Abstract
Anthocyanins are natural colorants are synthesized in a branch of the flavonoid pathway. chalcone isomerase gene HvCHI catalyzes the conversion of chalcones to flavanones which is a key regulatory enzyme of anthocyanin biosynthesis in plants. Hosta ventricosa is an ornamental plant with elegant flowers and rich colorful leaves. How the function of HvCHI contributes to the anthocyanins biosynthesis is still unknown. In this study, the CHI homolog was identified from H. ventricosa and sequence analysis showed that HvCHI possessed the conserved substrate binding and catalytic domains. A phylogenetic analysis showed HvCHI had a strong sister relationship with Dracaena cambodiana CHI. Gene expression analysis revealed that HvCHI was constitutive expressed in all tissues and expressed highly in flower as well as was positively correlated with anthocyanin content. In addition, the subcellular location of HvCHI showed that is in cytoplasm. Overexpression of HvCHI in transgenic tobacco lines enhanced the anthocyanins accumulation along with the key genes upregulated, such as NtF3H, NtF3′H, NtF3′5′H, NtDFR, NtUFGT, and NtANS. Our results indicated a functional activity of the HvCHI, which provide an insight into the regulation of anthocyanins content in H. ventricosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Flavonoids are important secondary metabolites in plants, with a wide variety and wide distribution, which participate in many physiological and biochemical processes in plants, including photosynthesis, respiration, growth and development, and plant defenses against various stresses. Anthocyanins and pro-anthocyanins are a class of flavonoids, which are one of the most abundant groups of secondary metabolites in plants (Xu et al. 2015). Anthocyanins have a variety of pharmacological activities, such as anticancer, anti-inflammatory, and neuroprotective, but also have commercial value of plant natural products (Szymanowska and Baraniak 2019; Chen et al. 2021; Khan et al. 2021).
Anthocyanin biosynthesis and accumulation are influenced by both hereditary and environmental influences. Among them, the inherent factors include structural and regulatory genes. Structural genes encode enzymes, including genes involving in general phenylpropanoid pathway such as phenylalanine ammonia lyase (PAL), cinnamic acid-4-hydroxylase (C4H), and 4-coumaric acid-CoA ligase (4CL), etc. (Jaakola 2013; Tanaka and Brugliera 2013; Davies et al. 2020). Regulatory genes containing transcription factors, transcriptional regulator complex and regulation network regulate in the anthocyanin biosynthesis. MYB, basic-helix-loop-helix (bHLH) and WD-repeat family member WD40, form a ternary MBW complex to regulate anthocyanins accumulation (An et al. 2020; Qiao et al. 2021). A R2R3-MYB family gene SlMYB72 is responsible for pigment formation in tomato fruit (Wu et al. 2020). In addition, overexpression of AtMYB75 makes accumulation of anthocyanins in Solanum nigrum (Chhon et al. 2020). Besides, C1 encodes a R2R3-MYB transcription factor, interacting with S1 and activating A1 to promote anthocyanin accumulation, the C-S-A system was well documented in maize, rice and Arabidopsis (Sun et al. 2018; Qiao et al. 2021). As a regulating spot in the anthocyanin synthesis pathway, CHI is under the transcriptional regulation of MYB transcription factor. For instance, NtMYB4a could upregulate anthocyanin biosynthetic pathway genes including NtPAL, Nt4CL, NtCHS, NtCHI, NtF3H, NtDFR, NtANS, and NtUFGT, which resulted in increased anthocyanin content in the tobacco corolla and darker colors (Luo et al. 2020). Structural gene chalcone isomerase (CHI) encodes the second enzyme in the anthocyanin biosynthetic pathway, catalyzing the stereospecificity of naringenin and chalcone and intramolecular isomerization to its corresponding (2S)-flavanone (Sun et al. 2019). Chalcone isomerase is the first isolated key enzyme related to the flavonoids biosynthesis pathway. Now, CHI has been characterized and functional verified from crops, ferns, and medical herbs (Grotewold and Peterson 1994; Deng et al. 2018; Sun et al. 2019; Ni et al. 2020b; Zhu et al. 2021). For example, in economic crop plant mulberry, two MmCHIs are responsible for the anthocyanins accumulation in fruits (Chao et al. 2021). And expression of type I CHI group member OjCHI in Arabidopsis tt5 mutant could restore the anthocyanins and flavonols phenotype (Sun et al. 2019). Miyahara et al. reported two Carnations CHI genes had functional differentiation and played an important regulatory role in the synthesis of flavonoids and anthocyanins (Miyahara et al. 2018). Similarly, chalcone isomerase is also associated with anthocyanin synthesis in sweet potatoes, radishes, and strawberries (Li et al. 2001; Park et al. 2011; Zhang et al. 2011b). These studies provide theoretical basis and clues for molecular breeding of these horticultural plants.
Hosta ventricosa, a species in the family Liliaceae, is an important landscaping plant and herbaceous ornamental flower (Zhang et al. 2021). H. ventricosa is cold-resistant and shade-loving, and mainly distributed in temperate and subtropical regions of Asia (Liu et al. 2013; Aelenei et al. 2020). The wild type H. ventricosa flowers come in only two colors, purple and white. They are good materials to study the mechanism of flower color formation. In our previous study, we sequenced the two types H. ventricose with different flower colors and screened thousands DEGs related to the purple color formation (Zhang et al. 2019a). However, the key genes involved in anthocyanins biosynthesis still need to be experimentally identified. In this study, the CHI homolog was isolated and characterized from H. ventricosa, and overexpression HvCHI in tobacco promoted the accumulation of flavonoids and anthocyanins. Our results highlighted the importance of HvCHI in anthocyanin biosynthesis. The aim of our work is to provide a theoretical basis for the application of CHI in molecular color breeding in ornamental plants.
2 Materials and methods
Plant materials and growth conditions – H. ventricosa plantlets were collected from the Greenhouse A5 in Jilin Agricultural University Experimental Garden, in Jilin province, China. The tissues of roots, stems, leaves, and flowers in different flowering stages (Green bud, purple bud, initial flowering, middle flowering, and blooming flowering) were quickly frozen with liquid nitrogen and stored at − 80 °C for later use. N. tabacum “NC89” was planted for the transformation. The seedlings were cultured in a growth chamber with growing condition: temperature, 25 °C, humidity, 70%, illumination, 6000 Lx, and light cycle: 16/8 h.
Cloning and bioinformatic analysis of HvCHI – Total RNA was extracted using RNAprep Pure Extraction Kit (TIANGEN biochemical Technology, Beijing, China) and the quality and integrity of the RNA were determined by the 1.0% agarose gel electrophoresis. And the first strand of cDNA was synthesized by PrimeScript RT reagent Kit with gDNA (TaKaRa, Tokyo, Japan) according to the instructions. The specific primers were designed using Permier Primer 6.0 based on the sequences from the transcriptome data (Table S1). The HvCHI gene was amplified from the cDNA with primers using TaKaRa Taq™ (TaKaRa, Tokyo, Japan), with the following cycle conditions: initial denaturation at 94 °C for 3 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s, and extension at 72 °C for 25 s, and a final extension at 72 °C for 10 min. The PCR fragments were obtained and purified with Gel Extraction Kit (CW2302 CWBIO, JiangSu, China), and then cloned into a pMD™ 18-T Vector Cloning Kit (TaKaRa, Tokyo, Japan) and sequenced by GENE Biotechnology company (Beijing, China). The conserved domain of the candidate gene was predicted on the NCBI Conserved Domain Database (https://www.ncbi.nlm.nih.gov/structure/cdd/wepsb.cgi). The multi-alignment analysis of CHIs was employed with DNAMAN and a phylogenetic tree was constructed with MEGA 7.0 using the Nerghbor-Joining method and 1000 bootstrap replicates.
Quantitative PCR analysis – The first strand of cDNA for quantitative PCR was synthesized using PrimeScript RT reagent Kit with gDNA Eraser (RR047, TaKaRa, Japan). The reaction was performed on BioRad IQ5 (Biorad, USA) with the SYBR Prime Ex Tap II (RR420, TaKaRa, Japan) according to the user’s manual. The reaction procedure was as follows: pre-denaturation at 95 °C for 30 s; 98 °C 5 s, 60 °C 34 s, 40 cycles; 95 °C 15 s, 60 °C 1 min, 95 °C 15 s. The relative expression level of HvCHI in different tissues and different development stages of flower was measured with HvActin as the reference gene (Zhang et al. 2019a).
The key genes involved in anthocyanin biosynthesis in tobacco were also determined by quantitative PCR. The reaction system and procedures were as same as above, and the primers were listed in Table S1. And the relative expression levels of the key genes were compared with the reference gene in control with the 2−∆∆CT method (Sun et al. 2020).
The subcellular localization of HvCHI – According to protocol of polyethylene glycol (PEG)-mediated DNA transformation into protoplasts (Shen et al. 2014), the 20-day-old leaves of Arabidopsis thaliana seedlings were chosen, and the supernatant was digested by enzymes, then treated with W5 and MMG solution to obtain a protoplast suspension. The protoplast concentration was adjusted to 2 × 106–2 × 107 with MMG solution. 20 μg recombinant plasmid (35S::HvCHI::GFP) and 200 μl protoplasts were mixed lightly in a 2-ml centrifuge tube. Then 250 μl 50% PEG 4000 was added and induced transformation for 30 min in darkness. Addition added 900 μl W5 solution to stop transfection. The sample was centrifuged at 100 g for 3 min and discarded the supernatant. The protoplasts were resuspended with 1 ml W5 and cultured in darkness at 26–28 °C for 2 days. Then, observe the fluorescence signal in the protoplasts using a laser confocal scanning microscope (Nikon C2-ER, Japan).
Overexpression vector construction and Agrobacterium-mediated transformation – The PCR products were digested with Bstp I and Bgl II before being cloned into the pCAMBIA3301 vector with the same restriction sites (Fig. 3a). The recombinant plasmids pCAMBIA3301-HvCHI was transferred into A. tumefaciens GV3101 by the freeze–thaw method and verified with HvCHI specific PCR analysis. Tobacco transformation was carried out using the leaf plate method, as previously described. The regenerated plantlets were screened on MS plates-containing hygromycin (50 mg/ml). The resistant shoots were regenerated from the cut surface of the explants and these shoots were separated from the mother explants and roots induced. The introduction of HvCHI gene was confirmed by gDNA PCR from leaves of transgenic plants with gene-specific primers. A specific band of ~ 600 bp indicated the introduction of HvCHI in transgenic tobacco. The plants transformed with an empty vector pCAMBIA3301were served as control. By qRT-PCR confirmation, the independent transgenic lines with the highest HvCHI gene expression level were chosen for further experiments.
Total flavonoids and anthocyanin determination – Total flavonoids were estimated according to the aluminum chloride method (Zhang et al. 2011a). Briefly the flash tobacco flowers from transgenic and wildtype lines were collected and ground to a fine powder with liquid nitrogen. The powders were extracted with 10 ml of 95% methanol under sonication for 5 h at 60 °C and then centrifuged at 10,000 g for 15 min. The supernatant was transferred to add 1 ml 10% AlCl3, and constant volume to 10 ml. The absorbance for each sample was measured at 420 nm. Rutin concentrations ranging from 0 to 9 μg/ml were prepared, and the standard calibration curve was obtained using a linear fit (R2 = 0.9995). Samples from flowers were extracted for anthocyanins measurement, and quantification of anthocyanins was performed according to the protocols of Ni et al. (Ni et al. 2020a). The process was repeated at least three times for each sample.
3 Results
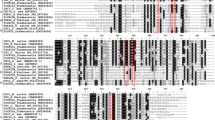
Molecular characterization of CHI from H. ventricosa – The ORF of CHI (EC5.5.1.6) from H. ventricosa was successfully isolated and named as HvCHI (the accession number in GenBank, OM632675), which encodes a polypeptide of 211 amino acids, with a predicted molecular mass of 23.44 kDa. A phylogenetic tree was established to investigate the homology amino acid sequences of HvCHI to other CHI from H. ventricosa and 10 other plants. HvCHI showed close relationship with CHI from Dracaena cambodiana based on the sequence alignment. (Fig. 1A). The highly conserved homology indicated the importance of CHI catalyzing function. Sequence alignment of HvCHI with CHIs in other plant species revealed that HvCHI shared many conserved catalytic and binding residues with other CHI proteins at the amino acid level, which indicating HvCHI was the member of CHI family (Fig. 1B).
Molecular characteristics of CHI proteins. A sequence alignment of HvCHI with others from Actinidia chinensis, Albuca bracteate, Camellia fraterna, Carthamus tinctorius, Dracaena cambodiana, Iris domestica, Pyrus pyrifolia, and Tulipa fosteriana. Red and blue dots represent the active site and critical active site residues, respectively. The red box indicates residues proposed to affect substrate preference. B phylogenetic tree of CHI proteins from different plant species. The tree was constructed by Neighbor-Joining method and CHI from Arabidopsis thaliana and Nicotiana tabacum were set as outgroup with 1000 bootstrap replications using MEGA 7.0. C the subcellular localization of HvCHI. The first line, observation of GFP empty vector introduced in Arabidoposis protoplast. The second line, observation of HvCHI-GFP recombinant vector introduced in Arabidoposis protoplast
Anthocyanin analysis and expression pattern of HvCHI – To understand the dynamic change trends of anthocyanin in flowering period in H. ventricosa, anthocyanin in flowers at 5 different developmental stages were identified and quantified (Fig. 2A, B). The results showed that the anthocyanin level was variational with flowering time and the highest anthocyanin content was 0.452 ± 0.0039 mg/g in the Flower S4 stage. Furthermore, to study the expression profiles of HvCHI gene, the quantitative PCR analysis of HvCHI in tissues was preformed (Fig. 2C). The HvCHI was expressed in root, stem, leaf, and flower tissues, and it maintained a high level in flower. Therefore, we also detected the HvCHI expression in flowering developmental stages, which showed that the expression level of HvCHI was increased with flowering development and reached the highest expression in the Flower S3 when the flower is not open. Besides, it is positively correlated with the anthocyanin content and HvCHI expression level (Fig. 2B, C). These results indicated that a high level of HvCHI expression enhanced anthocyanin biosynthesis in flowers of H. ventricosa.
Anthocyanin content and relative gene expression level of HvCHI in different tissues and flowering stages in H. ventricosa. A the photo of five flowering developmental stages of H. ventricose. B anthocyanin contents in flowering developmental stages. C the relative gene expression level of HvCHI in different tissues and flowering stages in H. ventricosa. Note, Data represent means ± SD of three biological replicates (**p < 0.01). a, b, c, and d represent significant differences between samples, ns no significant
Subcellular localization of HvCHI – To investigate the subcellular localization of HvCHI, the HvCHI coding sequence was cloned and fused to the GFP reporter under the CaMV35S promoter and then was transient to Arabidoposis protoplast. The free GFP fluorescence signal were appeared in the nucleus, cytoplasm, and cell membrane, while the 35S::HvCHI::GFP fluorescence was observed in the cytoplasm, which indicated that HvCHI might be located in cytoplasm agreeing with its catalyze function (Fig. 1C).
Overexpression of HvCHI transgenic tobacco lines were generated by Agrobacterium-mediated transformation – The transgenic tobacco lines overexpressing HvCHI under the control of 35S promoter were developed through Agrobacterium tumefaciens-mediated transformation. The introduction of HvCHI gene was confirmed by PCR with gene-specific primers (Fig. 3B, C). We isolated 13 individual transgenic plants following A. tumefaciens-mediated genetic transformation and further verified by qRT-PCR (Fig. 3D). We selected overexpression tobacco lines CHI-OE5, CHI-OE9 and CHI-OE10 for further examination due to HvCHI highest expressed, while no visible phenotypes were observed in the transgenic lines.
Construction of the overexpression vector and genetic transformation procedure. A the schematic of the HvCHI gene overexpression cassette. B agarose gel showing the HvCHI in the Agrobacterium tumefaciens harbored the recombinant vectors. C agarose gel showing the specific fragment for the HvCHI in the transgenic tobacco lines. D the relative expression level of HvCHI in the transgenic tobacco lines
Total flavonoids and anthocyanin contents measurement in transgenic tobacco lines – CHI plays a key role in anthocyanin biosynthesis in plant. To study the functions of HvCHI we generated the overexpression tobacco lines and measured the total flavonoids and anthocyanins content in our transgenic tobacco lines CHI-OE5, CHI-OE9 and CHI-OE10. Figure 4A showed that the flower colors of OE lines were darker pink than the wild type. In the results, the flavonoids concentrations in wildtype NC89 and OE lines were 1.42 ± 0.0025, 2.20 ± 0.0067, 2.14 ± 0.0019, and 2.18 ± 0.0077 mg/g, respectively, which observed significantly increased in overexpression of HvCHI lines. In addition, the results showed that the anthocyanin contents in OE tobacco lines were 0.134 ± 0.0033, 0.288 ± 0.0052, 0.284 ± 0.0069, and 0.241 ± 0.0074 mg/g which were 2.14-fold, 2.12-fold, and 1.79-fold compared with the wildtype NC89, respectively (Fig. 4B, C). Meanwhile, we determined the tobacco endogenous genes involved in anthocyanin biosynthesis by qRT-PCR in tobacco overexpression lines. As expected, the relative expression of HvCHI in OE lines had exceeded 5.12-fold than that of the control (Fig. 3D). It also showed that the expression levels of NtF3H, NtF3′H, NtF3′5′H, NtDFR, NtUFGT, and NtANS were significantly increased in overexpression tobacco lines compared to the control. While the NtCHS, and NtFLS were no significance in non-/transgenic lines (Fig. 4D).
The phenotype and flavonoids, anthocyanins and related genes expression level in OE tobacco lines compared with the control. A the flowers of transgenic tobacco and the wildtype. B total flavonoids and C anthocyanin contents in OE lines and NC89. D the key genes involved in anthocyanin biosynthesis in flowers of transgenic lines compared with the control
4 Discussion
In ornamental plants, flower color is an essential economic characteristic, and the richness and varieties hues are traits that breeding scientists strive for. Multiple studies have shown that flowers color determines by the concentration and composition of flavonoids, such as flavonols, flavones, and anthocyanins (Lou 2014; Wang 2017). It is found that cyanidin and peonidin were responsible for red color, whereas flavonols and flavones play a role in yellow and bule color in ornamental flowers (Liu 2016; Xue 2016). In this study, flower color was influenced by the content of total flavonoids and total anthocyanins, particularly anthocyanin. We found the petals of transgenic tobacco lines were darker pink (Fig. 4A–C), which could be due to greater total flavonoids and anthocyanins concentrations.
CHI encodes the essential enzyme in anthocyanin synthesis pathway. Our results and other previous studies share the common conclusion that increasing HvCHI expression level could improve anthocyanins content (Guo et al. 2018; Morimoto et al. 2019; Zhang et al. 2019b; Leng et al. 2020). Therefore, we hypothesize that HvCHI may alter the expression of genes downstream of anthocyanins synthesis pathway, resulting an increase in anthocyanins. In this study, we determined the expression levels of genes involved in anthocyanins pathway. It shown that the expression levels of the most genes involved in anthocyanins pathway were significantly increased in overexpression tobacco lines, such as F3H, F3′H, F3′5′H, DFR, ANS, and UFGT, and only the expression of CHS and FLS were not insignificantly changed in transgenic lines. It suggested CHI could influence all genes located in its downstream of anthocyanins pathway, while it could not alter the expression level of its upstream gene, CHS, and the far flavonol branch biosynthesis gene, FLS. Our results also believe CHI is an important regulatory site for its expression increased influences on the most downstream genes’ expression levels to change accordingly. Also, other plants have been shown to use CHI to modulate anthocyanin levels and downstream genes expression. For example, overexpression of SmCHI makes the higher anthocyanin accumulation in Arabidopsis stems and siliques (Jiang et al. 2016). Overexpression CtCHI1 in safflower significantly upregulated main secondary metabolites accumulation as well as CtPAL3, Ct4CL3, CtF3H and CtDFR2, which positively regulated flavanols and chalcone biosynthesis pathway while the results were opposite in tobacco (Guo et al. 2019). Our findings suggest a possible regulatory spot for anthocyanin production in plants, however further research determining catalytic activity of HvCHI in vivo or in vitro may be required to explore HvCHI’s catalytic function and transcriptional control mechanism in transgenic tobacco lines. The conserved amino acid sequence of protein is responsible for its crucial catalytic function. In this study, we found that amino acid sequence of HvCHI was highly conserved, especially at substrate binding sites and catalytic sites. This result is consistent with the other research on CHI enzymes in plants like maize and sweet potato (Grotewold and Peterson 1994; Li et al. 2006; Zhang et al. 2011b). Furthermore, CHI gene expresses specifically in various organs that anthocyanins accumulated. For example, the expression pattern of OjCHI were flower development-dependent in rice (Sun et al. 2019). In mulberry, CHI accompanied with other anthocyanin biosyntheitc genes were expressed highly during fruit ripen (Qi et al. 2014). We found similar results in H. ventricose. HvCHI is broadly expressed in tissues including roots, stems, leaves, and different stages of flowers, which is positively correlated with anthocyanins accumulation. and HvCHI has the highest expression level in flowers. We also discovered that the HvCHI protein was detected in the cytoplasm, which is compatible with SmCHI's subcellular location in eggplant (Jiang et al. 2016).
In the herbaceous peony, Brunfelsia Acuminata and Phalaenopsis, the anthocyanin synthesis pathways have been thoroughly studied, which have provided theoretical basis for the molecular breeding and germplasm innovation of those ornamental plants (Ma et al. 2009; Li et al. 2020; Tang et al. 2020). However, in H. ventricosa the research is relatively lagging for the mining of gene/genome information, and color decision genes and their regulatory mechanisms are yet to be studied. Therefore, as the key gene for anthocyanin synthesis, the isolated and characterized HvCHI in this study could increase the content of anthocyanins and flavonoids in overexpression tobacco lines. It is a potential entry point for the germplasm improvement of H. ventricosa and provides a new insight for germplasm innovation and further breeding.
5 Conclusions
We identified the CHI homolog from H. ventricosa, and the sequence analysis showed that HvCHI possessed the conserved catalyze and binding sites. A phylogenetic analysis showed that HvCHI was close to Dracaena cambodiana CHI. Gene expression analysis revealed that HvCHI was constitutive expressed in all tissues and expressed highly in flower as well as was positively correlated with anthocyanin content. In addition, the subcellular location of HvCHI showed that is in cytoplasm. Overexpression of HvCHI in transgenic tobacco lines enhanced the flavonoids and anthocyanins accumulation along with the key genes upregulated, such as NtF3H, NtF3′H, NtF3′5′H, NtDFR, NtUFGT, and NtANS. Our results indicated a functional activity of the HvCHI, which provide a new insight into the regulation of anthocyanins content as well as for germplasm innovation and further breeding in H. ventricose.
Abbreviations
- 4CL:
-
4-Coumaric acid-CoA ligase
- ANS:
-
Anthocyanin synthase
- C4H:
-
Cinnamic acid-4-hydroxylase
- CHI:
-
Chalcone isomerase
- CHS:
-
Chalcone synthase
- DFR:
-
Dihydroflavonol 4-reductase
- F3H:
-
Flavanone-3-hydroxylase
- F3′H:
-
Flavonoid 3′-hydroxylase
- F3′5′H:
-
Flavonoid 3′5′-hydroxylase
- UFGT:
-
Flavonoid glucosyltransferase
- MT:
-
Methyltransferase
- PAL:
-
Phenylalanine ammonia lyase
References
Aelenei RI, Petra S, Toma F (2020) Studies and research on the species and varieties of Hosta in cultivation. Sci Pap Ser B 64:511–518
An J-P, Wang X-F, Hao Y-J (2020) BTB/TAZ protein MdBT2 integrates multiple hormonal and environmental signals to regulate anthocyanin biosynthesis in apple. J Integr Plant Biol 62:1643–1646. https://doi.org/10.1111/jipb.12940
Chao N, Wang R-f, Hou C, Yu T, Miao K, Cao F-y, Fang R-j, Liu L (2021) Functional characterization of two chalcone isomerase (CHI) revealing their responsibility for anthocyanins accumulation in mulberry. Plant Physiol Biochem 161:75–83. https://doi.org/10.1016/j.plaphy.2021.01.044
Chen J, Xu B, Sun J, Jiang X, Bai W (2021) Anthocyanin supplement as a dietary strategy in cancer prevention and management: a comprehensive review. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.1913092
Chhon S, Jeon J, Kim J, Park SU (2020) Accumulation of anthocyanins through overexpression of AtPAP1 in Solanum nigrum Lin. (Black Nightshade). Biomolecules. https://doi.org/10.3390/biom10020277
Davies KM, Jibran R, Zhou Y, Albert NW, Brummell DA, Jordan BR, Bowman JL, Schwinn KE (2020) The evolution of flavonoid biosynthesis: a bryophyte perspective. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00007
Deng Y, Li C, Li H, Lu S (2018) Identification and characterization of flavonoid biosynthetic enzyme genes in Salvia miltiorrhiza (Lamiaceae). Molecules. https://doi.org/10.3390/molecules23061467
Grotewold E, Peterson T (1994) Isolation and characterization of a maize gene encoding chalcone flavonone isomerase. Mol Gen Genet MGG 242:1–8
Guo X, Wang Y, Zhai Z, Huang T, Zhao D, Peng X, Feng C, Xiao Y, Li T (2018) Transcriptomic analysis of light-dependent anthocyanin accumulation in bicolored cherry fruits. Plant Physiol Biochem 130:663–677. https://doi.org/10.1016/j.plaphy.2018.08.016
Guo DD, Gao Y, Liu F, He BX, Jia XL, Meng FW, Zhang H, Guo ML (2019) Integrating molecular characterization and metabolites profile revealed CtCHI1’s significant role in Carthamus tinctorius L. BMC Plant Biol. https://doi.org/10.1186/s12870-019-1962-0
Jaakola L (2013) New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci 18:477–483. https://doi.org/10.1016/j.tplants.2013.06.003
Jiang M, Liu Y, Ren L, Lian H, Chen H (2016) Molecular cloning and characterization of anthocyanin biosynthesis genes in eggplant (Solanum melongena L.). Acta Physiol Plant. https://doi.org/10.1007/s11738-016-2172-0
Khan J, Deb PK, Priya S, Medina KD, Devi R, Walode SG (2021) Dietary flavonoids: cardioprotective potential with antioxidant effects and their pharmacokinetic. Toxicol Ther Concerns Mol 26:4021. https://doi.org/10.3390/molecules26134021
Leng F, Cao J, Ge Z, Wang Y, Zhao C, Wang S, Li X, Zhang Y, Sun C (2020) Transcriptomic analysis of root restriction effects on phenolic metabolites during grape berry development and ripening. J Agric Food Chem 68:9090–9099. https://doi.org/10.1021/acs.jafc.0c02488
Li YJ, Sakiyama R, Maruyama H, Kawabata S (2001) Regulation of anthocyanin biosynthesis during fruit development in “Nyoho” strawberry. J Jpn Soc Hortic Sci 70:28–32
Li F, Jin Z, Qu W, Zhao D, Ma F (2006) Cloning of a cDNA encoding the Saussurea medusa chalcone isornerase and its expression in transgenic tobacco. Plant Physiol Biochem 44:455–461. https://doi.org/10.1016/j.plaphy.2006.08.006
Li M, Yang H, Kui X, Sun Y, Chen J, Qiu D (2020) Molecular and metabolic analysis of color change in brunfelsia acuminata flower. HortScience 55:S214–S214
Liu N, Sun G, Xu Y, Luo Z, Lin Q, Li X, Zhang J, Wang L (2013) Anthocyanins of the genus of Hosta and their impacts on tepal colors. Sci Hortic 150:172–180. https://doi.org/10.1016/j.scienta.2012.10.030
Liu L, Zhang LY, Wang SL, Niu XY (2016) Analysis of anthocyanins and flavonols in petals of 10 Rhododendron species from the Sygera Mountains in Southeast Tibet. Plant Physiol Biochem 104:250–256
Lou Q, Liu Y, Qi Y, Jiao S, Tian F, Jiang L, Wang Y (2014) Transcriptome sequencing and metabolite analysis reveals the role of delphinidin metabolism in flower colour in grape hyacinth. J Exp Bot 65:3157–3164
Luo Q, Liu R, Zeng L, Wu Y, Jiang Y, Yang Q, Nie Q (2020) Isolation and molecular characterization of NtMYB4a, a putative transcription activation factor involved in anthocyanin synthesis in tobacco. Gene. https://doi.org/10.1016/j.gene.2020.144990
Ma H, Pooler M, Griesbach R (2009) Anthocyanin regulatory/structural gene expression in phalaenopsis. J Am Soc Hortic Sci 134:88–96. https://doi.org/10.21273/jashs.134.1.88
Miyahara T, Sugishita N, Ishida-Dei M, Okamoto E, Kouno T, Cano EA, Sasaki N, Watanabe A, Tasaki K, Nishihara M, Ozeki Y (2018) Carnation I locus contains two chalcone isomerase genes involved in orange flower coloration. Breed Sci 68:481–487. https://doi.org/10.1270/jsbbs.18029
Morimoto H, Narumi-Kawasaki T, Takamura T, Fukai S (2019) Analysis of flower color variation in carnation (Dianthus caryophyllus L.) cultivars derived from continuous bud mutations. Hortic J 88:116–128. https://doi.org/10.2503/hortj.UTD-007
Ni J, Ruan RJ, Wang LJ, Jiang ZF, Gu XJ, Chen LS, Xu MJ (2020a) Functional and correlation analyses of dihydroflavonol-4-reductase genes indicate their roles in regulating anthocyanin changes in Ginkgo biloba. Ind Crop Prod 152:10. https://doi.org/10.1016/j.indcrop.2020.112546
Ni R, Zhu T-T, Zhang X-S, Wang P-Y, Sun C-J, Qiao Y-N, Lou H-X, Cheng A-X (2020b) Identification and evolutionary analysis of chalcone isomerase-fold proteins in ferns. J Exp Bot 71:290–304. https://doi.org/10.1093/jxb/erz425
Park NI, Xu H, Li X, Jang IH, Park S, Ahn GH, Lim YP, Kim SJ, Park SU (2011) Anthocyanin accumulation and expression of anthocyanin biosynthetic genes in radish (Raphanus sativus). J Agric Food Chem 59:6034–6039. https://doi.org/10.1021/jf200824c
Qi X, Shuai Q, Chen H, Fan L, Zeng Q, He N (2014) Cloning and expression analyses of the anthocyanin biosynthetic genes in mulberry plants. Mol Genet Genomics 289:783–793. https://doi.org/10.1007/s00438-014-0851-3
Qiao W, Wang Y, Xu R, Yang Z, Sun Y, Su L, Zhang L, Wang J, Huang J, Zheng X, Liu S, Tian Y, Chen L, Liu X, Lan J, Yang Q (2021) A functional chromogen gene C from wild rice is involved in a different anthocyanin biosynthesis pathway in indica and japonica. Theor Appl Genet 134:1531–1543. https://doi.org/10.1007/s00122-021-03787-1
Shen J, Fu J, Ma J, Wang X, Gao C, Zhuang C, Wan J, Jiang L (2014) Isolation, culture, and transient transformation of plant protoplasts. Curr Protoc Cell Biol 63:281–2817. https://doi.org/10.1002/0471143030.cb0208s63
Sun X, Zhang Z, Chen C, Wu W, Ren N, Jiang C, Yu J, Zhao Y, Zheng X, Yang Q, Zhang H, Li J (2018) The C-S-A gene system regulates hull pigmentation and reveals evolution of anthocyanin biosynthesis pathway in rice. J Exp Bot 69:1485–1498
Sun W, Shen H, Xu H, Tang X, Tang M, Ju Z, Yi Y (2019) Chalcone isomerase a key enzyme for anthocyanin biosynthesis in Ophiorrhiza japonica. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00865
Sun J, Wu Y, Shi M, Zhao DQ, Tao J (2020) Isolation of PlANS and PlDFR genes from herbaceous peony (Paeonia lactiflora Pall) and its functional characterization in Arabidopsis and tobacco. Plant Cell Tissue Organ Cult 141:435–445. https://doi.org/10.1007/s11240-020-01802-9
Szymanowska U, Baraniak B (2019) Antioxidant and potentially anti-inflammatory activity of anthocyanin fractions from pomace obtained from enzymatically treated raspberries. Antioxidants 8:299
Tanaka Y, Brugliera F (2013) Flower colour and cytochromes P450. Philos Trans R Soc B. https://doi.org/10.1098/rstb.2012.0432
Tang Y, Fang Z, Liu M, Zhao D, Tao J (2020) Color characteristics, pigment accumulation and biosynthetic analyses of leaf color variation in herbaceous peony (Paeonia lactiflora Pall). 3Biotech. https://doi.org/10.1007/s13205-020-2063-3
Wang L, Pan D, Liang M, Abubakar YS, Li J, Lin JK, Chen SP, Chen W (2017) Regulation of anthocyanin biosynthesis in purple leaves of Zijuan tea (Camellia sinensis var. kitamura). Int J Mol Sci 18:833
Wu M, Xu X, Hu X, Liu Y, Cao H, Chan H, Gong Z, Yuan Y, Luo Y, Feng B, Li Z, Deng W (2020) SlMYB72 regulates the metabolism of chlorophylls, carotenoids, and flavonoids in tomato fruit(1). Plant Physiol 183:854–868. https://doi.org/10.1104/pp.20.00156
Xu W, Dubos C, Lepiniec L (2015) Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci 20:176–185. https://doi.org/10.1016/j.tplants.2014.12.001
Xue LW, Zhang W, Li YX, Wang J, Lei JJ (2016) Flower pigment inheritance and anthocyanin characterization of hybrids from pink-flowered and white-flowered strawberry. Sci Hortic 200:143–150
Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, Smith PT, Bartlett J, Shanmugam K, Muench G, Wu MJ (2011a) Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J Agric Food Chem 59:12361–12367. https://doi.org/10.1021/jf203146e
Zhang Z, Qiang W, Liu X, Yang C, Fu Y, Chen M, Zeng L, Wang W, Liao Z (2011b) Molecular cloning and characterization of the chalcone isomerase gene from sweetpotato. Afr J Biotech 10:14443–14449
Zhang J, Sui C, Wang Y, Liu S, Liu H, Zhang Z, Liu H (2019a) Transcriptome-wide analysis reveals key DEGs in flower color regulation of Hosta plantaginea (Lam.) aschers. Genes. https://doi.org/10.3390/genes11010031
Zhang L, Sun X, Wilson IW, Shao F, Qiu D (2019b) Identification of the genes involved in anthocyanin biosynthesis and accumulation in Taxus chinensis. Genes. https://doi.org/10.3390/genes10120982
Zhang J, Sui C, Liu H, Chen J, Han Z, Yan Q, Liu S, Liu H (2021) Effect of chlorophyll biosynthesis-related genes on the leaf color in Hosta (Hosta plantaginea Aschers) and tobacco (Nicotiana tabacum L.). BMC Plant Biol. https://doi.org/10.1186/s12870-020-02805-6
Zhu J, Zhao W, Li R, Guo D, Li H, Wang Y, Mei W, Peng S (2021) Identification and characterization of chalcone isomerase genes involved in flavonoid production in Dracaena cambodiana. Front Plant Sci. https://doi.org/10.3389/fpls.2021.616396
Acknowledgements
This research was funded by Project of science and Technology Department of Jilin Province (2019031046NY).
Author information
Authors and Affiliations
Contributions
QS designed and performed most of the experiments and written the manuscript, ZX contributed to flavonoids and anthocyanins content analysis, CB performed PCR and qRT-PCR, CJ constructed the vectors, LS and LH modified the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shijie, Q., Xue, Z., Baiqi, C. et al. Functional characterization of chalcone isomerase gene HvCHI revealing its role in anthocyanin accumulation in Hosta ventricosa. Braz. J. Bot 45, 635–643 (2022). https://doi.org/10.1007/s40415-022-00805-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-022-00805-4