Abstract
Wheat seeds were pretreated with 0.01 µM cysteine for 24 h and then exposed to salinity (300 mM NaCl) or osmotic (1.8 MPa polyethylene glycol) stress for 2 weeks. The results showed that salinity stress reduced the growth of seedlings and increased the oxidative damages. Salinity increased the proline and soluble sugar content. Salinity response could be attributed to more ion toxicity than dehydration effect, as dehydration due to PEG showed little response. According to the results of this investigation pretreatment of seed with cysteine can be used as a method to ameliorate salinity induced damages in plants. It can be concluded that the maximum effect of cysteine on salinity stress was to alleviate the oxidative damages through the increase in activity of antioxidant enzymes, glutathione content, and decrease the ion toxicity through increase in K+/Na+ ratio and inhibition of Na+ uptake. However this aspect requires further confirmation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity and drought are the most important environmental factors that limit crop productivity, mainly due to alterations in water relations, ionic imbalance, metabolic perturbations, generation of reactive oxygen species (ROS) and tissue damage (Bartels and Sunkar 2005). An important consequence of salinity stress in plants is the excessive generation of reactive oxygen species (ROS) such as superoxide anion (O ·−2 ), hydrogen peroxide (H2O2) and the hydroxyl radicals (OH·), particularly in chloroplasts and mitochondria (Masood et al. 2006). Reactive oxygen species attack pigments, carbohydrates, proteins, lipids and nucleic acids, and the degree of damage depends on the balance between formation of ROS and its removal by the antioxidative scavenging system (Noreen and Ashraf 2009). To maintain a high K+/Na+ ratio in the cytosol, plant cells employ primary active transport, mediated by channels and co-transporters for Na+ extrusion and/or the intracellular compartmentalization of Na+ into the vacuole (Blumwald 2000). However, under salt stress, ion ratios are altered by the uncontrolled influx of Na+ through K-channels/transporters.
A number of nitrogen-containing compounds accumulate in plants exposed to saline stress. The most frequently accumulating compounds are amino acids, amides, imino acids, proteins, quaternary ammonium compounds, and polyamines, sugars and sugar alcohols. The specific compound that accumulates under saline environments varies with plant species (Robe 1990).
Accumulation of amino acids has been observed in many studies on plants exposed to abiotic stresses (Lugan et al. 2010). Cysteine is the only sulfide donor for all reduced sulfur containing cell constituents in plants (Leustek and Saito 1999). The level of free cysteine in plants is very low (<10 µM) but its production is quite high due to high requirements, both under optimal and stress conditions, and due to its fast utilization in subsequent synthetic processes. Cysteine is required for the synthesis of protein, glutathione, a tripeptide, and hydrogen sulfide (Sekiya et al. 1982) and functions as an S-donor for methionine and secondary metabolite biosynthesis (Leustek and Saito 1999). As part of the cysteine-molecule, the sulfur group called a ‘thiol’ is strongly nucleophilic, making it ideally suitable for biological redox processes. Redox control regulates enzymes and protects against oxidative damage (Leustek and Saito 1999). The thiol group of Cys and GSH is often involved in the redox cycle by two thiol ↔ disulphide conversions. This interchange is versatile for redox control and mitigation against oxidative stress in nearly all aerobic organisms, including plants (Leustek and Saito 1999). High demand for cysteine under salinity stress is also in agreement with the role of sulfuric compounds as osmolytes or antioxidants (Barroso et al. 1999).
It has been reported that seed priming (pre-sowing seed treatment) is an easy, low cost and low risk technique, and this approach has recently been used to overcome the salinity problem in agricultural lands. There are few researches on the effect of exogenous amino acids, particularly cysteine, in the possible antioxidative responses of plants against abiotic stress such as salinity. The present study was conducted to evaluate the effects of seed priming with cysteine on growth and alleviation of oxidative damages in shoot and root of wheat plant under salinity and osmotic stresses at early stages of growth.
Materials and methods
Seeds of wheat (Triticum aestivum L.) cv. Moghan were pretreated with 0.01 µM cysteine (concentration was optimized in preliminary experiment). After 24 h, seeds were grown in pots filled with perlite and transferred to green house with day/night temperature of 22/18 °C and a 16 h photoperiod, with a relative humidity of 50 %. These plants were irrigated with water and Hoagland nutrient solution for 5 days, and thereafter plants were subjected to salt stress by 300 mM NaCl and −1.8 MPa osmotic stress using PEG-6000. After 2 weeks of treatment, root and shoot of plants were harvested and immediately frozen in liquid nitrogen and stored at −80 °C for future analysis.
Measurement of growth parameters
Fresh and dry weights of shoot and root of plants were measured as growth parameters. For determination of dry weight, samples were oven dried at 70 °C for 24 h and then weighed.
Photosynthetic pigment estimation
Chlorophyll (Chl) and carotenoid (Car) were estimated by extracting the leaf material in 80 % acetone. Absorbance was recorded at 665, 645 and 470 nm. Chlorophyll and carotenoid contents were estimated according to the method of Lichtenthaler (1987).
Hydrogen peroxide content
Hydrogen peroxide (H2O2) content was measured colorimetrically after reaction with potassium iodide (KI) according to method of Alexieva et al. (2001).
Measurement of lipid peroxidation
The level of lipid peroxidation in plant tissues was measured by determination of malondialdehyde (MDA) content (Heath and Packer 1968). For the calculation of MDA, an extinction coefficient (ε) of 1.55 × 105 M−1 cm−1 was used at 532 nm. Results were expressed as nmol g−1 fresh weight.
Antioxidant enzyme extraction and activity assay
Leaves (500 mg) were homogenized in 50 mM potassium phosphate buffer (pH 7.0) containing 1 % soluble PVP, 1 mM EDTA and 1 mM PMSF. The homogenate was centrifuged at 10,000×g for 20 min, and the supernatant used for assay of the activity of enzymes and protein content.
Catalase activity assay
Catalase (CAT) activity was assayed by measuring the initial rate of H2O2 disappearance at 240 nm using the extinction coefficient of 40 mM−1 cm−1 for H2O2 (Dhindsa et al. 1981).
Ascorbate peroxidase activity assay
Ascorbate peroxidase (APX) was determined spectrophotometrically according to the oxidation of ASA. Hydrogen peroxide-dependent oxidation of ascorbic acid (ASA) was followed by measuring the decrease in absorbance due to ascorbic acid for 1 min at 290 using extinction coefficient of 2.8 mM−1 cm−1 (Nakano and Asada 1981).
Peroxidase activity assay
Peroxidase (POX) activity was determined using the method of Plewa et al. (1991) following the formation of tetra guaiacol by measuring the absorbance at 470 nm and using an extinction coefficient of 25.5 mM−1 cm−1.
Determination of glutathione and proline content
Reduced GSH contents were estimated using the method of Ellman (1959), and determination of free proline content was done according to Bates et al. (1973).
Determination of soluble sugar
Total soluble sugar content was determined using anthrone reagent by using glucose as standard (Roe 1955).
Sodium and potassium content
For the determination of K+ and Na+ content, dry shoot and root (0.02 g) material was treated with nitric acid, and diluted with distilled water. Total K+ and Na+ contents were directly measured by flame photometry.
Statistical analysis
Data were subjected to analysis of variance according to the MSTATC software. In order to identify the significant differences among the effects, LSD values were calculated for main effects (Priming) as A, and treatment as B) and interaction effect respectively, the attributed differences higher than the LSD value means significant difference (p < 0.05).
Results
Growth parameters
Salinity stress decreased only the root fresh weight and had no effect on other growth parameters in comparison with control condition. Osmotic stress also had non-significant effect on growth parameters of wheat seedlings. Pretreatment of seed with cysteine increased all the growth parameters except chla and carotenoids in control plants when compared with non-pretreatment plants. Cysteine pretreatment increased the root fresh and dry weight, and photosynthetic pigments under salinity stress when compared with non treated plants (Table 1). Under osmotic stress cysteine seed priming increased only root fresh and dry weight and total chlorophyll, while carotenoids content did not change significantly under osmotic stress condition and cysteine pretreatment also had non-significant effects on carotenoid content.
Hydrogen peroxide content
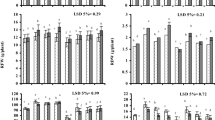
Salinity stress increased the hydrogen peroxide content in both shoot and roots of seedlings (Table 2). However PEG treatment caused an increase in H2O2 content only in shoot tissue and had non-significant effect on root tissue when compared with control plants. When seeds were pretreated with cysteine, hydrogen peroxide content decreased in shoot and roots of plant under both salinity and osmotic stress condition when compared with cysteine untreated plants.
Glutathione content
Following salinity and osmotic stresses, the contents of glutathione (GSH) in root increased, while shoot remained unchanged under both stresses (Table 2). Pretreatment of seed with cysteine increased the GSH content in root and shoots of seedlings under both salinity and osmotic stresses in comparison with non cysteine treated seedlings.
Proline content
Results showed that salinity stress increased the proline content in roots and shoot tissue, while osmotic stress caused increase in proline content only in root tissue (Table 2). Cysteine pretreatment of seed decreased the proline content of shoot and roots in both stressed and non-stressed plants.
Soluble sugar content
A significantly higher level of soluble sugar was observed in root tissue of seedlings under salinity and osmotic stresses in comparison with control seedlings, while sugar content of shoot tissue was unchanged both in salinity and osmotic stressed seedlings (Table 2). Cysteine pretreatment of seed had no effect on soluble sugar content in control and stressed seedlings.
Antioxidant enzyme activities
Salinity stress increased the activity of all the antioxidant enzymes (Table 3). However, osmotic stress increased the APX activity in both shoot and root tissue of seedlings, while activity of POX and CAT increased only in root tissue as compared to control seedlings. Cysteine pretreatment decreased the activities APX, POX and CAT in root tissue of control seedlings, while only in shoot tissue the activity of POX increased when compared with non pretreated seedlings. Under salinity stress cysteine pretreatment increased the activity of all enzymes in root and shoot tissues of seedlings. However under osmotic stress condition, cysteine pretreatment increased the activity of antioxidant enzymes in shoot, while decreased the activity of all antioxidant enzymes in root tissues when compared with non pretreated plants.
Sodium and potassium content
Results showed significant increase in Na+ content and decrease in K+ content in root and shoots of plants under salinity stress. Pretreatment of seeds with cysteine decreased the Na+ and increased the K+ content in root and shoot of stressed plants (Table 4). Potassium/sodium ratio decreased under salinity stress both in shoot and root tissue and pretreatment of seed with cysteine caused an increase in K+/Na+ ratio.
Discussion
The deleterious effects of salinity on plant growth are associated with low osmotic potential of soil solution, ion imbalance, specific ion toxicity or a combination of these factors. All of these cause adverse pleiotropic effects on plant growth and development at physiological, biochemical and at the molecular levels (Ashraf and Harris 2004). The inhibitory effects of salt stress on growth parameters have also been reported by other investigators in various plant species (Chaparzadeh et al. 2004). The reduction in growth not only helps the plant in saving the energy for defense purposes and reducing water use but also limits the risk of heritable damage (May et al. 1998). Pretreatment of seed with cysteine had non-significant effect on shoot fresh and dry weights but increased root fresh and dry weights. Similar results have been reported by Sotiropoulos et al. (2005) who showed that cysteine did not significantly alter growth of in vitro cultured apple rootstock EM 26. Reactive oxygen species lead to oxidative injuries and result in the reduction of plant growth and development (Ogawa and Iwabuchi 2001). It has been reported that role of GSH in ROS detoxification starts at an early stage of plant development. Thus, cysteine’s role in improvement of growth parameters could be attributed to GSH synthesis from cysteine. Decrease in chlorophylls in salinized plants could be attributed to increase in the activity of the chlorophyll-degrading enzyme, chlorophyllase, and ion accumulation in leaves (Sultana et al. 1999). Similar to our results Lu et al. (2002) observation no change in chlorophyll and carotenoid contents in salinity stressed Sueda salsa plants. Pretreatment of seed with cysteine increased content of chla, chlb and total chl in seedlings under salinity stress. Chen et al. (2011) has reported increase in chlorophyll content in Spinacia oleracea when treated with l00 µM NaHS (H2S donor).
Proline and soluble sugar content increased in roots of plants under salinity and osmotic stresses, confirming the findings of other researchers (Farouk, 2005; Kholova et al. 2009 Accumulation of organic compounds such as sugars and amino acids, mainly proline has been reported to contribute in maintaining the turgor pressure (Martinez et al. 2004; Farouk 2005). Proline is also considered to be involved in the protection of enzymes (Solomon et al. 1994) and cellular structures (Van Rensburg et al. 1993) and to act as a free radical scavenger (Alia et al. 1995). Our results showed that cysteine pretreatment alleviated stress and caused a decrease in proline content.
Increase of H2O2 level in seedlings under salinity stress compared to those seedlings which were under osmotic stress could be attributed to the ion toxicity of salinity rather than its dehydration effect. Salt stress causes oxidative stress, thus increasing the ROS formation in chloroplasts (Parida and Das 2005). Malondialdehyde (MDA) content, a product of lipid peroxidation, has been considered as an indicator of oxidative stress, leading to change in cell membrane permeability, which has been widely used to differentiate salinity-tolerant and sensitive plants (Azevedo Neto et al. 2006). Data of the present investigation showed that MDA content increased in stress condition, and pretreatment of seed with cysteine alleviate the adverse effect of NaCl and PEG on MDA and H2O2 content in wheat plants. Beneficial effect of cysteine in reduction of MDA and H2O2 contents also could be attributed to its product, such as glutathione, which has antioxidant activity. For example, it has been reported that exogenous glutathione increased phenol peroxidase activity with the reduction of deleterious effect of salinity in (Brassica napus L.) as well as it directly scavenge H2O2 and maintain the membrane integrity (Kattab 2007). Li et al. (2012) reported that H2S alleviated Cd toxicity in alfalfa seedling by the alleviation of oxidative damage.
Under normal growth conditions, production of reactive oxygen species (ROS), such as superoxide, hydrogen peroxide, and hydroxyl radicals, which are responsible for cellular damage under stress conditions (Mitler 2002) is generally at low level. Antioxidant enzymes CAT, POXX, and APX are important H2O2 scavengers. Results showed that activity of antioxidant enzymes were significantly affected under salinity stress. Our results are in agreement with Mandhania et al. (2006) and Xu et al. (2008) who reported increase in the activity of antioxidant enzymes under drought and salinity stress in wheat and barely plants, respectively. Pretreatment of seed with cysteine changed the antioxidant enzymes activity in plants. It has been reported that NaHS (H2S donor) treatment differently prevented the salinity induced decreases in APX, SOD, POX and CAT activities in alfalfa seedling (Wang et al. 2012). Therefore, changes in antioxidant enzymes activity in wheat plants pretreated with cysteine could be attributed to glutathione and hydrogen sulfide (May et al. 1998; Chen et al. 2011). In the present investigation, glutathione content increased in plants pretreated with cysteine.
Salt stress disturbs intracellular ion homeostasis of plants, which leads to membrane dysfunction perturbations of metabolic activity and the secondary effects that cause growth inhibition (Véry et al. 1998). Under salinity stress sodium influx may be facilitated by K+ inward rectifying channels, outward rectifying channels, high affinity K+ transporter (HKT), and low affinity cation transporter (LCT) (Tester and Davenport 2003). Accumulation of Na+ results in ionic imbalance, specific ion effects, nutrient deficiency symptoms and disturbed metabolism by the toxic effects of accumulated ions (Tester and Davenport 2003). When seeds were pretreated with cysteine Na+ content decreased while K+ content increased significantly in shoot and root tissue of plants. Wang et al. (2012) showed that NaHS (H2S donor) treatment resulted in a higher K+/Na+ ratio in root of alfalfa seedling 4 days after salt stress.
Conclusion
Results of this investigation showed that pretreatment of seed with cysteine can be used to ameliorate injurious effects of salinity stress s in plants. It can be concluded that the maximum effect of cysteine on salinity stress are alleviation of oxidative damages through the increase in antioxidant enzyme activity, and decrease in the ion toxicity through increase in K+/Na+ ratio and inhibition of Na+ uptake. It seems that protective role of cysteine may be attributed to either glutathione or H2S production, as antioxidant roles of these molecules is widely known. However this aspect requires further investigation.
References
Alexieva, V., Sergiev, I., Mapelli, S., & Karanov, E. (2001). The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environment, 24, 1337–1344.
Alia, K. V., Prasad, S. K., & Saradhi, P. P. (1995). Effect of zinc on free radicals and proline in Brassica and Cajanus. Phytochem, 39, 45–47.
Ashraf, M., & Harris, P. G. (2004). Potential biochemical indicators of salinity tolerance in plants. Plant Science, 166, 3–16.
Azevedo Neto, A. D., Prisco, J. T., Eneas-Filho, J., De Abreu, C. E. B., & Gomes Filho, E. (2006). Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt- sensitive maize genotypes. Environmental and Experimental Botany, 56, 87–94.
Barroso, C., Romero, L. C., Cejudo, F. J., Vega, J. M., & Gotor, C. (1999). Salt-specific regulation of the cytosolic O-acetylserine (thiol) lyase gene from Arabidopsis thaliana is dependent on abscisic acid. Plant Molecular Biology, 40, 729–736.
Bartels, D., & Sunkar, R. (2005). Drought and salt tolerance in plants. Plant Science, 24, 23–58.
Bates, L. S., Waldern, R. P., & Tare, I. D. (1973). Rapid determination of free proline for water stress studies. Plant and Soil, 29, 205–207.
Blumwald, E. (2000). Sodium transport and salt tolerance in plants. Current Opinion in Cell Biology, 12, 431–434.
Chaparzadeh, N., Amico, M. L. D., Khavari-Najad, R. A., Izzo, R., & Navarizzo, F. (2004). Antioxidative responses of Calendula officinolis under salinity conditions. Plant Physiology and Biochemistry, 42, 695–701.
Chen, J., Wu, F. H., Wang, W. H., Zheng, C. J., Lin, G. H., Dong, X. J., et al. (2011). Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. Journal of Experimental Botany, 62, 4481–4493.
Dhindsa, R. S., Dhindsa, P., & Thorpe, A. T. (1981). Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decrease levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32, 93–101.
Ellman, G. L. (1959). Tissue sulfydryl groups. Archive of Biochemistry and Biophysics, 82, 70–77.
Farouk, S. (2005). Response of Pisum sativum L. to some osmoregulators and plant growth substances under salt stress. Ph.D thesis, Faculty of Agriculture, Mansoura University of Egypt.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplast, kinetics and stoichiometry of fatty acid peroxidation. Archive of Biochemistry and Biophysics, 125, 189–198.
Kattab, H. (2007). Role of glutathione and polyadenylic acid on the oxidative defense systems of two different cultivars of Canola seedlings grown under saline condition. Australian Journal of Basic Applied Sciences, 1, 323–332.
Kholova, J., Sairam, R. K., Meena, R. C., & Srivastava, G. C. (2009). Response of maize genotypes to salinity stress in relation to osmolytes and metal ions contents, oxidative stress and antioxidant enzymes activity. Biology of Plant, 53(2), 249–256.
Leustek, T., & Saito, K. (1999). Sulfate transport and assimilation in plants. Plant Physiology, 120, 637–643.
Li, L., Wang, Y., & Shen, W. (2012). Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. Biometabolism, 25, 617–631.
Lichtenthaler, H. K. (1987). Chlorophyll and carotenoids: pigments photosynthetic of biomembranes. Methods in Enzymology, 148, 350–382.
Lu, C., Qiu, N., Lu, Q., Wang, B., & Kuango, T. (2002). Does salt stress lead to increased susceptibility of photosystem II to photoinhibition and changes in photosynthetic pigment composition in halophyte Sueda salsa grown outdoors? Plant Science, 163, 1063–1068.
Lugan, R., Niogret, M. F., Leport, L., Guegan, J. P., Larher, F. R., Savoure, A., et al. (2010). Metabolome and water homeostasis analysis of Thellungiella salsuginea suggests that dehydration tolerance is a key response to osmotic stress in this halophyte. The Plant Journal, 64, 215–229.
Mandhania, S., Madan, S., & Sawhney, V. (2006). Antioxidant defense mechanisms under salt stress in wheat seedlings. Biology of Plant, 50(2), 227–231.
Martinez, J. P., Lutts, S., Schanck, A., Bajji, M., & Kinet, J.-M. (2004). Is osmotic adjustment required for water stress resistance in the Mediterranean shrub Atriplex halimus L. Journal of Plant Physiology, 161, 1041–1051.
Masood, A., Shab, N. A., Zeeshan, M., & Abraham, G. (2006). Differential response of antioxidant enzymes to salinity stress in two varieties of Azolla (Azolla pinnata and Azolla filiculcides). Environmental and Experimental Botany, 58, 216–222.
May, M. J., Vernoux, T., Leaver, C., Van Montagu, M., & Inze, D. (1998). Glutathione homeostasis in plants: implications for environmental sensing and plant development. Journal of Experimental Botany, 49, 649–667.
Mitler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7, 405–410.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach choloroplast. Plant Cell Physiology, 22, 867–880.
Noreen, Z., & Ashraf, M. (2009). Changes in antioxidant enzymes and some key metabolites in some genetically diverse cultivars of radish (Raphanus sativus L.). Environmental and Experimental Botany, 67, 395–402.
Ogawa, K., & Iwabuchi, M. (2001). A mechanism for promoting the germination of Zinnia elegans seeds by hydrogen peroxide. Plant Cell Physiology, 42, 286–291.
Parida, A. K., & Das, A. B. (2005). Salt tolerance and salinity affects on plants. Ecotoxicology and Environmental Safety, 60, 324–349.
Plewa, M. J., Smith, S. R., & Wanger, E. D. (1991). Diethyl dithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase. Mutation Research, 247, 57–64.
Robe, E. (1990). Stress physiology: the functional significance of the accumulation of nitrogen-containing compounds. Journal of Horticultural Sciences, 65, 231–243.
Roe, J. H. (1955). The determination of sugar in blood and spinal fluid with anthrone reagent. Journal of Chemistry & Biology, 212, 335–343.
Sekiya, J., Schmidt, A., Wilson, L. G., & Filner, P. (1982). Emission of hydrogen sulfide by leaf tissue in response to l-cysteine. Plant Physiology, 70, 430–436.
Solomon, A., Beer, S., Waisel, Y., Jones, G. P., & Paleg, L. G. (1994). Effects of NaCl on the carboxylating activity of Rubisco from Tamarix jordanisin the presence and absence of proline-related compatible solutes. Plant Physiology, 90, 198–204.
Sotiropoulos, T. E., Dimassi, K. N., & Therios, I. N. (2005). Effects of l-arginine and l-cystein on growth, and chlorophyll and mineral contents of shoots of the apple rootstock EM26 cultured in vitro. Biologia Plantarum, 3, 443–445.
Sultana, N., Ikeda, T., & Itoh, R. (1999). Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environmental and Experimental Botany, 42(3), 211–220.
Tester, M., & Davenport, R. (2003). Na+ tolerance and transport in higher plants. Annuals of Botany, 91, 503–527.
Van Rensburg, L., Krüger, G. H. J., & Krüger, H. (1993). Proline accumulation as drought-tolerance selection criterion: Its relationship to membrane integrity and chloroplast ultrastructure in Nicotiana tabacum L. Journal of Plant Physiology, 141, 188–194.
Véry, A. A., Robinson, M. F., Mansfield, T. A., & Sanders, D. (1998). Guard cell cation channels are involved in Na+ induced stomatal closure in a halophyte. The Plant Journal, 14, 509–521.
Wang, Y., Li, L., Cui, W., Xu, S., Shen, W., & Wang, R. (2012). Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant and Soil, 351, 107–119.
Xu, Q., Xu, X., Zhao, Y., Jiao, K., Herbert, S. J., & Hao, L. (2008). Salicylic acid, hydrogen peroxide and calcium induced saline tolerance associated with endogenous hydrogen peroxide homeostasis in naked oat seedlings. Plant Growth Regulation, 54, 249–259.
Acknowledgments
The authors would like to acknowledge contributions made by Iranian Center of Excellence for Environmental Stresses in Cereal Crops, Shahid Bahonar University of Kerman, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasibi, F., Kalantari, K.M., Zanganeh, R. et al. Seed priming with cysteine modulates the growth and metabolic activity of wheat plants under salinity and osmotic stresses at early stages of growth. Ind J Plant Physiol. 21, 279–286 (2016). https://doi.org/10.1007/s40502-016-0233-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-016-0233-4