Abstract
Orostachys cartilaginous A. Bor. is a high-value medicinal plant, whereas flavonoids are important secondary metabolites. Bioreactor cell culture is an alternative method for the mass production of flavonoids in O. cartilaginous. This study investigated the adaption of culture conditions using bioreactors with different sizes to provide a reference for the pilot-scale culture of O. cartilaginous cells in the future. Results showed that cell fresh and dry weights per culture medium among the balloon-type airlift bioreactors of 3, 5, and 10 L did not change. Moreover, approximately equal amounts of total flavonoids were synthesized in bioreactors with different sizes, indicating that the culture conditions optimized in a bioreactor of certain size can be used in bioreactors of other sizes. This study used methyl jasmonate (MeJA) as an abiotic elicitor to treat 25-day-old O. cartilaginous cells, and an event whether nitric oxide (NO) was involved in flavonoid synthesis in MeJA-induced flavonoid synthesis was investigated to improve flavonoid accumulation. The contents of total flavonoids and flavonoid monomers including quercetin, kaempferide, epicatechin gallate, quercetin-3-O-glucose, and kaempferol-3-rutinoside, were significantly improved by MeJA treatment, reaching the maximum value at 48 h after elicitation. During the MeJA elicitation, NO burst in the early stage and NO content peaked at 6 h. In addition, nitrate reductase (NR) inhibitors of tungstate and glutamine blocked NO generation and inhibited flavonoid synthesis in MeJA-stimulated cells. However, such inhibition of flavonoid synthesis was relieved by a NO donor (sodium nitroprusside), thereby suggesting that NO was involved in MeJA-induced flavonoid synthesis through NR pathway. The present finding has a critical significance for understanding the mechanism of the defense response stimulated by the MeJA elicitor and can provide a new strategy that regulates NO burst and flavonoid synthesis by controlling the NR activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orostachys cartilaginous A. Bor. is a high-value medicinal plant of the Crassulaceae family, which is mainly distributed in the Changbai Mountain area of China (Zhang et al. 2017). The whole plants of O. cartilaginous contain various bioactive compounds, among which flavonoids are important secondary metabolites (Li et al. 2015). However, the shortage of plant resource has restricted the production of O. cartilaginous (Piao et al. 2017; Zhang et al. 2017). Plant cell culture is recognized as a promising alternative approach obtaining raw materials for plant species with natural resource shortage (Efferth 2019; Georgiev et al. 2009; Thanh et al. 2014). Therefore, the resource problem of O. cartilaginous could be solved by the plant cell culture using bioreactor systems (Piao et al. 2017). However, the relatively low yield of secondary metabolites has often occurred during plant cell culture, thereby restricting its application in industrial production (Xu et al. 2008). To overcome this problem, the use of elicitation methods with biotic or abiotic elicitors during plant cell culture is considered the most effective approach (Baldi et al. 2009). Methyl jasmonate (MeJA) is an effective abiotic elicitor that has been applied in numerous plant species, that are involved in plant defense response pathways and triggers plant metabolite biosynthesis (Wang et al. 2015; Kang et al. 2006; Lee et al. 2014).
The coordinated and synchronized response of plants to the elicitor involves the generation of several signaling molecules, including nitric oxide (NO), reactive oxygen, salicylic acid, and other molecules (Gao et al. 2012). NO is a free radical gas formed endogenously in many biological systems. The most possible and prominent role of NO is signaling and regulating plant defense or stress responses (Wang and Wu 2004). In addition, Modolo et al. (2002) reported the earliest study on NO that was involved in the secondary metabolite synthesis; subsequently, a similar viewpoint was suggested by various researchers (Shan and Yang 2017; Wang and Wu 2004; Xu et al. 2008). These findings indicated that involvement of NO in the secondary metabolite synthesis is probably related to the activated defense response in plant cells attributed from stresses. In addition, various defense responses, such as defense gene expression and hypersensitive response, were induced by elicitors during plant cell culture (Modolo et al. 2002). However, those responses can be blocked by the NO scavenger, thereby confirming that NO is the necessary signaling molecule for the elicitor-induced defense response (Xu et al. 2005). In general, plant cells synthesize NO through non-enzymic and enzymic pathways. In the enzymic pathway, NO synthase (NOS) is the key enzyme. However, some investigations found that NO burst was not completely inhibited by the NOS scavenger (Foissner et al. 2010), indicating that other enzymes affect NO generation. Moreover, NR displays a nitrite reductase activity that leads to the production of NO from nitrite (Yamasaki and Sakihama 2000).
Over the last decade, NO has been extensively investigated as an important signaling molecule in elicitor-induced metabolite synthesis during plant cell culture (Xu and Dong 2005; Foissner et al. 2010). However, its role in O. cartilaginous cell culture has not been investigated yet. Therefore, this study used MeJA as the elicitor to treat 25-day-old bioreactor cultured O. cartilaginous cells and investigated an event whether NO was involved in MeJA-induced flavonoid synthesis through NR pathway.
Materials and methods
Maintenance of O. cartilaginous cells
O. cartilaginous cells were induced and cultured in vitro using the methods of Zhang et al. (2017) and used as plant material in the experiment of bioreactor culture.
Cell culture in bioreactors of different sizes
The in vitro cultured cells of 12.5 g/L (fresh weight, FW) were separately inoculated into 3, 5, and 10 L balloon-type airlift bioreactors, where 2, 4, and 8 L culture medium was poured, respectively. The culture medium was Murashige and Skoog (MS) (Murashige and Skoog 1962) medium supplemented with 3.5 mg/L benzylaminopurine (BA) (Shanghai YuanYe Bio-Tech Co., Ltd. Shanghai, China), 0.1 mg/L a-naphthalene acetic acid (NAA) (Aoboxing Bio-Tech Co., Ltd., Beijing, China), and 30 g/L sucrose (Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China); the medium pH was adjusted to 5.8 prior autoclaving. The bioreactor was maintained at 25 °C under 30 μmol/m2/s light intensity, with a 16 h photoperiod, and aerated at 100 mL/min air. After 25 days of bioreactor culture, cell FW and dry weight (DW), and the contents of total flavonoids and five flavonoid monomers of quercetin (Qc), kaempferide (Ke), epicatechin gallate (Egc), Qc-3-O-glucose (Qc-3-glc), and kaempferol-3-rutinoside (Ke-3-rut) were determined.
Effect of MeJA on flavonoid synthesis during cell suspension culture
On the basis of the O. cartilaginous cell growth kinetic study conducted by our research team (Piao et al. 2017), the cells were harvested from 5 L bioreactors after 25 days of culture and the culture medium was also collected. The collected medium of 100 mL was poured into a 150 mL Erlenmeyer flask and 15 g of harvested fresh cells were added. These operations were same in following experiments. The flask was added with 100 μM of the filter-sterilized liquid MeJA (Sigma, USA). Then, flasks were underwent continuous shaking (Jintan Science Analysis Instrument Co. Ltd., Jintan, China) at 100 rpm at 25 °C under 30 μmol/m2/s light intensity, with a 16 h photoperiod (as same in the following elicitation experiments). The cell samples were collected from the flask at 12 h intervals over a 72 h elicitation period and the contents of total flavonoids and flavonoid monomers in cells were determined.
Investigation of NO involvement in MeJA-induced flavonoid synthesis
To understand whether the NO involves in MeJA-induced flavonoid synthesis, the changes of NR and NO in cells were investigated in the first experiment. In brief, a total of 100 μM MeJA was added in the flask, and equal amounts of sterilized water were added in the control group. The cell samples were collected from the flask at 2 h intervals over a 24-h elicitation period, and the NO content and NR activity in cells were determined. In the second experiment, to explore whether the NO synthesizes though the NR pathway, the effect of NR inhibitors on NO production and NR activity in cells were examined. The experimental groups were designed as follows. (1) Control group, in which sterilized water (equal amounts with 100 μM MeJA) was added to the flask. (2) MeJA group, in which 100 μM MeJA was added. (3) MeJA + TUN group, in which 100 μM MeJA and 0.5 mM of filter-sterilized NR inhibitor, tungstate (TUN) were added. (4) MeJA + Gln group, in which 100 μM MeJA and 0.5 mM of filter-sterilized NR inhibitor, glutamine (Gln) were added. After 6 h of treatment, cell samples were collected, and NO contents and NR activities were determined. Finally, the cells were treated with NR inhibitors and a NO donor for verifying that the NO affected MeJA-induced flavonoid synthesis. The experiment was designed six groups. (1) Control group, in which sterilized water (equal amounts with 100 μM MeJA) was added to the flask. (2) MeJA group, in which 100 μM MeJA was added. (3) MeJA + TUN group, in which 100 μM MeJA and 0.5 mM TUN were added. (4) MeJA + Gln group, in which 100 μM MeJA and 0.5 mM Glu were added. (5) MeJA + TUN + SNP group, in which 100 μM MeJA, 0.5 mM TUN, and 0.5 mM of filter-sterilized sodium nitroprusside (SNP, a NO donor) were added. (6) MeJA + Gln + SNP, in which 100 μM MeJA, 0.5 mM Gln, and 0.5 mM SNP were added. After 48 h of treatment, the cells were collected, and contents of five flavonoid monomers were determined. In aforementioned experiments, the concentrations of NR inhibitors or NO donor were confirmed by the results of the preliminary test.
Determination of biomass
Cells were harvested from the culture medium and washed with tap water for several times. The FW of cells was determined after removing the surface water. The dry weight (DW) of cells was recorded after the fresh cells were dried in the dry oven (Tianjin North China Experimental Instrument Co., Ltd.) to a constant weight at 50 °C for 48 h.
Determination of flavonoid content
The total flavonoids was extracted by the method of Jiang et al. (2017) and the content was determined by the aluminum chloride colorimetric method (Wang et al. 2013), rutin (purity > 98%) (Beijing Notlas BioScience Co. Ltd, Beijing, China) was used as the standard to prepare the calibration curve. The absorbance was measured at 510 nm with a UV spectrophotometer (UV-2600; Shimadzu Corporation).
For determination of the flavonoid monomer contents, cells were extracted and determined according to the method described by Piao et al. (2017) using high-performance liquid chromatography (HPLC). HPLC analysis used an ODS-C18 reverse phase column (4.6 mm × 250 mm, 5 μm; Thermo Fisher Scientific, Waltham, MA, USA). The Qc and Ke contents were determined with a UV detector (SPD-15C, Shimadzu Corporation. Kyoto, Japan) at 366 nm. The mobile phase was 0.1% (v/v) phosphoric acid (A) and methanol (B). The gradient elution profile was: 0–15 min, 45% B; 16–30 min, 80% B. The flow rate of the mobile phase was 0.8 mL/min. The Ecg, Qc-3-glc, and Kp-3-rut contents were determined with a UV detector (SPD-15C, Shimadzu Corporation) at 280 nm. The mobile phase was 0.05 M ammonium formate (A) and methanol (B), with a linear gradient of 10–90% B over 40 min. The flow rate was 0.8 mL/min. The standards of Qc (purity > 98%), Ke (purity > 98%), Ecg (purity > 98%), Qc-3-glc (purity > 98%), and Ke-3-rut (purity > 98%) were purchased from Chengdu Mansite Bio-Technology Co., Ltd (Chengdu, China).
Determination of NO content and NR activity
The NO content was determined with the method described by Zhou et al. (2005). The fresh cells sample of 2 g was ground with a mortar in 8 mL of 50 mM glacial acetic acid buffer (pH 3.7, containing 10% sodium acetate). The homogenates were centrifuged at 10,000 rpm (CR22G, Hitachi Koki Co. Ltd., Tokyo, Japan) for 15 min at 4 °C, and the supernatant was collected. The remaining residue was washed twice with 2 mL of extraction buffer and centrifuged as previously described. The two supernatants were combined and 0.2 g of activated charcoal was added. After vortexing and filtration, the filtrate was collected. A mixture of 2 mL filtrate and 2 mL Greiss reagent was incubated at room temperature for 30 min before the absorbance was quantified by spectrophotometry (UV-2600, Shimadzu Corporation) at 540 nm. The NO content was calculated by comparison to a standard curve of NaNO2.
The fresh cell sample (0.5 g) was ground with a mortar and pestle in 5 mL of 100 mM PBS (pH 7.4) on an ice bath. The homogenate was centrifuged at 10,000 rpm for 15 min at 4 °C (CR22G; Hitachi Koki Co., Ltd.), and the supernatant was collected. The NR activity was determined using the NR assay kits (Nanjing JianCheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. The optical density (iMark; Bio-Rad Laboratories, Inc., CA, USA) was measured at 540 nm.
Experimental design and data analysis
Data were collected from three experimental replicates. Statistical calculations were carried out using GraphPad Prism 5 (GraphPad Software, Inc.). The results are presented as mean ± standard deviation. The mean values were subjected to Duncan’s multiple-range test and Student’s t-test with the SPSS statistical software 22.0 (SPSS Inc, Chicago, USA). Values of p < 0.05 were considered significant.
Results
Biomass and flavonoid contents in O. cartilaginous cells cultured in difference size bioreactors
To confirm the adaptability of cell biomass and flavonoid accumulation in different size bioreactors, 3, 5, and 10 L bioreactors with 2, 4, and 8 L working volumes were used to culture cells, respectively. The cell growth pattern was similar to those in different size bioreactors. Cells floated up and down in bioreactors before 15 days of culture. Then, cells floated on the upper part of the medium in all size bioreactors, indicating that cells were at the exponential growth phase during the bioreactor culture of O. cartilaginous cells. After 25 days of culture, cells were harvested according to the result of the kinetic experiment conducted by Piao et al. (2017). Table 1 shows that cell FW and DW per 1 L culture medium did not change and increased with bioreactor sizes accordingly (Fig. 1). For per bioreactor, cell FW of 345.6, 698.1, and 1355.2 g, as well as DW of 18.1, 36.8, and 72.1 g were obtained in 3, 5, and 10 L bioreactors, respectively (Table 1). The flavonoid accumulation in cells were similar to cell growth, in which the contents and productivities of total flavonoids were not affected by the bioreactor size. Approximately 200 mg/L total flavonoids were produced in each bioreactor (Fig. 2). The finding demonstrated that bioreactor culture conditions were applicable among different size bioreactors during the cell culture of O. cartilaginous. This result indicated a feasibility of using the current culture conditions in further pilot-scale culture.
Total flavonoid contents and productivities in Orostachys cartilaginous cells harvested from different size bioreactors after 25 days of culture. Data are the mean ± standard deviation (n = 3). The different letters within the same column indicate significant difference by Duncan’s multiple test at 5% level
Effect of MeJA on flavonoid accumulation
In our preliminary test, the elicitation effect of MeJA on flavonoid accumulation of O. cartilaginous cells was investigated and found that the 100 μM was the suitable MeJA concentration. Thus, the100 μM of MeJA was used to treat the 25-day-old cells in the present experiment. After MeJA treatment, cell FW and DW did not significantly change within 72 h (Table 2), but flavonoid accumulation obviously changed with elicitation times. Figure 3 exhibits that the total flavonoid contents increased with the prolonged duration of MeJA treatment from 0 to 48 h, peaked (31.6 mg/g DW) at 48 h, and then decreased afterwards. However, the flavonoid contents in the control group did not exert change. The contents of five flavonoid monomers (e.g., Qc, Ke, Egc, Qc-3-O-glc, and Ke-3-rut) between groups of MeJA and control at 48 h were compared. Table 3 shows that contents of Qc, Ke, Egc, Qc-3-O-glc, and Ke-3-rut in the MeJA group were significantly higher than those in the control group. Moreover, relatively high contents of Qc-3-O-glc and Ke-3-rut were determined after MeJA treatment. The data demonstrated that MeJA could improve flavonoid accumulation during the cell culture of O. cartilaginous and 48 h-elicitation was the most efficient for flavonoid accumulation.
Changes of total flavonoid contents in Orostachys cartilaginous cells with duration of methyl jasmonate (MeJA) treatment. The 25-day-old bioreactor cultured cells were treated with filter-sterilized MeJA (100 μM) in MeJA group and equal amount of sterilized water was added to culture medium in control group. Data are the mean ± standard deviation (n = 3). *Indicates statistically significant difference between groups of MeJA and control at 48 h by Student’s t-test at 5% level
Changes of NO and NR in cells
One of the early responses of cells to elicitor treatment was the rapid production of NO (Xu and Dong 2005). Therefore, this experiment investigated the kinetic of NO generation in cells after MeJA treatment. Figure 4a shows that MeJA-induced NO burst, and the burst mainly occurred at the initial 14 h. Within 6 h after MeJA treatment, NO contents in cells elevated with the duration of MeJA treatment, thereby reaching the maximum value (3.5 μg/g) at 6 h, which was about approximately threefold more than the control. In the control group, NO contents of cells did not change obviously.
Changes of nitric oxide (NO) and nitrate reductase (NR) in Orostachys cartilaginous cells with duration of methyl jasmonate (MeJA) treatment. The 25-day-old bioreactor cultured cells were treated with filter-sterilized MeJA (100 μM) in MeJA group and equal amount of sterilized water was added to culture medium in control group. Data are the mean ± standard deviation (n = 3). *Indicates statistically significant difference between groups of MeJA and control at 6 h by Student's t-test at 5% level
The kinetics of NR activity were investigated to further understand whether NO burst was affected by the NR pathway. Result showed that NR activity increased with the duration of MeJA treatment, peaked at 6 h, decreased from 6 to 20 h, and then remained stable afterwards (Fig. 4b). This pattern matched with the NO generation.
Effect of NR inhibitors on NO content and NR activity
NR inhibitors (TUN and Gln) together with MeJA were used to treat O. cartilaginous cells for 6 h, and NR activity and NO content in cells were determined. Figure 5 shows that NO content (Fig. 5a) and NR activity (Fig. 5b) increased after MeJA treatment. However, they were blocked by TUN and Gln, indicating that NO generated by the NR catalyzes during MeJA elicitation in O. cartilaginous cell culture.
Effects of NR inhibitors [(tungstate (TUN) and glutamine (Gln)] on nitric oxide (NO) contents and nitrate reductase (NR) activities and in methyl jasmonate (MeJA)-induced cells of Orostachys cartilaginous. The filter-sterilized MeJA (100 μM), TUN (0.5 mM), and Gln (0.5 mM) were used to treat 25-day-old bioreactor cultured cells; an equal amount of sterilized water was added to the culture medium in control group. Data are the mean ± standard deviation (n = 3). The different letters within the same column indicate significant difference by Duncan’s multiple test at 5% level
Verification of NO involvement in MeJA-induced flavonoid synthesis through NR pathway
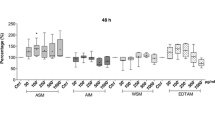
The occurrence of NO burst by NR catalysis in MeJA-stimulated cells of O. cartilaginous was investigated in the aforementioned experiment. To prove the involvement of NO in MeJA-induced flavonoid synthesis through NR pathway, O. cartilaginous cells were treated with MeJA, NR inhibitors (TUN and Gln), and the NO donor (SNP), as well as their combinations, and effects on flavonoid contents were determined. Figure 6 shows that contents of the five flavonoid monomers increased in MeJA group. However, this increment effect was eliminated by the TUN (MeJA + TUN) or Gln (MeJA + Gln). An elevation of flavonoid contents was found again when MeJA + TUN/Gln was combined with a NO donor of SNP. Consequently, the involvement of NO in MeJA-induced flavonoid synthesis through NR pathway was verified.
Effect of the NR inhibitors [(tungstate (TUN) and glutamine (Gln)] and NO donor [sodium nitroprusside (SNP)] on methyl jasmonate (MeJA)-induced flavonoid synthesis in Orostachys cartilaginous cells. The filter-sterilized MeJA (100 μM), TUN (0.5 mM), Gln (0.5 mM), and SNP (0.5 mM) were used to treat 25-day-old bioreactor cultured cells; an equal amount of sterilized water was added to the culture medium in control group. Data are the mean ± standard deviation (n = 3). The different letters within the same column indicate significant difference by Duncan’s multiple test at 5% level
Discussion
The ultimate aim of bioreactor culture experiments in the laboratory is to apply the optimized conditions in the further pilot-scale culture. Therefore, data from many laboratorial experiments could serve as reference in the pilot-scale culture to maximize target metabolite production (Murthy et al. 2014). Therefore, studies have often conducted bioreactor size experiments, among which a good adaptation was found in different size bioreactors and have even successfully operated the large-scale bioreactor culture. A typical example is Thanh et al. (2014), where ginseng cells were cultured in 500 L and 1,000 L airlift bioreactors depending on the results of 5 L and 10 L bioreactor culture, and 6.2 kg DW with a total saponin production of 7.86 mg/g DW was obtained in 500 L bioreactor. Similarly, 13.2 kg DW with a total saponin production of 7.75 mg/g DW were also obtained in 1,000 L bioreactor. In this study, the cell biomass correspondingly increased with bioreactor sizes and flavonoid content was equal. This finding agrees with the result of Thanh et al. (2014) and proposes a feasibility of the laboratorial culture conditions used in the pilot-scale culture of O. cartilaginous cells in the future.
MeJA, as an abiotic elicitor, has been extensively used to improve secondary metabolite production during plant cell culture. For instance, Mendoza et al. (2018) treated 4-day-old cell cultures of Thevetia peruviana with 3 μM MeJA and produced the highest contents of phenolics and flavonoids. Wang et al. (2015) observed that the highest flavonoid production was found when 100 μM MeJA was added to the culture medium after 15 days of cell culture of Hypericum perforatum and treated for 5 days. In this study, the maximum flavonoids were obtained when 25-day-old cells were treated with 100 μM MeJA for 48 h. These findings suggested that the times responded to the MeJA differ depending on the culture systems, a result that requires the selection of the suitable times of MeJA treatment to achieve the maximum production of the desired compounds.
At present, although elicitation effects of MeJA during the plant cell culture have been repeatedly reported, signaling molecules regarding MeJA-induced metabolite synthesis have rarely been investigated, and its molecular mechanism is unclear. Studies proved that secondary metabolites were accumulated in stress-induced plant cells by the effects of various endogenous signaling molecules (Wang and Wu 2005). NO burst is the common defense of plant cells against biotic or abiotic elicitor stresses (Gupta et al. 2013; Li et al. 2017; Modolo et al. 2002; Mostofa et al. 2015). Studies have indicated that elicitor can induce many defense responses, such as the expression of defense gene and hypersensitive response in plant cells of numerous species (Hu et al. 2003; Modolo et al. 2002), and the elicitation effect can be blocked by the NO scavengers (Delledonne et al. 2001; Hu et al. 2003), thereby suggesting that NO is the necessary molecule in the elicitor-induced defense response of the plant cells. Similar result was found in the study of O. cartilaginous cell culture, that is, NO burst appeared at an early stage and peaked 6 h after MeJA treatment. Flavonoid contents increased with the MeJA treatments and peaked at 48 h.
Various pathways of NO generation have been presented in plant cells, including NR. The NR can convert nitric acid to NO via the NR activation by stresses (Yamasaki et al. 1999), thereby indicating that NR is the important source of NO generation. To date, studies regarding MeJA-induced metabolite synthesis reported that NO production was affected by NOS activation (Wang and Wu 2005). However, the effect of NR has not been investigated yet. In this study, we investigated kinetics of NR activity and effects of NR inhibitors (TUN and Gln) on NR activity and NO content in MeJA-stimulated cells to clarify whether NR is the necessary factor for NO generation in MeJA-induced flavonoid synthesis during the O. cartilaginous cell culture. Our result showed that the kinetics of NR activity were similar to the NO content, that is, NR activity increased with MeJA treatment times and peaked 6 h after MeJA treatment, indicating that NR was activated by the MeJA stimulation. However, NR activity in MeJA-treated cells was inhibited by TUN and Gln, which were NR inhibitors that simultaneously inhibited MeJA-induced NO burst. This finding suggests that NR is the necessary pathway of NO generation during the culture of MeJA-stimulated cells of O. cartilaginous. Furthermore, the effects of NR inhibitors (TUN and Gln) and NO donor (SNP) were investigated to prove whether the NO was the necessary molecule in MeJA-induced flavonoid synthesis. The result showed that TUN and Gln blocked MeJA-induced flavonoid synthesis, which is matched with their effects on NO generation. In addition, the inhibited flavonoid synthesis by NR inhibitors was restored by the SNP. Similar result was found in an elicitation study of Lu et al. (2011), who demonstrated that NR was involved in the fungal elicitor-triggered NO generation and fungal elicitor-induced camptothecin production of Camptotheca acuminata cells dependently on NR-mediated NO generation. Consequently, this study speculated that NR-mediated NO generation and involved in MeJA-induced flavonoid synthesis during O. cartilaginous cell culture. However, the further experiments should be employed using various test tools for validation of the finding of the present study.
Conclusion
Cell biomass increased correspondingly with bioreactor sizes and total flavonoid contents were approximately equal in 3, 5, and 5 L bioreactors, thereby indicating a feasibility of using the current culture conditions in further pilot-scale culture. MeJA improved flavonoid synthesis, and the maximum flavonoid content was determined at 48 h of MeJA treatment. The burst of NO was identified at the early stage of MeJA elicitation, peaked at 6 h. In MeJA-stimulated cells, NR inhibitors (TUN and Glu) blocked NO generation, and the flavonoid synthesis was also inhibited. However, such inhibition of flavonoid synthesis was relieved by a NO donor (SNP). Therefore, this study suggested that NO was involved in MeJA-induced flavonoid synthesis through NR pathway during O. cartilaginous cell culture. Our finding has a critical significance in understanding the defense mechanism response stimulated by an abiotic elicitor of MeJA and provides a new strategy that regulates NO burst and flavonoid synthesis by controlling the NR activity.
Author contribution statement
All authors contributed extensively to the work presented in this paper (Cell bioreactor culture of Orostachys cartilaginous A. Bor. and involvement of nitric oxide in methyl jasmonate-induced flavonoid synthesis). YJH conducted bioreactor culture experiment and JRL conducted signaling molecular experiment. XHC and XLA contributed to component analysis. HDS was responsible for statistical analysis. XCP and MLL designed the experiments and wrote the paper.
References
Baldi A, Srivastava AK, Bisaria VS (2009) Fungal elicitors for enhanced production of secondary metabolites in plant cell suspension cultures. In: Varma A, Kharkwal AC (eds) Symbiotic Fungi, Soil Biology. Springer Verlag, Berlin, pp 373–380
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98:13454–13459
Efferth T (2019) Biotechnology applications of plant callus cultures. Engineering 5:50–59
Foissner I, Wendehenne D, Langebartels C, Durner J (2010) In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J 23:817–824
Gao FK, Ren CG, Dai CC (2012) Signaling effects of nitric oxide, salicylic acid, and reactive oxygen species on isoeuphpekinensin accumulation in Euphorbia pekinensis suspension cells induced by an endophytic fungal elicitor. J Plant Growth Regul 31:490–497
Georgiev MI, Weber J, Maciuk A (2009) Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl Microbiol Biotechnol 83:809–823
Gupta NS, Banerjee M, Basu SK, Achaya K (2013) Involvement of nitric oxide signal in Alternaria alternata toxin induced defense response in Rauvolfia serpentina Benth. ex Kurz calli. Plant Omics 6:157–164
Hn M, Georgiev MI, Kim YS, Jeong CS, Kim SJ, Park SY, Paek KY (2014) Ginsenosides: perspective for sustainable biotechnological production. Appl Microbiol Biotechnol 98:7319–7329
Hu X, Neill S, Cai W, Tang Z (2003) NO-mediated hypersensitive responses of rice suspension cultures induced by incompatible elicitor. Chin Sci Bull 48:358–363
Jiang XL, Piao XC, Gao R, Jin MY, Jiang J, Jin XH, Lian ML (2017) Improvement of bioactive compound accumulation in adventitious root cultures of an endangered plant species, Oplopanax elatus. Acta Physiol Plant 39:226–235
Kang SM, Min JY, Kim YD, Kang YM, Park DJ, Jung HN, Kim SW, Choi MS (2006) Effects of methyl jasmonate and salicylic acid on the production of bilobalide and ginkgolides in cell cultures of Ginkgo biloba. Vitro Cell Dev Biol Plant 42:44–49
Lee EJ, Park SY, Paek KY (2014) Enhancement strategies of bioactive compound production in adventitious root cultures of Eleutherococcus koreanum Nakai subjected to methyl jasmonate and salicylic acid elicitation through airlift bioreactors. Plant Cell Tiss Organ Cult 120:1–10
Li CF, Wang SH, Li DH (2015) Antioxidant activities from Orostachys cartilaginous extracts. Food Sci Technol 40:285–288
Li X, Zhang L, Ahammed GJ, Li ZX, Wei JP, Shen C, Yan P, Zhang LP, Han WY (2017) Nitric oxide mediates brassinosteroid-induced flavonoid biosynthesis in Camellia sinensis L. J Plant Physiol 214:145–151
Lu D, Dong JF, Jin HH, Sun LN, Xu XB, Zhou T, Zhu Y, Xu MJ (2011) Nitrate reductase-mediated nitric oxide generation is essential for fungal elicitor-induced camptothecin accumulation of Camptotheca acuminata suspension cell cultures. Appl Microbiol Biotechnol 90:1073–1081
Mendoza D, Cuaspud O, Arias JP, Ruiz O, Arias M (2018) Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol Rep 19:e00273–e00273
Modolo LV, Cunha FQ, Braga MR, Salgado I (2002) Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorumf. sp. meridionalis Elicitor. Plant Physiol 130:1288–1297
Mostofa MG, Fujita M, Tran LSP (2015) Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul 77:265–277
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Piao XC, Zhang WB, Jiang J, Jin YH, Park PJ, Kim SE, Lian ML (2017) Cell suspension culture of Orostachys cartilaginous in bioreactor systems for bioactive compound production and evaluation of their antioxidant properties. Acta Physiol Plant 39:70–79
Shan C, Yang T (2017) Nitric oxide acts downstream of hydrogen peroxide in the regulation of ascorbate and glutathione metabolism by jasmonic acid in Agropyron cristatum leaves. Biol Plant 61:779–784
Thanh NT, Murthy HN, Paek KY (2014) Ginseng cell culture for production of ginsenosides. In: Paek KY, Murthy HN, Zhong JJ (eds) Production of biomass and bioactive compounds using bioreactor technology. Dordrecht, New York, pp 121–142
Wang JW, Wu JY (2004) Involvement of nitric oxide in elicitor-induced defense responses and secondary metabolism of Taxus chinensis cells. Nitric Oxide Biol Chem 11:298–306
Wang JW, Wu JY (2005) Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol 46:923–930
Wang J, Zhang J, Gao WY, Wang Q, Yin SS, Liu H, Man SL (2013) Identifi cation of triterpenoids and flavonoids, step-wise aeration treatment as well as antioxidant capacity of Glycyrrhiza uralensis Fisch. cell. Ind Crops Prod 49:675–681
Wang J, Qian J, Yao LY, Lu YH (2015) Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresource Bioproc 2:5–13
Xu MJ, Dong JF (2005) Elicitor-induced nitric oxide burst is essential for triggering catharanthine synthesis in Catharanthus roseus suspension cells. Appl Microbiol Biotechnol 67:40–44
Xu MJ, Dong JF, Zhu MY (2005) Nitric oxide mediates the fungal elicitor-induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic-acid-dependent signal pathway. Plant Physiol 139:991–998
Xu MJ, Dong JF, Zhang XB (2008) Signal interaction between nitric oxide and hydrogen peroxide in heat shock-induced hypericin production of Hypericum perforatum suspension cells. Sci China 51:676–686
Yamasaki H, Sakihama Y (2000) Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. Febs Lett 468:89–92
Yamasaki H, Sakihama Y, Takahashi S (1999) An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci 4:128–129
Zhang WB, Piao XC, Wu CH, Li JR, Jin YH, Lian ML (2017) Optimized culture medium for the production of flavonoids from Orostachys cartilaginea V.N. Boriss. callus cultures. Vitro Cell Dev Biol Plant 53:1–11
Zhou BY, Guo ZF, Xing JP, Huang BR (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31660080).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by H. Peng.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hao, YJ., Cui, XH., Li, JR. et al. Cell bioreactor culture of Orostachys cartilaginous A. Bor. and involvement of nitric oxide in methyl jasmonate-induced flavonoid synthesis. Acta Physiol Plant 42, 9 (2020). https://doi.org/10.1007/s11738-019-3008-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-3008-5