014) Genetic analysis of salt tolerance at seedling and reproductive stages in rice (Oryza sativa). Plant Breed 133:548–559
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Omisun T, Sahoo S, Saha B, Panda SK (2018) Relative salinity tolerance of rice cultivars native to North East India: a physiological, biochemical and molecular perspective. Protoplasma 255:193–202
Polle A, Chen S (2015) On the salty side of life: molecular, physiological and anatomical adaptation and acclimation of trees to extreme habitats. Plant Cell Environ 38:1794–1816
Pongprayoon W, Cha-um S, Pichakum A, Kirdmanee C (2008) Proline profiles in aromatic cultivars photoautotrophically grown in responses to salt stress. Int J Bot 4:276–282
Qadir M, Quillérou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Drechsel P, Noble AD (2014) Economics of salt-induced land degradation and restoration. Nat Resour Forum A U N Sust Dev J 38:282–295
Ravikiran RT, Krishnamurthy SL, Warraich AS, Sharma PC (2018) Diversity and haplotypes of rice genotypes for seedling stage salinity tolerance analyzed through morpho-physiological and SSR markers. Field Crop Res 220:10–18
Sakina A, Ahmed I, Shahzad A, Iqbal M, Asif M (2015) Genetic variation for salinity tolerance in Pakistani rice (Oryza sativa L.) germplasm. J Agron Crop Sci 202:25–36
Shabala SN, Shabala SI, Martynenko AI, Babourina O, Newman IA (1998) Salinity effect on bioelectric activity, growth, Na+ accumulation and chlorophyll fluorescence of maize leaves: a comparative survey and prospects for screening. Aust J Plant Physiol 25:609–616
Vajrabhaya M, Vajrabhaya T (1991) Somaclonal variation of salt tolerance in rice. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry 14. Springer, Berlin, pp 368–382
Wanichananan P, Kirdmanee C, Vutiyano C (2003) Effect of salinity on biochemical and physiological characteristics in correlation to selection of salt-tolerance in aromatic rice (Oryza sativa L.). Sci Asia 29:333–339
Wankhade SD, Cornejo MJ, Aateu-Andres I, Sanz A (2013) Morpho-physiological variaations in response to NaCl stress during vegetative and reproductive development of rice. Acta Physiol Plant 35:323–333
Zeng L (2005) Exploration of relationships between physiological parameters and growth performance of rice (Oryza sativa L.) seedlings under salinity stress using multivariate analysis. Plant Soil 268:51–59
Zeng L, Shannon MC, Grieve CM (2002) Evaluation of salt tolerance in rice genotypes by multiple agronomic parameters. Euphytica 127:235–245
Zeng L, Poss JA, Wilson C, Draz ASE, Gregorio GB, Grieve CM (2003) Evaluation of salt tolerance in rice genotypes by physiological characters. Euphytica 129:281–292
Acknowledgements
The authors are grateful to Suphan Buri Rice Research Center (Rice Research Institute, Department of Agriculture, Ministry of Agriculture and Cooperative, Thailand) for providing rice seeds. We would like to thank Faculty of Science, Burapha University and National Center for Genetic Engineering and Biotechnology (BIOTEC) for funding support and lab facilities.
Author information
Authors and Affiliations
Contributions
WP conducted the research project, report to funding agency, analyzed the data and wrote a first draft of manuscript, CT performed the experiment layout, free proline assay and analysis, RT analyzed the cluster ranking, critical revision of the data and performed the experiment, and SC performed the secondary data analysis and finished the final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pongprayoon, W., Tisarum, R., Theerawittaya, C. et al. Evaluation and clustering on salt-tolerant ability in rice genotypes (Oryza sativa L. subsp. indica) using multivariate physiological indices. Physiol Mol Biol Plants 25, 473–483 (2019). https://doi.org/10.1007/s12298-018-00636-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-018-00636-2