Abstract
In this study, the effect of methyl jasmonate (MeJA) incorporation on in vitro propagation, metabolic profiling, and production of the bioactive compound, proximadiol, in the medicinally important herb Cymbopogon schoenanthus subsp. proximus has been investigated. The propagation approaches involved both somatic embryogenesis and direct organogenesis. All studied concentrations (10–400 µM) have significantly improved somatic embryogenesis at different developmental stages, as indicated by higher numbers of somatic and mature embryos as well as the embryogenic shoots. In contrast, the studied concentrations have negatively affected organogenic shoot and root regeneration. Metabolic profiling of polar extracts from the direct regenerated shoots was analyzed based on NMR measurements. The results showed that 200 µM MeJA increased production of trigonelline by tenfold. However, the concentrations of several amino acids including alanine were decreased. Based on gas chromatography and mass spectrometry (GC/MS) data, proximadiol concentrations significantly decreased with 10 and 100 µM MeJA. Proximadiol production improved by using 200 µM MeJA, although the data were non-significant. Our findings suggested that, while the addition of MeJA to embryogenic calli improved somatic embryo induction, maturation, and germination, it suppressed organogenic shoot and root formation. MeJA at a particular concentration (200 µM) enhanced the accumulation of trigonelline and osmoprotectant amino acids, while their effect on proximadiol production was statistically non-significant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methyl jasmonate (MeJA) is a methyl ester of jasmonic acid. It is a phytohormone that is widely distributed in the plant kingdom and reportedly proven to enhance plant regeneration and bioactive metabolite elicitation. It plays a pivotal role in protecting plants from biotic (e.g. pathogen attacks) and abiotic stress (e.g. salinity, drought) (Pan et al., 2020; Taheri et al., 2020; Tayyab et al., 2020). Jasmonates (Jasmonic acid and MeJA) were proven to improve the shoot and root regeneration of several plants. They promoted the de novo root and shoot regeneration in Arabidopsis (Park et al., 2019; Zhang et al., 2020); induced adventitious root formation in petunia cuttings (Lischweski et al., 2015); and increased shoot and bulb development in Allium sativum (Ravnikar et al., 1993). They also enhanced the photosynthetic activity and productivity of three Brassica oleracea varieties (Sirhindi et al., 2020).
The elucidating role of MeJA in accumulation of therapeutically important metabolites in plants has been previously reported. Exogenous application of MeJA enhanced the production of glucosinolates and phenolic metabolites from broccoli cell culture (Sánchez-Pujante et al., 2020) and upregulated the production of phenolics and monoterpenes from Mentha x piperita (Cappellari et al., 2020). It also improved the accumulation of flavonoids in Orostachys cartilaginous (Hao et al., 2020).
Methyl jasmonate has been reported to down regulate amino acids which are known to be involved in secondary metabolite biosynthesis in green, oolong, and black tea (Shi et al., 2019). Concentration of the amino acids histidine, threonine, arginine, leucine, and lysine decreased when using 100 μM MeJA on Scenedesmus quadricauda culture (Kováčik et al., 2011).
Genus Cymbopogon belongs to the family Poaceae; is distributed in the tropical and subtropical regions of Africa, Asia, and America (Avoseh et al., 2015). It comprises numerous aromatic species, and many of them possess therapeutic activities (Akhila, 2009). Cymbopogon schoenanthus (L.) Spreng. subsp. proximus (also known as Halfa barr) grows as wild aromatic grass in the desert of Southern Egypt and Northern Sudan (Bolous, 1999). Plant extract from aerial plant parts is used for treatment of renal calculi-related spasms and available in pharmacies under different dosage forms such as tablets, capsules, and effervescent powders for this therapeutic purpose. Additionally, the arial parts are of importance in traditional medicine (usually used as herbal infusions) as an efficient curative for many other ailments, e.g. inflammatory disorders, urinary tract infection, and diabetes (Batanouny et al., 1999; Taeckholm, 1974). The antimicrobial and antioxidant activities of the plant extract have also been documented (Selim, 2011). Terpenoids make up most of the chemical constituents in the plant extract (El-Askary et al., 2003). Proximadiol, an eudesmane sesquiterpene, is the principle bioactive compound present in the plant. Pharmaceutical industries, as well as folk medicine societies still depend on the wild species as their main source of plant material and therefore are bringing the wild population under an accelerated threat of extinction due to the uncontrolled collection. Thus, alternative intervening approaches of plant mass propagation and/or bioactive metabolite elicitation are in high demand. In vitro propagation protocol for Cymbopogon schoenanthus subsp. proximus has been previously established (El-Bakry and Abdelsalam, 2012; Abdelsalam et al., 2017a), and the differences between the wild and the in vitro propagated tissues at the metabolite level were investigated (Abdelsalam et al., 2017b). However, the possibility of mass propagation of the species under the influence of exogenous elicitors application has not been studied.
Therefore, this study was undertaken with the aim of studying the influences of MeJa exogenous supplementation on the mass production of plants of Cymbopogon schoenanthus subsp. proximus, metabolite changes within each propagation system (i.e., embryogenesis and organogenesis), as well as its impact on the accumulation of the bioactive principle from the plant.
Materials and methods
Plant material
Mature inflorescences of Cymbopogon schoenanthus subsp. proximus were collected during April 2015 from the botanical garden of Aswan University in Egypt. The plant material was authenticated by Prof Hasnaa Hosni, Professor of Plant taxonomy, Herbarium of Cairo University and a voucher specimen was deposited at the Herbarium of the Faculty of Science at Helwan University in Egypt.
Chemicals
Murashige and Skoog modified basal medium with Gamborg vitamins was obtained from Phytotech Lab (Lenexa, KS, USA). Proximadiol reference standard (5 mg) was purchased from BOC Sciences (NY, USA). Methyl jasmonate, Benzyl adenine, 2, 4-Dichlorophenoxyacetic acid, 1-Naphthaleneacetic acid, sucrose, phytagel powder, solvents and NMR standard components were purchased from Sigma Aldrich (St. Louis, Mo., USA).
Seed sterilization and culture conditions
Healthy, mature seeds were collected from the inflorescence one day before culturing. Seeds were washed under running tap water for 15 min followed by dist. H2O for 5 min. Surface sterilization was performed using 95% ethanol for 1 min followed by 1.0% NaOCl for 20 min. Residual disinfectants were removed from the surface of sterilized seeds by washing with sterile dist. H2O for 15 min under aseptic conditions. Murashige and Skoog (1962) with B5 vitamins (Gamborg et al., 1968) medium (MSB5) was used as the basic culturing media, which was further fortified with other regulators according to the intended propagation protocol. As a general culturing condition, the pH of the media was adjusted to 5.8 and supplemented with phytagel (2.0 g/L) before sterilization at 121 °C for 20 min. The cultures were incubated at 25 ± 1 °C under cool white fluorescent light (3000 lx). Somatic embryogenic tissues were maintained at 8/16 h light/dark photoperiod in the first 12 weeks, then the photoperiod increased to 16/8 h light/dark until the end of the experiment. The direct regenerated plants were incubated under 16/8 h light/dark photoperiod.
Effect of MeJA on in vitro regeneration through somatic embryogenesis
Seeds were cultured in Petri dishes on MSB5 supplemented with 4.0 mg/L 2, 4-Dichlorophenoxyacetic acid (2, 4-D) and 0.5 mg/L Benzyl adenine (BA). The first subculture was carried out under similar conditions four weeks post culturing. Six weeks after the first subculture, the second subculturing was carried out by transferring embryogenic calli to MSB5 media containing 1.0 mg/L 2,4-D, 0.125 mg/L BA and different concentrations of MeJA (10.0, 100.0, 200.0, 300.0, 400.0 µM), while control cultures were MeJA-free. After 8 weeks from the second subculture, the explants were screened for the number of embryos (globular), mature embryos (scutellar), as well as the number of embryogenic shoots. For each MeJA concentration 20 replicates were used (5 plates, 4 seeds/plate).
Effect of MeJA on in vitro regeneration through direct organogenesis
Surface sterilized seeds were cultured in Magenta vessels (4 vessels for each MeJA concentration, 4 seeds/vessel) on MSB5 supplied with 7.0 mg/L BA and 0.05 mg/L 1-Naphthaleneacetic acid (NAA). After four weeks of seed culturing, 10.0 mL of MSB5 liquid media containing MeJA at a final different concentration (10.0, 100.0, 200.0, 300.0, 400.0 µM) and 0.2 mg/L BA were added in each culturing magenta box. After two weeks of liquid media addition, 10.0 ml MSB5 hormone-free media were added into each magenta box. Two weeks later, each explant was screened for the number of the organogenic shoots and roots.
Metabolic profiling of direct regenerated shoots using NMR spectroscopy
Sample collection
Only plantlets produced via organogenesis were examined for accumulation of proximadiol, as embryogenic shoots only were produced in two of the studied treatments. Six replicates from the control and MeJA-treated shoots (10.0, 100.0, 200.0 µM) were randomly collected from nine different Magenta vessels. Plants of the other two concentrations i.e. 300.0 and 400.0 µM were completely brown at the time of sample collection, and therefore were not included. Collected plantlets were immediately immersed in liquid nitrogen, lyophilized for 48 h, and shoot parts were separated and finely powdered to be used in metabolic profiling analysis.
Polar metabolite extraction and NMR data collection
Twenty milligrams of the finely powdered samples were extracted using methanol: chloroform: water in a volumetric ratio of 2:2:1.8 (Kim et al., 2011) based on dry mass and water loss ratio reported by Bligh and Dyer (1959) and Wu et al. (2008). The extraction process was proceeded by vortex-mixing the calculated methanol and water volumes with the powder for 30 s, then placed on ice for 10 min to allow the solid matter to precipitate. The supernatant was then separated in 10.0 mL glass tubes containing the calculated amount of ice-cooled chloroform, vortex-mixed for 30 s, and kept on ice for 5 min. For bilayer separation, samples were centrifuged at 4 °C (2000 × g) for 5 min. The polar layers were separated and dried under vacuum at 30 °C for 24 h using a CentriVap vacuum concentrator (Labcocno, Kansas City, MO, USA).
The obtained (mostly brownish) residues were dissolved in 620 μL of NMR buffer containing 100.0 mM sodium phosphate buffer, pH 7.3, 1.0 mM 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid as internal standard (TMSP), and 0.1% sodium azide, in 99.9 atom % D2O. NMR data were acquired at 700 MHz (BrukerAvance™ III spectrometer, Bruker Biospin GmbH, Rheinstetten, Germany).
Data were obtained with a spectral width of 16.0 ppm and 64 K points resulting in an acquisition time of 2.9 s. On-resonance pre-saturation was used for solvent suppression during a 3 s recycle delay. The first increment of the presat-noesy spectra was collected with 120 scans, 4 dummy scans, 3 s relaxation delay, and pre-saturation at the residual water frequency. The 90º pulse widths were measured for each sample using the automatic pulse calculation experiment (pulsecal) in TopSpin 2.1.1 (BrukerBioSpin, Billerica, MA). Two dimensional 1H-13C HSQC data were collected at 700 MHz using a Bruker hsqcedetgpsisp 2.2 pulse sequence. The 1H was observed in the F2 channel with a spectral width of 11 ppm while the 13C was observed in the F1 channel with a spectral width of 180 ppm.
Metabolite identification and statistical analysis of NMR data
Polar metabolites were annotated by inputting the acquired 1H NMR spectra into Chenomx NMR Suite (Chenomx Inc., Edmonton, Alberta, Canada) and using the 700 MHz data library. For further metabolite confirmation, 2D 1H-13C HSQC experiments were carried, and the observed cross peaks were matched with those reported for the same compounds in Madison Metabolomics Consortium Database (MMCD) (Cui et al., 2008). Spectra were labeled using Mnova software.
NMR data analysis was carried out using MetaboAnalyst version 4.0 (MetaboAnalyst 4.0—a comprehensive server for metabolomic data analysis) with 95% confidence intervals according to the bucket tables created by AMIX software. Chemometrics analysis including the Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA), and one way analysis of variance (ANOVA) were performed with a spectral region ranging from 0.5 to 10.0 ppm with 0.01 ppm bucket widths and advance bucketing, while water region (4.833–4.833 ppm) was specified for exclusion. The spectral bins were normalized to total intensity (Xia et al., 2012). Hierarchical cluster analysis was carried out using Ward’s linkage as a clustering algorithm and Euclidean distance as a similarity measurement. Boxplots were generated for the most significant metabolites selected based on p values calculated using one way ANOVA. ANOVA was adjusted to p‹0.05 value and Fisher’S LSD post-hoc analysis.
Proximadiol identification and quantification in direct regenerated shoots
Sample collection
Similarly, plantlets resulting from the organogenic experiments were used. Three replicates were sampled from the control and each MeJa-treated plant (10.0, 100.0, 200.0 µM). Any remaining solid media were carefully removed using forceps and samples were immediately immersed in liquid nitrogen to quench any possible enzymatic reactions. Samples were then lyophilized (Labcocno, Kansas City, MO, USA) for 48 h. Afterwards, aerial parts were separated and powdered in a mortar, then used for proximadiol analysis.
Extract preparation, qualitative and quantitative analysis of proximadiol
Non-polar soluble fraction preparation, as well as procedures for qualitative and quantitative analysis of proximadiol have been carried out as we described previously (Abdelsalam et al., 2017b). Briefly, 100.0 mg dry powder from each sample were extracted using methanol: water (1: 1, V/V, 2.0 mL) in 10.0 mL glass reaction tubes. The mixture was then sonicated for 20 min at 40 °C. Metabolite portioning was performed by adding 2.0 mL chloroform, and the mixture was vigorously shaken for 5 min. Following bilayer separation, the non-polar organic phase was separated. The partition process was repeated by adding another 2.0 mL chloroform and steps proceeded as mentioned. Collected organic phases were pooled, filtered through 0.22 µM syringe filters, dried under vacuum, and redissolved in 1.0 mL chloroform. This solution was kept at – 20 °C until GC analysis of proximadiol.
GC–MS measurements were recorded on an HP Agilent 5890 GC instrument equipped with Restek fused silica RtX-5 column (30 m × 0.25 mm i.d.; film thickness 0.25 µm) and connected to VG 70 S mass unit. Tested samples and standard solution were injected (1.0 µL) in splitless mode and spectra were collected over a mass range 50–450 m/z. Helium was used as a carrier gas and worked at a flow rate of 1.0 mL/min. The injector temperature was set to 250 °C and oven temperature ramp was programmed as follows: 70 °C for 0 min, (70–150 °C) at 10 °C/min, (150–210 °C) at 5 °C/min and finally (210–300 °C) at 10 °C/min for 10 min. Proximadiol stock solution was prepared at 1.0 mg/mL in chloroform. The standard calibration curve was constructed using four dilution points (0.5, 1.0, 10.0, and 50.0 µg/mL). The proximadiol peak in each sample was determined by matching the retention indices as well as mass fragmentation behavior with those of the proximadiol standard. The area under the base ion peak at 149.0 was used for construction of the calibration curve. Proximadiol concentration was calculated from the generated linear regression equation.
Statistical design and data analysis
For in vitro culture studies, a completely randomized experimental design was performed. For each methyl jasmonate treatment, 4–5 replicates have been carried out. Each replicate was represented by one container (petri-dish or magenta box) with 4 explants cultured in each container. All replicates came from different seeds.
Proximadiol concentration data was collected using 3 explants for each MeJA treatment selected randomly from 3 different containers.
Data was analyzed using Minitab 17 software by one way ANOVA. Fisher least significant difference (LSD) method with 95% confidence level was used to compare treatment means.
Results and discussion
Effect of MeJA on in vitro regeneration through somatic embryogenesis
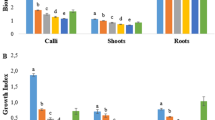
MeJA at five different concentration levels (10.0–400.0 µM) was externally applied to the embryogenic calli. Under the applied MeJA concentrations, the total number of somatic shoots significantly increased compared to the control cultures (Fig. 1). Although, there was no significant difference in the number of mature embryos, the numbers were enhanced (10.9 and 12.0 embryos/explant) by using 200 and 300 µM MeJA, respectively. MeJA at 300 µM concentration improved the number of somatic embryos, mature embryos, and embryogenic shoots (Fig. 2). The enhancing effect of MeJA on embryo induction and maturation is supported by earlier studies. It induced the embryogenesis-related proteins and mRNAs in leaf tissues of Nicotiana plumbaginifolia (Reinbothe et al., 1994). Moreover, MeJA at concentrations of 10 and 100 µM has improved somatic embryo maturation in Lithospermum erythrorhizon plant (Mariani et al., 2004). Jasmonic acid enhanced the biosynthesis of indole acetic acid in Arabidopsis explants, which is known to induce the production of somatic embryos (Mira et al., 2016).
Effect of different MeJA concentrations on mean number of somatic embryos, mature embryos, and embryogenic shoots. *Different letters indicate statistically significant difference within embryos at P-value = 0.022; within mature embryos at P-value = 0.00; within shoots at P-value = 0.001, according to Fisher test (n = 20)
Effect of MeJA on in vitro regeneration through direct organogenesis
In contrast to regeneration through somatic embryogenesis, the applied MeJA concentrations (10.0–400.0 µM) have significantly reduced the number of de novo regenerated shoots and roots (Fig. 3). MeJA at 10.0 and 100.0 µM reduced the shoot number by approximately tenfold compared with the control cultures by dramatically decreasing the mean number of adventitious roots. Moreover, organogenic shoots and roots were completely inhibited when 300 and 400 µM of MeJA were used (Fig. 4E). Based on the results, we conclude that the regeneration process via direct organogenesis was significantly retarded by exogenous MeJA application under the studied concentrations. Earlier studies showed that MeJA led to reduction in the height and biomass of Helianthus annuus, Solanum lycopersicum, and Glycine max (Lie et al., 2018), decreased the number of floral buds and inhibited the growth of shoots and roots in Pharbitis nil (Maciejewska & Kopcewicz, 2002). Moreover, MeJA decreased the bulb formation in in vitro regenerated Tulip plants (Podwyszyn´ska et al., 2015). Also, it has resulted in root suppression of Phaseolus coccineus, Allium cepa and Zea mays (Maksymiec & Krupa, 2007). Therefore, the application of exogenous MeJA in the present study adds evidence to the previous studies reporting the negative effects of MeJA on plant organogenesis.
Effect of MeJA on metabolic profiling of organogenic shoots
Metabolic profiling of the polar extracts of organogenic shoots was performed using NMR spectroscopy. Metabolite annotation was facilitated by Chenomx NMR Suite and, for confirmation, compared with corresponding metabolite spectra on Madison Metabolomics Consortium Database (MMCD). In total, 34 metabolites were identified in both the control and MeJA-fortified shoot samples (Table S1 in supplementary data). The identified compounds included different classes like alkaloids (e.g. trigonelline), amino acids (e.g. alanine, arginine, asparagine, threonine, and valine), carbohydrates (e.g. fructose, glucose, sucrose), and organic compounds (e.g. formic acid).
Scores plot analysis generated using PCA showed that samples corresponding to each MeJA treatment were clustered together, whereas different treatments were clearly separated based on the metabolite variabilities (Fig. 5A and B). Samples treated with 10 and 200 µM MeJA are close to each other and varied from 200 µM MeJA treated samples. The metabolites responsible for the separation and sample grouping in PCA are shown in the score plot (Fig. 5C). Hierarchical clustering (Fig. 5D) showed two main clusters, control cultures in one cluster and the MeJA-treated cultures in the other one. The cluster including MeJA-treated samples are divided into two subclusters one for 10 and 200 µM MeJA-treated samples and the other for 10 µM MeJA-treated samples. This could indicate that the observed increase in proximadiol concentration in both samples of the former subcluster compared to the later subcluster could be attributed to the favorable alteration effects of MeJA on the primary precursors upstreaming to the sesquiterpenes biosynthesis in the former.
Statistical analysis of the control as well as the MeJA-treated organogenic shoots (6 replicates from each treatment) of normalized data generated by MetaboAnalyst 4.0. A & B 2-D and 3-D PCA scores plots, the ovals in the 2D score plot indicate 95% Hotellings confidence intervals, PC1and PC2 explained the total of 62.2% variance and PC3 explained 79.4% of total variance C loadings plot of the two-principal component analysis and D hierarchical cluster analysis. MJ0 = MeJA-free cultures, MJ10 = samples treated with 10 µM MeJA, MJ100 = samples treated with 100 µM MeJA, D MJ200 = samples treated with 200 µM MeJA
Boxplots of twelve metabolites that were selected from the loading plot analysis are shown in (Fig. 6). Alanine, 4-hydroxybenzoate is downregulated sharply by using 10 and 200 µM MeJA. Also, fructose and threonine concentrations decreased by nearly 3- and 10-folds, respectively, by using 200 µM. On the other hand, glucose-6-P was upregulated twofold by using 200 µM-treated samples. Exogenous application of MeJA significantly decreased the concentrations of fructose and glucose in Manduca sexta leaves (Machado et al., 2015). Also, decreased fructose, mannose, and sucrose concentrations were reported in Agastache rugosa cell cultures (Kim et al., 2013) and decreased alanine and glutamine levels in Codonopsis pilosula (Ji et al, 2019).
Boxplots of relative concentrations of metabolites that were significantly different in PCA loading plot analysis. The black dots represent the concentration of the selected metabolite in each replicate. The notch indicates the 95% confidence interval around the average of six replicates, defined as ± 1.58* IQR/sqrt (n). The mean concentration of each treatment is indicated by the yellow diamond. The Y axis defines the relative abundances of the specific metabolite and X axis defines the treatment group. MJ0 = MeJA-free cultures, MJ10 = samples treated with 10 µM MeJA, MJ100 = samples treated with 100 µM MeJA, D MJ200 = samples treated with 200 µM MeJA
The alkaloid, trigonelline accumulated in MeJA-treated cultures tenfold compared to the control culture. Trigonelline is a medicinal compound with anti-diabetic, anti-viral and anti-tumor activities (Qiu et al., 2020; Zhou et al., 2012). MeJa at concentration of 100 μM improved trigonelline production in Trigonella foenumgraecum suspension culture and hairy root culture (Abdel Mawla, 2011; Qaderi et al., 2016).
Heat map dendrogram (Fig. 7) confirmed the Boxplot findings and showed that sucrose and glucose were downregulated in 200 µM MeJA treatments compared with control. Pyroglutamate and arginine have been accumulated in 100 μM MeJA treated samples.
Heat map analysis showing the effect of different concentrations of MeJA (0.0, 10, 100, and 200 µM) on the metabolite concentrations in the polar extract of direct regenerated shoots. Colour scale is relative to the abundance of each metabolite. Each row represents a metabolite, and each column represents a sample
Two hundred µM MeJA induced the highest concentration of the amino acids betaine, proline and glutamate. Betaine has a role in cell protection under stress conditions (Naidu et al., 1991). Exogenous application of jasmonate resulted in accumulation of betaine in pear leaves and improved its tolerance to drought stress (Gao et al., 2004). Glutamate is the precursor of several amino acids, e.g. arginine, proline and 4-aminobutyrate (δ-aminobutyric acid), proline, and δ-aminobutyric acid and is reported to accumulate in plants cultured under stress conditions (Forde & Lea, 2007). Proline is an osmolyte, which accumulates in plants under abiotic stress to maintain cell functions (Dar et al., 2016). The effect of jasmonates in downregulating amino acids threonine, isoleucine, leucine, and serine has been reported in Brassica oleracea (Tytgat et al., 2013).
Effect of MeJA on proximadiol production
Proximadiol is the principle active metabolite in the Cymbopogon schoenanthus subsp. proximus. The therapeutic potential of the plant extract as a renal calculi repellent has been traced to its proximadiol content. Qualitative analysis of proximadiol in the non-polar extract of direct organogenic shoots was performed using GC–MS. Confirmation was done by matching the obtained measurements with a standard proximadiol solution (Fig S1 & S2 in supplementary data). Quantitative measurements showed that all samples variably accumulated proximadiol. Statistical analysis showed that the concentration of proximadiol was significantly lower (12.08 ± 0.11 µg/100 mg dry weight, DW) when using 100 µM MeJA (Fig. 8), while its concentration has increased to 20.3 ± 0.19 and 25.9 ± 0.21 µg/100 mg DW in 10.0 and 200.0 µM MeJA-treated cultures, respectively in comparison with 100 µM MeJA-treated samples.
MeJA is capable of modulating the biosynthetic pathways in plants and could therefore shift the carbon backbones to a certain pathway (Ji et al., 2019). Our results indicate that the concentration of proximadiol significantly decreased in 100 µM MeJa-treated cultures in comparison with the control and 200 µM MeJA-treated samples. Also, proximadiol was down regulated further in 100 µM MeJa-treated cultures compared to 10 µM MeJa-treatment (results were non-significant), which suggests that the elicitation potential of MeJa is concentration dependent. Notably, MeJa at 100 µM resulted in morphologically different shoots as compared to other treatments. These shoots were dwarf, thin, pale green, or purple in color. The effect of MeJA on terpenoid biosynthesis and plant development is controversial. For example, MeJA has been shown to induce accumulation of terpenoids in many plants as in Centella asiatica and Galphimia glauca plantlets (Mangas et al., 2006) and in Rhazya stricta hairy root cultures (Akhgari et al., 2019). However, it did not affect the terpenoid production in Eucalyptus grandis leaves (Henery et al., 2008). Moreover, Yousefian et al., (2020) reported the fluctuation in the concentrations of some phenolic compounds from Mentha spicata hairy root cultures exposed to MeJA at different time courses. Also, the seedling biomass was shown to fluctuate with different MeJA concentrations in three varieties of Brassica oleracea (Sirhindi et al., 2020).
In the present study, 100 μM MeJA treatment significantly decreased organogenic shoot and root numbers and induced the upregulation of lactate and glycolate. Lactate accumulation in the plant is considered unfavorable for the plant development because it rapidly leads to cytosolic acidosis (Ricard et al., 1994). Exogenous lactate was reported to have negative effects on seedling development of Arabidopsis thaliana (Wienstroer et al., 2012). Glycolate has been shown to have a negative effect on plant development (South et al., 2019). It was reported to decrease metabolic activities in maize (González-Moro et al., 1997) and decrease root growth of Arabidopsis plant (Engqvist et al., 2015).
The 200 µM MeJA treatment induced the accumulation of the bioactive secondary metabolites trigonelline (an alkaloid) and proximadiol (a sesquiterpene), while down regulated the amino acids arginine, lysine, leucine, and valine. Arginine and lysine are precursors to a variety of alkaloids (Bunsupa et al., 2017; Lichman et al., 2021). Leucine is involved in sesquiterpenoids biosynthesis (Zhang et al., 2015).
Our data suggest that Cymbopogon schoenanthus subsp. proximus cultures require a high concentration of MeJA (200 µM) to induce the production of bioactive secondary metabolites, as well as, for the accumulation of osmoprotectant amino acids betaine and proline.
Conclusion
The effect of MeJA on somatic embryogenesis and organogenesis, and metabolite accumulation in Cymbopogon schoenanthus subsp. proximus is concentration dependent; however, it does not exhibit a direct proportional correlation. MeJa at certain concentrations improved somatic embryo maturation and germination, promoted direct organogenesis and enhanced the accumulation of some metabolites as trigonelline, as well as, osmoprotectant amino acids. Proximadiol concentration increased significantly using 10 and 200 μM MeJA in comparison with 100 μM concentration. Further studies are needed to study the application of MeJA for a targeted improvement of in vitro propagation system and for the enhancement of specific metabolite production.
Data Availability
The data that support the findings of this study are available from the corresponding author (AB) upon reasonable request.
References
Abd-El Mawla, M. A. A., & Osman, E. H. (2011). Elicitation of trigonelline and 4-hydroxyisoleucine with hypoglycemic activity in cell suspension cultures of Trigonella foenum graecum L. The Open Conference Proceedings Journal, 2(1), 80–87.
Abdelsalam, A., Chowdhury, K., & El-Bakry, A. (2017a). Micropropagation through in vitro tillering from seed cultures of the medicinal plant Cymbopogon schoenanthus subsp. proximus. Asian Journal of Applied Science, 5(1), 31–40.
Abdelsalam, A., Mahran, E., Chowdhury, K., Boroujerdi, A., & El-Bakry, A. (2017b). NMR-based metabolomic analysis of wild, greenhouse, and in vitro regenerated shoots of Cymbopogon schoenanthus subsp. proximus with GC–MS assessment of proximadiol. Physiology and Molecular Biology of Plants, 23(2), 369–383.
Akhgari, A., Laakso, I., Maaheimo, H., Choi, Y. H., Seppänen-Laakso, T., Oksman-Caldentey, K. M., & Rischer, H. (2019). Methyl jasmonate elicitation increases terpenoid indole alkaloid accumulation in Rhazya stricta hairy root cultures. Plants, 8(12), 538–534.
Akhila, A. (2009). Essential oil-bearing grasses: The genus Cymbopogon. CRC Press.
Avoseh, O., Oyedeji, O., Rungqu, P., Nkeh-Chungag, B., & Oyedeji, A. (2015). Cymbopogon species; ethnopharmacology, phytochemistry and the pharmacological importance. Molecules, 20(5), 7438–7453.
Batanouny, K.H., Aboutabl, E., Shabana, M., & Soliman, F. (1999). Wild medicinal plants in Egypt. With contribution of: E. Aboutabl, M. Shabana, & F. Soliman). With support of the Swiss Development Co-operation (SDC). Academy of Scientific Research and Technology, Egypt. The World Conservation Union (IUCN), Switzerland. 60-4
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917.
Boulos, L. (1999). Flora of Egypt. Al Hadara publishing.
Bunsupa, S., Yamazaki, M., & Saito, K. (2017). Lysine-derived alkaloids: Overview and update on biosynthesis and medicinal applications with emphasis on quinolizidine alkaloids. Mini Reviews in Medicinal Chemistry, 17(12), 1002–1012.
Cappellari, L. D. R., Santoro, M. V., Schmidt, A., Gershenzon, J., & Banchio, E. (2020). Improving phenolic total content and monoterpene in Mentha x piperita by using salicylic acid or methyl jasmonate combined with Rhizobacteria inoculation. International Journal of Molecular Sciences, 21(1), 50.
Cui, Q., Lewis, I. A., Hegeman, A. D., Anderson, M. E., Li, J., Schulte, C. F., Westler, W. M., Eghbalnia, H. R., Sussman, M. R., & Markley, J. L. (2008). Metabolite identification via the madison metabolomics consortium database. Nature Biotechnology, 26(2), 162–164.
Dar, M.I., Naikoo, M.I., Rehman, F., Naushin, F., & Khan, F.A. (2016). Proline accumulation in plants: roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies (pp. 155–166). Springer, New Delhi.
El-Askary, H. I., Meselhy, M. R., & Galal, A. M. (2003). Sesquiterpenes from Cymbopogon proximus. Molecules, 8(9), 670–677.
El-Bakry, A. A., & Abdel-Salam, A. M. (2012). Regeneration from embryogenic callus and suspension cultures of the wild medicinal plant Cymbopogon schoenanthus. African Journal of Biotechnology, 11(43), 10098–10107.
Engqvist, M. K., Schmitz, J., Gertzmann, A., Florian, A., Jaspert, N., Arif, M., Balazadeh, S., Mueller-Roeber, B., Fernie, A. R., & Maurino, V. G. (2015). GLYCOLATE OXIDASE3, a glycolate oxidase homolog of yeast L-lactate cytochrome c oxidoreductase, supports L-lactate oxidation in roots of Arabidopsis. Plant Physiology, 169(2), 1042–1061.
Forde, B. G., & Lea, P. J. (2007). Glutamate in plants: Metabolism, regulation, and signaling. Journal of Experimental Botany, 58(9), 2339–2358.
Gamborg, O. L., Miller, R. A., & Ojima, K. (1968). Nutrient requirements of suspension culture of soybean root cells. Experimental Cell Research, 50(1), 151–158.
Gao, X. P., Wang, X. F., Lu, Y. F., Zhang, L. Y., Shen, Y. Y., Liang, Z., & Zhang, D. P. (2004). Jasmonic acid is involved in the water-stress-induced betaine accumulation in pear leaves. Plant Cell & Environment, 27(4), 497–507.
González-Moro, B., Lacuesta, M., Becerril, J. M., Gonzalez-Murua, C., & Muñoz-Rueda, A. (1997). Glycolate accumulation causes a decrease of photosynthesis by inhibiting RUBISCO activity in maize. Journal of Plant Physiology, 150(4), 388–394.
Hao, Y. J., Cui, X. H., Li, J. R., An, X. L., Sun, H. D., Piao, X. C., & Lian, M. L. (2020). Cell bioreactor culture of Orostachys cartilaginous A. Bor. and involvement of nitric oxide in methyl jasmonate-induced flavonoid synthesis. Acta Physiologiae Plantarum, 42(1), 1–10.
Henery, M. L., Wallis, I. R., Stone, C., & Foley, W. J. (2008). Methyl jasmonate does not induce changes in Eucalyptus grandis leaves that alter the effect of constitutive defenses on larvae of a specialist herbivore. Oecologia, 156(4), 847–859.
Ji, J. J., Feng, Q., Sun, H. F., Zhang, X. J., Li, X. X., Li, J. K., & Gao, J. P. (2019). Response of bioactive metabolite and biosynthesis related genes to methyl jasmonate elicitation in Codonopsis pilosula. Molecules, 24(3), 533.
Kim, H. K., Choi, Y. H., & Verpoorte, R. (2011). NMR-based plant metabolomics: Where do we stand, where do we go? Trends in Biotechnology, 29(6), 269–275.
Kim, Y. B., Kim, J. K., Uddin, M. R., Xu, H., Park, W. T., Tuan, P. A., Li, X., Chung, E., Lee, J. H., & Park, S. U. (2013). Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE, 8, e64199.
Kováčik, J., Klejdus, B., Štork, F., Hedbavny, J., & Bačkor, M. (2011). Comparison of methyl jasmonate and cadmium effect on selected physiological parameters in scenedesmus quadricauda (chlorophyta, chlorophyceae) 1. Journal of Phycology, 47(5), 1044–1049.
Li, C., Wang, P., Menzies, N. W., Lombi, E., & Kopittke, P. M. (2018). Effects of methyl jasmonate on plant growth and leaf properties. Journal of Plant Nutrition and Soil Science, 181(3), 409–418.
Lichman, B. R. (2021). The scaffold-forming steps of plant alkaloid biosynthesis. Natural Product Reports, 38(1), 103–129.
Lischweski, S., Muchow, A., Guthorl, D., & Hause, B. (2015). Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biology, 15(1), 229.
Machado, R. A., Arce, C. C., Ferrieri, A. P., Baldwin, I. T., & Erb, M. (2015). Jasmonate-dependent depletion of soluble sugars compromises plant resistance to M anduca sexta. New Phytolgist, 207(1), 91–105.
Maciejewska, B., & Kopcewicz, J. (2002). Inhibitory effect of methyl jasmonate on flowering and elongation growth in Pharbitis nil. Plant Growth Regultion, 1(3), 216–223.
Maksymiec, W., & Krupa, Z. (2007). Effects of methyl jasmonate and excess copper on root and leaf growth. Biologia Plantarum, 51(2), 322–326.
Mangas, S., Bonfill, M., Osuna, L., Moyano, E., Tortoriello, J., Cusido, R. M., Piñol, M. T., & Palazón, J. (2006). The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry, 67(18), 2041–2049.
Mariani, T. S., Ramayanti, O., Yazaki, K., & Miyake, H. (2004). Development of somatic embryo in Lithospermum erythrorhizon Siebb. et Zucc and the study on the effect of methyl jasmonate on its maturation. Annales Bogorienses, 9(2), 72–79.
Mira, M. M., Wally, O. S., Elhiti, M., El-Shanshory, A., Reddy, D. S., Hill, R. D., & Stasolla, C. (2016). Jasmonic acid is a downstream component in the modulation of somatic embryogenesis by Arabidopsis Class 2 phytoglobin. Journal of Experimental Botany, 67(8), 2231–2246.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassay with tobacco tissue culture. Plant Physiology, 15(3), 473–497.
Naidu, B. P., Paleg, L. G., Aspinall, D., Jennings, A. C., & Jones, G. P. (1991). Amino acid and glycine betaine accumulation in cold-stressed wheat seedlings. Phytochemistry, 30(2), 407–409.
Pan, L., Zhao, X., Chen, M., Fu, Y., Xiang, M., & Chen, J. (2020). Effect of exogenous methyl jasmonate treatment on disease resistance of postharvest kiwifruit. Food Chemistry, 305, 125483.
Park, O. S., Bae, S. H., Kim, S. G., & Seo, P. J. (2019). JA-pretreated hypocotyl explants potentiate de novo shoot regeneration in Arabidopsis. Plant Signaling & Behavior, 14(8), 1618180.
Podwyszyńska, M., Kosson, R., & Treder, J. (2015). Polyamines and methyl jasmonate in bulb formation of in vitro propagated tulips. Plant Cell, Tissue and Organ Culture, 123(3), 591–605.
Qaderi, A., Akbari, Z., Kalateh-jari, S., Fatehi, F., Tolyat, M., Jalali, M. M., & Naghdi, B. H. (2016). Improving trigonelline production in hairy root culture of fenugreek (Trigonella foenum-graecum). Journal of Medicinal Plants., 15(59), 73–80.
Qiu, Z., Wang, K., Jiang, C., Su, Y., Fan, X., Li, J., Xue, S., & Yao, L. (2020). Trigonelline protects hippocampal neurons from oxygen-glucose deprivation-induced injury through activating the PI3K/Akt pathway. Chemico-Biological Interactions, 317, 108946.
Ravnikar, M., Žel, J., Plaper, I., & Špacapan, A. (1993). Jasmonic acid stimulates shoot and bulb formation of garlic in vitro. Plant Growth Regulations, 12(2), 73–77.
Reinbothe, S., Mollenhauer, B., & Reinbothe, C. (1994). JIPs and RIPs: The regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. The Plant Cell, 6(9), 1197.
Ricard, B., Couee, I., Raymond, P., Saglio, P. H., Saint-Ges, V., & Pradet, A. (1994). Plant metabolism under hypoxia and anoxia. Plant Physiology and Biochemistry, 32, 1–10.
Sánchez-Pujante, P. J., Gionfriddo, M., Sabater-Jara, A. B., Almagro, L., Pedreño, M. A., & Diaz-Vivancos, P. (2020). Enhanced bioactive compound production in broccoli cells due to coronatine and methyl jasmonate is linked to antioxidative metabolism. Journal of Plant Physiology, 248, 153136.
Selim, S. A. (2011). Chemical composition, antioxidant and antimicrobial activity of the essential oil and methanol extract of the Egyptian lemongrass Cymbopogon proximus Stapf. Grasas y Aceites, 62(1), 55–61.
Shi, J., Xie, D., Qi, D., Peng, Q., Chen, Z., Schreiner, M., Lin, Z., & Baldermann, S. (2019). Methyl jasmonate-induced changes of flavor profiles during the processing of green, oolong, and black tea. Frontiers in Plant Science, 10(2019), 781.
Sirhindi, G., Mushtaq, R., Gill, S. S., Sharma, P., Abd-Allah, E. F., & Ahmad, P. (2020). Jasmonic acid and methyl jasmonate modulate growth, photosynthetic activity and expression of photosystem II subunit genes in Brassica oleracea L. Scitific Reports, 10(1), 1–14.
South, P. F., Cavanagh, A. P., Liu, H. W., & Ort, D. R. (2019). Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science, 363, 6422.
Taeckholm, V. (1974). In Students flora of Egypt. Cairo University, Cooperative Printing.
Taheri, Z., Vatankhah, E., & Jafarian, V. (2020). Methyl jasmonate improves physiological and biochemical responses of Anchusa italica under salinity stress. South African Journal of Botany, 130(2020), 375–382.
Tayyab, N., Naz, R., Yasmin, H., Nosheen, A., Keyani, R., Sajjad, M., Hassan, M. N., & Roberts, T. H. (2020). Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. PLoS ONE, 15, e0232269.
Tytgat, T. O., Verhoeven, K. J., Jansen, J. J., Raaijmakers, C. E., Bakx-Schotman, T., McIntyre, L. M., van der Putten, W. H., Biere, A., & van Dam, N. M. (2013). Plants know where it hurts: root and shoot jasmonic acid induction elicit differential responses in Brassica oleracea. PLoS ONE, 11, e65502.
Wienstroer, J., Engqvist, M. K., Kunz, H. H., Flügge, U. I., & Maurino, V. G. (2012). d-Lactate dehydrogenase as a marker gene allows positive selection of transgenic plants. FEBS Letters, 586(1), 36–40.
Wu, H., Southam, A. D., Hines, A., & Viant, M. R. (2008). High-throughput tissue extraction protocol for NMR- and MS-based metabolomics. Analaytical Biochemistry, 372(2), 204–212.
Xia, J., Mandal, R., Sinelnikov, I. V., Broadhurst, D., & Wishart, D. S. (2012). MetaboAnalyst 2.0—a comprehensive server for metabolomics data analysis. Nucleic Acids Research, 40(W), 127-W133.
Yousefian, S., Lohrasebi, T., Farhadpour, M., & Haghbeen, K. (2020). Effect of methyl jasmonate on phenolic acids accumulation and the expression profile of their biosynthesis-related genes in Mentha spicata hairy root cultures. Plant Cell, Tissue and Organ Culture, 142, 285–297.
Zhang, F., Fu, X., Lv, Z., Lu, X., Shen, Q., Zhang, L., Zhu, M., Wang, G., Sun, X., Liao, Z., & Tang, K. (2015). A basic leucine zipper transcription factor, AabZIP1, connects abscisic acid signaling with artemisinin biosynthesis in Artemisia annua. Molecular Plant, 8(1), 163–175.
Zhang, J., Liu, R., Zhu, Y., Gong, J., Yin, S., Sun, P., Feng, H., Wang, Q., Zhao, S., Wang, Z., & Li, G. (2020). Identification and characterization of circRNAs responsive to methyl jasmonate in Arabidopsis thaliana. International Journal of Molecular Sciences, 21(3), 792.
Zhou, J., Chan, L., & Zhou, S. (2012). Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Current Medicinal Chemistry, 19(21), 3523–3531.
Funding
This work was funded by the Culture Affairs and Missions Sector, Ministry of Higher Education, Egypt. NMR facilities were provided by SC-INBRE (2 P20 GM103499), NSF HBCU-UP (HRD-1332516), and NSF MRI (1429353).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AA, EM. The first draft of the manuscript was written by AA and all authors edited the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
We declare that all authors comply with Springer’s ethical policies.
Human and animal rights
No human participants or animals were involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdelsalam, A., Mahran, E., Chowdhury, K. et al. Effect of exogenous methyl jasmonate on in vitro propagation, metabolic profiling and proximadiol production from Cymbopogon schoenanthus subsp. proximus. Plant Physiol. Rep. 26, 548–560 (2021). https://doi.org/10.1007/s40502-021-00608-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-021-00608-x