Abstract
This study investigated iron (II) sulfate (FeSO4) effects in two forms on some antioxidant enzyme activity, osmoregulators and sunflower growth under salt stress. Treatments included five cultivars (Alstar, Olsion, Yourflor, Hysun36 and Hysun33), two salinity levels (control and 100 mM NaCl), and three foliar applications (none-sprayed, FeSO4 normal and nano-particles at a rate of 2 g L−1). As a results, salt stress increased superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), polyphenol oxidase (PPO), peroxidase (POX), pyrroline-5-carboxylate synthase (P5CS) and invertase activities; and also proline, soluble carbohydrates, malondialdehyde (MDA) content and reactive oxygen species (ROS) generation; however, reduced shoot dry weight and seed yield. Foliar spray of FeSO4 in two forms enhanced CAT, PPO, POX, invertase activity, soluble carbohydrates and decreased MDA content and ROS generation compared to non-sprayed plants under saline condition. In general, FeSO4 in nano was more effective than normal form in improving the growth of sunflower. As an average, the spray of FeSO4 increased proline content of Alestar cultivar under 100 mM salinity, which related with P5CS activity but reduced leaf proline content of Olsion and Hysun33 under non-saline condition. In addition, shoot dry weight and seed yield of sunflower were increased by FeSO4. Leaf Fe concentration of cultivars was strongly intensified by FeSO4 foliar spray under two conditions compared to non-sprayed treatment. Overall, the usage of FeSO4 alleviated the negative impact of salt stress, although the positive effects of nano-particles were more than the normal form.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity stress creates oxidative stress by inducing the generation of reactive oxygen species (ROS) such as singlet oxygen, superoxide radical, hydroperoxy radical, hydrogen peroxide and hydroxyl radical (Lekshmy et al. 2013; Yadav et al. 2014). Also, salinity causes a significant reduction in plant water content and cell turgor potential, which in most cases results in reducing growth and yield (Akram et al. 2007; Nasibi et al. 2016). Plants under this condition by synthesis of some organic materials such as proline, glycine-betaine, soluble carbohydrates and proteins adjust water potential of cells. The organic solutes, collectively referred to as compatible osmolytes or compatible solutes, not only contribute to osmoregulation but they may also protect the structure of different biomolecules and membranes (Murata et al. 1992) or act as free-radical scavengers that protect DNA from the damaging effects of ROS (Ashraf and Akram 2009). In summary, compatible solutes play major roles in plant stress tolerance. Their principal role is the maintenance of osmoregulation (turgor) in plants exposed to stress conditions.
Micronutrients including iron (Fe), zinc (Zn), cupper (Cu), manganese (Mn), boron (B) and molybdenum (Mo) can mitigate stress effects. Ionic forms of these elements act as co-factors in many antioxidant enzymes. Under micronutrient deficiency the activity of antioxidant enzymes decreases, which in turn increases plant sensitivity to environmental stresses (Cakmak 2000). Due to Fe redox properties and its ability to form complexes with diverse ligands, this element is constituent of many electron carriers and enzymes, thus playing an important role in plant metabolism. Iron in the free or in the loosely bound form, as a pro-oxidant factor, catalyses the free radical generation through the Fenton reaction. It works as a co-factor of key enzymes that plays a role in plant hormone synthesis and is engaged in many electron transportation reactions (Kerkeb and Connolly 2006). The relationship between the decrease of iron availability in the nutrient media and the possible onset of oxidative stress is becoming more evident, because of the dual role played by iron in cell metabolism as either an antioxidant or a pro-oxidant factor. However, it has been shown that many enzymes require iron in order to function correctly in particular iron is present in the active sites of catalase (CAT) and superoxide dismutase (SOD) involved in the scavenging of ROS. Because Fe is a constituent of the major antioxidant enzymes associated with the detoxification of O ·−2 and H2O2 (Bybordi and Mamedov 2010; Sinha and Saxena 2006), Fe-starved plants are more susceptible to oxidative damage.
Materials with a particle size less than 100 nm in at least one dimension are generally classified as nano-particles (NPs). The quantum effects that appear in this size range can drastically modify the physical, chemical and electrical characteristics of NPs as compared to larger particles (Gonzalez-Melendi et al. 2008). The development of nanotechnology in conjunction with biotechnology has significantly expanded the application domain of NPs in various fields. The majority of applications in these areas have focused on the significance of the nano-materials for improved efficiency and productivity. The effect of NPs on plants can be positive or negative, which varies greatly depending on NPs composition, size, concentration and plant species (Monica and Cremonini 2009). For instance, ZnO NPs caused a dose-dependent inhibition in seed germination of cabbage (Brassica oleracea var. capitata L.), while it showed no negative effects on germination of maize (Zea mays L.) seeds (Pokhrel and Dubey 2013). Iron oxide (FeSO4) NPs produced a significant positive effect on root elongation of soybean, particularly when compared to the bulk counterpart suspensions of concentrations greater than 500 mg L−1 (Alidoust and Isoda 2013). In addition, they observed more pronounced positive effects of FeSO4 NPs via foliar application than by soil treatment on the photosynthetic rate enhancement. In recent years, NPs are being used as a critical tool for improving growth and productivity of crop plants under adverse environmental conditions including salt stress (Nasir Khan et al. 2017). NPs have been implicated in the protection of plants against oxidative stress as they mimic the role of antioxidative enzymes such as SOD, CAT and POX and increasing osmoregulators (Rico et al. 2013; Wei and Wang 2013, Siddiqui et al. 2015). Exposure of plants to NPs stimulates antioxidant system in plants, perhaps as an adaptive response to alleviate oxidative stress (Li et al. 2016). Foliar application of zinc, iron and silicon oxide in nano forms alleviated the harmful effects of salt stress on different plants (Soliman et al. 2015; Torabian et al. 2016a; Fathi et al. 2017a, b; Farhangi-Abriz and Torabian 2018). Torabian et al. (2017) stated that FeSO4 NPs increased biomass production of sunflower plants greater compared to normal form under saline condition.
Iron deficits in plants under salt stress is a common effect of this environmental stress, and foliar application of iron sulphate (FeSO4) could be useful in improving iron content and increasing plant tolerance to the salt stress. On the other hand, The NPs mimic the activities of antioxidative enzymes and scavenge ROS. Hence, the objective of this study was to investigate (1) the effects of FeSO4 in two forms on different antioxidant enzymes activity, osmoregulators content and ROS generation in sunflower plant, (2) response of different sunflower cultivars to Fe nano-particles under salinity stress and (3) the alleviation effects of Fe, especially nano-particles on sunflower, which exposed to salt stress.
Materials and methods
Experimental conditions
A pot experiment was conducted in the greenhouse of the Agriculture college, Isfahan University of Technology, Isfahan, Iran. The experiment was laid out in a complete randomized block design with three replications. Surface soil samples (0–30 cm) were collected from Golpaygan, Isfahan, Iran. The characteristics of used soil are given in Table 1. Five sunflower cultivars (Helianthus Annuus L. cvs. Alstar, Olsion, Yourflor, Hysun36 and Hysun33) were tested under two levels of salinity (0 and 100 mM of NaCl) and three spray treatments (FeSO4 normal and NPs at a rate of 2 g l−1 and control containing distilled water). Before foliar spray, solution pH was reached to almost 7 with methylated potassium hydroxide (KOH). The bulk FeSO4 of 99.9% purity as a normal form was purchased from Merck Ltd. FeSO4 NPs prepared by mechanical alloying (ball mill) for 15 h. The speed of rotation was 200 rpm and the ball-to-powder weight ratio was 10:1. The physical structure of particles was evaluated by scanning electron microscopy (SEM model Philips XL30, Netherlands). Additionally, the mean diameter of FeSO4 NPs was evaluated by transmission electron microscopy (TEM, JEOL 3010; Jeol Ltd, Peabody, MA, USA).
Seeds were provided by Seed and Plant Improvement Institute, Karaj, Iran. Seeds were planted in plastic pots filled with 15 kg soil (with 8% moisture). The pot size was 30 cm (height) × 30 cm (diameter). The plants were kept under controlled conditions of a greenhouse with a 12 h light/dark cycle at a light intensity of 390 µmol photons m−2 s−1, 25/20 °C day/night temperature and 65–75% relative humidity. Four sunflower plants were maintained in each pot. Plants were irrigated daily with tap water (EC = 0.48 dS m−1) during the period of emergence and seedling establishment to keep the soil water content near the field capacity. Nutrient solution containing potassium nitrate (KNO31 ppm in 500 cc water) was applied to each pot at two fully expanded. Salt was added to irrigation water supplied to saline treatments with step-wise increasing of salt aliquots, beginning from 18 days after sowing (four fully expanded leaf grow stage). Foliar application of FeSO4 was done twice. The first one was applied 1 week (25 days after sowing) and the second one 2 weeks after the application of a final aliquot of salt (85 days after sowing). A portion of fresh leaf samples was harvested 2 days after second foliar spray, and then was frozen at − 80 °C for laboratory analysis.
Shoot dry weight and seed yield
Plants were harvested at the seed maturation stage for measuring shoot dry weight and seed yield. Shoot samples were dried at 70 °C for 72 h and dry weights were measured.
Antioxidant enzymes assay
The leaf samples (500 mg) were ground with 3.0 ml of potassium phosphate buffer, centrifuged at 2000 G for 10 min and the supernatants were used for the assay. Peroxidase (POX) activity assay was monitored by changes in absorbance at 420 nm and activity was expressed as Ug−1 FW (Gueta-Dahan et al. 1997). Ascorbate peroxidase (APX) activity was assayed by the methods of Nakano and Asada (1981) with monitoring the decrease in absorbance at 290 nm due to ascorbate oxidation. One unit of superoxide dismutase (SOD) activity was defined as the amount of enzyme affecting 50% of the maximum inhibition of nitro blue tetrazolium reduction (Nishikimi et al. 1972). Catalase (CAT) activity was assayed by monitoring the disappearance of H2O2 at 240 nm (Aebi 1984). The activity of polyphenol oxidase (PPO) was analyzed by the method of Kumar and Khan (1982).
Assaying Lipid peroxidation, hydrogen peroxide and singlet oxygen generations
Lipid peroxidation in plant cells was assayed by the methods of Stewart and Bewley (1980) with measuring malondialdehyde content in leaves (MDA). The leaf samples (200 mg) were ground with trichloroacetic acid (2.5 ml, 0.1%, w/v) and centrifuged at 15,000g for 15 min at 4 °C. Then, an equal volume of supernatant and 0.5% thiobarbituric acid (TBA) were added to 20% TCA, the produced mixture heated at 96 °C for 30 min, and after that cooled in an ice-water 4 °C for 10 min. The Absorbance was read at 532 and 600 nm. The hydrogen peroxide was determined spectrophotometrically after reaction with potassium iodide (KI) according to methods of Velikova et al. (2000). The absorbance reading was taken at 390 nm. The content of O ·−2 generation was measured as referring to Wang and Jiao (2000).
Pyrroline-5-carboxylate synthase and invertase activities
Pyrroline-5-carboxylate synthase (P5CS) extracted according to the method of Kishor et al. (1995) with the extraction buffer (pH = 7.2) containing: 100 mM Tris–HCl buffer, 20 mM MgCl2, 1 mM DTT and 1 mM PMSF. Reaction was started with added 1.6 ml reaction buffer, including 100 mM Tris–HCl buffer, 2, 75 mM sodium glutamate, 20 mM MgCl and 5 mM ATP into the 0.2 ml of 0.4 mM NADPH, reducing absorption was monitored at 340 nm with a spectrophotometer. The activity of invertase was determined by the Tsai et al. (1970) method. Invertase extracted (10 μl) with a buffer containing 50 mM sodium acetate, 15 mM magnesium chloride, and 100 mM sucrose with pH 4.5. Reduced carbohydrates were quantified by spectrometry according to the Nelson (1944) with a glucose standard.
Proline and soluble sugar content in leaves
For measuring proline content of leaves, we used the methods of Bates (1973). With reading absorbance at 520 nm, using a spectrophotometer (PG instruments T80). Proline content was calculated as μmol g−1 FW (Fresh weight) by the calibration curve. The content of soluble sugar of sunflower leaves was measured with the phenol sulphuric acid method (Kochert 1978) and stated as mg g−1 FW, using a calibration curve.
Fe concentration
For determination of leaf Fe concentration, we used an atomic absorption spectrophotometer (Perkin-Elmer, Analyst 200, Perkin Elmer, Waltham, MA). A 100 mg of dried leaves was weighed and used for determination of iron (Fe) concentration. Primarily leaves were dried at 500 °C for 12 h and then, digested in 5 M HNO3; finally, Fe concentration was described by mg g−1 dry weight (DW) (Chapman and Pratt 1961).
Statistical analysis
Main and interaction effects of experimental factors were defined from analysis of variance (ANOVA) using the generalized linear model (GLM) of SAS (SAS Institute, Cary, NC, USA). The suitable error term for testing significance of each effect was determined based on the expected mean square from the output of PROC GLM. The mean values were compared by least significant difference (LSD) test at 0.05 level of probability.
Results
Mean particle diameter of NPs
The TEM image of the FeSO4 NPs revealed their spherical, truncated, and uneven nature with an average size of approximately 90 nm; however, the mean particle diameter of bulk FeSO4 as a normal form was 10 µm (Fig. 1).
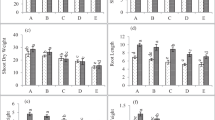
Shoot dry weight and seed yield
Shoot dry weight and seed yield of sunflower cultivars were reduced differently at salinity stress. The lowest reduction in shoot dry weight and seed yield was observed in cv. Olsion. Averaged over the salinity and cultivar, usage of FeSO4 resulted in considerable enhance in shoot dry weight (17%) and seed yield (22%) of sunflower plant (Fig. 2). Except cv. Yourflor, shoot dry weight and seed yield of all cultivars increased by use of FeSO4 under saline and non-saline conditions. The extent of the increase by foliar spray under control was more than a saline condition in all cultivars (Fig. 2). Shoot dry and yield of Yourflor had a different trend in comparison with other cultivars when exposed to FeSO4. Under control condition, these parameters were diminished but under saline media, were increased. Moreover, nano form exposed better effect than normal form in rising seed yield of sunflower cultivars. The highest shoot dry weight and seed yield were belonged to Hysun36 by 5.23 g and 2.48 g plant−1, respectively, by spray of FeSO4 NPs.
Antioxidant enzyme activity
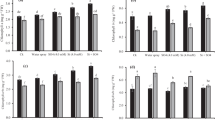
The result presented in Table 2 showed that the effect of salinity was remarkable on all of the antioxidant enzyme activity. POX activity was increased with rising salinity. Foliar application of FeSO4 in regular and nano form significantly raised POX activity. Regardless of the cultivar and salinity treatment, foliar spray of FeSO4 increased CAT activity, so that there is no considerable difference between two forms of FeSO4. Salt stress resulted in an increase in CAT activity under all spray treatments. In fact, FeSO4 significantly intensified CAT activity of all cultivars under saline condition. The leaf CAT activity of cultivars had not significant differences in all spray treatments under none saline condition. Although salinity enhanced the SOD activity of all cultivars, the extent of the increase was different (Table 2). When the estimation of SOD enzyme activity was done, it was found that the highest activity was recorded in cv. Olsion (averaged 25.1 U g−1 FW) at the saline condition, followed by cv. Hysun33; in contrast, the lowest SOD activity was observed in cv. Yourflor (around 5.3 U g−1 FW) at control treatment. A spray of FeSO4 did not change SOD activity of sunflower cultivars under saline and none-saline conditions. The amount of APX and PPO were increased by salinity in all cultivars, but the degree of enhancing was different between cultivars. The maximum APX, POX and PPO activity was obtained in cv. Hysun33 at salt stress treatment. Foliar application of FeSO4 did not alter the APX activity of sunflower cultivars under two conditions, but improved PPO activity in all cultivars of sunflower under salt stress (Table 2).
Proline content and P5CS activity
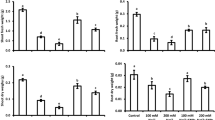
The statistical analysis of the data revealed that the effects of salinity, cultivar and interaction of salinity × cultivar were remarkable on proline content and P5CS activity of sunflower plants. According to the result, salt stress significantly increased leaf proline content and P5CS activity of all cultivars (Fig. 3). The amount of increase in proline content was diverse between cultivars under saline condition. The highest increase of leaf proline content and P5CS activity in the saline condition were recorded in cv. Hysun33 and Alstar when reached around 7 μmol g−1 FW and 600 U mg−1 protein. On the other hand, FeSO4 application improved proline content of Alestar cultivar under 100 mM NaCl; however, reduced leaf proline content of Olsion and Hysun33 under non-saline treatment. The P5CS activity of Alstar and Yourflor cultivars when subjected to salinity and FeSO4 increased. Olsion P5CS activity was decreased by FeSO4 spray at control condition (Fig. 3).
Soluble sugar content and invertase activity
Soluble sugar content and invertase activity of sunflower considerably were affected by the interaction of salinity, cultivar and FeSO4 treatments. Concerning soluble sugar content and invertase activity, a significant increase was noticed in the saline condition in comparison to the control (Fig. 4). Foliar spray of FeSO4 in nano and normal forms significantly improved sugar content and invertase activity of cultivars under saline medium. It was interesting that soluble sugar content and invertase activity were stable at non-saline condition by foliar spray of FeSO4 (Fig. 4). Hysun33 with rising salt stress showed the highest of soluble sugar when exposed to nano FeSO4. Moreover, the highest of soluble sugar was recorded in cv. Yourflor under 100 mM NaCl and nano FeSO4 application.
MDA content and ROS generation
MDA content, H2O2 and O ·−2 generation of sunflower leaves were considerably influenced by salinity, foliar spray, cultivar and interaction of salinity × cultivar and salinity × foliar spray. MDA content, H2O2 and O ·−2 generation of sunflower cultivars were increased by salt stress differently. Among cultivars, cv. Yourflor had the greatest increase in MDA content under 100 mM NaCl treatment (as an average from 3.74 to 11.2 mmol g−1 FW) (Table 2). The lowest increase in MDA content was observed in cv. Olsion when exposed to salinity with application of nano-particle. Foliar spray of FeSO4 in two forms decreased MDA and ROS of all cultivars under saline condition compared to none-spray treatment. There was no difference between spray treatments at the none-saline condition in terms of MDA content and ROS generation of sunflower cultivars (Table 2).
Fe concentration
The result showed that the effects of salinity, cultivars and foliar spray, and interaction of foliar spray × cultivar and salinity × foliar spray was significant on leaf Fe concentration. The amount of Fe concentration was reduced by salinity in all sunflower cultivars sprayed with FeSO4 (Fig. 5). Usage of FeSO4 resulted in an improve considerably in leaf Fe concentration under both saline and none-saline conditions, but the extent of increase in leaf Fe concentration was greater under the non-saline than saline treatment. Although there were no significant differences between two forms of FeSO4, accumulation of Fe by the spray of nano-particles was higher than normal. Between cultivars, leaf Fe concentration of cv. Olsion was significantly higher than other tested cultivars when FeSO4 was sprayed under the non-saline condition when increased from 457 to 6570 mg kg−1 DW. No considerable difference was found among cultivars in the leaf Fe concentration under none-sprayed treatment. The most important factor in increase of leaf Fe concentration was FeSO4 sprayed; however, salt stress caused degrade (Fig. 5).
Discussion
According to the results, the salinity caused a significant decrease in shoot dry weight, seed yield, Fe concentration and increase in the proline, soluble sugar, and MDA content, the activity of antioxidant enzymes of sunflower cultivars. Decreased in shoot dry weight and seed yield may be a consequence of the generation of ROS that is evident from significant increases in antioxidant enzyme activity in leaves of sunflower plants under salinity. The production of toxic oxygen formative is increased as a result of all types of environmental stresses. Plants possess efficient systems for scavenging active oxygen species that defend them against destructive oxidative reactions (Farhangi-Abriz and Torabian 2017). As part of this system, antioxidative enzymes are main factors in the defense mechanisms such as SOD, APX, CAT, POX and PPO. Increase of antioxidant enzyme activities under salinity showed that they were functioning concurrently to remove ROS. Plants are able to maintain the turgor potential at the same value for lower values of leaf water potential owing to their osmotic adjustment. The compounds mainly involved in osmotic adjustment are the soluble sugars, organic acids, free amino acids and potassium ion. Accumulation of proline is a main factor that supports plants to sustain growth under saline conditions. Proline accumulation may be due to the increased activity of P5CS and reduced level of proline oxidase observed during episodes of salinity (Nounjan et al. 2012). Proline induces the stress tolerance through scavenging hydroxyl radicals (Chen and Dickman 2005). The change in total soluble sugar contents of rapeseed under salt stress has already been reported (Khattab 2007). Increase in total soluble sugar contents with increasing salinity level could be due to the accumulation of starch and total soluble sugar in the plant under the stressed condition. In fact, the increase in proline content in sunflower cultivars is attributed to the rising P5CS activity under salt stress. Also, enhancing soluble sugar content in the saline condition is related to rising invertase activity.
Salt stress increased leaf MDA content of sunflower cultivars as a peroxidation lipid compared to control. Membranes are primary targets of salinity injury to cell and cellular organelles due to the fact that ROS has high potential to react with unsaturated fatty acids resulting in peroxidation of essential membrane lipids in the membranes of cell and intercellular organelles (Ahmad et al. 2011). Peroxidation of membranes leads to leaky membranes, leading to loss of electrochemical gradient, loss of homeostasis, loss of cellular contents, rapid desiccation and cell death. In this study, cv. Hysun33 had the highest extent of increase in the leaf proline content and cv. Olsion had a highest increase in SOD activity the lowest extent of reduction in leaf MDA content, which caused to lowest reduction in shoot dry weight and seed yield in comparison with other cultivars under saline condition. Additionally, salinity can affect nutrient uptake directly. As an average, Fe concentration of leaf sunflower declined by salinity in five cultivars. Salt stress decreased Fe concentration in the shoots of barley and corn (Yousfi et al. 2007; Sun et al. 2007). This reduction in Fe concentration may be associated with diminishing nutrient uptake under salt stress.
Foliar application of micronutrients such as Fe and Zn might prove advantageous for supplying plants with sufficient levels of these nutrients and ameliorating, at least in part, the adverse effects of drought and salinity. In the present study, the spray of FeSO4 in both of normal and nano form increased seed yield, soluble sugar content, Fe concentration, and activities of POX, CAT and PPO; while, decreased leaves MDA content of sunflower cultivars via decreasing ROS generation. Plant cells have an antioxidant defense mechanism against ROS consisting of (1) nonenzymatic low-molecular-weight antioxidants, such as ascorbate, α-tocopherol, glutathione, and carotenoids, and (2) protective Fe-containing enzymes, including Fe-SOD that catalyzes the surplus of (O ·−2 ) to H2O2 and O2, and CAT and POD, which transform H2O2 to H2O (Bybordi and Mamedov 2010). CAT has iron in its structure and catalyses the turn of hydrogen peroxide to oxygen and water. CAT reduces hydrogen peroxide by reduced gluthatione, resulting in the protection of cellular damage from oxidative processes (Cakmak 2000). Movahed Dehnavi et al. (2002) indicated that application of micronutrient fertilizers could enhance plants resistance to environmental stresses such as drought and salinity. The deficiency of micronutrient can influence cell mitochondria activity through inducing oxidative stress on the metal binding proteins (Tan et al. 2010; Sajedi et al. 2011). Increase in POX, CAT and PPO activities, and diminution in MDA content with the foliar spray of FeSO4 is indicative of a correlation between ROS generation and ROS scavenging by antioxidant enzyme activity. It is usual for enzyme activities to be induced or enhanced when their metal cofactors have been supplied. No significant change of SOD activity in the leaf of sunflower cultivars subjected to Fe application was observed. SOD has different isoforms including Cu/Zn-SOD, Fe-SOD and Mn-SOD, which may be cause different response to Fe. Tewari et al. (2005) have mentioned that an increase active Fe content along with a lower SOD activity might potentially be indicative of the induction of new isoforms of SOD in Fe-treated plants. Indeed, based on the preceding studies, we expected that using foliar Fe might boost the activity of SOD. Conversely, SOD activity remained without change after foliar application. It has proposed that this may support a compensatory improvement in the expression of another SOD isoform when the expression of one isoform of SOD is decreased (Sharma et al. 2004). Improving soluble sugar content of leaves with FeSO4 clearly related to the enhancing invertase activity in plants. The spray of FeSO4 in two forms improved leaf Fe concentration related to non-sprayed plants. Moreover, sunflower cultivars were different in their ability to accumulate Fe both nano and normal FeSO4 forms. Olsion cv. accumulated much more Fe in their leaves than other cultivars. In other research of Torabian et al. (2017), the application of FeSO4 diminished (15%) the leaf Na concentration under saline condition. Therefore, it concludes that Fe usage can reduced Na uptake and accumulation.
As an average, shoot dry weight and seed yield of all cultivars intensified under two conditions when NPs of FeSO4 was sprayed. Fe and Zn adequate supply enhances crop productivity and their excess can be toxic to plant species (Kabir et al. 2014). NPs interaction with plants causing many morphological and physiological changes, depending on the properties of NPs such as chemical composition, size, surface covering, reactivity, and the dose (Khodakovskaya et al. 2012). They have large specific surface areas and a large proportion of atoms are available for chemical reactions. Today, several researchers have studied the effects of nano-materials on plant growth. Applications of nano-materials can help faster plant germination/production, effective plant protection with reduced environmental impact (Khot et al. 2012). Various researchers reported alleviating effects of NPs on abiotic stresses. A spray of zinc oxide (ZnO) NPs increased shoot dry weight and SOD activity of sunflower in comparison with normal form under saline condition (Torabian et al. 2016b). Moreover, foliar spray of ZnO and FeSO4 as nano-fertilizers mitigated the effect of salt stress on Moringa peregrina plants (Soliman et al. 2015). NPs at one end induce the generation of ROS (Simon et al. 2013), while on the other scavenge ROS by mimicking the activities of antioxidative enzymes (Rico et al. 2013). In addition, phytotoxicity studies on nano-materials exhibited increased production of ROS that plays a dual role in plants as toxic compounds and signaling molecules. The dual role of ROS delicately depends on their generation and scavenging; any imbalance between these two processes will lead to either excessive accumulation or reduced availability of ROS that may cause oxidative stress or signaling failure respectively. Previously, changes of antioxidant enzyme activities by nono-particles application were observed. Lu et al. (2002) indicated that SOD and CAT activities of germinating seeds of soybean treated by a mixture of nano-SiO2 and nano-titanium dioxide (TiO2) significantly increased. Rao and Shekhawat (2014) stated that ZnO nano-particle improved shoot and leaf SOD activity of Brassica juncea. In this study, we observed that nano-particle of FeSO4 has clearly a role of ROS scavenger. Increase in CAT, PPO, POX, and invertase activity with decreasing MDA content are indicative of a correlation between ROS generation (H2O2 and O ·−2 ) and ROS scavenging by FeSO4 nano-particle.
Conclusion
Sunflower cultivars showed different response to FeSO4, especially nano-particle. Olsion accumulated Fe in its leaf more than other cultivars and finally had the lowest shoot dry weight and seed yield. It seems that there is a positive relationship between Fe accumulation and tolerance to salt stress. Foliar spray of FeSO4 in two forms of nano and normal form improved yield and physiological performance of sunflower cultivars under salt stress via improving antioxidant enzyme activity, invertase activity, soluble sugar content, Fe concentration and decreasing lipid peroxidation, ROS generation. Based on our result, Fe under stress can help sunflower to utilize the mechanisms, which eventually result in the production of antioxidants, more effectively. We think the dose of nano-particle that applied in our study is recommendable for increased sunflower production under salinity stress as such use can enhance the activities of antioxidants and hence protect the plant from oxidative damage. Concern about the use of nano-materials and their potential environmental risks and toxic impacts has arisen among many scientists. Despite the rapid progress in the study of positive and negative effect, uptake and accumulation of NPs, we confront numerous questions and need to more test and experiment.
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Ahmad, P., Nabi, G., Jeleel, C. A., & Umar, S. (2011). Free radical production, oxidative damage and antioxidant defense mechanisms in plants under abiotic stress. In P. Ahmad & S. Umar (Eds.), Oxidative stress: Role of antioxidants in Plants (pp. 19–53). New Delhi: Studium Press.
Akram, M. S., Athar, H. R., & Ashraf, M. (2007). Improving growth and yield of sunflower (Helianthus annuus L.) by foliar application of potassium hydroxide (KOH) under salt stress. Pakistan Journal of Botany, 39(3), 769–776.
Alidoust, D., & Isoda, A. (2013). Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiologiae Plantarum, 35, 3365–3375.
Ashraf, M., & Akram, N. A. (2009). Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnology Advances, 27, 744–752.
Bates, L. S. (1973). Rapid determination of free proline for water stress studies. Plant and Soil, 39, 205–207.
Bybordi, A., & Mamedov, G. (2010). Evaluation of application methods efficiency of zinc and iron for canola (Brassica napus L.). Notulae Scientia Biologicae, 2, 94–103.
Cakmak, I. (2000). Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytologist, 146, 85–200.
Chapman, H. D., & Pratt, P. F. (1961). Methods of analysis for soil, plant and water. Riverside, CA: University of California, Division of Agriculture Science.
Chen, C., & Dickman, M. B. (2005). Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proceedings of the National Academy of Sciences, 102, 3459–3464.
Farhangi-Abriz, S., & Torabian, S. (2017). Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicology and Environmental Safety, 137, 64–70.
Farhangi-Abriz, S., & Torabian, S. (2018). Nano-silicon alters antioxidant activities of soybean seedlings under salt toxicity. Protoplasma, 255, 953–962.
Fathi, A., Zahedi, M., & Torabian, S. (2017a). Effect of interaction between salinity and nanoparticles (Fe2O3 and ZnO) on physiological parameters of Zea mays L. Journal of Plant Nutrition, 40(19), 2745–2755.
Fathi, A., Zahedi, M., Torabian, S., & Khoshgoftar, A. (2017b). Response of wheat genotypes to foliar spray of ZnO and Fe2O3 nanoparticles under salt stress. Journal of Plant Nutrition, 40(10), 1376–1385.
Gonzalez-Melendi, P., Fernandez-Pacheco, R., Coronado, M. J., Corredor, E., Testillano, P. S., Risueno, M. C., et al. (2008). Nanoparticles as smart treatment-delivery systems in plants: assessment of different techniques of microscopy for their visualization in plant tissues. Annals of Botany, 101, 187–195.
Gueta-Dahan, Y., Yaniv, Z., Zilinskas, B. A., & Ben-Hayyim, G. (1997). Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in citrus. Planta, 203, 460–469.
Kabir, A. H., Swaraz, A. M., & Stangoulis, J. (2014). Zinc-deficiency resistance and biofortification in plants. Journal of Plant Nutrition and Soil Science, 177, 311–319.
Kerkeb, L., & Connolly, E. (2006). Iron transport and metabolism in plants. Genetic Engineering, 27, 119–140.
Khattab, H. (2007). Role of glutathione and polyadenylic acid on the oxidative defense systems of two different cultivars of canola seedlings grown under saline conditions. Australian Journal of Basic and Applied Sciences, 1, 323–334.
Khodakovskaya, M. V., de Silva, K., Biris, A. S., Dervishi, E., & Villagarcia, H. (2012). Carbon nanotubes induce growth enhancement of tobacco cells. American Chemical Society Nano, 6, 2128–2135.
Khot, L. R., Sankaran, S., Maja, J. M., Ehsani, R., & Schuster, E. W. (2012). Applications of nanomaterials in agricultural production and crop protection: A review. Crop Protection, 35, 64–70.
Kishor, P. K., Hong, Z., Miao, G. H., Hu, C., & Verma, D. (1995). Overexpression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiology, 108, 1387–1394.
Kochert, G. (1978). Carbohydrate determination by the phenol-sulfuric acid method. Handbook of Phycological Methods, 2, 95–97.
Kumar, K. B., & Khan, P. A. (1982). Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Indian Journal of Experimental Biology, 20, 412–416.
Lekshmy, S., Sairam, R. K., & Kushwaha, S. R. (2013). Effect of long-term salinity stress on growth and nutrient uptake in contrasting wheat genotypes. Indian Journal of Plant Physiology, 18, 344–353.
Li, M., Ahammed, G. J., Li, C., Bao, X., Yu, J., Huang, C., et al. (2016). Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Frontiers in Plant Science, 7, 1–13.
Lu, C. M., Zhang, C. Y., Wen, J. Q., & Wu, G. R. (2002). Effects of nano material on germination and growth of soybean. Soybean Science, 21, 168–171. (in Chinese).
Monica, R. C., & Cremonini, R. (2009). Nanoparticles and higher plants. Caryologia, 62, 161–165.
Movahed Dehnavi, M., Modarres Sanavi, A. M., Soroushzadae, A., & Jalali, M. (2002). Effect foliar application of zinc and manganese on yield and yield components of three safflower cultivars under drought stress in Isfahan. In Proceeding book of 8th congress of agronomy and plant breeding. Rasht, Iran.
Murata, N., Mohanty, P. S., Hayashi, H., & Papageorgiou, G. C. (1992). Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Letters, 296, 187–189.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiology, 22, 867–880.
Nasibi, F., Kalantari, K. M., Zanganeh, R., Mohammadinejad, G., & Oloumi, H. (2016). Seed priming with cysteine modulates the growth and metabolic activity of wheat plants under salinity and osmotic stresses at early stages of growth. Indian Journal of Plant Physiology, 21, 279–286.
Nasir Khan, M., Mobin, M., Abbas, Z. K., AlMutairi, K. A., & Siddiqui, Z. H. (2017). Role of nanomaterials in plants under challenging environments. Plant Physiology and Biochemistry, 110, 194–209.
Nelson, N. (1944). A photometric adaptation of the Somogyi method for the determination of glucose. Journal of Biological Chemistry, 153, 375–380.
Nishikimi, M., Rao, N. A., & Yagi, K. (1972). The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochemical and Biophysical Research Communications, 48, 849–854.
Nounjan, N., Nghia, P. T., & Theerakulpisut, P. (2012). Exogenous proline and trehalose promote recovery of rice seedling from salt-stress and differentially modulate antioxidants enzymes and expression of related genes. Journal of Plant Physiology, 169, 596–604.
Pokhrel, L. R., & Dubey, B. (2013). Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Science of the Total Environment, 452, 321–332.
Rao, S., & Shekhawat, G. S. (2014). Toxicity of ZnO engineered nanoparticles and evaluation of their effect on growth, metabolism and tissue specific accumulation in Brassica juncea. Journal of Environmental Chemical Engineering, 2, 105–114.
Rico, C. M., Hong, J., Morales, M. I., Zhao, L., Barrios, A. C., Zhang, J. Y., et al. (2013). Effect of cerium oxide nanoparticles on rice: A study involving the antioxidant defense system and in vivo fluorescence imaging. Environmental Science and Technology, 47, 5635–5642.
Sajedi, N. A., Ardakani, M. R., Madani, H., Naderi, A., & Miransari, M. (2011). The effects of selenium and other micronutrients on the antioxidant activities and yield of corn (Zea mays L.) under drought stress. Physiology and Molecular Biology of Plants, 17(3), 215–222.
Sharma, P. N., Kumar, P., & Tewari, R. K. (2004). Early signs of oxidative stress in wheat plants subjected to zinc deficiency. Journal of Plant Nutrition, 27, 449–461.
Siddiqui, M. H., Al-Whaibi, M. H., Firoz, M., & Al-Khaishany, M. Y. (2015). Role of nanoparticles in plants. In M. Siddiqui, M. Al-Whaibi, F. Mohammad (Eds.), Nanotechnology and plant sciences. Cham: Springer, pp. 19–35.
Simon, D. F., Domingos, R. F., Hauser, C., Hutchins, C. M., Zerges, W., & Wilkinson, K. J. (2013). Transcriptome sequencing (RNA-seq) analysis of the effects of metal nanoparticle exposure on the transcriptome of Chlamydomonas reinhardtii. Applied and Environmental Microbiology, 79, 4774–4785.
Sinha, S., & Saxena, R. (2006). Effect of iron on lipid peroxidation and enzymatic and non-enzymatic antioxidants and bacoside-A content in medicinal plant Bacopamonnieri L. Chemosphere, 62, 1340–1350.
Soliman, A. S., El-feky, S. A., & Darwish, E. (2015). Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. Journal of Horticulture and Forestry, 7, 36–47.
Stewart, R. C., & Bewley, J. D. (1980). Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiology, 65, 245–248.
Sun, B., Jing, Y., Chen, K., Song, L., Chen, F., & Zhang, L. (2007). Protective effect of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays). Journal of Plant Physiology, 164, 536–543.
Tan, Y., O’Toole, N., Taylor, N., & Millar, A. (2010). Divalent metal ions in plant mitochondria and their role in interactions with proteins and oxidative stress-induced damage to respiratory function. Plant Physiology, 152, 747–761.
Tewari, R. K., Kumar, P., & Sharma, P. N. (2005). Sign of oxidative stress in the chlorotic leaves of iron starved plants. Plant Science, 169, 1037–1045.
Torabian, S., Zahedi, M., & Khoshgoftar, A. H. (2016a). Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. Journal of Plant Nutrition, 39(2), 172–180.
Torabian, S., Zahedi, M., & Khoshgoftar, A. H. (2016b). Effect of foliar spray of zinc oxide on some antioxidant enzymes activity of sunflower under salt stress. Journal of Agricultural Science and Technology, 18, 1013–1025.
Torabian, S., Zahedi, M., & Khoshgoftar, A. H. (2017). Effects of foliar spray of nano-particles of FeSO4 on the growth and ion content of sunflower under saline condition. Journal of Plant Nutrition, 40(5), 615–623.
Tsai, C. Y., Salamini, F., & Nelson, O. E. (1970). Enzymes of carbohydrate metabolism in the developing endosperm of maize. Plant Physiology, 46, 299–306.
Velikova, V., Yordanov, I., & Edreva, A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Science, 151, 59–66.
Wang, S. Y., & Jiao, H. (2000). Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. Journal of Agricultural and Food Chemistry, 48, 5677–5684.
Wei, H., & Wang, E. (2013). Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chemical Society Reviews, 42, 6060–6093.
Yadav, T., Mungray, A. A., & Mungray, A. K. (2014). Fabricated nanoparticles: Current status and potential phytotoxic threats”. In D. M. Whitacre (Ed.), Reviews of environmental contamination and toxicology. Cham: Springer.
Yousfi, S., Mahmoudi, H., Abdelly, C., & Gharsalli, M. (2007). Effect of salt on physiological responses of barley to iron deficiency. Plant Physiology and Biochemistry, 45, 309–314.
Acknowledgements
The authors would like to thank Department of Agronomy, College of Agriculture, Isfahan University of Technology, Isfahan, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torabian, S., Farhangi-Abriz, S. & Zahedi, M. Efficacy of FeSO4 nano formulations on osmolytes and antioxidative enzymes of sunflower under salt stress. Ind J Plant Physiol. 23, 305–315 (2018). https://doi.org/10.1007/s40502-018-0366-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-018-0366-8